Introduction
Defense reactions are triggered when the body
suffers damage or pathogen infection and is characterized by
redness, swelling, pain, fever and functional disorders, and this
defensive reaction is known as inflammation (1). When inflammation occurs, the first
type of leukocyte to be recruited to the infection site is the
neutrophil. Neutrophils, as the first line of defense in the body
against external stimuli, have a critical role in the early
inflammatory reaction (2,3). The recruitment and function of
neutrophils at inflammatory sites requires cell migration (4). The migratory process of neutrophils
in inflammatory site requires β2 integrin, a specific type of
integrin family exclusively expressed on leukocyte membranes, and
the natural ligand is fibrinogen (5). When neutrophils are activated,
clustering of β2 integrin occurs and this further promoted the
binding of β2 integrin with ligand to regulate neutrophil migration
(6).
The lipid raft is a micro-area in the cell membrane,
which becomes a large area through diffusion or recruitment under
certain stimuli (7). The presence
of several types of proteins on the lipid raft structure is the
basis of the recruitment of the lipid raft. Among these proteins,
adducin is one which is associated with the cellular cortical
skeleton (8,9). The cellular cortical skeleton and
cellular skeletal network are closely associated with each other,
suggesting that the cytoskeletal network has an important role in
the function of the lipid raft structure (10,11).
Adducin is a type of lipid raft-associated protein that is thought
to regulate the assembly of the cell membrane and cell skeletal
network through directly connecting with the cellular cortical
skeleton and cellular skeleton network (12–15).
The adducin family has three members, α-adducin, β-adducin and
γ-adducin (16,17). Our previous study demonstrated that
β-adducin has an important role in the process of leukocyte rolling
and β-adducin as a membrane skeleton protein, acts as a link
between the cell membrane and cytoskeleton system. β-adducin can
transfer from the lipid raft to the cytoplasm, leading to the cell
membrane becoming temporarily disconnected from the cytoskeleton
(18).
During the process of neutrophil migration, the
formation of filamentous and lamellar pseudopodia requires the cell
membrane to become disconnected from the cytoskeleton to promote
cell movement (2). Therefore, the
cytoskeleton and lipid raft-associated protein β-adducin may have a
role in the process of neutrophil migration. In the present study,
the function of β-adducin in neutrophil migration was investigated
using neutrophils and neutrophil-like differentiated HL-60 cells
(dHL-60 cells). The results from immunofluorescence, Transwell
assay and the Living cell Imaging System demonstrated that lipid
rafts have crucial role in neutrophil migration. There is a
translocation of β-adducin prior to and following
N-formylmethionyl-leucyl-phenyl-alanine (fMLP) treatment, and when
β-adducin was knocked-down in dHL-60 cells, the ability of
neutrophil to migrate was reduced. Notably, the phosphorylation of
β-adducin was required for the relocation. The results of the
present study are the first to demonstrate, to the best of our
knowledge, that the lipid raft-associated protein β-adducin
participates in the regulation of neutrophil migration.
Materials and methods
Reagents and antibodies
Methyl-β-cyclodextrin (MβCD), soluble cholesterol,
mouse anti-human actin monoclonal antibody (AC-40),
anti-phosphotyrosine monoclonal antibody (PY20; P4110), inhibitor
of Src-family tyrosine kinase (PP2), non-conjugated F (ab')2
fragment of goat anti-mouse IgG (M0659) and anti-tubulin monoclonal
antibody (T4026) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Anti-human β-adducin antibody (SC-376063) and
antibody to flotillin-2 (SC-28320) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). AlexaFluor-488-conjugated
cholera toxin (CTxB) was obtained from Molecular Probes (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Iscove's modified
Dulbecco's medium was from Gibco (Thermo Fisher Scientific, Inc.).
The β-adducin truncated mutant plasmid was produced in our
laboratory.
Neutrophil isolation
Peripheral blood from randomly selected healthy
adult volunteers (age was 25–40 years old, 2 male and 3 female) was
drawn into heparinized syringes (10 U/ml). The healthy blood
samples were obtained from the Jilin/Changchun Blood Center between
January 2014 and December 2017. The collection of samples took
place according to the principles of the Declaration of Helsinki.
When donors provided blood, they signed informed consent with the
Blood Center and the authors also signed an agreement with Blood
Center for use of the blood. Peripheral blood (5 ml) and 6% Dextran
T-500 balance fluid (1.5 ml) was gently mixed and immobilized at
4°C for 1 h, then the white blood cells were separated to the top
layer and the red blood cells in the bottom layer. The top layer of
white cells and lymphocyte separation medium were mixed in a new
centrifuge tube at 1:1 ratio. Then the mixture was centrifuged at
500 × g at 4°C for 20 min and then the supernatant was aspirated
and discarded. The sediment was suspended with red blood cell lysis
buffer (NH4Cl 8.29 g/l, EDTA
Na2.2H2O 37.2 mg/l and potassium bicarbonate
1.0 g/l) and the suspension was immobilized at 4°C for 10 min, then
centrifuged at 200 × g at 4°C for 5 min. Cell viability was
evaluated using trypan blue exclusion test. The cells and 0.4%
trypan blue were mixed in equal proportions, and the mixture
allowed to incubate at room temperature for 3 min. A drop of the
trypan blue/cell mixture was added to a hemocytometer. The
hemocytometer was placed on the stage of a binocular microscope and
the unstained (viable) and stained (nonviable) cells counted. The
morphological alterations were visually assessed using
Giemsa-Wright's staining (19,20).
The collected cells were resuspended in 1 ml methanolic-acetic acid
buffer and fixed for 5 min. A drop of the cell suspension was
placed on a low-temperature pre-cooled slide glass, excess liquid
removed and the slide allowed to dry naturally. The slides were
immersed in a freshly prepared Giemsa-Wright's stain solution: 0.5
g Giemsa stain and 1 g Wright stain were added to 33 ml glycerin
and then 500 ml methanol was added once the glycerin was fully
dissolved. The slides were immersed for 15–20 min, then rinsed with
tap water and allowed to dry. The slide was examined using a bright
field microscope (Eclipse80i; Nikon Corporation, Tokyo, Japan).
