Introduction
Cardiac fibrosis is a major aspect in the remodeling
of diverse cardiovascular diseases, including atherosclerosis,
hypertension, arrhythmias, ischemic and dilated cardiomyopathy
(1). Excessive cardiac fibrosis
can lead to cardiac dysfunction, interstitial remodeling,
structural disorder, and eventually progressive heart failure
(1). Cardiac fibrosis is
characterized by the net accumulation of extracellular matrix in
the cardiac interstitium (2), and
cardiac fibroblasts (CFs), main effector cells in cardiac fibrosis,
play an important role in the formation of cardiac fibrosis
(3). Transforming growth factor β1
(TGF-β1) is a potent profibrotic cytokine and initiates and
maintains fibrotic responses (4).
CFs that are induced by TGF-β1 can transform into myofibroblasts
that exhibit augmented proliferative, migratory, contractile, and
collagen-producing abilities (2,5). A
better understanding of the regulation of CFs would help ameliorate
the deleterious effects of cardiac fibrosis.
Curcumin (diferuloylmethane) has been reported to
exhibit several beneficial properties, including anti-inflammatory,
anti-oxidative, anti-proliferative, and anti-apoptotic activities
(6). Accumulating evidence has
demonstrated preventive effects of curcumin in fibrotic diseases,
including oral submucosal (7),
liver (8), lung (9), and kidney fibrosis (10). In a previous study, the cardiac
protective effect of curcumin was demonstrated in several heart
diseases, such as hypertension, myocardial infarction, and diabetic
cardiomyopathy (11–13). The protective effects of curcumin
on myocardial injury have been reported to be anti-inflammatory,
however, the anti-fibrotic properties of curcumin on cardiac
fibrosis have yet to be elucidated.
To determine effects of curcumin on CFs, we
evaluated the proliferation, cell cycle phase, and collagen
deposition in CFs. Our results revealed that curcumin inhibited
TGF-β1-induced cardiac fibroblast proliferation, differentiation,
and collagen production, and may be mediated by inhibiting the Smad
and p38 MAPK pathways.
Materials and methods
Cells and reagents
Normal human CFs were obtained from ScienCell
Research Laboratories (San Diego, CA, USA). TGF-β1 was obtained
from R&D Systems, Inc., (Minneapolis, MN, USA). Antibodies
directed against α-smooth muscle actin (α-SMA), collagen type Iα
(COLA)-1, COLA3, cyclin-dependent kinase 1 (CDK1), cyclin B,
phospho-smad2/3 (p-smad2/3), phospho-p38 mitogen-activated protein
kinase (p-p38 MAPK), phospho-extracellular regulated protein
kinases (p-ERK), and GAPDH were purchased from Bioss Antibodies
(Beijing, China).
Cell treatment
CFs were cultured in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), supplemented with 10% fetal bovine serum (Hangzhou
Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou,
China), 100 U/ml penicillin and 100 mg/ml streptomycin. Cells were
cultured at 37°C in a 5% CO2 atmosphere. CFs were
treated with/without TGF-β1 (10 ng/ml) for 24 h or pretreated with
curcumin (20 µmol/l) for 1 h prior to stimulation with TGF-β1.
Proliferation assay
The proliferation of CFs was evaluated by a
commercial Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). A total of 3×103
CFs were seeded in each well of a 96-well plate and 10 µl CCK-8
solution was added to each well w for 1, 2, 3, 4, 5, 6, or 7 days.
Then, the optical density (at 450 nm) was measured using a
microplate reader (Thermo Fisher Scientific, Inc.), and the cell
viability was calculated.
Cell cycle assay
CFs were seeded in 6-well plates at a density of
4×104 cells/well and cultured for 24 h at 37°C. Next,
CFs were treated with/without TGF-β1 (10 ng/ml) for 24 h or
pretreated with curcumin (20 µmol/l) for 1 h prior to stimulation
with TGF-β1. Cells were washed with phosphate-buffered saline
(PBS), fixed with 70% ethanol for 2 h at 4°C and then the cells
were incubated for 30 min with 10 mg/ml propidium iodide (PI) and
2.5 mg/ml DNase-free RNase (Beyotime Institute of Biotechnology,
Haimen, China) for staining of DNA. A total of 2×104
cells were analyzed by flow cytometry on a BD FACSCanto flow
cytometer. FlowJo software was used for data analysis (Tree Star,
Inc., Ashland, OR, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from cells by TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA by a M-MLV Reverse Transcriptase kit
(Promega, Madison, WI, USA). qPCR was performed on an Applied
Biosystems 7500 Real-Time PCR system (Thermo Fisher Scientific,
Inc.). mRNA levels of α-SMA, COLA1, and COLA3 were determined by
RT-qPCR using GAPDH as an internal control. Primer sequences are
shown in Table I. PCR
amplification was carried out by denaturation at 94°C for 5 sec
followed by 40 cycles of annealing and extension (62°C for 40 sec).
