Introduction
Sepsis is the third most common disease in the USA,
causing ~300,000 cases of mortality annually (1). Despite the availability of various
potent antibiotics, the mortality rates of sepsis have failed to
reduce (2), suggesting that a more
comprehensive approach is required in its management. Genome wide
expression studies indicated that pro- and anti-inflammatory
cytokines encoding genes were upregulated during the host immune
response (3), and the disruption
of this balance may lead to hyper-inflammation (4) and immunosuppression (5).
Autophagy is an evolutionary conservative process
critical for cell survival under stress (6). A microarray assay demonstrated that
autophagy related gene lysosomal associated membrane protein 1 was
upregulated in patients with sepsis compared with patients with
non-infectious systemic inflammatory response syndrome (SIRS),
which is defined as the SIRS associated with previously existing
non-infectious diagnosis, while no sign of ongoing infection was
observed (7). Polymorphisms of the
immunity related GTPase M gene, which is autophagy-associated, was
additionally associated with mortality due to sepsis, suggesting a
protective role for autophagy (8).
To investigate this hypothesis, various factors potentially
regulating autophagic activities were studied, including microRNA
(miRNA).
miRNAs are a group of short single-stranded RNA, ~21
nucleotides in length. miRNAs cause the degradation of target mRNA
by selectively binding to its 3′untranslated region (UTR),
fine-tuning gene expression post-transcriptionally (9). Previous studies demonstrated that the
expression profile of microRNAs differs significantly between
patients with sepsis and patients with non-infectious SIRS
(10). A similar alteration was
additionally confirmed in microarray studies focused on macrophages
stimulated by lipopolysaccharide (LPS) (11). The two aforementioned studies
demonstrated miR-23a downregulation in sepsis; however, the
underlying mechanism for the involvement of miR-23a in sepsis
response remains unclear.
In the present study, microRNA expression profiling
data from previous studies were adopted and analyzed. Additionally,
the downregulation of miR-23a during septic insult was confirmed
with in vivo and in vitro experiments. Further study
on the acting mechanism of miR-23a was conducted, aiming to better
understand the molecular mechanism for sepsis response.
Materials and methods
Gene expression omnibus (GEO) data
analysis
To identify a group of microRNAs that shared a
similar expression pattern following LPS stimulation, clustering
analysis on a dataset from the GEO database (12) was conducted. The dataset (accession
no. GSE55414) contains expression profile from a previous study
(11) was adopted in the present
study. This dataset (accession no. GSE55414) evaluated miRNA levels
at different time points following LPS stimulation. The initial
screening of data was performed using Linear Models of Microarray
Analysis 3.3 in R 3.4.2 (13),
with a false discovery rate and minimal log fold change set as 0.05
and 1, respectively. Clustering analysis of the filtered microarray
expression data was conducted with heatmap 1.0.8 (14) utilizing the Euclidean distance
algorithm.
Cell culture
To conduct the in vitro study on macrophages,
the RAW264.7 macrophage cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA) and cultured in
high-glucose Dulbecco's modified Eagle's medium (DMEM, Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA, Grand Island, NY,
USA) supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo
Fisher Scientific, Inc.) at 37°C. The 3rd passage of cells were
used for further experiments.
Cell transfection
Manipulation of the miRNA (miR)-23a expression level
was achieved by oligonucleotide transfection. RAW264.7 cells were
cultured in six-well plates at a concentration of
2×105/well prior to transfection. miR-23a mimics (100
nM; 5′-AUCACAUUGCCAGGGAUUUCC-3′) and miR-23a inhibitor (100 nM;
5′-GUGGUAAUCCCUGGCAAUGUGAU-3′) were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China), and a miRNA negative
control was purchased from Exiqon, Inc. (10 nM, www.qiagen.com/us/shop/; cat. no. 479903; Woburn,
MA, USA). A Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) system was employed for
microRNA transfection following the manufacturer's protocol.
Following incubation at 37°C for 1 day, three groups of cells were
generated, denoted as the control, miR-23a mimics and miR-23a
inhibitor groups, respectively. The expression level of miR-23a was
confirmed in all three groups of cells with a reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), the
detailed protocol was described later in this article.
Immunofluorescence staining
To study the influence of the miR-23a expression
level on autophagy activity, immunofluorescence imaging of
autophagy markers was employed. RAW264.7 macrophages were fixed
with 4% formaldehyde for 15 min at room temperature and incubated
in 5% Tris buffered saline with Tween-20 (TBS-T; pH 8.3) diluted
non-fat dry milk for 1 h. Immunofluorescence staining was performed
using Microtubule-associated protein light chain 3 (LC-3) primary
antibody (1:100; Abcam, Cambridge, UK; cat. no. ab62720) incubation
overnight at 4°C and subsequent secondary antibody (1:200; Alexa
Fluor 488 anti-rabbit IgG; Thermo Fisher Scientific, Inc.; cat. no.
A10235) incubation at room temperature for 1 h. Cells were
incubated using DAPI (100 ng/ml) for nuclear staining at room
temperature for 30 min. Images were obtained from a confocal laser
scanning microscope (LSM710; Zeiss, Oberkochen, Germany) at
magnification, ×600. LC3 puncta were quantified with Image-J 1.51
(National Institutes of Health, Bethesda, MD, USA) (15).