Differentiation of HL-60
HL-60 cells were purchased from the cell bank of the
type culture collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were placed into a cell culture bottle
(density: 5×105/ml) and 1.3% DMSO was added, then the
mixture was incubated at 37°C in CO2 incubator for 96 h.
Finally, the artificially differentiated HL-60 (dHL-60) cells were
obtained (21).
RNA interference
For short hairpin RNA (shRNA) preparation, annealed
ds-shRNA oligonucleotides 5′GCA AGA TCA GCA GTG TCT A3′ and control
shRNA oligonucleotides 5′GCT CTA GTA CAG AAT CGCT3′ were cloned
into the HpaI and XhoI cloning sites of the
lentiviral pLL3.7 vector (11795; Addgene, Inc., Cambridge, MA,
USA). A total of 4 µg β-adducin shRNAs was transfected into
5×106 293T cells (Cell Bank, Shanghai Institutes for
Biological Sciences, Chinese Academy of Sciences, Shanghai, China),
together with packaged mix (sPAX2 and pMD2; at a ratio of 4:3:1),
to generate the respective lentiviruses. Viral concentrates were
prepared and were used to infect dHL-60 cells, according to the
manufacturer's protocol (VPK-091; Cell Biolabs, Inc., San Diego,
CA, USA). The extent of suppression and specificity for β-adducin
were evaluated by western blotting with anti-β-adducin antibody and
an anti-actin antibody was used as a control.
Transwell assay
The fMLP treated cells (100 µl IMDM containing
1×106 cells) were placed in 5-µm pore polyester membrane
Transwell inserts (Corning Incorporated, Corning, NY, USA) in
24-well plate. IMDM, with 2.5% FCS containing 100 nm fMLP as a
chemoattractant, was added to the lower wells. Then the plate was
placed in the CO2 incubator at 37°C for 90 min. The
inserts were removed from the plate and the cells in each well were
counted using a hemocytometer under bright field microscopy.
(Eclipse80i; Nikon Corporation) The ratio of cells that penetrated
into the well to the number of total cells added in the insert was
used to determine the migratory ability of cells.
Cell migration assay
Fibrinogen (10 µg/ml) was spread evenly over the
central part of a coverslip and immobilized at 4°C overnight. The
neutrophils from human peripheral blood were suspended in migration
buffer [1 mM Tris, 0.14 M NaCl, 1 mM Hepes (pH 7.2), 5.4 mM KCl,
1.1 mM CaCl2, 0.4 mM MgSO4, gelatin (1 mg/ml)
and 5 mg/ml bovine serum albumin (BSA; Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China). Then, 1×106
dHL-60 cells were suspended in 1 ml migration buffer and added to
22×40 mm coverslips at 37°C for 10 min. The coverslips were washed
with PBS and the cell-covered side of the coverslip was put on the
top of a Zigmond chamber. The chamber has two grooves and the
migration buffer was added to one groove, then the migration buffer
with 100 nM fMLP was added to the other groove. The Zigmond chamber
was put in the Live Cell Imaging System, which comes with its own
tracking command (Ultra VIEW VoX, PerkinElmer, Inc., Waltham, MA,
USA) and the temperature was set to 37°C. Images were captured
using a Nikon microscope (Eclipse 80i; Nikon Corporation) under
bright field with a CCD camera for 4 min at 60 sec intervals. The
migratory behavior of the neutrophils was recorded by the Live Cell
Imaging System using the tracking command and analyzed by with
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA.
Double-labeled immunofluorescence
fMLP (100 nM) was dropped on one side of a coverslip
covered with neutrophils (1×106), which were then
allowed to migrate for a certain time. Then 4% paraformaldehyde was
added to the coverslip to fix the cells at room temperature for 10
min. The cells were washed with PBS three times, then 0.2%
TritonX-100 was dropped onto the coverslip and the cells were
incubated at room temperature for 2 min. The cells were treated
with the primary antibody of β-adducin (1:100 dilution) at room
temperature for 1 h. Then the cells were treated with the secondary
antibody TRITC-conjugated goat anti-mouse secondary antibody
(ZF-0313; OriGene Technologies, Beijing, China) at room temperature
for 1 h. To stain monosialotetrahexosylganglioside (GM1) (lipid
raft maker), the cells were treated with Alexa Fluor 488 conjugated
CTx B subunit (2 µg/ml) at 4°C for 30 min. The treated cells were
observed under a laser scanning confocal microscope (FluoView
FV1000; Olympus Optical, Tokyo, Japan). The photos were analyzed
with Photoshop-CS5 (Adobe Systems, Inc., San Jose, CA, USA).
Immunoprecipitation and
immunoblotting
The neutrophils (1×107 per sample)
collected from the peripheral blood of healthy donors were washed
twice with PBS. The collection of samples took place according to
the principles of the Declaration of Helsinki. The cells were
suspended with lysis buffer [150 mM NaCl, 50 mM Tris-HCl (pH 7.5),
1 mM EDTA, 1 mM EGTA, 1% NP-40, 2.5 mM
Na4P2O7•10 H2O, 1 mM
NaF, 1 mM Na3VO4, 1 mM β-glycerophosphate and
20 µg/ml aprotin/leupeptin/PMSF]. The lysate was centrifuged at
13,000 × g, 4°C for 20 min and the supernatant was kept.
Determination of protein concentration was performed using the BCA
method. The lysates (1 ml) were added to new tubes and then the 2
µg antibody of β-adducin was added. The tubes were incubated on the
rotor at 4°C for 1 h, then 15 µl Protein G Agarose/ Salmon Sperm
DNA beads (EMD Millipore, Billerica, MA, USA) were added. Then the
tubes were incubated on the rotor at 4°C overnight. The mixture was
centrifuged at 13,000 × g for 1 min at 4°C, then the beads were
washed once with the high-concentration solution (500 mM NaCl, 50
mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1% NP-40, 2.5 mM
Na4P2O7•10 H2O, 1 mM
NaF, 1 mM Na3VO4, 1 mM β-glycerophosphate and
20 µg/ml aprotin/leupeptin/PMSF), medium-concentration solution
(250 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1%
NP-40, 2.5 mM Na4P2O7•10
H2O, 1 mM NaF, 1 mM Na3VO4, 1 mM
β-glycerophosphate and 20 µg/ml aprotin/leupeptin/PMSF) and then
the low-concentration solution (150 mM NaCl, 50 mM Tris-HCl (pH
7.5), 1 mM EDTA, 1 mM EGTA, 1% NP-40, 2.5 mM
Na4P2O7•10 H2O, 1 mM
NaF, 1 mM Na3VO4, 1 mM β-glycerophosphate and
20 µg/ml aprotin/leupeptin/PMSF) on ice successively. Following
centrifugation at 500 × g for 5 min at 4°C, the beads were
suspended with loading buffer and boiled in water bath for 10 min.
