Introduction
Scoparone is obtained from Artemisiae Scopariae
Herba, which is a traditional Chinese medicine. Artemisiae
Scopariae Herba refers to the aerial parts of Artemisia
capillaris Thunb. or Artemisia Scoparia Waldst. et Kit.,
which are mainly distributed in China, Japan, Korea and Mongolia
(1). Artemisiae Scopariae Herba
has numerous effects, and has been reported to possess
anti-inflammatory, antioxidant, antiviral and antitumor activities.
In addition, it regulates immunity, blood sugar, blood lipid levels
and blood pressure (1). Previous
experimental and clinical studies have reported that Artemisiae
Scopariae Herba exerts therapeutic effects against hepatobiliary
disease, postoperative sequelae of gynecological diseases,
maternal-fetal blood group incompatibility, severe acute
pancreatitis, pneumonia, diabetes, oral ulcers, acute
conjunctivitis and cancer (2–7).
Scoparone, which is also known as
6,7-dimethoxycoumarin, is a potent anti-inflammatory agent that has
been reported to exert anti-inflammatory effects via inhibition of
the transcriptional activity of nuclear factor-κB (8). Scoparone has previously been reported
to inhibit interleukin (IL)-8 and monocyte chemoattractant protein
1 production in U937 cells, and tumor necrosis factor-α, IL-6 and
IL-1β production in lipopolysaccharide-stimulated RAW264.7 cells
(9). Scoparone also possesses
antitumor activity in DU145 androgen-independent prostate cancer
cells via the inhibition of signal transducer and activator of
transcription 3 activity (10).
Furthermore, scoparone may enhance bilirubin clearance in the liver
by activating constitutive androstane receptor, which is a nuclear
receptor that acts as a transcription factor to upregulate the
expression of bilirubin glucuronyl transferase and other components
of the bilirubin metabolism pathway (11). In addition, scoparone exerts
protective effects against alterations in plasma lipoproteins,
vascular morphology and vascular reactivity in hyperlipidaemic
diabetic rabbits, which may be partly due to its free radical
scavenging abilities (12).
However, to the best of our knowledge, there are currently no
studies regarding the protective effects of scoparone against
ischemia-reperfusion (I/R)-induced cardiac myocyte injury, and the
associated mechanisms have not been reported. The present study
aimed to investigate the protective effects and molecular
mechanisms of scoparone on I/R-induced myocardial injury in an
in vitro primary cultured cardiac myocyte model of
oxygen-glucose deprivation/reoxygenation (OGD/R) and an in
vivo rat model of I/R.
Materials and methods
Reagents
Scoparone was purchased from Dalian Meilun Biology
Technology Co., Ltd., (Dalian, China).
Primary cultures of neonatal rat
cardiac myocytes
Cardiac myocytes were prepared from 20 neonatal
Sprague-Dawley rats (Male, 1–3 days-old, 10 g) as previously
described (13). Rats were
obtained from Hebei Medical University. Rats were housed under the
same standard environmental conditions of light (a 12-h light/dark
cycle), temperature (22±2°C), and ambient humidity of 50±10% with
free access to food and water. Briefly, the obtained ventricles
were cut into sections, which were digested with trypsin at 37°C
for 8 min. Subsequently, the supernatant was added to Dulbecco's
modified Eagle's medium (DMEM; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS,
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
digestion step was repeated 6–8 times, until the tissue sections
were digested. Cell suspensions were centrifuged at 800 × g for 5
min at 37°C, and the cells were cultured with DMEM containing 10%
FBS at 37°C in an atmosphere containing 5% CO2 for 2 h.
The non-adherent cell suspension was collected to separate
fibroblast cells and cardiac myocytes based on the varying
durations of adherence. The fibroblast cells and cardiac myocytes
were separated by its adherent at different time. The cardiac
myocytes (1×105 cells/ml) were cultured in DMEM
containing 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin and
2 g/ml vitamin B12. After 48 h, dead cells were removed, and the
medium was replaced with fresh medium containing 0.1 mM
bromodeoxyuridine. The cardiac myocytes were assessed by
immunofluorescence staining with α-actin (cat. no. sc-58670; Santa
Cruz Biotechnology, Inc., Dallas, Texas, USA) as previously
described (13); myocardial cell
purity was confirmed at >95%.
OGD/R model
OGD/R was performed on primary cultured neonatal rat
cardiac myocytes according to previously described methods
(14). Briefly, primary cultured
neonatal rat cardiac myocytes were randomly divided into five
groups: Control group, OGD/R group, scoparone low-dose group (S-L),
scoparone mid-dose group (S-M) and scoparone high-dose group (S-H).