Blood sampling and miRNA
isolation
To further confirm the association between miR-23a
levels and the host response to sepsis in the clinical setting,
blood samples were collected from 27 patients with sepsis and 22
patients with non-infectious SIRS admitted to the SICU department
of The First Affiliated Hospital of Sun Yat-sen University
(Guangzhou, China) during the period of June 2016 to February 2017.
Patients under the age of 18 or for whom it was impossible to
obtain informed consent within 6 h of admission were excluded.
Patients were categorized as sepsis or non-infectious SIRS using
standard criteria (16), and a
blood microbial culture assay result was obtained from all patients
as required by the standard criteria (17). Demographic data and Sequential
Organ Failure Assessment scores are listed in Table I, revealing no statistically
significant difference. miRNA isolation from plasma samples was
conducted using a mirVana kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Informed consent was
obtained from all individual participants included in the present
study. The present study was approved by the Ethics Committee of
The First Affiliated Hospital of Sun Yat-sen University.
 | Table I.Demographic data and SOFA scores of
patients included in the present study. |
Table I.
Demographic data and SOFA scores of
patients included in the present study.
| Group | Age, years | Statistic | P-value | Male/female
ratio | Statistic | P-value | SOFA score | Statistic | P-value |
|---|
| Sepsis, n=27 | 62.3±10.3 | −1.768a | 0.107 | 13:14 | 0.025b | 0.874 | 8.1±2.3 | 1.299a | 0.215 |
| Non-infectious
SIRS, | 64.2±11.5 |
|
| 12:10 |
|
| 7.92±2.6 |
|
|
| n=22 |
ELISA
An ELISA was employed to evaluate the inflammatory
response of macrophages subjected to LPS stimulation. RAW264.7
cells were seeded on 24-well plates at 4×104/well.
Following LPS (10 µg/ml, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) stimulation for 4 h in room temperature, the cell culture
supernatant was collected. Subsequently, inflammatory cytokine
levels of interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α
were measured with ELISA kits (cat. nos. 550950 and 560478; BD
Biosciences, Franklin Lakes, NJ, USA), following the manufacturer's
protocol.
Western blot analysis
Protein expression levels were evaluated with
western blot analysis. Cells were seeded on 6 well plates at a
density of 1×106 per well. Western blotting was
conducted following the protocol reported in a previous study
(18). Total protein extracts were
obtained with a radioimmunoprecipitation assay and transferred to
nitrocellulose membranes. The membranes were incubated with
blocking solution overnight, and primary antibodies were
subsequently applied and incubated at room temperature for 2 h.
Then the membranes were incubated with secondary antibody at room
temperature for 1h. Primary antibodies employed in the present
study included LC3 (1:1,000; cat. no. ab48394; Abcam), Beclin-1
(1:1,000; cat. no. 3495; Cell Signaling Technology, Inc., Danvers,
MA, USA), ATG12 (1:1,000; cat. no. ab155589) and Sequestosome-1
(p62; 1:1,000; cat. no. ab91526; both Abcam), and β-actin (1:1,000;
cat. no. sc-130657; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) was used as a loading control. HRP-conjugated anti-rabbit
(1:5,000; cat. no. ab205718) was used as secondary antibody.
Image-J 1.51 was used for densitometry analysis.
RT-qPCR analysis
RNA expression levels were evaluated with RT-qPCR.
Total RNA was extracted with TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed to cDNA with the QuantiTect RT kit (Qiagen, Inc.,
Valencia, CA, USA). RT-qPCR was performed with a SYBR Mix (Qiagen,
Inc) and the Bio-Rad Real-time PCR System (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Following a protocol used in a previous
study (19), these cycling
parameters were used: initial denaturation at 95°C for 30 sec,
followed by 35 cycles: denaturation at 95°C for 10 sec, annealing
at 55°C for 10 sec and extension at 72°C for 30 sec. The expression
level of mRNAs were first normalized to the GAPDH mRNA level, and
the 2−ΔΔCq method (20)
was used for quantification. The relative expression level was
compared between the three groups of cells. For microRNAs, the U6
level was used as the internal control. AlleleID 6 (Palo Alto, CA,
USA, www.premierbiosoft.com/) was used for
primer design and sequences are provided in Table II.
 | Table II.Primers for reverse
transcription-quantitative polymerase chain reaction analysis with
the SYBR green system. |
Table II.
Primers for reverse
transcription-quantitative polymerase chain reaction analysis with
the SYBR green system.
| mRNA | Sense (5′-3′) | Anti-sense
(5′-3′) |
|---|
| TNF-α |
GTGAGGAGGACGAACATC |
GAGCCAGAAGAGGTTGAG |
| IL-6 |
TGACCCAACCACAAATGC |
TGACCAGAAGAAGGAATGC |
| GAPDH |
TCATCCCTGCCTCTACTG |
TGCTTCACCACCTTCTTG |
Luciferase reporter assays
To confirm the targeting association between miR-23a
and ATG12 mRNA, luciferase reporter assays were conducted. The
PsiCHECK-2 system (Promega Corporation, Madison, WI, USA) was used
for the construction of the dual-luciferase assay plasmid.
