Introduction
Keloid is a type of abnormal scar tissue, which is
caused by excessive proliferation of fibroblasts, massive
deposition of collagen and extracellular matrix, and deposition of
core protein (1). However, keloid
is difficult to completely cure, therefore it is necessary to find
a more efficient strategy prevent and treat keloid.
The current study demonstrated that ~2/3 of the
human protein encoding genome could be regulated by microRNAs
(miRNAs). It has been reported that in keloid, miRNAs affect the
proliferation, metastasis and differentiation of fibroblasts, and
may also adjust the deposition of the extracellular matrix, which
may affect the development of keloid (2).
The mothers against decapentaplegic homolog (Smad)3
mediated signaling pathway is closely associated with wound healing
and scar formation (3). Previous
studies have shown that Smad3 is highly expressed in pathological
scar tissues, which induces the transition of fibroblasts to
myofibroblasts (4). Inhibiting the
expression of Smad3 can inhibit the formation of keloid (5).
In the current research, we aimed to investigate the
differential expression of miRNAs in the keloid epidermis compared
with that in the normal skin epidermis. For the selected,
significantly lower expressed miR-637, the effects on the
proliferation and metastasis of human keloid fibroblast cells were
evaluated. The examinations were carried out to confirm the roles
of miR-637 in the regulation of proliferation and metastasis of
human keloid fibroblast cells. The involvement of miR-637 in the
regulation of Smad3 signaling pathway in keloid fibroblast cells
was additionally investigated.
Materials and methods
Tissue samples and cell lines
We enrolled keloid patients at the General Hospital
of Shenyang Military Region (Shenyang, China) between January 2012
and January 2015. We obtained 30 paired samples of keloid and the
excess normal tissue from the partial skin flap (control). All
patients (14 female and 16 male, average age 31.25±7.39) had not
received topical and systemic therapy for at least 2 months prior
to undergoing a skin biopsy. We also retained three normal skin
tissues from trauma patients as normal controls (normal). Written
consent was gathered from all participants before the study was
performed. The protocols were approved by the Ethics Committee of
General Hospital of Shenyang Military Command.
Human keloid fibroblasts (HKF) were purchased from
Bioleaf Corporation (Shanghai, China), and human embryonic skin
fibroblasts obtained from Beijing Union Medical College cell bank.
Cells were cultured in Dulbecco's modified Eagle's medium
supplemented with 10% FBS (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), at 37°C in an environment containing 5%
CO2.
RNA isolation
Tissues were stored in liquid nitrogen. Total RNA
was extracted from tissues and cells using mirVana®
miRNA isolation kit (Ambion; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following the manufacturer's instructions. The
quantity and quality of the extracted RNA were measured with a
spectrophotometer (Thermo Fisher Scientific, Inc.). Finally, the
sample was stored at −80°C.
MicroRNA microarray analysis
Agilent's Human miRNA Microarray Release 18.0
(Shanghai Biochip Co., Ltd., Shanghai, China) was used to detect
the RNA from 3 keloids and 3 adjacent tissues. The slides were
scanned by an Agilent Microarray Scanner with Feature Extraction
Software 10.7, and raw data were normalized by Quantile algorithm,
Gene Spring Software 11.0 (all by Agilent Technologies, Inc., Santa
Clara, CA, USA). Differentially expressed microRNAs were identified
by significance analysis of microarrays (SAM, http://www-stat.stanford.edu/tibs/SAM/index.html).
Quantitative polymerase chain reaction
(qPCR)
qPCR was conducted with qPCR Universal Reagent and
the MX3000P qPCR instrument following the protocols. U6 small
nuclear RNA was used as an internal control. The expression of
miR-637 was detected using Stem-Loop RT-PCR assay, as previously
described (6,7). Primer sequences were synthesized as
follows (Table I). All the
reactions were carried out as described previously (8).
 | Table I.Primers of RNAs. |
Table I.
Primers of RNAs.
| Name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-637 |
ACACTCACTGGGGGCTTT |
GCAGAGCCCGTTGAGAGTACA |
| miR-487 |
ACACTCAATCATACAGGGA |
AACTGGATGTTGAGAGTACAT |
| miR-154 |
ACACTCAATCATACACGGT |
AATAGGTCTTGAGAGTACAT |
| miR-582 |
ACACTCTAACTGGTTGAAC |
GGTTCAGTTTTGAGAGTACAT |
| miR-194 |
ACACTCCCAGTGGGGCTG |
CAGATAACAGTTGAGAGTACAT |
| miR-21 |
ACACTCCAACACCAGTCG |
ACAGCCCATTTGAGAGTACAT |
| miR-503 |
CACTCGGGGTATTGTTTCC |
CCTGGCAGCTTGAGAGTACAT |
| miR-550 |
ACACTCAGTGCCTGAGGGA |
CTCTTACTTGAGAGTACAT |
| U6 |
CTCGCTTCGGCAGCACA |
ACGCTTCACGAATTTGCGT |
| Smad3 |
GGGCTTTGAGGCTGTCTAC |
GTCCACGCTGGCATCTTCTG |
| Cyclin D1 |
CCAACCTCCTCAACGACC |
TGGCACAGAGGGCAACGAAG |
| MMP2 |
GATCTTGACCAGAATACCAT |
GGCTTGCGAGGGAAGAAGTT |
| GAPDH |
CATCCCTTCTCCCCACAC |
GTCCCAGGGCTTTGATTTG |
Western blot analyses
Cells and tissues were lysed and the protein was
extracted using radioimmunoprecipitation assay buffer. Samples were
resolved by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride PVDF membranes. Target proteins were probed with
specific antibodies. Relative expression of relevant proteins were
quantified and normalized to GAPDH. Antibodies used for western
blotting were: anti-Smad3 (sc-101154, 1:500), anti-Cyclin D1
(sc-70899, 1:300), anti-matrix metallopeptidase (MMP)2 (sc-13594,
1:300), anti-GAPDH (sc-51631, 1:1,000) and horseradish
peroxidase-conjugated anti-mouse (sc-2005, 1:5,000) secondary
antibodies (Santa Cruz, USA).