The control group was incubated without any treatment. As for the
OGD/R group, the cell medium was replaced with DMEM (glucose-free),
which was preincubated with a mixture of 5% CO2 and 95%
N2 for 20 min to remove O2. Subsequently,
cells were cultured in an incubator containing 5% CO2
and 95% N2 at 37°C. After 3 h, the medium was replaced
with DMEM containing glucose, and cardiac myocytes were transferred
into an incubator containing 5% CO2 at 37°C for 1 h. The
cells in the S-L, S-M and S-H groups were pretreated with scoparone
at 100, 500 and 1,000 mg/ml, respectively, for 1 h prior to OGD/R,
which was conducted as described for the OGD/R group.
I/R rat model
Male Wistar rats with body weight ranging from
240–260 g, 7-weeks old were used in the present study. Rats were
obtained from Hebei Medical University and were housed under the
same standard environmental conditions of light (12-h light/dark
cycle), temperature (22±2°C), and ambient humidity of 50±10% with
free access to food and water. A total of 120 Wistar rats were
randomly divided into five groups: Sham-operated group (sham), I/R
group, scoparone low-dose group (S-L), scoparone mid-dose group
(S-M) and scoparone high-dose group (S-H) (n=24 rats/group).
Briefly, the rats were anesthetized with ether and an incision was
made in the chest to expose the heart. The left anterior descending
branch of the coronary artery was then isolated. With the exception
of the sham group, in the other groups, the coronary artery was
immediately ligated with line 0 (15); the groups underwent ischemia for 30
min, followed by 120 min of reperfusion. Scoparone was administered
1 h prior to ligation. The rats in the sham and I/R groups were
intravenously injected with 2 ml/kg normal saline, whereas the rats
in the S-L, S-M and S-H groups were intravenously injected with 25,
50, 100 mg/kg scoparone, respectively. The rats were sacrificed
after reperfusion and myocardial tissues were collected. The
present study was approved by the Ethics Committee of Hebei Medical
University (Shijiazhaung, China).
Cell viability assay
Following OGD/R in vitro, cell viability was
determined using MTT reagent. Cells (1×105/ml) were
cultured with MTT reagent (final concentration, 0.5 mg/ml) for 4 h
at 37°C. The medium was then removed and dimethyl sulfoxide (150
µl) was added to each well for 15 min at 37°C, in order to
solubilize formazan. The absorbance of formazan was measured at 492
nm, which is directly proportional to cell viability.
Cell apoptosis assay
The apoptotic rates of cardiac myocytes subjected to
OGD/R injury in vitro and I/R injury in vivo were
measured by terminal deoxynucleotidyl-transferase-mediated dUTP
nick end labeling (TUNEL) assay using a TUNEL kit (In situ
Cell Death Detection kit, fluorescein; Roche Diagnostics,
Indianapolis, IN, USA) according to manufacturer's protocols. The
cell nucleus was stained with DAPI (Roche Diagnostics) according to
manufacturer's protocols. Positive TUNEL staining was observed
under a fluorescence microscope (TE2000U; Nikon Corporation, Tokyo,
Japan) using a B-2A filter (450–490 nm excitation filter, 505 nm
dichroic mirror, 520 nm bandpass filter). The ratio was determined
by calculating the number of TUNEL-positive cells to the total
number of cells in each of the 10 fields of view.
ELISA assay
Following OGD/R in vitro, cell culture medium
was collected. The levels of lactate dehydrogenase (LDH) and
creatine kinase (CK) were measured using commercial ELISA kits
(cat. nos. JL13677 and 0-025486; Shanghai Jiang Lai Biological
Technology Co., Ltd., Shanghai, China), according to the
manufacturer's protocols. In addition, the cells were collected by
centrifugation (1,000 × g, 5 min, 37°C), and the levels of
malondialdehyde (MDA) and superoxide dismutase (SOD) were measured
using commercial ELISA kits (cat. no. JL13297 and JL11065; Shanghai
Jiang Lai Biological Technology Co., Ltd.) according to the
protocols recommended by the manufacturer.