Bioinformatics analysis tool miRanda (microrna.org/)
revealed a potential binding sequence for miR-23a in the 3′UTR of
ATG12 mRNA. The predicted target sequence was cloned into the
PsiCHECK-2 plasmid (Promega), generating an ATG12 wild-type (wt)
dual-luciferase reporter plasmid. Subsequently, the mutated target
sequence was generated with site-directed mutagenesis and was
additionally cloned into the PsiCHECK-2 plasmid system, creating an
ATG12 mutant (mut) reporter plasmid. RAW264.7 cells with miR-23a
mimics, miR-23a inhibitor and negative controls were first seeded
on 96-well plates at a concentration of 1×104/well and
transfected with ATG12 wt or ATG12 mut (200 ng/ml) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Luciferase activities were evaluated with the
Modulus™ dual-luciferase reporter assay system (Turner Designs,
Sunnyvale, CA, USA) following 48 h incubation, normalized to
Renilla activity. and all assays were performed in triplicate.
Cell viability assay
A cell viability assay was performed to study the
influence of the miR-23a expression level on cell survival
subsequent to LPS stimulation. All three groups of cells were
seeded on 96-well plates at 5×103 cells per well. A Cell
Counting kit-8 (Engreen Biosystem, Ltd., Auckland, New Zealand) was
used for cell viability evaluation, and an absorbance value at 450
nm was used for the quantification of cell viability.
Statistical analysis
All experiments were repeated three times and data
are expressed as the mean ± standard deviation. Comparisons between
groups were made using one-way analysis of variance, and Fisher's
Least Significant Difference test was used for post hoc analysis.
Baseline data for patients in sepsis or Non-infectious SIRS groups
was compared, independent t-test was used for SOFA score and age
comparison, while χ2 test was used for sex ratio
comparison. P<0.05 was considered to indicate a statistically
significant difference. R studio 1.1.383 (RStudio, Inc., Boston,
MA, USA) was used for statistical analysis.
Results
miRNA expression levels in
LPS-stimulated macrophages
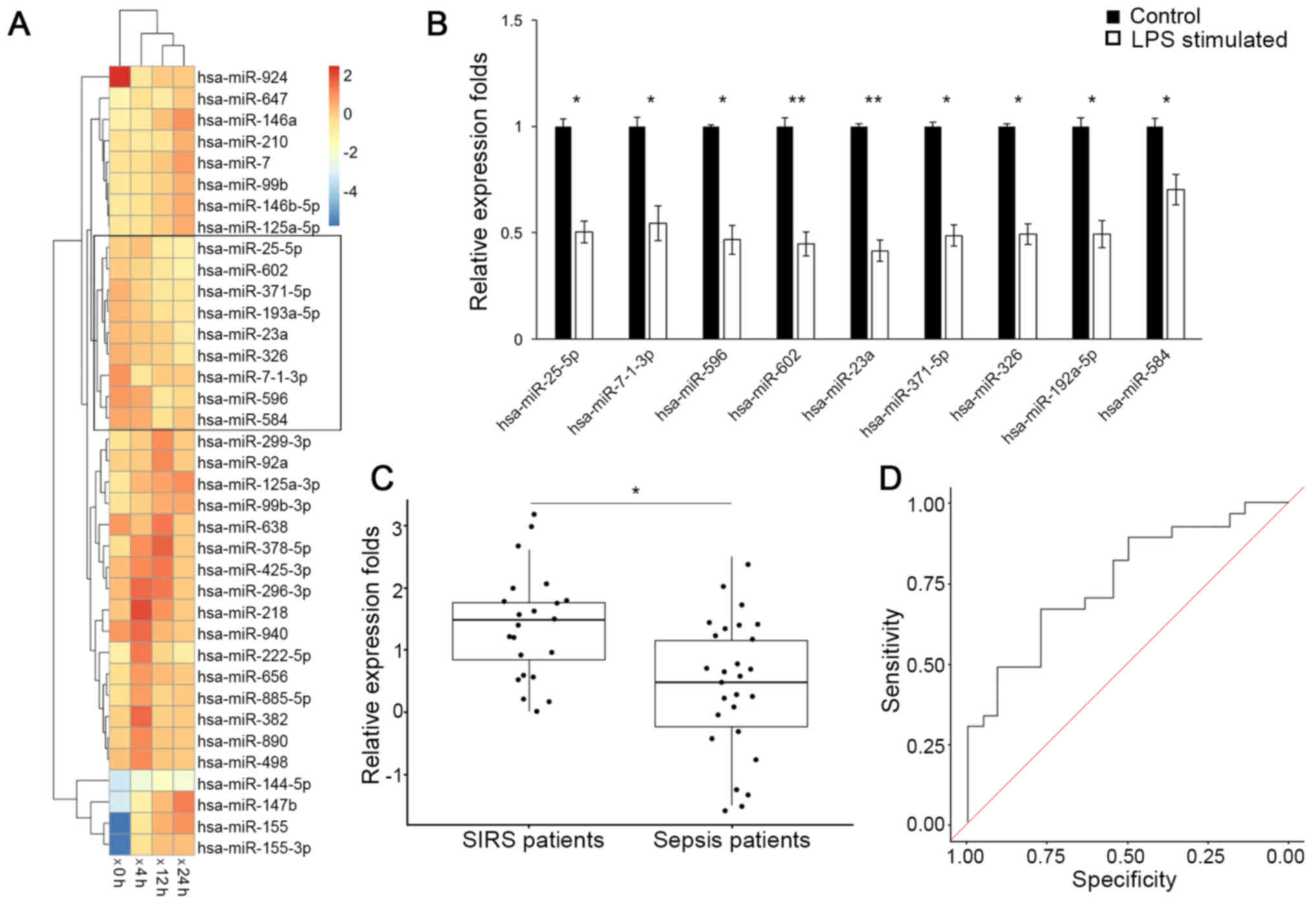
A group of miRNAs that were consistently down
regulated following LPS stimulation were identified (Fig. 1A) via clustering analysis on the
GEO dataset (GSE55414). Expression levels of these miRNAs were
further validated by RT-qPCR analysis in RAW264.7 cells (Fig. 1B), among which the down regulation
of miR-23a was the most significant (P<0.01). Consistently,
RT-qPCR analysis of circulating miRNAs (Fig. 1C) additionally demonstrated that
the miR-23a serum level was decreased in patients with sepsis
(0.524; 95% confidence interval (CI), 0.036–1.012), compared with
patients with non-infectious SIRS (1.557; 95% CI, 1.191–1.923) with
a statistical significance (P<0.05). The receiver operating
characteristic curve analysis on the miR-23a level yielded an area
under the curve value of 0.758 (Fig.