Dual luciferase reporter assay
Dual luciferase activity assays were performed as
previously described (9). The
Smad3 3′-untranslated region (UTR) was PCR amplified and cloned
into the pMIR-REPORT™ vector (Ambion; Thermo Fisher Scientific,
Inc.). The primers were: Smad3-WT, F: 5′-AGGGCTTTGAGGCTGTCTACC-3′,
R: 5′-GTCCACGCTGGCATCTTCTG-3′, Smad3-mut (the site of miR-637
binding was mutated), F: 5′-AAACCAGGCGGCTAAACAAGTG-3′, R:
5′-GCAACAGCAGCAGTGAAGGTG-3′. HKF cells were seeded and
co-transfected with the above constructs and miR-637 mimic
(Guangzhou Ribobio Co., Ltd, Guangzhou, China), miR-637 antisense
(AS) (Guangzhou Ribobio Co., Ltd) or control (Guangzhou Ribobio
Co., Ltd). Luciferase activity was determined with the dual
luciferase reporter assay system after 36 h transfection; the
luciferase activity was measured using the Dual Luciferase Reporter
Assay System (Promega Corporation, Madison, WI, USA).
MTT assays
Cell proliferation activity was detected using an
MTT assay (Solarbio, China). Cells were seeded at a density of
1×103 cells per well of 96-well plates, and transfected
with miR-637 mimic, miR-637 inhibitor (Guangzhou Ribobio Co., Ltd)
or negative control; then the cell proliferation was evaluated by
MTT assay. A microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to measure the optical densities at a
wavelength of 490 nm.
Transwell assay
A total of 12 h following transfection with miR-637
mimic, miR-637 inhibitor or negative control, 1×105
cells in serum-free media were seeded in transwell chambers with or
without Matrigel coating, and then transwell assays were conducted.
After 8 h cells were fixed by 4% paraformaldehyde, and stained with
0.4% trypan blue. Numbers of cells in different groups were
determined using Image-Pro Plus 6.0 software (Nikon Corporation,
Tokyo, Japan).
Construction of stable
siRNA-expressing cell lines
To stably silence Smad3, cells were transfected with
the pRS-si-Smad3 plasmid (Shanghai GeneChem Company) and were
selected with G418 (400 µg/ml). After 3 weeks, stable cells were
selected, cultured and amplified.
Statistical analysis
All data (showed as mean ± standard deviation) were
analyzed with SPSS software, version 17.0 (SPSS Inc., Chicago, IL,
USA). Statistical significance between two groups of data was
evaluated by Student's t-test (two-tailed) and one-way analysis of
variance following Fisher's Least Significant Difference post hoc
test. Statistical significance was defined as P<0.05. All
experiments were repeated three times.
Results
Differential expression profile of
miRNAs in keloid tissues
The miRNA microarray revealed 15 downregulated
(P<0.05) and 3 upregulated miRNAs (P<0.05) in keloid tissues
compared with adjacent tissues (Table
II). There were 8 miRNAs tat exhibited a fold change >x30.
Among these miRNAs, we found that the change in miR-637 was the
greatest. The expression levels of these miRNAs in keloid and
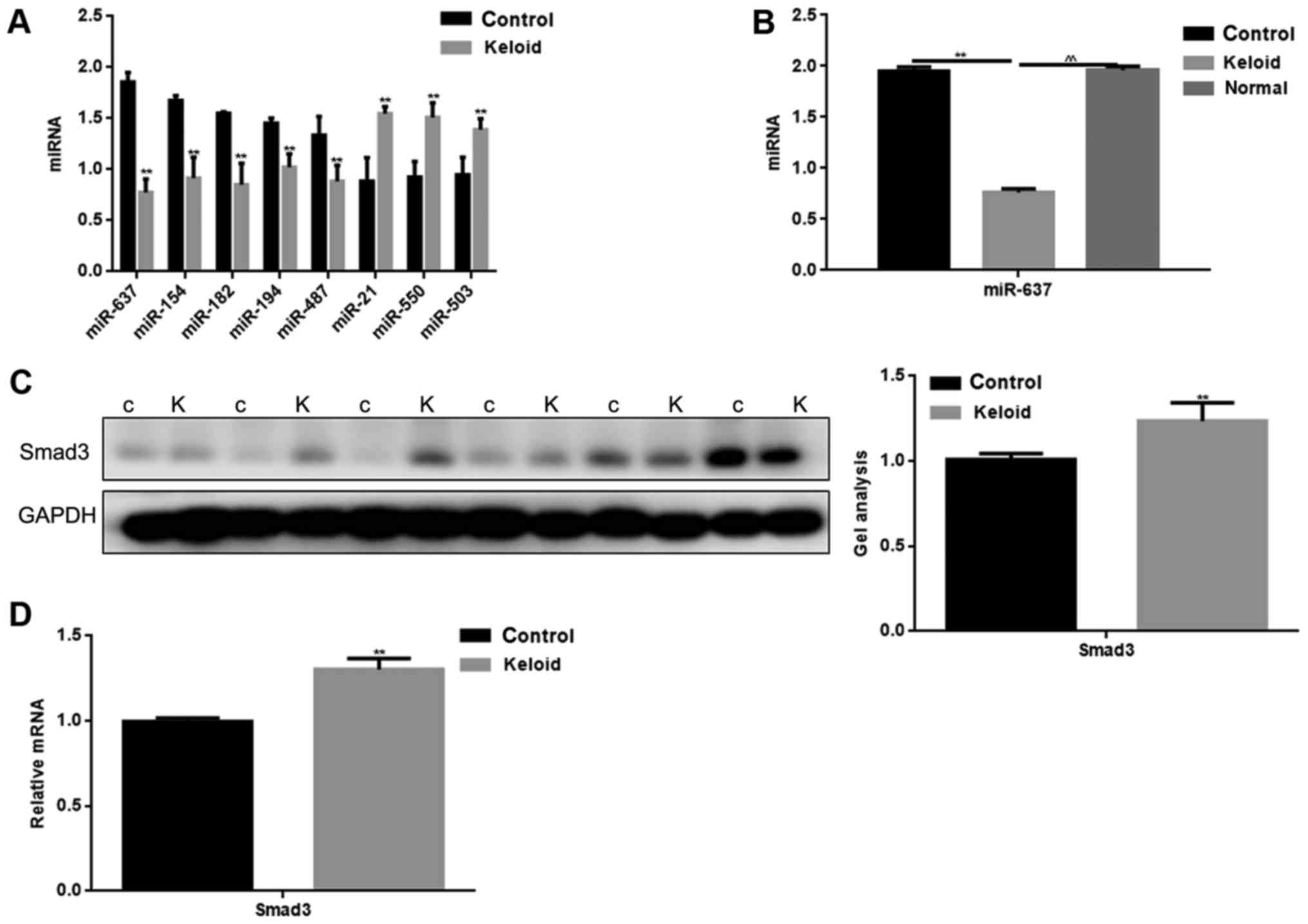
adjacent tissues were detected by qPCR (Fig. 1A). The expression of miR-637 was
verified to be significantly lower in keloid tissues. We compared
the expression of miR-637 in keloid, normal tissues from keloid
patients and normal skin tissues from trauma patients (Fig. 1B). It was found that miR-637
expression was low in keloid. It can be seen that miR-637 may play
an important role in keloid.