The supernatant was the solution with the target protein.
The supernatant (25 µl) was added to the wells of an
10% SDS-PAGE gel. At the beginning of the blotting, the voltage was
set to 80 V, then the voltage was adjusted to 120 V until the red
protein maker appeared. Following protein transfer the
nitrocellulose membrane was blocked in 5% BSA-TBST (0.05% Tween-20)
on the table concentrator at room temperature for 1 h. The film was
placed into the primary antibody dilution and incubated on the
table concentrator at 4°C for 3 h. Then the film was washed 3 times
with TBST and incubated with the horseradish-peroxidase-conjugated
secondary antibodies at 37°C for 1 h. Following washing 3 times
with TBST, the film was developed with ECL Plus western blotting
reagents, according to the manufacturer's protocol (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) and imaged using an image
analysis system (Tanon 5500; Tanon Science & Technology Co.,
Ltd. Shanghai, China). The following primary antibodies were used
for analysis: anti-adducin (1:1,000), anti-PY20 (1:2,000),
anti-flotillin2 (1:1,000) and anti-tubulin (1:1,000). Anti-β-actin
(1:4,000) served as the loading control.
Cholesterol depletion and
repletion
Cells (1×106) were treated with 5 mM MβCD
for 30 min at 37°C. The cell viability was assessed by trypan blue
exclusion. To replenish membrane cholesterol, the MβCD treated
cells were incubated with 25 mM cholesterol for 30 min at 37°C.
Sucrose density gradient
centrifugation
Cells were collected and washed with PBS
(5×107 dHL-60 or neutrophils) and suspended with IMDM.
Then the cells were stimulated with fMLP (100 nM) and washed with
PBS. The cells were collected by centrifugation and homogenized
with 1 ml cool lysis buffer using tissue homogenizer. The lysate
was centrifuged at 12,000 × g at 4°C for 10 min. The supernatant
was kept, and 20 µl of each sample removed to mix with loading
buffer, which was then added the wells with 10% SDS-PAGE and
Coomassie blue staining performed. The remaining supernatant was
mixed with 1 ml 80% sucrose solution. Then the mixture was
transferred to a 5 ml ultracentrifugation tube and 2 ml 30% sucrose
solution was added, followed by 1 ml 5% sucrose solution. The
discontinuous gradient was spun for 18 h at 200,000 × g at 4°C in
Beckman MLS50 rotors (Beckman Coulter, Inc., Brea, CA, USA).
Fractions collected from the gradient top were used immediately or
kept frozen at −80°C until use. The tubes were centrifuged at
200,000 × g for 18 h at 4°C in Beckman MLS50 rotors (Beckman
Coulter, Inc.). The tubes were put on ice and 12 layers in each
sample were collected. The following primary antibodies were used
for analysis: anti-adducin (1:1,000), anti-flotillin2 (1:1,000) and
anti-tubulin (1:1,000). The flotillin2 served as a raft marker and
tubulin served as the non-raft marker.
Statistical analysis
Data are representative of 3 independent