1D), and at the threshold of 1.01 for miR-23a relative
expression level, a sensitivity and specificity of 70.3 and 68.3%,
respectively, was achieved for differentiating between patients
with sepsis and patients with non-infectious SIRS. These results
demonstrated that miR-23a is downregulated in response to sepsis
insult.
Downregulation of miR-23a protects
cells by modulating inflammatory mediators
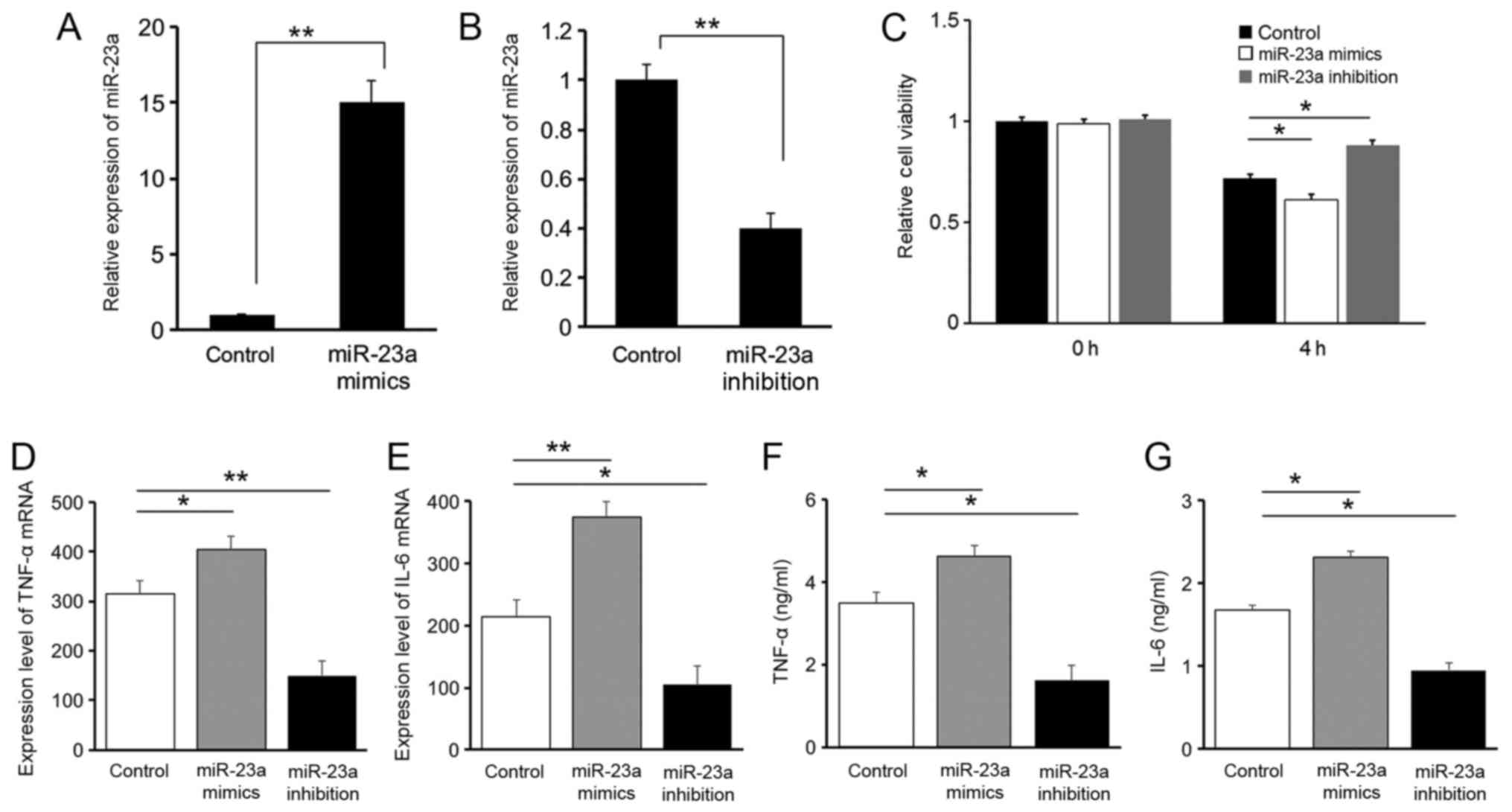
Results of the RT-qPCR analysis on miR-23a
expression levels suggested that oligonucleotide transfection was
successfully conducted in all three groups of RAW264.7 cells
(Fig. 2A and B). The Cell Counting
kit-8 result suggested that 4 h following LPS stimulation, the cell
viability of RAW264.7 cells decreased in all three groups of cells.