Relative gene expression was determined using the 2−ΔΔCq
method.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequence
(5′-3′) |
|---|
| GAPDH | F:
ACATCATCCCTGCCTCTACTG |
|
| R:
CCTGCTTCACCACCTTCTTG |
| α-SMA | F:
TTCCTTCGTGACTACTGCTGAG |
|
| R:
GAAAGATGGCTGGAAGAG |
| COLA1 | F:
GAATATGTATCACCAGACGCAGAAG |
|
| R: AGACCA
CGAGGACCAGAAGG |
| COLA3 | F:
CTGGAGTCGGAGGAATGG |
|
| R:
GCCAGATGGACCAATAGCA |
Western blot analysis
Total protein was extracted from cells using RIPA
buffer (Thermo Fisher Scientific, Inc.) and quantified using the
BCA Protein Assay Kit following the manufacturer's instructions
(Thermo Fisher Scientific, Inc.). A total of 20 µg protein was
loaded onto SDS-PAGE gels, proteins were separated by
electrophoresis, electrotransferred onto polyvinylidene difluoride
(PVDF) membranes (EMD Millipore, Billerica, MA, USA), and blocked
for 1 h at room temperature using tris-buffered saline
(TBS)-Tween-20 (TBST) (J&K Scientific, Ltd., Shanghai, China)
containing 5% non-fat dry milk. Subsequently, membranes were probed
overnight at 4°C with rabbit polyclonal antibodies (1:500) directed