experiments. Data were analyzed by one-way analysis of variance
followed by a Tukey post hoc test using the SPSS statistics version
22 (IBM, Corp., Armonk, NY, USA). Quantitative data are expressed
as the mean ± standard deviation. P<0.01 was considered to
indicate a statistically significant difference.
Results
Integrity of lipid rafts is required
for neutrophil migration
The lipid raft is a specialized micro-area structure
in the plasma membrane, which consists of a rich of sheath
phospholipids and cholesterol (22,23).
It has been reported that lipid rafts can regulate cellular signal
transduction and the cell membrane dynamics (18). To investigate whether the integrity
of the lipid raft is required for neutrophil migration, cell
migratory ability was analyzed with or without integrated lipid
raft structure. The peptide chemotaxin fMLP was selected as the
inducer of neutrophil migration in vitro. The results
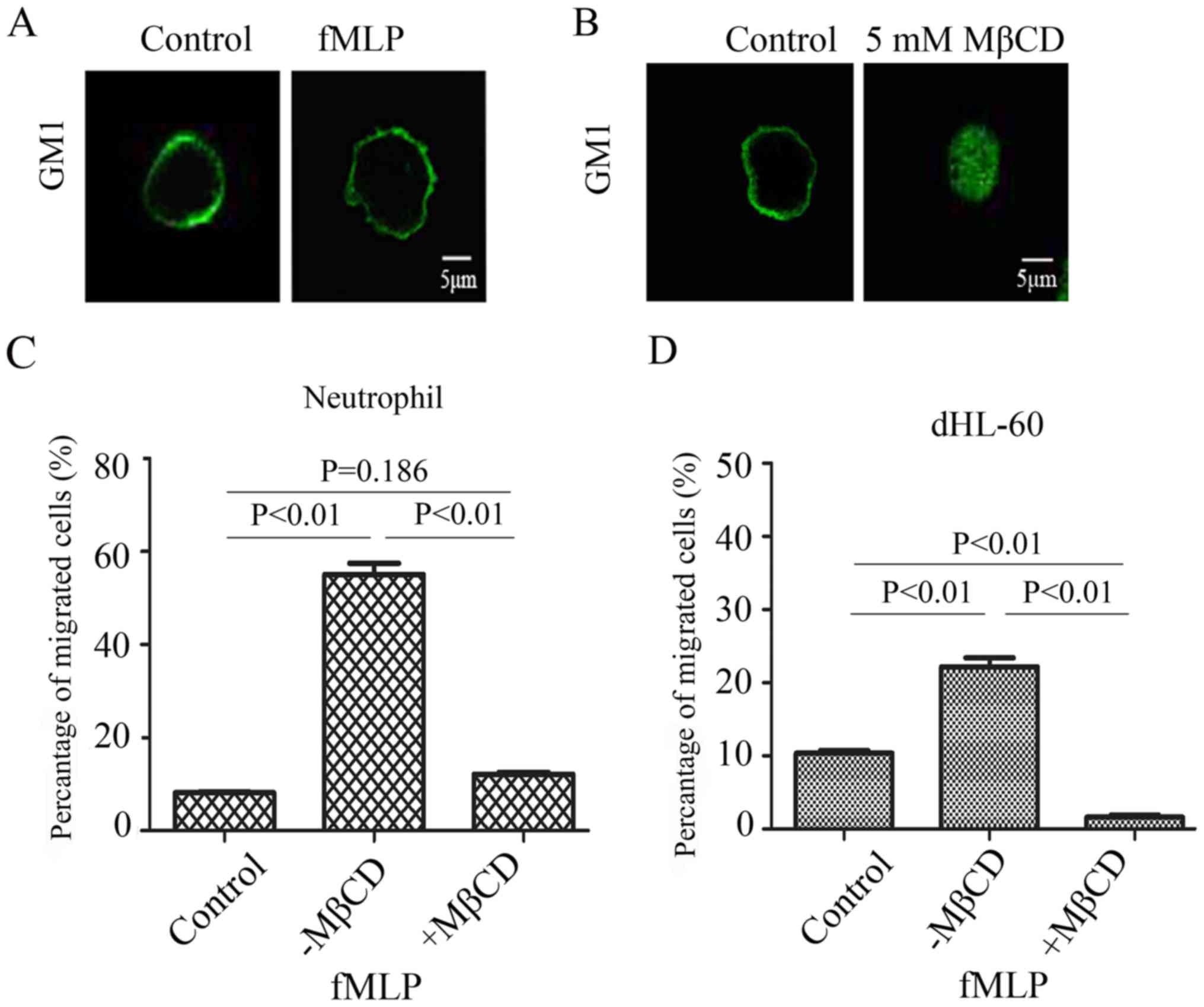
demonstrated that fMLP could induce deformation of the cell
membrane (Fig. 1A), suggesting
that fMLP can effectively induce the migration of neutrophils.
Based on the existing literature, 5 mM MβCD was chosen as an agent
for disrupting the lipid raft structure and GM1 was chosen as a
lipid raft marker (24). The
results demonstrated that MβCD treatment blurred the boundary of
the cell membrane, demonstrating that the integrity of the cell
membrane is disrupted (Fig. 1B).
To investigate the effect of the disruption of lipid raft integrity
on the migration of neutrophils in vitro, Transwell assays
were performed using fMLP as a chemotaxin. The results demonstrated
that when the cells were treated with 5 mM MβCD was their migratory
ability was significantly inhibited (P<0.01; Fig. 1C). The same assay was repeated
using dHL-60 (Fig. 1D). The
results indicated that the migration of neutrophils in vitro
relies on the integrity of the lipid raft structure.
Migratory behavior of neutrophils is
dependent on the integrity of lipid rafts
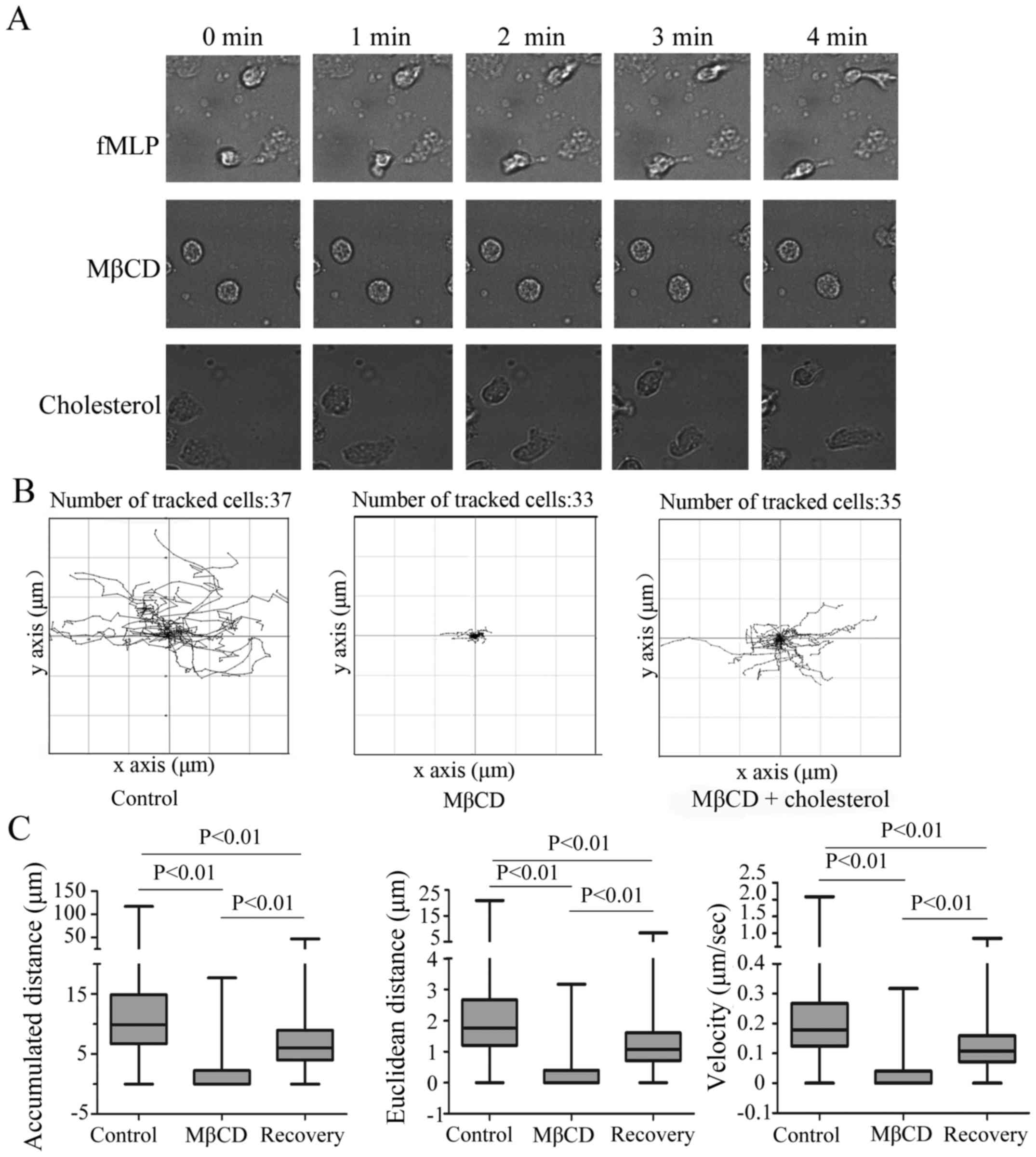
Subsequently a real-time observation of neutrophil
migration was performed in a Zigmond chamber to further confirm the
finding that neutrophil migration depends on the integrity of the
lipid raft structure. The Live Cell Imaging System was used in this
experiment and neutrophil migration was quantitatively analyzed
using the Live Cell Imaging System, GraphPad prism 5, and
Chemotaxis Tool software. The migratory ability of peripheral blood
neutrophils is strong, which is demonstrated by rapid membrane
deformation (Fig. 2A). Unlike the
cells in the control group, the cells in the MβCD treatment group
exhibited a weakly polarized state and the migratory speed was very
slow (Fig. 2). When cholesterol
was used to recover the integrity of the cell membrane, the cell
migratory ability was restored, with regards to deformation
velocity and polarization.