Compared with the control group, the cell viability in the miR-23a
mimics group was significantly decreased (P<0.05), while
inhibition of miR-23a demonstrated a protective effect with an
increased cell viability (P<0.05), suggesting that the
downregulation of miR-23a is protective for cells following LPS
stimulation (Fig. 2C).
Expression levels of the pro-inflammatory cytokines
IL-6 and TNF-α were evaluated with RT-qPCR (Fig. 2D and E) and ELISA (Fig. 2F and G). A positive association was
identified between miR-23a and IL-6 or TNF-α levels, suggesting a
pro-inflammatory role for miR-23a. These results indicated that the
protective effect of miR-23a downregulation following LPS
stimulation is associated with the regulation of inflammatory
factors.
Effects of mir-23a on autophagic
activity
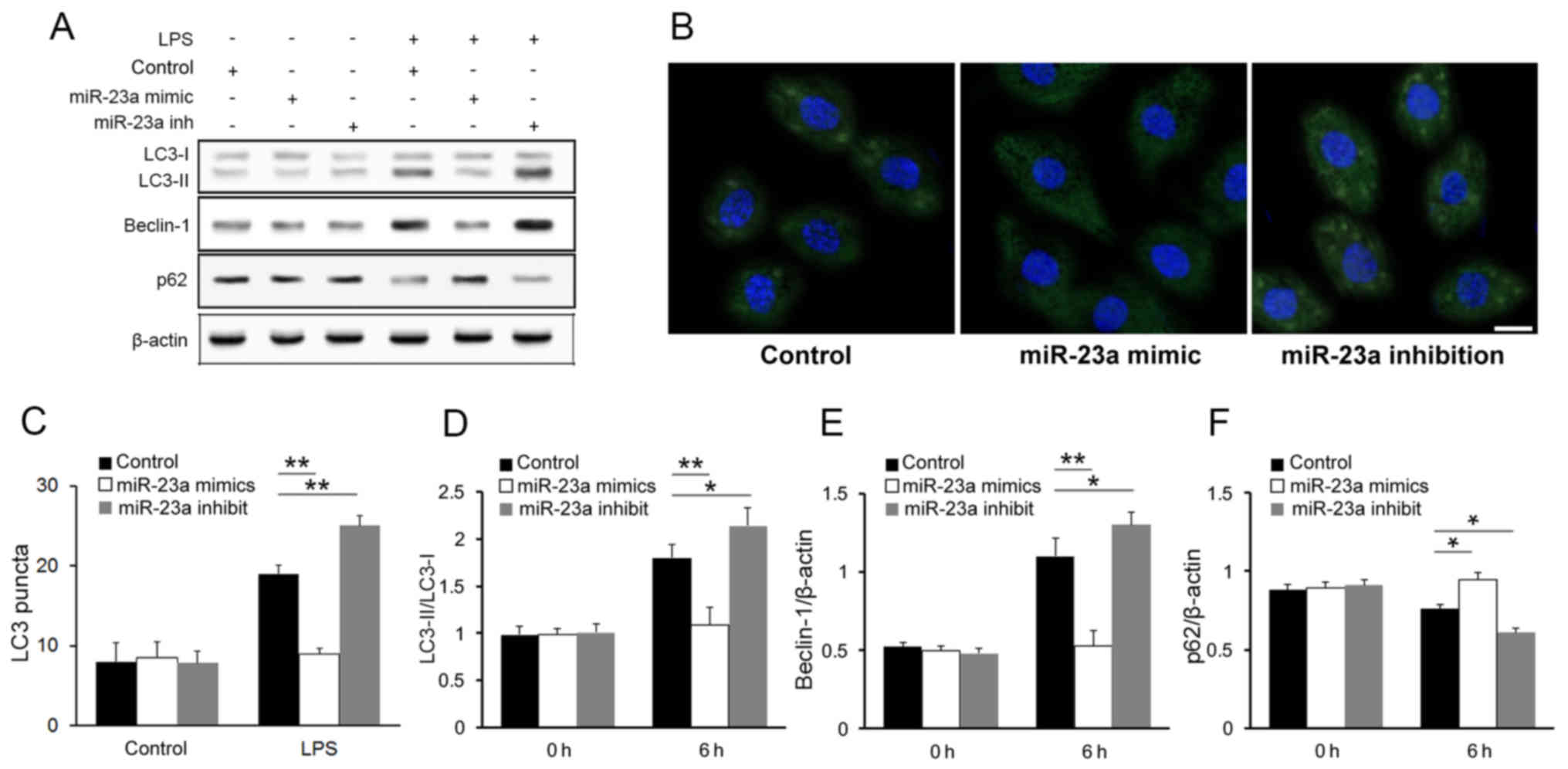
The western blot analysis of the autophagy markers
demonstrated an increase in the LC3 conversion (LC3-II/LC3-I ratio)
(Fig. 3A) in the miR-23a
inhibition group compared with the control group.