against α-SMA, COLA1, COLA3, Cyclin B, CDK1, p-Smad2/3, p-p38 MAPK,
p-ERK, and GAPDH in TBST. After incubation with horseradish
peroxidase (HRP)-conjugated secondary antibodies, proteins were
visualized using an enhanced chemiluminescence (ECL) substrate kit
(Thermo Fisher Scientific, Inc.). GAPDH was used as a loading
control.
Statistical analysis
Statistical analyses were carried out using IBM SPSS
Statistics software v20.0 (IBM Corp., Armonk, NY, USA). Data are
presented as the mean ± standard deviation of three independent
experiments, and compared using the post-hoc test used with
analysis of variance with Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumin inhibits TGF-β1-induced
differentiation of CFs
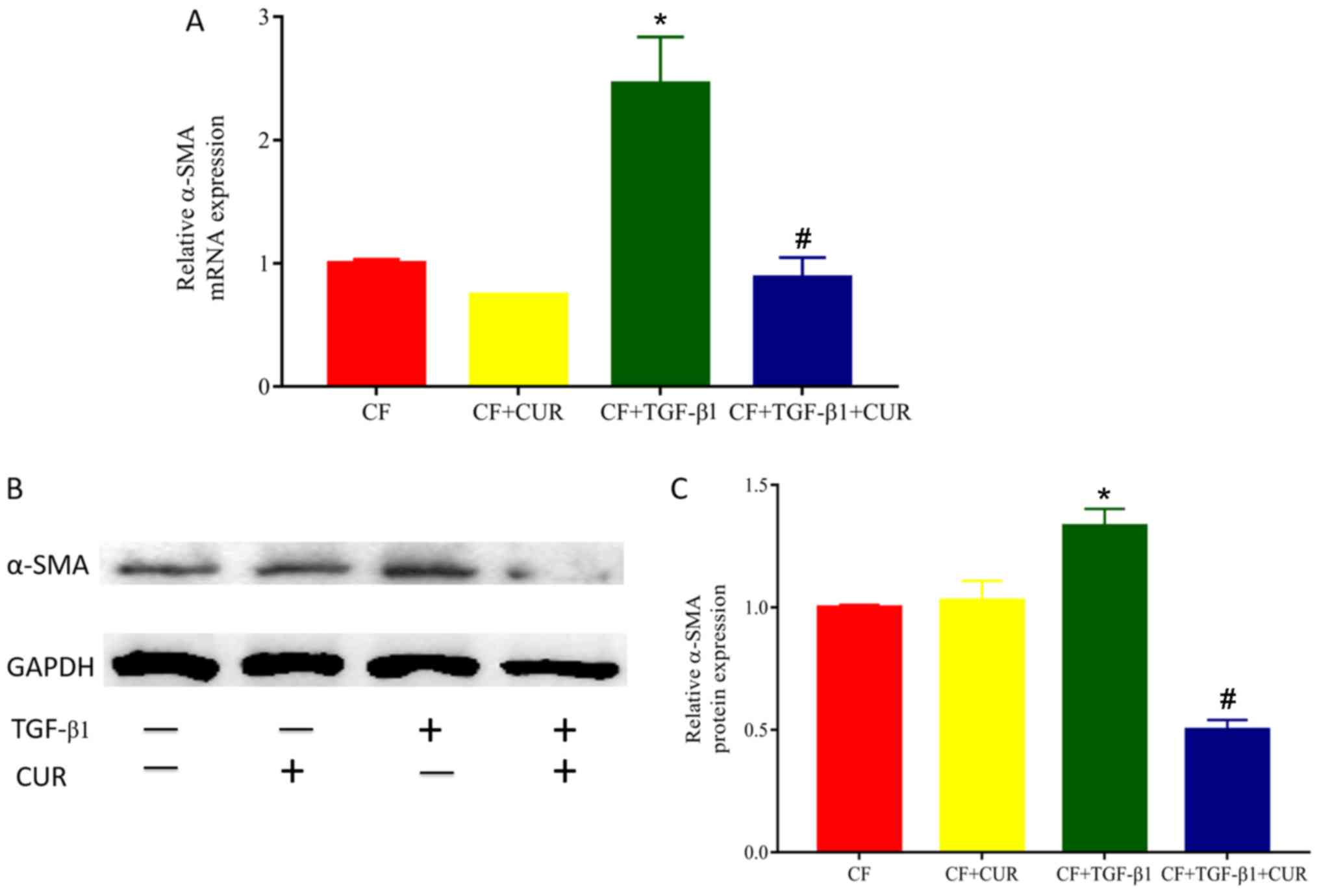
To determine the effects of curcumin on cardiac
fibrosis, we evaluated the level of α-SMA, a biomarker of
myofibroblast differentiation. As shown in Fig. 1, both gene and protein levels of
α-SMA in TGF-β1-stimulated CFs were significantly increased
compared with the controls, and elevated levels of α-SMA were
reduced in curcumin-treated CFs.
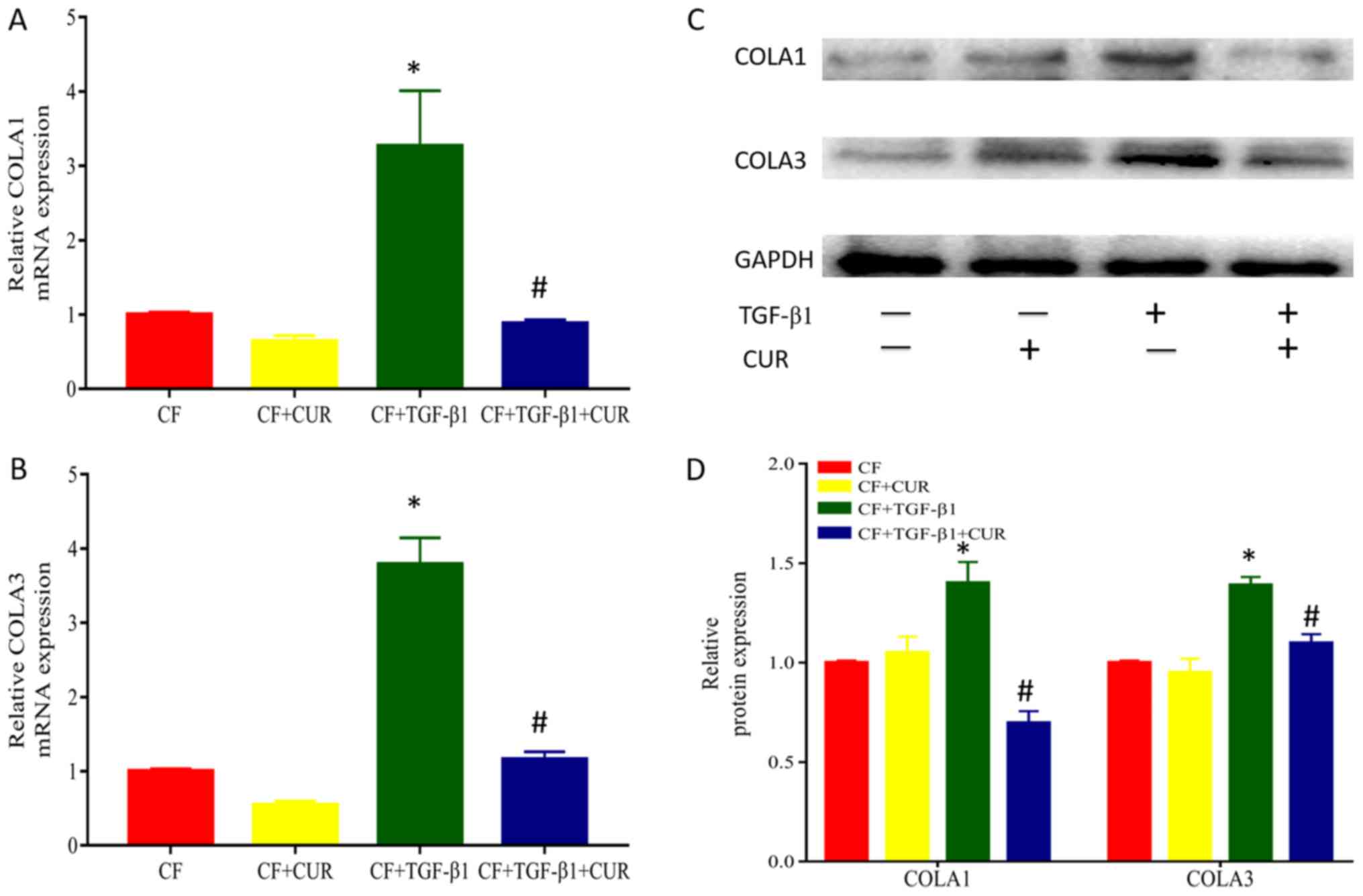
Curcumin reduces collagen deposition