Wind-rose plots of the tracked migration paths of
neutrophils were plotted using a manual tracking chemotaxis tool
plugin (Fig. 2B) and quantitative
assessment of migratory behavior, including Euclidean distance,
velocity and accumulated distance, was obtained by tracking
individual cells. Neutrophils without MβCD treatment migrated a
significantly greater distance during the 4 min observation period
compared with the treatment group and the average accumulated
distance, the Euclidean distance and the average velocity were
9.98, 1.76 and 0.18 µm/s, respectively. When treated with MβCD, the
migratory capacity of neutrophils was impacted significantly and
the accumulated distance, Euclidean distance, and average velocity
were reduced compared with that of the untreated neutrophils
(P<0.01). When cholesterol was used to recover the integrity of
the cell membrane, the migratory capacity of neutrophils was
significantly restored (P<0.01; Fig. 2C).
β-adducin, a lipid raft-associated
protein participates in neutrophil migration
Our previous study (18) reported that β-adducin, as membrane
skeleton protein, has an important role in the process of
neutrophil adhesion. In order to determine if β-adducin is involved
in the process of neutrophil migration, the distribution of
β-adducin was detected using sucrose density gradient
centrifugation prior to and following stimulation with fMLP. The
results demonstrated that no β-adducin was observed in the lipid
raft component in the control cells, but in the fMLP stimulated
cells, β-adducin appeared in the lipid raft component (Fig. 3A). The association of β-adducin and
lipid rafts was further examined by confocal microscopy. Results
demonstrated that, there was no colocalization between lipid rafts
and β-adducin when neutrophils were in a resting state (Fig. 3Ba1-a3). Following fMLP stimulation,
an obvious colocalization between lipid rafts and β-adducin was
observed (Fig. 3Bb1-b3). When MβCD
was used to disrupt the structure of lipid rafts, fMLP stimulation
did not induce the colocalization of lipid rafts and β-adducin
(Fig. 3Bc1-c3). In order to
investigate the role of β-adducin in the process of neutrophil
migration, the β-adducin was knocked down in dHL-60 cells. The
result demonstrated that the β-adducin-shRNA effectively inhibited
the expression of β-adducin (Fig.
3C). Transwell experiments were performed to determine the
effect of β-adducin interference on the migration of neutrophils.
The results demonstrated that, compared with the control group, the
migratory rate of the dHL-60 cells decreased by 40% when β-adducin
expression was knocked down (Fig.
3D). The results indicated that β-adducin is involved in