Immunofluorescence imaging and LC3 puncta quantification further
confirmed that miR-23a inhibited autophagic activity in macrophages
following LPS stimulation (Fig. 3B and
C). The result of western blot analysis also shown that other
autophagy markers were influenced by miR-23a level after LPS
stimulation (Fig. 3A). Compared
with control group, beclin-1 was upregulated while p62 was
downregulated in miR-23a inhibited cells. Such negative association
between miR-23a level and autophagy markers was further confirmed
with densitometry analysis (Fig.
3D-F; P<0.05), while no significant association was found
between them in macrophages prior to LPS stimulation. These result
is consistent with a previous study (21).
miR-23a targets ATG12 specifically to
modulate autophagic activity
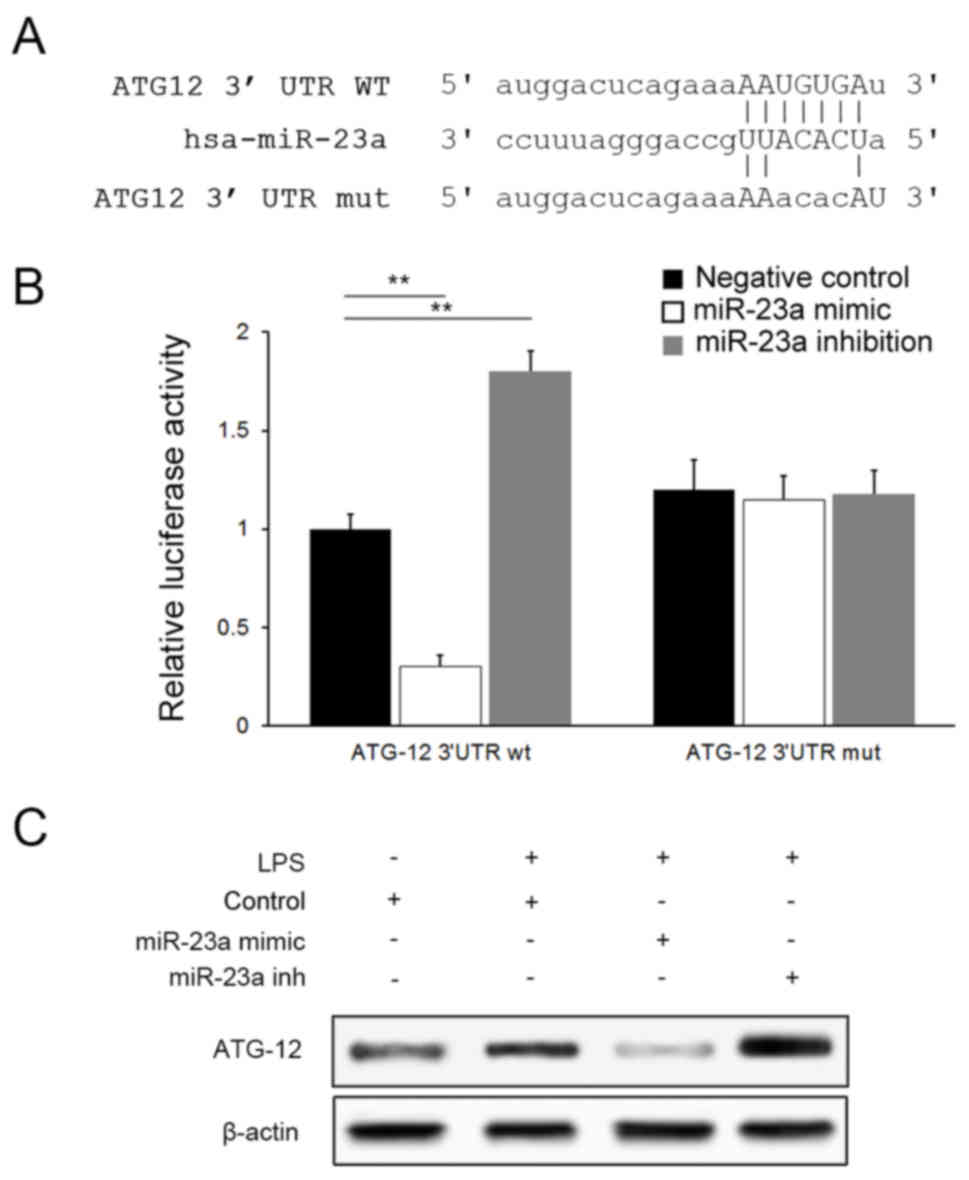
ATG12 serves an integral role in the autophagy
pathway by forming a complex with Autophagy protein 5(ATG5)
(22). Bioinformatics analysis
tools miRanda (microrna.org/) suggested that miR-23a
may bind to ATG12 selectively via a specific sequence in the 3′UTR
of ATG12 mRNA (Fig. 4A). A
dual-luciferase assay was conducted for confirmation and a negative
association was identified between luciferase activities and
miR-23a levels in cells transfected with the ATG12-wt plasmid;
however, no significant difference was observed between groups when
ATG12-mut plasmids were applied (P>0.05), suggesting that
miR-23a targets ATG12 specifically (Fig. 4B). Downregulation of ATG12
expression by miR-23a was further confirmed with western blot
analysis (Fig. 4C).
Discussion
Sepsis is a principal challenge in the management of
critically ill patients (1). The
incomplete understanding of mechanisms underlying organ damage and
immune defense during septic insult hinder the development of more
comprehensive management (23).