 | Table II.Differential miRNAs in keloids. |
Table II.
Differential miRNAs in keloids.
| miRNA | P-values | Fold change
(keloid/adjacent tissues) | Trend |
|---|
| Hsa-miR-34a | 0.001 | 27.265 | Down |
| Hsa-miR-499 | 0.031 | 2.905 | Down |
| Hsa-miR-582 | 7.69×10-05 | 54.608 | Down |
| Hsa-miR-154 | 9.53×10-06 | 65.257 | Down |
| Hsa-miR-126 | 0.023 | 4.167 | Down |
| Hsa-miR-194 | 1.04×10-04 | 50.137 | Down |
| Hsa-miR-101 | 0.011 | 6.072 | Down |
| Hsa-miR-98 | 0.001 | 29.150 | Down |
| Hsa-miR-940 | 0.001 | 29.152 | Down |
| Hsa-miR-200c | 0.009 | 7.689 | Down |
| Hsa-miR-101 | 0.006 | 9.871 | Down |
| Hsa-miR-646 | 0.003 | 13.509 | Down |
| Hsa-miR-106 | 0.004 | 11.097 | Down |
| Hsa-miR-487 | 2.76×10-05 | 31.825 | Down |
| Hsa-miR-637 | 4.05×10-06 | 69.303 | Down |
| Hsa-miR-21 | 6.61×10-06 | 67.042 | Up |
| Hsa-miR-503 | 0.001 | 39.842 | Up |
| Hsa-miR-550 | 9.75×10-06 | 54.187 | Up |
The expression of Smad3 in keloid
tissues
By using miRDB software, it was demonstrated that
miR-637 targeted multiple proteins. Smad3 is not only an important
regulatory protein in keloid, however also contains a binding site
to miR-637. The expression of Smad3 in keloid and adjacent tissues
was detected by western blotting and qPCR (Fig. 1C and D). The adjacent tissues were
used as a control. Results showed that there was a higher