Following I/R in vivo, serum samples were
collected by centrifugation (3,000 × g, 10 min, 4°C) from
myocardial tissues. The concentrations of LDH and CK in the serum
were detected using ELISA kits (cat. nos. JL13677 and 0-025486;
Shanghai Jiang Lai Biological Technology Co., Ltd.) according to
manufacturer's protocols. In addition, myocardial tissues were
collected from all rats to detect MDA and SOD levels. Ice
physiological saline is added to the myocardial tissues. A total of
10% myocardial tissue homogenate was made by using a high-speed
homogenizer and centrifuged 3,000 × g at 4°C. The supernatant was
collected. The levels of MDA and SOD in the supernatant were
determined using commercial kits (cat. nos. JL13297 and JL11065;
Shanghai Jiang Lai Biological Technology Co., Ltd.) according to
the manufacturer's protocols.
Reactive oxygen species (ROS)
assay
Intracellular ROS levels were measured using a
fluorescent carboxy-H2DCFDA probe, as previously
described (16).
Carboxy-H2DCFDA is hydrolyzed to H2DCF in
cells; H2DCF emits no fluorescence and cannot leave the
cell through the cell membrane. However, ROS can oxidize
H2DCF to DCF, which emits a green fluorescence;
therefore, detection of the fluorescence intensity of DCF can
reflect intracellular ROS levels; the fluorescence intensity is
proportional to the concentration of ROS. Following I/R injury, the
myocardium was homogenized in Hank's buffered salt solution and the
supernatant was collected. The samples were cultured with 10 µM
carboxy-H2DCFDA for 20 min at 37°C in the dark. The
fluorescence signal intensity of DCF was detected using a flow
cytometer with 488 nm excitation wavelength and 600 nm detection
wavelength. Expo 32 ADC (Beckman Coulter, Inc., Brea, CA, USA) was
used to analyze the fluorescence data.
Measurement of infarct area (IA)
Myocardial IA was determined using the triphenyl
tetrazolium chloride (TTC) staining method (17). Briefly, the heart samples were
frozen and sliced into 1 mm sections along the vertical axis. The
sections were incubated for 15 min at 37°C in 1% TTC, and were then
immersed in 4% formaldehyde for 12 h at 37°C. IA were determined
using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Western blotting
Following OGD/R in vitro, cardiac myocytes
were collected and lysed at 4°C with lysis buffer (Tiangen Biotech,
Co., Ltd., Beijing, China) containing 1 µM phenylmethylsulfonyl
fluoride. Protein concentration was determined according to the
bicinchoninic acid method. Equal amounts of protein (100 µg) were
separated by 10% SDS-PAGE and were electrotransferred onto a
polyvinylidene fluoride membrane. The membrane was blocked with
Tris-buffered saline (TBS) containing 5% skimmed milk for 1 h at
37°C, and was then incubated with primary antibodies against B-cell
lymphoma 2 (Bcl-2; cat. no. ab692), Bcl-2-associated X protein
(Bax; cat. no. ab77566), caspase-3 (cat. no. ab13585) and
cytochrome c (Cyt C; cat. no. ab110325; Abcam, Cambridge, MA
USA), all 1:100 dilution, at 4°C overnight. Subsequently, the
membrane was washed with TBST [TBS, (pH 7.5) containing 0.1%
Tween-20] and was incubated with an anti-Mouse immunoglobulin G
(IgG) H&L secondary antibody (1:10,000; cat. no. ab6785; Abcam)
for 1 h at 37°C. Signals were then developed using the Enhanced
Chemiluminescence Plus Western Blotting Detection system (Amersham;
GE Healthcare Life Sciences, Little Chalfont, UK). Signal
intensities were semi-quantified by densitometry using ImageJ v1.45
software (National Institutes of Health, Bethesda, MD, USA) and
were normalized against the corresponding β-actin (cat. no. ab8226;
Abcam) signals. The antibody was diluted by 1:1,000 and incubated
for 1 h at 37°C.
Immunohistochemical staining
Harvested myocardial tissues were fixed in formalin
overnight at 37°C, paraffin embedded; serial sections (3–5 µm)
obtained. The myocardial sections were blocked with 3% hydrogen
peroxide for 15 min at room temperature. Subsequently, the sections
were microwaved in 10 mmol/l (pH 8.0) EDTA (Sangon Biotech Co.,
Ltd., Shanghai, China) for 2 min, incubated with 5% goat serum
(Shanghai Haoran Bio, Shanghai, China) for 1 h at 37°C, and
incubated overnight at 4°C with various antibodies (Cyt c,
caspase-3, Bcl-2 and Bax) as aforementioned. Anti-Mouse IgG H&L
was used as aforementioned. To analyze staining, the following
systems were used: PicTure PV6000 and Elivision Plus (Fuzhou Maixin
Biotech Development Co., Ltd., Fuzhou, China). Finally, the
sections were counterstained with hematoxylin (3 min, 37°C). An
Olympus BX41 brightfield microscope (Olympus Corporation, Tokyo,
Japan) was used to observe sections.