Accumulating evidence suggests that autophagic activity is
increased during the initial phase of sepsis (24,25)
and LPS, a key bacterial product, may initiate autophagy through
toll-like receptor 2 and toll-like receptor 4 activation (26,27).
Two studies indicated a protective role for such a response
(24,28); however, the detailed underlying
mechanism requires further investigation.
miRNA are a group of small non-coding RNAs that act
as post-transcriptional regulators in various processes. The
results of the present study demonstrated that multiple microRNAs
inhibiting autophagy (29–32) were downregulated subsequent to LPS
stimulation. Among these, the downregulation of miR-23a was the
most significant. The in vivo study additionally
demonstrated that the miR-23a serum level in patients with sepsis
differed distinctly compared with patients with non-infectious
SIRS, a result consistent with previous studies (33,34).
These results collectively demonstrate that miR-23a is involved in
the host response to sepsis.
The present results from the western blot analysis
and immunofluorescence studies demonstrated for the first time, to
the best of the authors' knowledge, that miR-23a is negatively
associated with autophagic activities following septic insult.
Downregulation of miR-23a mitigates the inhibition of autophagy,
leading to the suppression of inflammatory mediators, a role
proposed in a previous study for autophagy in sepsis response
(35).
The present bioinformatics study and dual luciferase
analysis indicated that miR-23a suppressed autophagy via ATG12. The
targeting association was demonstrated with the dual-luciferase
study, and a negative association was revealed between the miR-23a
and ATG12 expression levels. The inhibitory effect that miR-23a
exerts on ATG12 was further demonstrated by the LC3-II/LC3-I ratio
and immunofluorescence imaging in the present study, since ATG12,
along with autophagy protein 5 and autophagy-related protein 16-1
comprise the E3 ubiquitin ligase, which facilitates LC3 family
conversion from LC3-I to LC3-II (22).
In conclusion, the present study demonstrated that
miR-23a was downregulated during initial septic insult. It was
demonstrated for the first time, to the best of the authors'
knowledge, that miR-23a downregulation promoted the autophagic
activity of macrophages in response to LPS stimulation, and this
consequently suppressed inflammatory mediators, preventing an
overwhelming inflammatory response. Finally, it was demonstrated
that miR-23a may selectively bind to the 3′UTR of ATG12 mRNA,
modulating the formation of the E3 ligase and the subsequent
facilitation of LC3 conversion.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Fundamental Research Funds for the Central Universities (grant no.
15ykpy14) and Sun Yat-sen University Clinical Research 5010 Program
(grant no. 2007015).
Availability of data and materials
The data and materials used and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
XS and DC carried out the statistical analysis and
drafted this article. JC and YN were responsible for blood sampling
and data analysis. ZJ, M-YC and J-FW carried out western blot and
RT-qPCR analyses. X-DG conceived the idea and provided
guidance.
Ethics approval and consent to
participate
Informed consent was obtained from all individual
participants included in the present study. The present study was
approved by the Ethics Committee of The First Affiliated Hospital
of Sun Yat-sen University [approval no. (2016)025].
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
GEO
|
gene expression omnibus
|
|
IL
|
interleukin
|
|
LPS
|
lipopolysaccharide
|
|
miRNA/miR
|
microRNA
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SIRS
|
systemic inflammatory response
syndrome
|
|
TNF
|
tumor necrosis factor
|
|
UTR
|
untranslated region
|
References
|
1
|
Vincent JL, Marshall JC, Namendys-Silva
SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli
M, Pickkers P, Njimi H, et al: Assessment of the worldwide burden
of critical illness: the Intensive Care Over Nations (ICON) audit.
Lancet Respir Med. 2:380–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Russell JA: Management of sepsis. N Engl J
Med. 355:1699–1713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao W, Mindrinos MN, Seok J, Cuschieri J,
Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al:
A genomic storm in critically injured humans. J Exp Med.
208:2581–2590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yadav H and Cartinceba R: Balance between
hyperinflammation and immunosuppression in sepsis. Semin Respir
Crit Care Med. 37:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis CG, Chang K, Osborne D, Walton AH,
Dunne WM and Muenzer JT: Increased susceptibility to Candida
infection following cecal ligation and puncture. Biochem Biophys
Res Commun. 414:37–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi H, Zhang Z, Wang X, Li R, Hou W, Bi W
and Zhang X: Inhibition of autophagy induces IL-1β release from
ARPE-19 cells via ROS mediated NLRP3 inflammasome activation under
high glucose stress. Biochem Biophys Res Commun. 463:1071–1076.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McHugh L, Seldon TA, Brandon RA, Kirk JT,
Rapisarda A, Sutherland AJ, Presneill JJ, Venter DJ, Lipman J,
Thomas MR, et al: A molecular host response assay to discriminate
between sepsis and infection-negative systemic inflammation in
critically ill patients: discovery and validation in independent
cohorts. PLoS Med. 12:e10019162015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kimura T, Watanabe E, Sakamoto T, Takasu
O, Ikeda T, Ikeda K, Kotani J, Kitamura N, Sadahiro T, Tateishi Y,
et al: Autophagy-related IRGM polymorphism is associated with
mortality of patients with severe sepsis. PLoS One. 9:e915222014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JF, Yu ML, Yu G, Bian JJ, Deng XM,
Wan XJ and Zhu KM: Serum miR-146a and miR-223 as potential new
biomarkers for sepsis. Biochem Biophys Res Commun. 394:184–188.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caserta S, Kern F, Cohen J, Drage S,
Newbury SF and Llewelyn MJ: Circulating plasma microRNAs can
differentiate human sepsis and systemic inflammatory response
syndrome (SIRS). Sci Rep. 6:280062016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie N, Cui H, Banerjee S, Tan Z, Salomao
R, Fu M, Abraham E, Thannickal VJ and Liu G: miR-27a regulates
inflammatory response of macrophages by targeting IL-10. J Immunol.