expression of Smad3 in keloid tissues, compared with adjacent
tissues (P<0.05).
Relationship between miR-637 and
Smad3
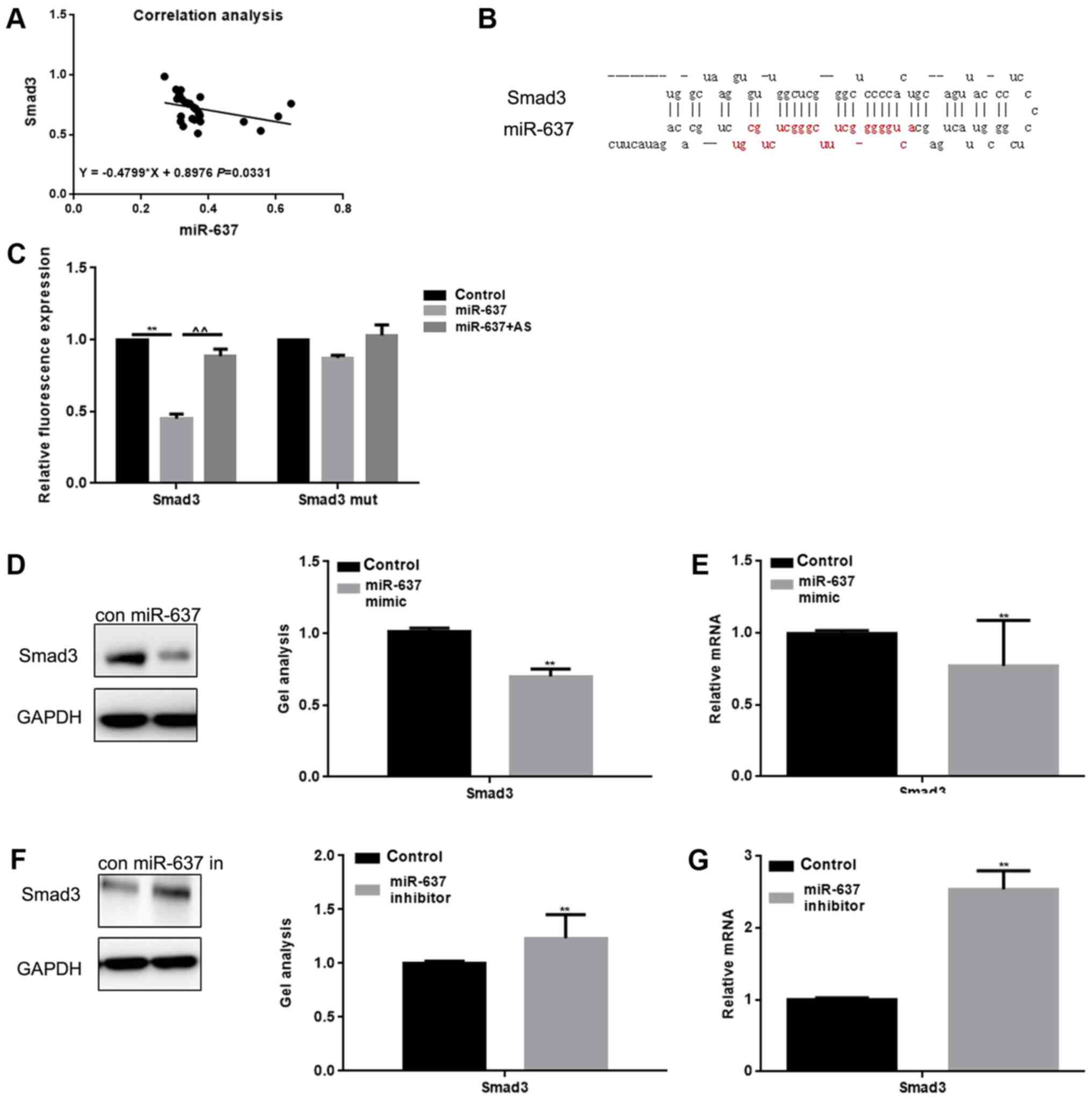
We analyzed the correlation between the expression
levels of miR-637 and Smad3 in keloid, and found that the
expression in keloid was negatively correlated (Fig. 2A). Through use of the miRDB tool,
we found that miR-637 was able to bind with the 3′-UTR of Smad3
(Fig. 2B). The Luciferase reporter
assay showed that miR-637 reduced the expression of Smad3 at the
transcriptional level (Fig. 2C).
Results showed that when cells were co-transfected with Smad3 and
miR-637, a decreased luciferase activity was observed (P<0.05),
however, the cells co-transfected with Smad3 mut or miR-637 AS
(Antisense) did not result in a significant variation. miR-637
mimic or miR-637 inhibitor were transfected into HKF cells, and
then western blot and qPCR were conducted in order to detect the
expression of Smad3 (Fig. 2D-G).
Results showed that the expression of Smad3 could be downregulated
by miR-637 (P<0.05).
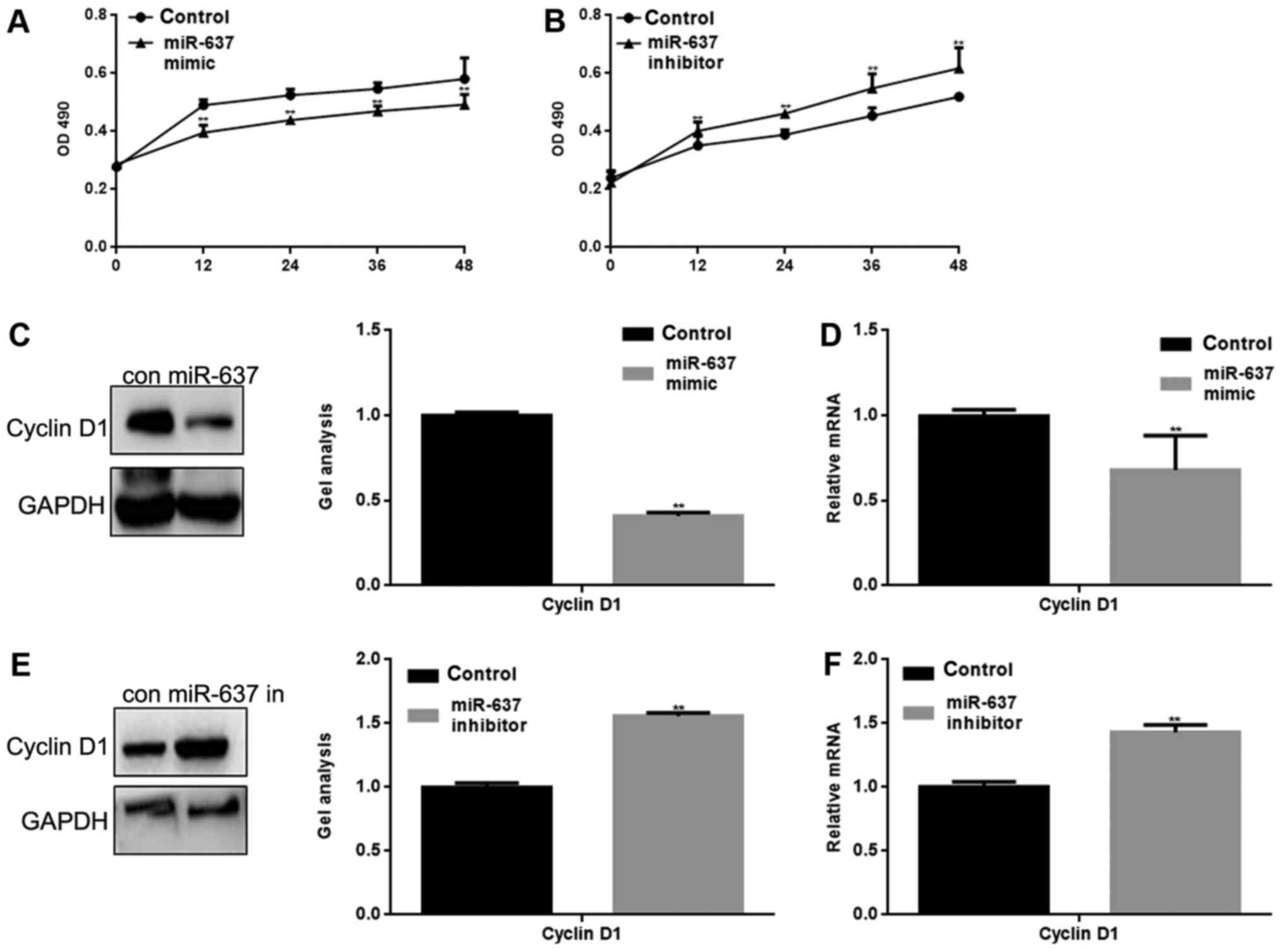
miR-637 inhibits the proliferation of
HKF cells by suppressing Cyclin D1
miR-637 mimic or inhibitor was transfected into HKF
cells. The proliferation of HKF cells was measured by MTT assay.