of TGF-β1-induced CFs
To investigate the effects of curcumin on collagen
accumulation, we determined the protein expression of collagen I
and III, which are major components of extracellular matrix. As
shown in Fig. 2, when compared to
TGF-β1-stimulated CFs, treatment with curcumin significantly
reduced protein expression of collagen I and III.
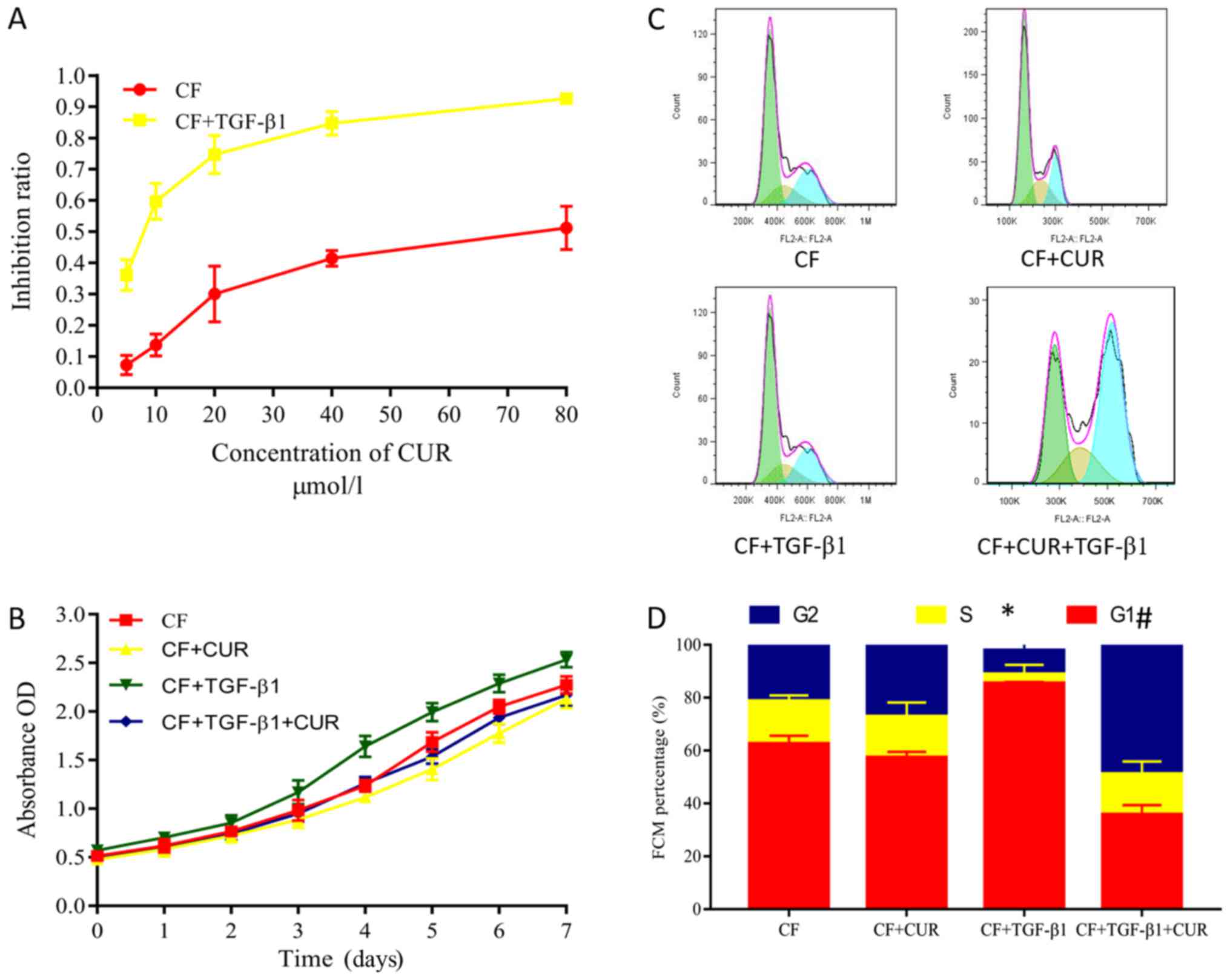
Curcumin inhibits cell proliferation
and promotes cell cycle arrest of TGF-β1-induced CFs
Excessive proliferation of CFs plays a key role in
myocardial fibrosis (14). Herein,
we evaluated the proliferation of CFs by using CCK-8 assays.
Curcumin pretreatment had no significant effect on the
proliferation of CFs. However, CFs displayed increased
proliferative abilities after 24 h of TGF-β1 stimulation. These
increased proliferative abilities were suppressed by curcumin
pretreatment in a dose-dependent manner (Fig. 3A), and were observed from 1 to 7
days after curcumin administration. Flow cytometry was performed to
evaluate the effects on the cell cycle phases of CFs. Our findings
demonstrated that curcumin significantly decreased the percentage
of TGF-β1-stimulated CFs in the G0/G1 phase but increased the
percentage of cells in the G2/M phase (Fig. 3C and D), indicating that curcumin
causes G2/M cell cycle arrest.
Curcumin affects cyclin B and CDK1
expression via inhibiting Smad2/3, p38 MAPK, and ERK signaling
activation in TGF-β1-induced CFs
To further explore the effect of curcumin on cell
cycle arrest, we determined the levels of cyclin B and CDK1 protein
expression. Our results revealed that the protein expression of
cyclin B and CDK1 was elevated after TGF-β1 stimulation, and this
effect was suppressed by curcumin pretreatment.
Given that TGF-β1 is the most important mediator in
cardiac fibrosis, we examined proteins involved in TGF-β1 signaling
in CFs. In the canonical signaling pathway, TGF-β1 activates
down-stream Smad2/3 pathways to exert its pro-fibrotic effects
(14). To determine the effect of
curcumin effect on Smad 2/3, we evaluated the protein expression of
phosphorylated Smad 2/3 in CFs. As shown in Fig. 4A and D, Smad2/3 phosphorylation was
significantly enhanced after TGF-β1 stimulation, and this effect
was significantly reduced by curcumin.