193:327–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:D991–D995. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kolde R: pheatmap: Pretty Heatmaps.
2015.
|
|
15
|
Schindelin J, Argandacarreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G:
SCCM/ESICM/ACCP/ATS/SIS: 2001 SCCM/ESICM/ACCP/ATS/SIS international
sepsis definitions conference. Crit Care Med. 31:1250–1256. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Llewelyn MJ, Berger M, Gregory M, Ramaiah
R, Taylor AL, Curdt I, Lajaunias F, Graf R, Blincko SJ, Drage S and
Cohen J: Sepsis biomarkers in unselected patients on admission to
intensive or high-dependency care. Crit care. 17:R602013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu S, Liu W and Lei Z: MiR-590-3p
regulates osteogenic differentiation of human mesenchymal stem
cells by regulating APC gene. Biochem Biophys Res Commun.
478:1582–1587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiang L, Chen M, He L, Cai B, Du Y, Zhang
X, Zhou C, Wang C, Mao JJ and Ling J: Wnt5a regulates dental
follicle stem/progenitor cells of the periodontium. Stem Cell Res
Ther. 5:1352014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oh JE and Lee HK: Pattern recognition
receptors and autophagy. Front Immunol. 5:3002014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chinatsu O, Zoltan M, Giichi T and
Takanori O: Structure of the human ATG12~ATG5 conjugate required
for LC3 lipidation in autophagy. Nat Struct Mol Biol. 20:59–66.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marik PE: Early management of severe
sepsis: Concepts and controversies. Chest. 145:1407–1418. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CW, Lo S, Perng DS, Wu DB, Lee PH,
Chang YF, Kuo PL, Yu ML, Yuan SS and Hsieh YC: Complete activation
of autophagic process attenuates liver injury and improves survival
in septic mice. Shock. 41:241–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi W, Watanabe E, Fujimura L,
Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S and
Hatano M: Kinetics and protective role of autophagy in a mouse
cecal ligation and puncture-induced sepsis. Crit Care. 17:R1602013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yunanto A, Endharti AT and Widodo A:
Neutrophil, TLR2, and TLR4 expression in newborns at risk of
sepsis. Paediatrica Indonesiana. 53:132–137. 2013. View Article : Google Scholar
|
|
27
|
Wang J, Feng X, Zeng Y, Fan J, Wu J, Li Z,
Liu X, Huang R, Huang F, Yu X and Yang X: Lipopolysaccharide
(LPS)-induced autophagy is involved in the restriction of
Escherichia coli in peritoneal mesothelial cells. Bmc Microbiol.
13:2552013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin CW, Lo S, Hsu C, Hsieh CH, Chang YF,
Hou BS, Kao YH, Lin CC, Yu ML, Yuan SS and Hsieh YC: T-Cell
autophagy deficiency increases mortality and suppresses immune
responses after sepsis. PLoS One. 9:e1020662014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie
T, Zhang J, Peng C, Lin Y and Chen J: MicroRNA-25 regulates
chemoresistance-associated autophagy in breast cancer cells, a
process modulated by the natural autophagy inducer
isoliquiritigenin. Oncotarget. 5:7013–7026. 2014.PubMed/NCBI
|
|
30
|
Yang YX and Li L: Identification of
potential biomarkers of sepsis using bioinformatics analysis. Exp
Ther Med. 13:1689–1696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou W, Wang J, Li Z, Li J and Sang M:
MicroRNA-205-5b inhibits HMGB1 expression in LPS-induced sepsis.
Int J Mol Med. 38:312–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo W, Wang H, Yang Y, Guo S, Zhang W, Liu
Y, Yi X, Ma J, Zhao T, Liu L, et al: Down-regulated miR-23a
contributes to the metastasis of cutaneous melanoma by promoting
autophagy. Theranostics. 7:2231–2249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ge QM, Huang CM, Zhu XY, Bian F and Pan
SM: Differentially expressed miRNAs in sepsis-induced acute kidney
injury target oxidative stress and mitochondrial dysfunction
pathways. PLoS One. 12:e01732922017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu S, Liu C, Wang Z, Huang J and Zeng Q:
microRNA-23a-5p acts as a potential biomarker for sepsis-induced
acute respiratory distress syndrome in early stage. Cell Mol Biol
(Noisy-le-grand). 62:31–37. 2016.PubMed/NCBI
|
|
35
|
Giegerich AK, Kuchler L, Sha LK, Knape T,
Heide H, Wittig I, Behrends C, Brüne B and von Knethen A:
Autophagy-dependent PELI3 degradation inhibits proinflammatory IL1B
expression. Autophagy. 10:1937–1352. 2014. View Article : Google Scholar : PubMed/NCBI
|