The result showed that miR-637 mimic significantly inhibited HKF
cell proliferation and conversely, miR-637 inhibitor significantly
promoted the proliferation of HKF cells (P<0.05) (Fig. 3A and B). Cyclin D1 is an important
protein in the regulation of the cell cycle, and is one of the
proteins involved in the Smad3 signaling pathway (10). We suspect that the inhibitory
effect on HKF cell proliferation may be mediated by regulation of
Cyclin D1. Western blotting and qPCR were used to detect the effect
of miR-637 on Cyclin D1. The results showed that miR-637
significantly inhibited the expression of Cyclin D1 (P<0.05)
(Fig. 3C-F).
miR-637 inhibits the metastasis of HKF
cells by suppressing MMP2
Smad3 can affect the metastasis of cells in many
ways, for example MMP2 is one of the downstream proteins of the
Smad3 pathway (11). Transwell
assays (with or without matrigel) were used to study whether
miR-637 is involved in metastasis of HKF cells (Fig. 4A-D). The present study demonstrated
that the migration and invasion of HKF cells was significantly
inhibited by miR-637 (P<0.05). In addition, western blotting and
qPCR showed that MMP2 was significantly downregulated by miR-637
(P<0.05) (Fig. 4E-H).
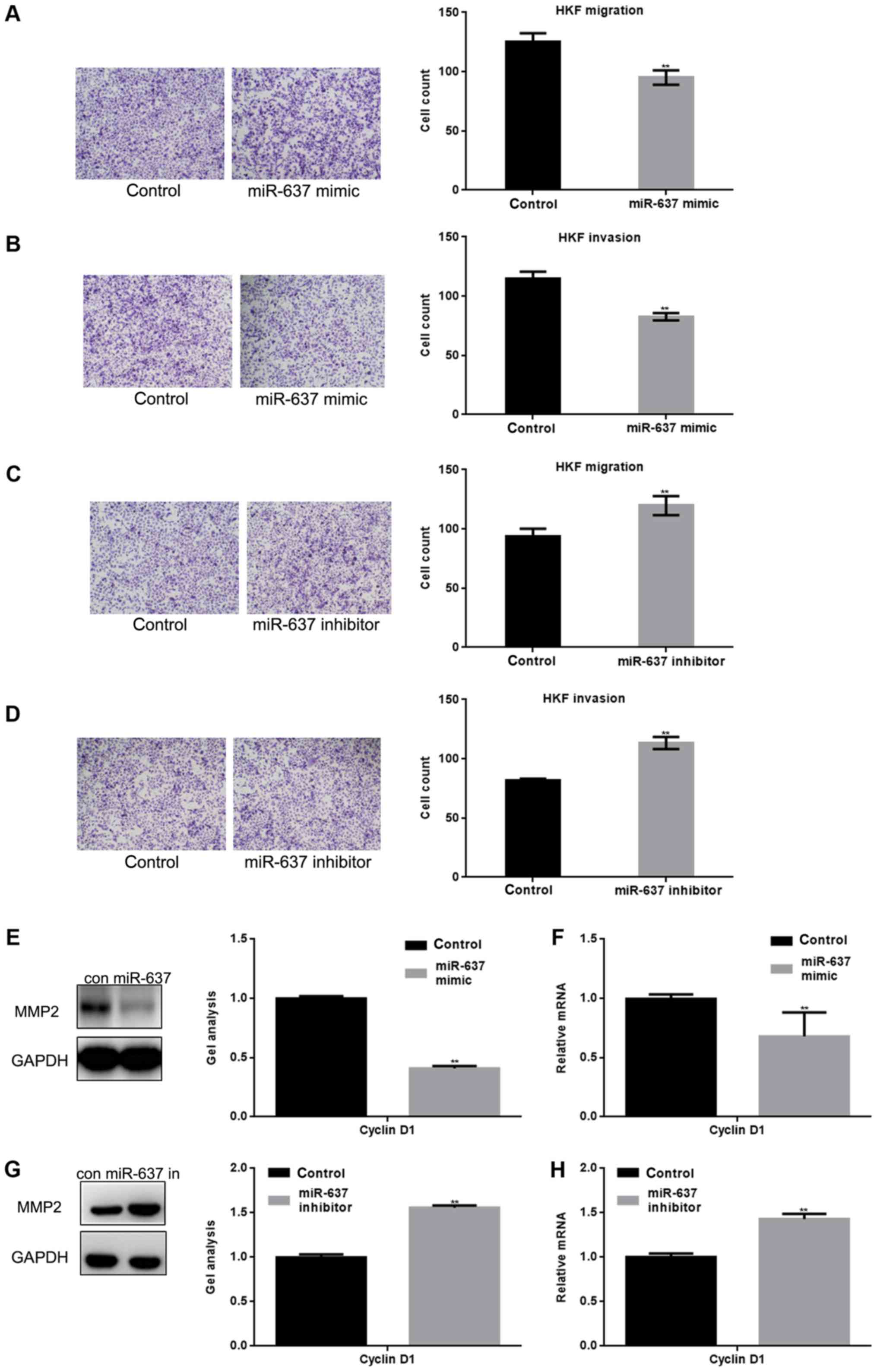 | Figure 4.miR-637 inhibits the metastasis of HKF
cells by suppressing MMP2. (A and B) After overexpression of
miR-637 in HKF cells, transwell assays with or without matrigel
were performed. Cells were counted and results represent the mean ±
standard deviation of three experiments (magnification, ×200).