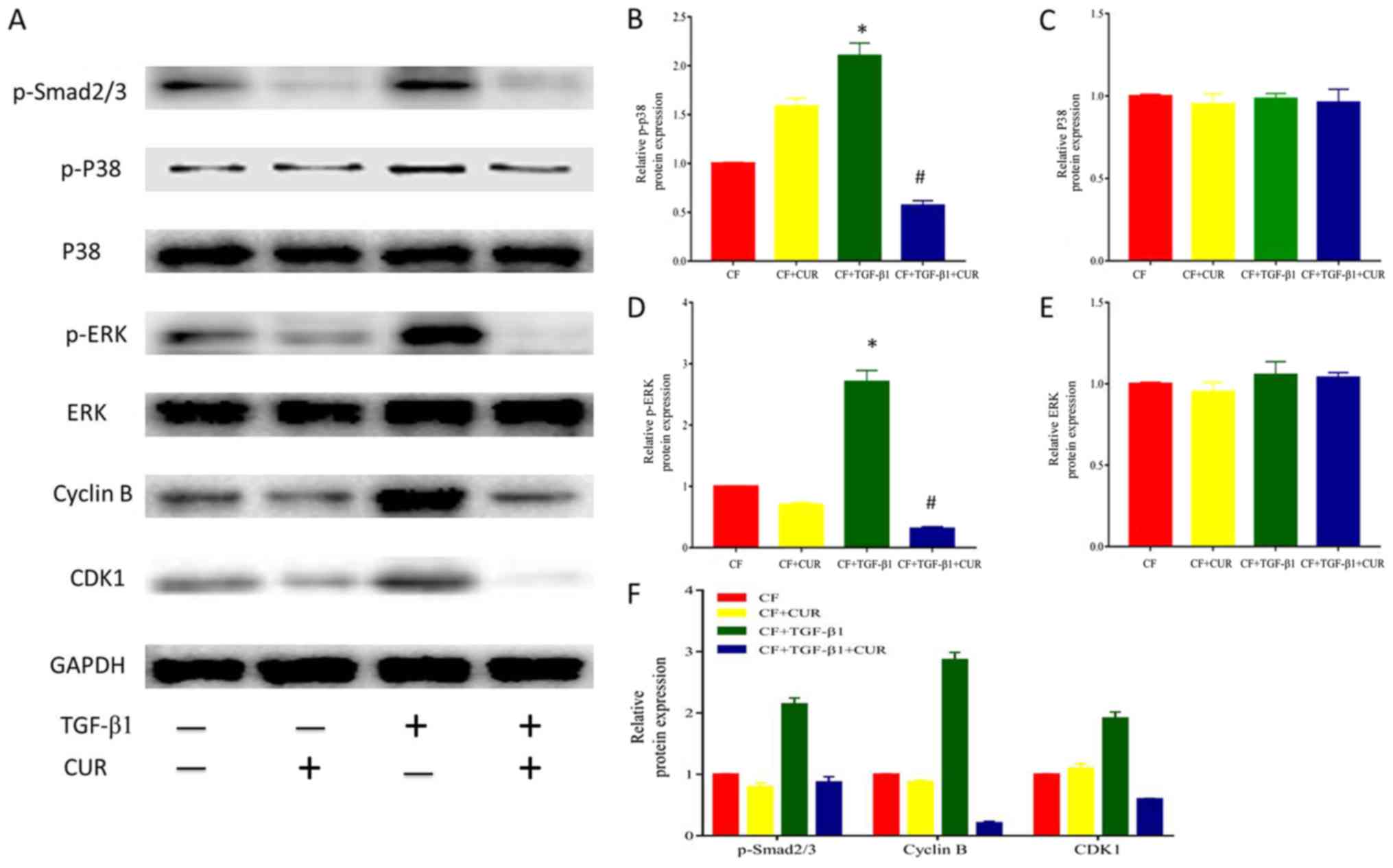 | Figure 4.CUR affects cell-cycle protein
expression by inhibiting p38 MAPK signaling activation in
TGF-β1-induced CFs. (A) Western blotting analysis of p-smad2/3,
P38, p-P38, ERK, p-ERK, Cyclin B, and CDK1 expression. Quantitative
analysis of (B) p-P38 protein expression using P38 as a control,
(C) P38 protein expression using GAPDH as a control, (D) p-ERK
protein expression using ERK as a control, (E) ERK protein
expression using GAPDH as a control, and (F) p-smad2/3, Cyclin B
and CDK1 protein expression using GAPDH as a control. Data are
presented as the mean ± standard deviation of triplicate
experiments. *P<0.05 vs. CF group; #P<0.05 vs. CF
+ TGF-β1 group. CUR, curcumin; MAPK, mitogen-activated protein
kinase; TGF, transforming growth factor; CFs, cardiac fibroblasts;
ERK, extracellular signal-regulated kinase; p-, phosphorylated;
CDK, cyclin dependent kinase; smad2/3, phosphorylation-mothers
against decapentaplegic homolog 2/3. |
In addition, it has been described that TGF-β1
activates the non-canonical MAPK pathway to modulate profibrotic
processes (14). As shown in
Fig. 4A-C, TGF-β1 did not affect
p38 and ERK expression, but enhanced p38 and ERK phosphorylation.
Curcumin pretreatment significantly reduced increased
phosphorylation of p38 MAPK and ERK. Thus, these findings indicated
that curcumin suppressed p38 MAPK and ERK activation in response to
TGF-β1 in CFs.