neutrophil migration.
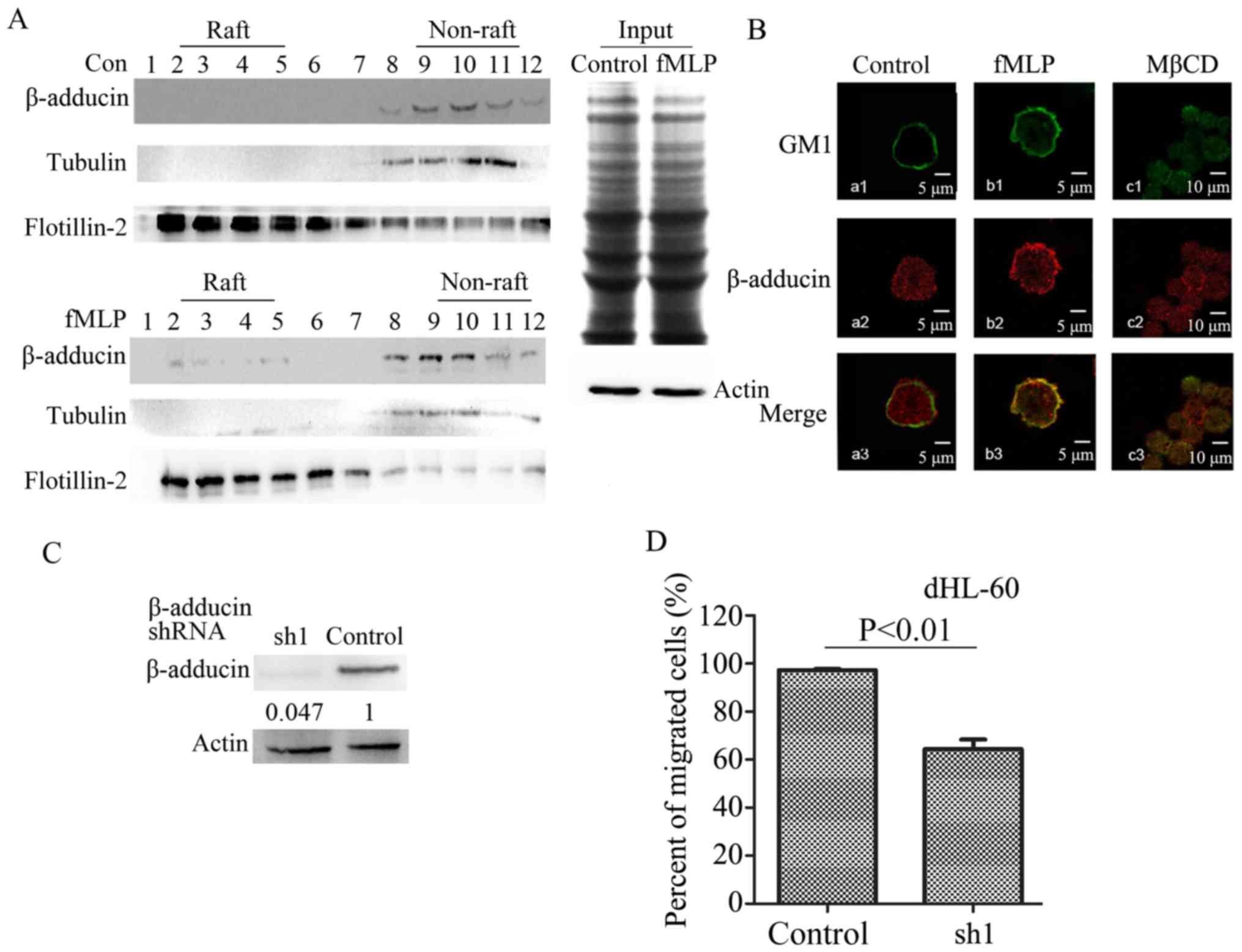 | Figure 3.The role of β-adducin in the
migration of neutrophils. (A) Sucrose density gradient
centrifugation was used to separate the lysates of control dHL-60
cells and fMLP-stimulated dHL-60 cells. β-adducin antibody was used
to detect β-adducin in the raft and the non-raft samples by western
blotting. Rows 2, 3, 4 and 5 represent raft components, and 9, 10,
11 and 12 represent the non-raft components. The raft marker was
flotillin and the non-raft marker was tubulin. The equivalent
amount of lysates from control and fMLP treated cells were stained
with Coomassie blue and immunoblotted with anti-actin antibody
(right). (B) The colocalization between β-adducin and lipid rafts
prior to and following fMLP stimulation were observed under a
confocal microscope. AlexaFluor-488-conjugated CTxB was used to
stain the specific lipid rafts marker GM1 and specific
immunofluorescence antibody was used to label β-adducin (red). (C)
Nonsense shRNA (control group) and β-adducin shRNA was used to
infect dHL-60 cells. The lysates of whole cells following 48 h of
culture were collected and analyzed by western blotting, and the
β-adducin antibody was used to detect the interference efficiency.
(D) The control cells and β-adducin knockdown cells in Transwell
experiments. Cell migratory rate was determined by calculating the
number of migratory cells/the number of total cells. The migratory
rate of the control group (nonsense shRNA sequences) was set as
100%. Con, control; fMLP, N-formylmethionyl-leucyl-phenyl-alanine;
MβCD, methyl-β-cyclodextrin; sh, short hairpin RNA. |
Role of β-adducin phosphorylation in
neutrophil migration
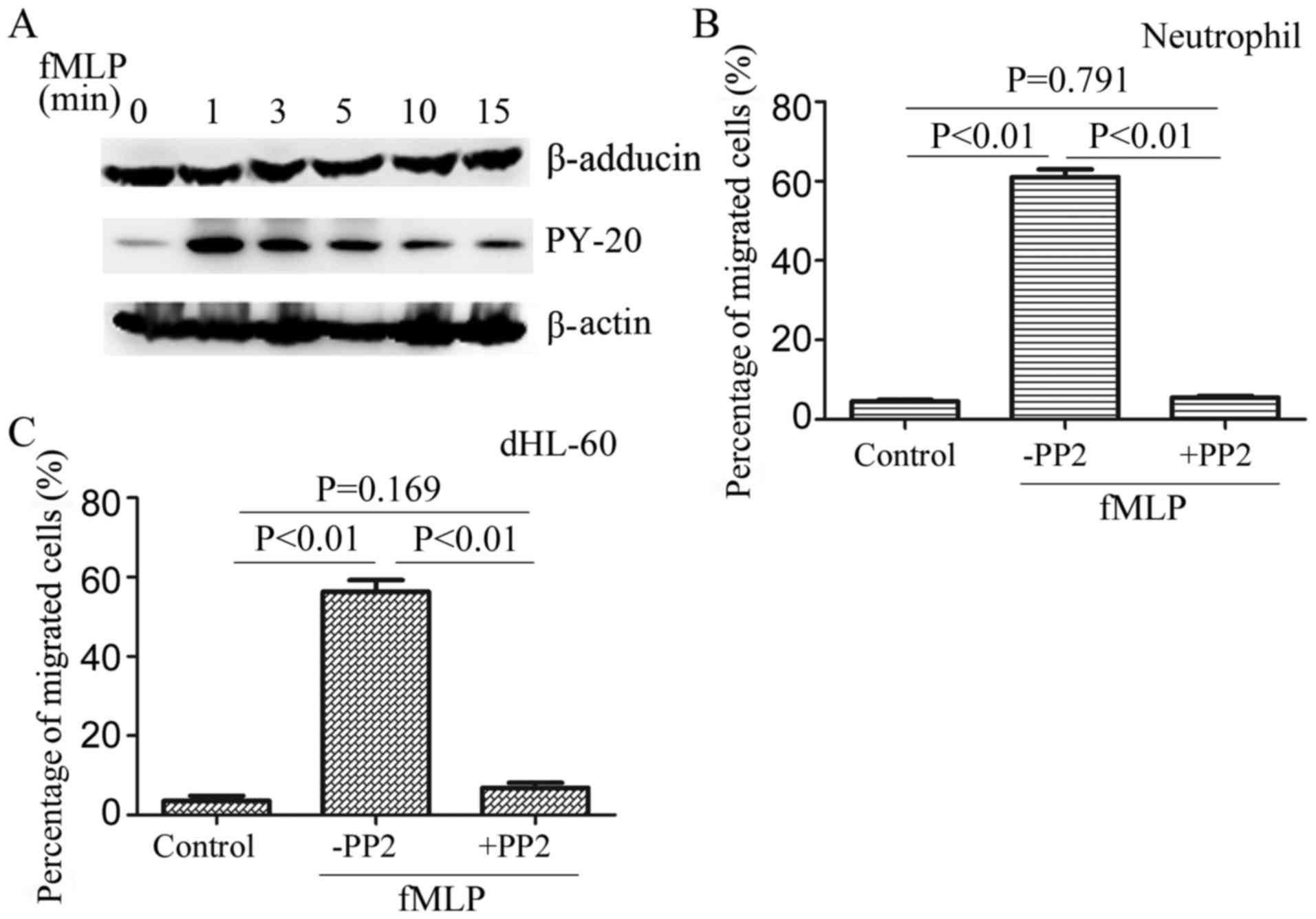
Subsequently, the expression alterations of
β-adducin in dHL-60 cells were detected prior to and following fMLP
stimulation. The results demonstrated that fMLP stimulation did not
lead to a change of β-adducin expression (Fig. 4A), indicating that β-adducin
expression is not the main reason for the inhibition of neutrophil
migration. It has been reported that there are four tyrosine
residues in the C-terminal of β-adducin and the activity of
β-adducin is associated with the phosphorylation of tyrosine
residues (25). The
phosphorylation of β-adducin prior to and following fMLP
stimulation was detected by immunoprecipitation of β-adducin and
immunoblotting with anti-phosphotyrosine antibody PY-20. The
results demonstrated that fMLP stimulation induced the
phosphorylation of β-adducin. The phosphorylation peak appeared at
1 min and was still detectable at 15 min after stimulation
(Fig. 4A).