Statistical analysis
Data are presented as the means ± standard
deviation. Experiments were repeated in triplicate. All data were
analyzed using one-way analysis of variance followed by Bonferroni
post hoc test (SPSS software package version 19.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Protective effects of scoparone on
OGD/R injury in cardiac myocytes
Following OGD/R injury in cardiac myocytes, cell
viability and SOD activity were significantly reduced, whereas LDH
release, MDA levels, CK activity and cell apoptosis were markedly
increased. Scoparone, at concentrations of 100, 500 and 1,000
mg/ml, attenuated these alterations in a dose-dependent manner
(Figs. 1–3). In cardiac myocytes treated with 100,
500 and 1,000 mg/ml scoparone prior to OGD/R injury, cell viability
was increased by 17.9, 28.7 and 65.7%, respectively; cell apoptosis
was decreased by 27.0, 41.7 and 62.2%, respectively; LDH levels
were decreased by 12.8, 32.1 and 52.8%, respectively; MDA
production was decreased by 13.7, 28.6 and 47.4%, respectively; SOD
activity was increased by 24.4, 56 and 82.4%, respectively; and CK
activity was decreased by 24.2, 47.2 and 62.7%, respectively.
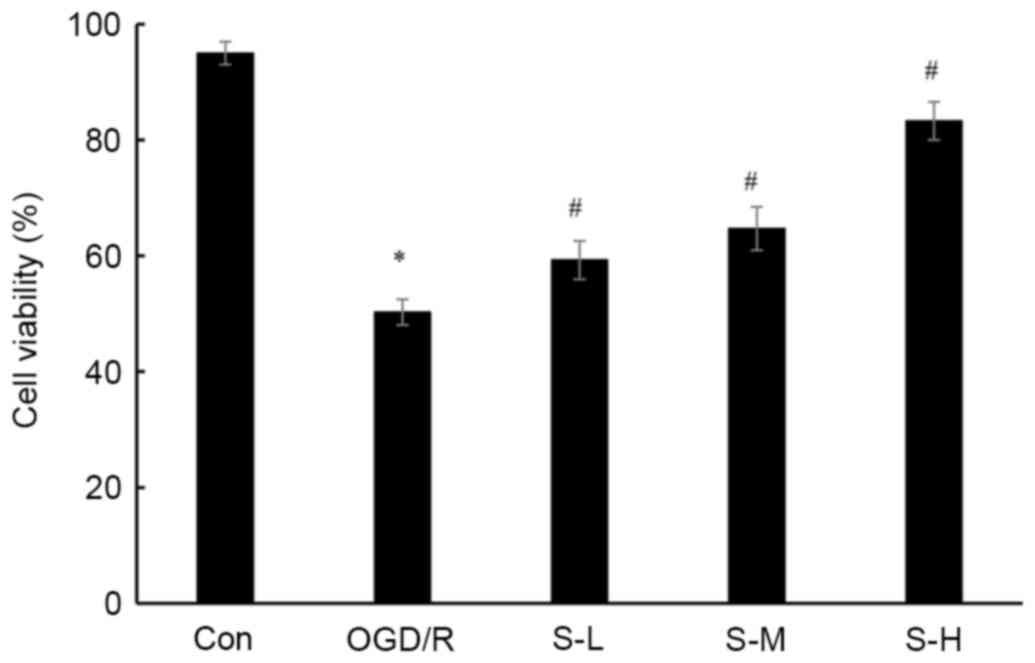 | Figure 1.Effects of scoparone on viability of
cardiac myocytes following OGD/R injury. Con group, cells were
cultured under normal conditions; OGD/R group, cells were subjected
to 3 h OGD and 1 h recovery; S-L, S-M and S-H groups, cells were
pretreated with scoparone at concentrations of 100, 500 and 1,000
mg/ml, respectively, for 1 h prior to OGD/R. Data are presented as
the means ± standard deviation of three independent experiments.