Discussion
Curcumin exhibits promising pharmacological
activities and has demonstrated to prevent properties in multiple
fibrotic diseases, such as oral submucosal (7), liver (8), lung (9), and kidney fibrosis (10). In the present study, we evaluated
the protective effect of curcumin on cardiac fibrosis. Curcumin
inhibited TGF-β1-induced proliferation, differentiation, and
collagen deposition of CFs, and promoted cell apoptosis. The
inhibitory effects of curcumin may be caused by inhibition of
Smad2/3, p38 MAPK, and ERK signaling pathways. Together, these
findings suggested that curcumin may be of potential therapeutic
use for the treatment of cardiac fibrosis.
Transformation of cardiac myofibroblasts is a
central event of cardiac fibrosis, which is featured by high
expression of α-SMA (15,16). Collagen I and III are major
components of the extracellular matrix, which are excessively
synthesized by cardiac myofibroblasts (17). It has been well documented that
TGF-β1 enhances the proliferative, migratory, and
collagen-producing properties of CFs by inducing myofibroblast
differentiation (4). In the
present study, we used TGF-β1 to stimulate CFs to possibly mimic
the ‘fibrotic’ situation. Herein, pretreatment with curcumin
significantly decreased mRNA and protein expression of α-SMA,
suggesting that curcumin-suppressed TGF-β1 induced cardiac
fibroblast differentiation. Moreover, TGF-β1 stimulation increased
protein levels of collagen I and III; however, these effects were
suppressed by curcumin. Taken together, these findings suggested
that curcumin attenuated cardiac fibrosis by inhibiting
myofibroblast differentiation, which is consistent with the
findings presented in previous studies (13,18).
Abnormal proliferation of cardiac fibroblast to
myofibroblasts causes excessive synthesis and accumulation of
extracellular matrix proteins, including collagen I and III,
eventually leading to cardiac fibrosis. Suppression of
proliferation and induction of apoptosis could help reduce
activated fibroblasts and thus alleviate profibrogenic effects. In
the present study, we demonstrated that curcumin regulated
proliferation and the cell cycle phase of CFs in vitro. Our
findings demonstrated that curcumin inhibited proliferation and
promoted G2/M phase arrest in TGF-β1-induces CFs, which constituted
one likely explanation of the cardiac protective effects of
curcumin on cardiac fibrosis. Similarly, cyclin B, and CDK1, key
modulators in G2/M checkpoint (19), were suppressed by curcumin
treatment. In addition, curcumin had no effect on proliferation and
cell cycle arrest in CFs that were not treated with TGF-β1,
indicating the safety in normal human CFs.
TGF-β1 is a potent pro-fibrotic cytokine that acts
through the canonical Smad pathway (20). After activation, p-Smad2 and 3
translocate into the nucleus to modulate the expression of
downstream genes, thereby exerting pro-fibrotic effects (4). In the present study, exogenous
treatment with of CFs with TGF-β1 significantly enhanced
phosphorylation of Smad2/3, and this effect was significantly
inhibited after administration of curcumin, which was in line with
the findings of a previous study (21). In addition, TGF-β1 can also act
through non-canonical pathways, such as the MAPK c-Jun N-terminal
kinase (c-JNK), ERK, and p38 MAPK pathways (22,23).
In this study, TGF-β1 promoted p38 MAPK and p-ERK phosphorylation,
while curcumin pretreatment markedly inhibited the increased
phosphorylation of p38 MAPK, and p-ERK. These results were
consistent with the data presented in a previous study (14). Taken together, these results
suggested that curcumin inhibited p38 MAPK and p-ERK
phosphorylation in TGF-β1-stimulated CFs.
In conclusion, curcumin inhibited TGF-β1-mediated CF
activities, including differentiation and collagen deposition by
inhibiting cell proliferation and promoting G2/M phase arrest.
Moreover, curcumin reduced cyclin B and CDK1 expression by
inhibiting smad2/3, p38 MAPK, and ERK signaling activation in
TGF-β1-induced CFs. Thus, curcumin presents a promising drug for
the treatment of cardiac fibrosis.
Acknowledgements
The authors would like to thank the Cardiac Surgery
Laboratory for their technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL and GF conceived and designed the study. GF, SC,
QH and LC performed the experiments and were major contributors in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schelbert EB, Piehler KM, Zareba KM, Moon
JC, Ugander M, Messroghli DR, Valeti US, Chang CC, Shroff SG, Diez
J, et al: Myocardial fibrosis quantified by extracellular volume is
associated with subsequent hospitalization for heart failure,
death, or both across the spectrum of ejection fraction and heart
failure stage. J Am Heart Assoc. 4:pii: e002613. 2015. View Article : Google Scholar
|
|
2
|
Kong P, Christia P and Frangogiannis N:
The pathogenesis of cardiac fibrosis. Cell Mol Life Sci.