**P<0.01 vs. control. (C and D) After downregulation of miR-637
in HKF cells, transwell assays with or without matrigel were
performed. Cells were counted and results represent the mean ± SD
of three experiments. **P<0.01 vs. control. (E and F) After
transfection with miR-637 mimic in HKF cells, the expression of
MMP2 was detected by western blotting and qPCR. Data are presented
as mean ± SEM. **P<0.01 vs. control. (G and H) After
downregulation of miR-637, the expression of MMP2 was detected by
western blotting and qPCR. Data are presented as mean ± SEM.
**P<0.01 vs. control. miRNA, microRNA; qPCR, quantitative
polymerase chain reaction; SEM, standard error of the mean; SD,
standard deviation; HKF, human keloid fibroblast; MMP, matrix
metallopeptidase. |
Smad3 is crucial to cell proliferation
and metastasis of HKF cells
Smad3 small interfering (si)RNA significantly
suppressed cell proliferation and metastasis ability of cells
(P<0.05) (Fig. 5A and B). To
explore the potential signaling pathways involved in the actions of
miR-637 and Smad3 in HKF cells, Cyclin D1 and MMP2 expression
levels were detected. Co-treatment with Smad3 siRNA and miR-637
inhibitor in cells significantly downregulated the level of Smad3
to a greater extent compared with miR-637 inhibitor alone
(P<0.05), and the same trend was observed in the expression
levels of Cyclin D1 and MMP2 (P<0.05) (Fig. 5C and D). The expression of tumor
growth factor-β1 was downregulated by miR-637 inhibitor
(P<0.05), however was not affected by Smad3 siRNA (Fig. 5C and D). These results suggested
that miR-637 may regulate the proliferation and metastasis of HKF
cells via Smad3 signaling pathway.
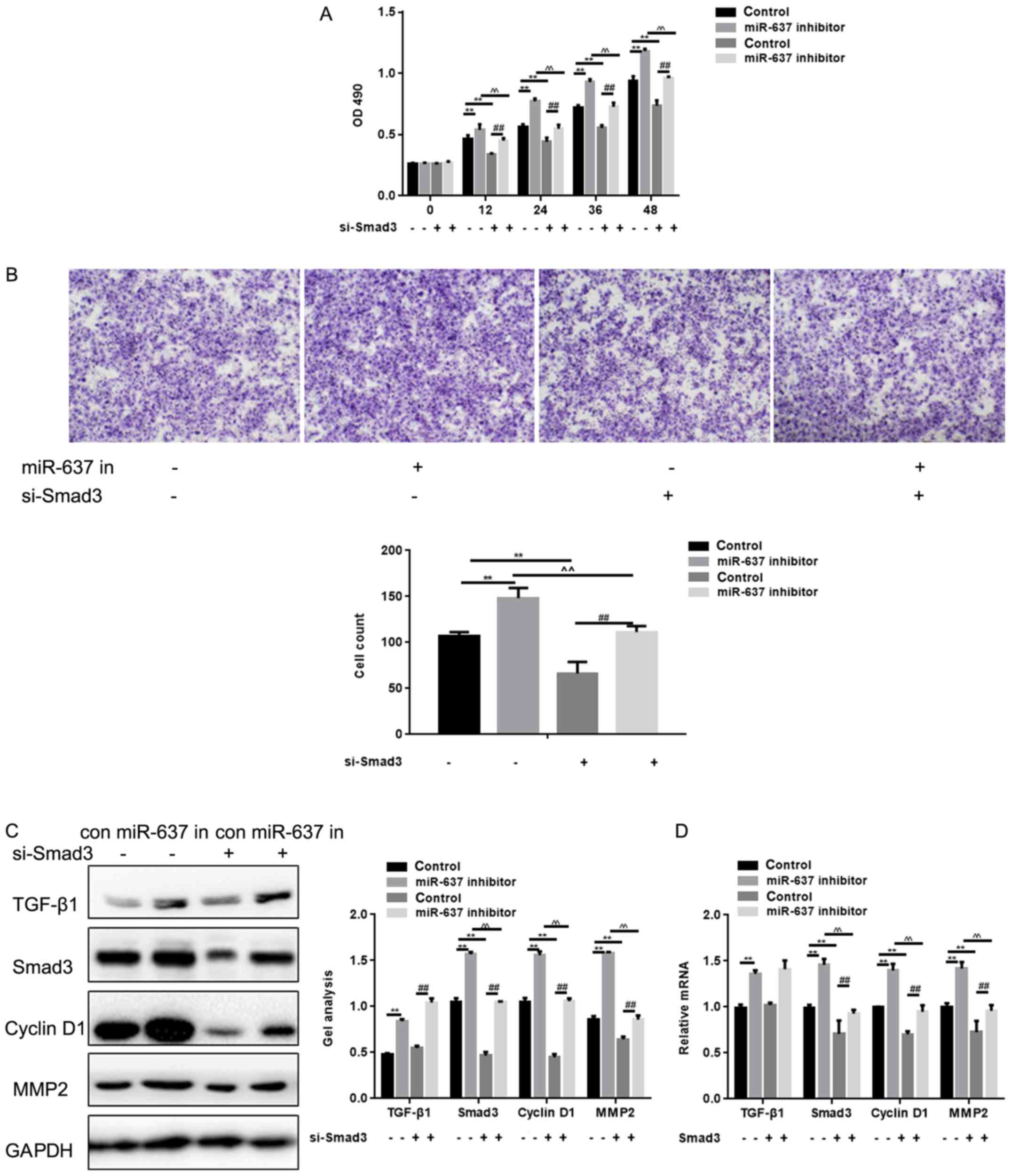 | Figure 5.Smad3 is crucial to cell proliferation
and metastasis of HKF cells. (A) Effects of miR-637 siRNA and Smad3
siRNA on cell proliferation ability using MTT. Data are presented
as mean ± SEM. **P<0.01, ^^P<0.01 ##P<0.01 as
indicated. (B) Effects of miR-637 siRNA and Smad3 siRNA on cell
proliferation ability using transwell assay. Cells were counted and
results represent the mean ± SD of three experiments
(magnification, ×200). **P<0.01, ^^P<0.01
##P<0.01 as indicated. (C and D) Effects of miR-637
siRNA and Smad3 siRNA on Smad3, Cyclin D1 and MMP2 were detected by
western blotting and qPCR. Data are shown as mean ± SEM. HKF, human
keloid fibroblast; MMP, Matrix Metallopeptidase; qPCR, quantitative
polymerase chain reaction; Smad, mothers against decapentaplegic
homolog; SEM, standard error of the mean. **P<0.01, ^^P<0.01
##P<0.01 as indicated. |
Discussion
A variety of miRNAs may be involved in the
proliferation and migration of fibroblasts, and many miRNAs have
been shown to regulate the formation of keloids (12). It has previously been demonstrated
that p63 is affected by a high expression of miR-205 in the skin,
and miR-205 influences cell differentiation of skin stem cells, and
regulates the proliferation and apoptosis of fibroblasts (13). miR-222 stimulates the growth factor
signaling pathway to promote cell cycle progression, thereby
regulating the formation of keloids (14). It has also been revealed that
miR-29b regulates collagen I in skin fibroblast cells, thereby
affecting the formation of keloid (15). In a mouse model, abnormal
expression of miR-31 and miR-21-5p has been revealed to be involved
in the formation of keloid (16).
The migration of fibroblasts may be affected by the regulation of
miR-21 on MMPs, and this regulation is crucial in the formation of
keloids (13,14,16,17).
These findings demonstrated that miRNAs exhibit a key role in the
healing of skin wounds, and predicted that they may play an
important role in scar formation and skin remodeling. Researchers
have reported that miR-637 inhibits the metastasis and growth of
glioma cells by targeting the AKT pathway (18). miR-637 has also been shown to
inhibit the formation of inflammation by regulating C-Reactive
Protein (19–21). However, the effect of miR-637 on
keloid has not been studied yet.
Overexpression of Smad3 in keloid significantly
upregulates pro-collagen gene expression, promotes deposition of
extracellular matrix, proliferation and metastasis of fibroblasts
(3,5,22).
Therefore, Smad3 is one of the potential targets for keloid
treatment (23).
Microarray analysis was used to screen abnormal
expression of miRNAs in keloid. The results showed that miR-637,