The phosphorylation of β-adducin is dependent on
Src-family tyrosine kinases. PP2, an effective inhibitor of
Src-family tyrosine kinase, was used in the Transwell assay. The
results demonstrated that the inhibition of β-adducin
phosphorylation resulted in a decrease in neutrophil migration
(Fig. 4B and C), indicating that
β-adducin phosphorylation is involved in regulating neutrophil
migration.
Discussion
Cell migration is a complex and precisely regulated
process, and cellular cortical skeleton and actin cytoskeleton
rearrangement have an important role in cell polarization and
migration by promoting membrane protrusion and providing the
driving force (26,27). Current evidence supports the
hypothesis that lipid rafts are involved in various types of cell
migration processes (28) and the
functions of lipid rafts in the neutrophil migration process have
been well established (8,10). However, there is no direct,
real-time evidence to demonstrate that the integrity of the lipid
raft is critical in highly polarized and rapidly moving
neutrophils. In the present study the essential role of the
integrity of lipid raft in regulating neutrophil migration was
demonstrated. The Transwell and time-lapse observation data
indicated that treatment with MβCD, a lipid raft disruption agent,
impacts membrane protrusion at the leading edge of migratory
neutrophils and resulted in a marked impairment of neutrophil
migration. On the basis of these results, it was concluded that the
migratory behavior of neutrophils is dependent on the integrity of
lipid rafts. Thus, the results of the present study have improved
upon our previous understanding of how the integrity of lipid rafts
affects neutrophil migration.
These results prompted investigation of lipid
raft-associated proteins and the mechanism by which the cellular
cortical skeleton and the actin cytoskeleton cooperate to regulate
the migration of neutrophils. It has been reported that β-adducin
is a lipid raft-associated cortical protein and a capping protein
of F-actin (14,16,29).
β-adducin was demonstrated to be involved in neutrophil migration.
The results of the present study demonstrated that when neutrophils
were treated with fMLP, β-adducin appeared in the lipid raft
component. In addition, fMLP stimulation could not induce the
colocalization of lipid rafts and β-adducin when the lipid rafts
were disrupted. When β-adducin was knocked down in dHL-60,
neutrophil migration was inhibited. Xu et al (18) reported that β-adducin can transfer
from the lipid raft area to the cytoplasm, which leads to the cell
membrane becoming temporarily disconnected from the actin. The
results of the present study demonstrated that during neutrophil
migration, β-adducin moves from the cytoplasm to lipid rafts. As a
capping protein of F-actin, the translocation of β-adducin to the
lipid raft may regulate the cluster polymerization of F-actin under
the membrane and this may provide a cytoskeleton for the stretching
of the membrane during the formation of protruding structures.
Previous studies have demonstrated that β-adducin is
phosphorylated during neutrophil rolling and the deformation of the
cytoskeleton in erythrocytes (13–16,18,30);
however, it is not yet known whether β-adducin regulates neutrophil
migration via phosphorylation. The results of the present study
demonstrated that tyrosine phosphorylation of β-adducin occurred
during neutrophil migration. The phosphorylation of β-adducin is
dependent on Src-family tyrosine kinases. Therefore, the function
of the Src-family tyrosine kinase was inhibited using PP2, and the
data indirectly demonstrated that the phosphorylation of β-adducin
is involved in neutrophil migration. Thus, in the present study it
was demonstrated that a Src-family tyrosine kinase is a regulator
of β-adducin in neutrophil migration.
In conclusion, a novel function of β-adducin in
neutrophil migration has been identified. The results of the
present study demonstrated that the integrity of lipid rafts and
the recruitment of β-adducin to lipid rafts are necessary for
membrane protrusion and migration of neutrophils. In addition, it
was demonstrated that phosphorylation of β-adducin is a key step in
the recruitment of β-adducin to lipid rafts. Following activation,
β-adducin transfers to lipid rafts and this may provide a
cytoskeletal basis for the membrane protrusion, which further
affects the migration of neutrophils.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31471332
and 81172014), and Jilin Province Department of Education (grant
no. 2012-221).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author contributions
CY, ZS, TX and WL performed experiments, data
analysis, drafted the paper and approved the final version. XZ, XW
and CY contributed to the study design and clinical study, drafted
the paper, and approved the final version.