71:549–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krenning G, Zeisberg E and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan R and Sheppard R: Fibrosis in heart
disease: Understanding the role of transforming growth factor-beta
in cardiomyopathy, valvular disease and arrhythmia. Immunology.
118:10–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ivey MJ and Tallquist MD: Defining the
cardiac fibroblast. Circ J. 80:2269–2276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hewlings SJ and Kalman DS: Curcumin: A
review of its' effects on human health. Foods. 6:pii: E92. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hazarey VK, Sakrikar AR and Ganvir SM:
Efficacy of curcumin in the treatment for oral submucous fibrosis-A
randomized clinical trial. J Oral Maxillofac Pathol. 19:145–152.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen YN, Hsu SL, Liao MY, Liu YT, Lai CH,
Chen JF, Nguyen MT, Su YH, Chen ST and Wu LC: Ameliorative effect
of curcumin-encapsulated hyaluronic acid-PLA nanoparticles on
thioacetamide-induced murine hepatic fibrosis. Int J Environ Res
Public Health. 14:pii: E11. 2016. View Article : Google Scholar
|
|
9
|
Tyagi N, Dash D and Singh R: Curcumin
inhibits paraquat induced lung inflammation and fibrosis by
extracellular matrix modifications in mouse model.
Inflammopharmacology. 24:335–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Y, Mou L, Yang F, Tu H and Lin W:
Curcumin attenuates cyclosporine A-induced renal fibrosis by
inhibiting hypermethylation of the klotho promoter. Mol Med Rep.
14:3229–3236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D,
Guo S, Ming Z and Liu C: Curcumin alleviates diabetic
cardiomyopathy in experimental diabetic rats. PLoS One.
7:e520132012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang NP, Wang ZF, Tootle S, Philip T and
Zhao ZQ: Curcumin promotes cardiac repair and ameliorates cardiac
dysfunction following myocardial infarction. Br J Pharmacol.
167:1550–1562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng Z, Yu XH, Chen J, Li L and Li S:
Curcumin attenuates cardiac fibrosis in spontaneously hypertensive
rats through PPAR-γ activation. Acta Pharmacol Sin. 35:1247–1256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung CC, Kao YH, Liou JP and Chen YJ:
Curcumin suppress cardiac fibroblasts activities by regulating
proliferation, migration, and the extracellular matrix. Acta
Cardiol Sin. 30:474–482. 2014.PubMed/NCBI
|
|
15
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van den Borne SW, Diez J, Blankesteijn WM,
Verjans J, Hofstra L and Narula J: Myocardial remodeling after
infarction: The role of myofibroblasts. Nat Rev Cardiol. 7:30–37.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Haas HJ, Arbustini E, Fuster V, Kramer
CM and Narula J: Molecular imaging of the cardiac extracellular
matrix. Circ Res. 114:903–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li HL, Liu C, de Couto G, Ouzounian M, Sun
M, Wang AB, Huang Y, He CW, Shi Y, Chen X, et al: Curcumin prevents
and reverses murine cardiac hypertrophy. J Clin Invest.
118:879–893. 2008.PubMed/NCBI
|
|
19
|
Canaud G and Bonventre JV: Cell cycle
arrest and the evolution of chronic kidney disease from acute
kidney injury. Nephrol Dial Transplant. 30:575–583. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma J, Ma S and Ding CH: Curcumin reduces
cardiac fibrosis by inhibiting myofibroblast differentiation and
decreasing transforming growth factor β1 and matrix
metalloproteinase 9/tissue inhibitor of metalloproteinase 1. Chin J
Integr Med. 23:362–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Voloshenyuk TG, Landesman ES, Khoutorova
E, Hart AD and Gardner JD: Induction of cardiac fibroblast lysyl
oxidase by TGF-β1 requires PI3 K/Akt, Smad3, and MAPK signaling.
Cytokine. 55:90–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang D, Liu X, Chen X, Gu J, Li F, Zhang
W and Zheng Y: Role of the MAPKs/TGF-β1/TRAF6 signaling pathway in
atrial fibrosis of patients with chronic atrial fibrillation and
rheumatic mitral valve disease. Cardiology. 129:216–223. 2014.
View Article : Google Scholar : PubMed/NCBI
|