miR-487, miR-154, miR-582 and miR-194 were significantly
downregulated in keloid, and the expression levels of miR-21,
miR-503 and miR-550 were upregulated. Furthermore, we found that
these miRNAs may play an important role in keloid. miR-637 was
selected as the primary factor for investigation in the present
study. It was proved that miR-637 directly affected the activity of
Smad3 through the targeting of Smad3.
A keloid scar is an overgrowth of dense fibrous
tissue that develops around a wound. These scars are raised scars
that spread beyond the margins of the original wound to normal skin
by invasion (24). The
downregulation of miR-637 may be responsible for the enhanced
capacity of keloid to proliferate and metastasise (25). miR-637 may affect the proliferation
and migration of cells by inhibiting the expression of Cyclin D1
and MMP2. These two proteins can be regulated by Smad3 (26,27),
leading to the hypothesis that regulation of miR-637 on
proliferation and migration of fibroblasts is partly mediated by
regulation of Smad3. The present study additionally demonstrated
that miR-637 can influence the expression of TGF-β1 to some extent,
as TGF-β1 has been proved to play an important role in keloid
(28), and can regulate Smad3
activity (18,22,28).
miR-637 has been shown to inhibit AKT pathway activity (18), and the inhibitory effect of miR-637
on Smad3 may be through its effects on TGF- β1 and targeting Smad3.
This experiment has some limitations, and further experimentation
is required in vivo to verify the results.
The results of the present study suggested that
miR-637 participated in the regulation of keloid development by
inhibition of Smad3, and may therefore act as a future potential
target for keloid treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ conceived of the present study and performed the
molecular studies. BG also performed the molecular studies. QH
participated in the design of the study and performed the
statistical analysis. WL participated in its design and
coordination of the study and helped to draft the manuscript. PC
assisted in the conception of the study. KT contributed to analysis
and interpretation of data.
Ethics approval and consent to
participate
Written consent was gathered from all participants
before the study was performed. The protocols were approved by the
Ethics Committee of General Hospital of Shenyang Military Command
(R201208).
Consent for publication
Patients have provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yao X, Cui X, Wu X, Xu P, Zhu W, Chen X
and Zhao T: Tumor suppressive role of miR-1224-5p in keloid
proliferation, apoptosis and invasion via the TGF-β1/Smad3
signaling pathway. Biochem Biophys Res Commun. 495:713–720. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Yang D, Xiao Z and Zhang M: miRNA
expression profiles in keloid tissue and corresponding normal skin
tissue. Aesthetic Plast Surg. 36:193–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin SE, Kim CK and Kim YB: Cellular
delivery of cationic lipid nanoparticle-based SMAD3 antisense
oligonucleotides for the inhibition of collagen production in
keloid fibroblasts. Eur J Pharm Biopharm. 82:19–26. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao B, Guan H, Liu JQ, Zheng Z, Zhou Q,
Zhang J, Su LL and Hu DH: Hypoxia drives the transition of human
dermal fibroblasts to a myofibroblast-like phenotype via the
TGF-β1/Smad3 pathway. Int J Mol Med. 39:153–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phan TT, Lim IJ, Aalami O, Lorget F, Khoo
A, Tan EK, Mukhopadhyay A and Longaker MT: Smad3 signalling plays
an important role in keloid pathogenesis via epithelial-mesenchymal
interactions. J Pathol. 207:232–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng J, Wang K, Liu X, Chen S and Chen J:
The quantification of tomato microRNAs response to viral infection
by stem-loop real-time RT-PCR. Gene. 437:14–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zelivianski S, Cooley A, Kall R and Jeruss
JS: Cyclin-dependent kinase 4-mediated phosphorylation inhibits
Smad3 activity in cyclin D-overexpressing breast cancer cells. Mol
Cancer Res. 8:1375–1387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Clutter S, Benincosa J, Fortney J
and Gibson LF: Activation of transforming growth
factor-beta1/p38/Smad3 signaling in stromal cells requires reactive
oxygen species-mediated MMP-2 activity during bone marrow damage.