Ethics approval and consent to
participate
The blood donors signed informed consent with the
Blood Center and the authors also signed an agreement with Blood
Center for using the blood.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
fMLP
|
N-formylmethionyl-leucyl-phenyl-alanine
|
|
MβCD
|
methyl-β-cyclodextrin
|
References
|
1
|
McEver RP and Zhu C: Rolling cell
adhesion. Annu Rev Cell Dev Biol. 26:363–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ley K, Laudanna C, Cybulsky MI and
Nourshargh S: Getting to the site of inflammation: The leukocyte
adhesion cascade updated. Nat Rev Immunol. 7:678–689. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zarbock A, Ley K, McEver RP and Hidalgo A:
Leukocyte ligands for endothelial selectins: Specialized
glycocon-jugates that mediate rolling and signaling under flow.
Blood. 118:6743–6751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan JR, Hyduk SJ and Cybulsky MI:
Chemoattractants induce a rapid and transient upregulation of
monocyte alpha4 integrin affinity for vascular cell adhesion
molecule which mediates arrest: An early step in the process of
emigration. J Exp Med. 193:1149–1158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giagulli C, Scarpin E, Ottoboni L,
Narumiya S, Butcher EC, Constantin G and Laudanna C: RhoA and zeta
PKC control distinct modalities of LFA-1 activation by chemokines:
Critical role of LFA-1 affinity triggering in lymphocyte in vivo
homing. Immunity. 20:25–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinashi T: Intracellular signalling
controlling integrin activation in lymphocytes. Nat Rev Immunol.
5:546–559. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Resh MD: Fatty acylation of proteins: New
insights into membrane targeting of myristoylated and palmitoylated
proteins. Biochim Biophys Acta. 1451:1–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanagida M, Nakayama H, Yoshizaki F,
Fujimura T, Takamori K, Ogawa H and Iwabuchi K: Proteomic analysis
of plasma membrane lipid rafts of HL-60 cells. Proteomics.
7:2398–2409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Haller PD, Donohoe S, Goodlett DR,
Aebersold R and Watts JD: Mass spectrometric characterization of
proteins extracted from Jurkat T cell detergent-resistant membrane
domains. Proteomics. 1:1010–1021. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Janes PW, Ley SC, Magee AI and Kabouridis
PS: The role of lipid rafts in T cell antigen receptor (TCR)
signaling. Semin Immunol. 12:23–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simons K and Toomre D: Lipid rafts and
signal transduction. Nat Rev Mol Cell Biol. 1:31–39. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hughes CA and Bennett V: Adducin: A
physical model with implications for function in assembly of
spectrin-actin complexes. J Biol Chem. 270:18990–18996. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barkalow KL, Italiano JE Jr, Chou DE,
Matsuoka Y, Bennett V and Hartwig JH: Alpha-adducin dissociates
from F-actin and spectrin during platelet activation. J Cell Biol.
161:557–570. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pariser H, Herradon G, Ezquerra L,
Perez-Pinera P and Deuel TF: Pleiotrophin regulates serine
phosphorylation and the cellular distribution of beta-adducin
through activation of protein kinase C. Proc Natl Acad Sci USA.
102:12407–12412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalfa TA, Pushkaran S, Mohandas N, Hartwig
JH, Fowler VM, Johnson JF, Joiner CH, Williams DA and Zheng Y: Rac
GTPases regulate the morphology and deformability of the
erythrocyte cytoskeleton. Blood. 108:3637–3645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naydenov NG and Ivanov AI: Adducins
regulate remodeling of apical junctions in human epithelial cells.
Mol Biol Cell. 21:3506–3517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CL, Lin YP, Lai YC and Chen HC:
α-Adducin translocates to the nucleus upon loss of cell-cell
adhesions. Traffic. 12:1327–1340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu T, Liu W, Yang C, Ba X, Wang X, Jiang Y
and Zeng X: Lipid Raft-associated β-adducin is required for PSGL-1
mediated neutrophil rolling on P-selectin. J Leukocyte Biol.
97:297–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Filippi MD, Szczur K, Harris CE and
Berclaz PY: Rho GTPase Rac1 is critical for neutrophil migration
into the lung. Blood. 109:1257–1264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nauseef WM: Isolation of human neutrophils
from venous blood. Method Mol Biol. 412:15–20. 2007. View Article : Google Scholar
|
|
21
|
Hauert AB, Martinelli S, Marone C and
Niggli V: Differented HL-60 cells are a valid model system for the
analysis of human neutrophil migration and chemotaxis. Int J
Biochem Cell Biol. 34:838–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rajendran L and Simons K: Lipid rafts and
membrane dynamics. J Cell Sci. 118:1099–1102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dinic J, Ashrafzadeh P and Parmryd I:
Actin filaments attachment at the plasma membrane in live cells
cause the formation of ordered lipid domainsc. Biochem Biophys
Acta. 1828:1102–1111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang R, Bi J, Ampah K, Ba X, Liu W and
Zeng X: Lipid rafts control human melanoma cell migration by
regulating focal adhesion disassembly. Biochim Biophys Acta.
1833:3195–3205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bednarek E and Caroni P: β-Adducin is
required for stable assembly of new synapses and improved memory
upon environmental enrichment. Neuron. 69:1132–1146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krause M, Dent EW, Bear JE, Loureiro JJ
and Gertler FB: Ena/VASP proteins: Regulators of the actin
cytoskeleton and cell migration. Annu Rev Cell Dev Biol.
19:541–564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruiz-Sáenz A, Kremer L, Alonso MA, Millán
J and Correas I: Protein 4.1R regulates cell migration and IQGAP1
recruitment to the leading edge. J Cell Sci. 124:2529–2538. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simons K and Sampaio JL: Membrane
organization and lipid rafts. Cold Spring Harb Perspect Biol.
3:3437–3451. 2011. View Article : Google Scholar
|
|
29
|
Gotoh H, Okumura N, Yagi T, Okumura A,
Shima T and Nagai K: Fyn-induced phosphorylation of beta-adducin at
tyrosine 489 and its role in their subcellular localization.
Biochem Biophys Res Commun. 346:600–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Henkels KM, Frondorf K, Gonzalez-Mejia ME,
Doseff AL and Gomez-Cambronero J: IL-8-induced neutrophil
chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Lett.
585:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|