Stem Cells. 23:1122–1134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Xu D, Li N, Li Y, He Y, Hu X, Lyu
L and He L: Downregulation of microRNA-31 inhibits proliferation
and induces apoptosis by targeting HIF1AN in human keloid.
Oncotarget. 8:74623–74634. 2017.PubMed/NCBI
|
|
13
|
An G, Liang S, Sheng C, Liu Y and Yao W:
Upregulation of microRNA-205 suppresses vascular endothelial growth
factor expression-mediated PI3K/Akt signaling transduction in human
keloid fibroblasts. Exp Biol Med (Maywood). 242:275–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Bai Y, Liu H, Zuo X, Yao H, Xu Y and
Cao M: Comparative study of microRNA profiling in keloid fibroblast
and annotation of differential expressed microRNAs. Acta Biochim
Biophys Sin (Shanghai). 45:692–699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang GY, Wu LC, Liao T, Chen GC, Chen YH,
Zhao YX, Chen SY, Wang AY, Lin K, Lin DM, et al: A novel regulatory
function for miR-29a in keloid fibrogenesis. Clin Exp Dermatol.
41:341–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Wang X, Yang D, Xiao Z and Chen X:
MicroRNA-21 affects proliferation and apoptosis by regulating
expression of PTEN in human keloid fibroblasts. Plast Reconstr
Surg. 134:561e–573e. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu ZY, Lu L, Liang J, Guo XR, Zhang PH and
Luo SJ: Keloid microRNA expression analysis and the influence of
miR-199a-5p on the proliferation of keloid fibroblasts. Genet Mol
Res. 13:2727–2738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Que T, Song Y, Liu Z, Zheng S, Long H, Li
Z, Liu Y, Wang G, Liu Y, Zhou J, et al: Decreased miRNA-637 is an
unfavorable prognosis marker and promotes glioma cell growth,
migration and invasion via direct targeting Akt1. Oncogene.
34:4952–4963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi W, Li D, Guo Y, Zhang Y, Huang B and Li
X: Sevoflurane inhibits the migration and invasion of glioma cells
by upregulating microRNA-637. Int J Mol Med. 38:1857–1863. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim Y, Hooten Noren N, Dluzen DF,
Martindale JL, Gorospe M and Evans MK: Posttranscriptional
regulation of the inflammatory marker C-reactive protein by the
RNA-binding protein HuR and MicroRNA 637. Mol Cell Biol.
35:4212–4221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang JF, He ML, Fu WM, Wang H, Chen LZ,
Zhu X, Chen Y, Xie D, Lai P, Chen G, et al: Primate-specific
microRNA-637 inhibits tumorigenesis in hepatocellular carcinoma by
disrupting signal transducer and activator of transcription 3
signaling. Hepatology. 54:2137–2148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang CJ, Yen YH, Hung LY, Wang SH, Pu CM,
Chien HF, Tsai JS, Lee CW, Yen FL and Chen YL: Thalidomide inhibits
fibronectin production in TGF-β1-treated normal and keloid
fibroblasts via inhibition of the p38/Smad3 pathway. Biochem
Pharmacol. 85:1594–1602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Gao Z, Shi Y, Sun Y, Lin Z, Jiang
H, Hou T, Wang Q, Yuan X, Zhu X, et al: Inhibition of Smad3
expression decreases collagen synthesis in keloid disease
fibroblasts. J Plast Reconstr Aesthet Surg. 60:1193–1199. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma X, Chen J, Xu B, Long X, Qin H, Zhao RC
and Wang X: Keloid-derived keratinocytes acquire a fibroblast-like
appearance and an enhanced invasive capacity in a hypoxic
microenvironment in vitro. Int J Mol Med. 35:1246–1256. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiao H, Dong P, Yan L, Yang Z, Lv X, Li Q,
Zong X, Fan J, Fu X, Liu X and Xiao R: TGF-β1 induces
polypyrimidine tract-binding protein to alter fibroblasts
proliferation and fibronectin deposition in keloid. Sci Rep.
6:380332016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia W, Lo CM, Poon RYC, Cheung TT, Chan
ACY, Chen L, Yang S, Tsao GSW and Wang XQ: Smad inhibitor induces
CSC differentiation for effective chemosensitization in cyclin D1-
and TGF-β/Smad-regulated liver cancer stem cell-like cells.
Oncotarget. 8:38811–38824. 2017.PubMed/NCBI
|
|
27
|
Xu C, Lu G, Li Q, Zhang J, Huang Z and Gao
X: Selenium modulates MMP2 expression through the TGFβ1/Smad
signalling pathway in human umbilical vein endothelial cells and
rabbits following lipid disturbance. J Trace Elem Med Biol.
42:59–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao X, Cui X, Wu X, Xu P, Zhu W, Chen X
and Zhao T: Tumor suppressive role of miR-1224-5p in keloid
proliferation, apoptosis and invasion via the TGF-β1/Smad3
signaling pathway. Biochem Biophys Res Commun. 495:713–720. 2018.
View Article : Google Scholar : PubMed/NCBI
|