*P<0.05 vs. the Con group; #P<0.05 vs. the OGD/R
group. Con, control; OGD/R, oxygen-glucose
deprivation/reoxygenation; S-H, scoparone high-dose; S-L, scoparone
low-dose; S-M, scoparone mid-dose. |
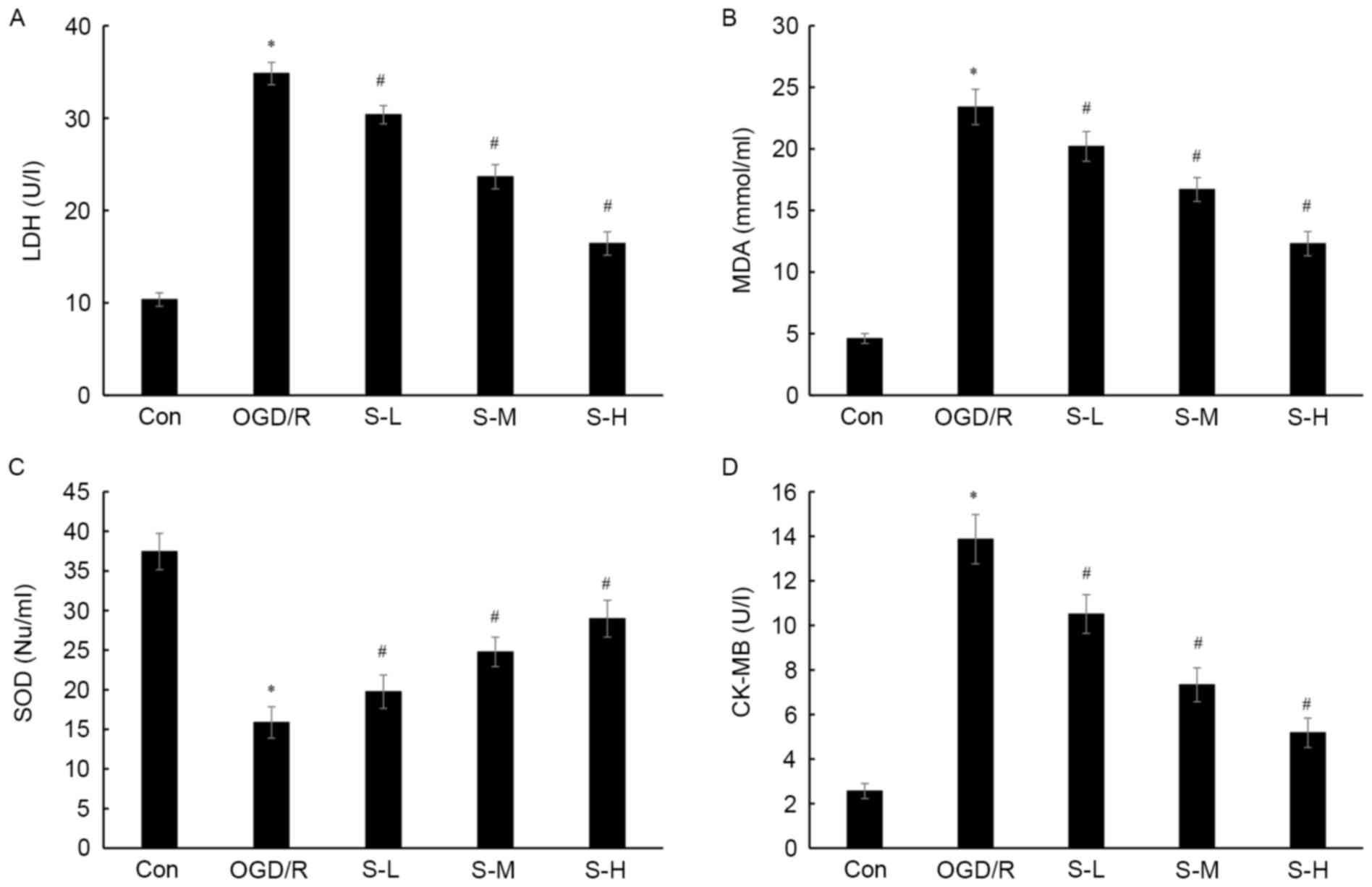 | Figure 3.Effects of scoparone on (A) LDH, (B)
MDA, (C) SOD and (D) CK levels in cardiac myocytes following OGD/R
injury. Con group, cells were cultured under normal conditions;
OGD/R group, cells were subjected to 3 h OGD and 1 h recovery; S-L,
S-M and S-H groups, cells were pretreated with scoparone at
concentrations of 100, 500 and 1,000 mg/ml, respectively, for 1 h
prior to OGD/R. Data are presented as the means ± standard
deviation of three independent experiments. *P<0.05 vs. the Con
group; #P<0.05 vs. the OGD/R group. CK, creatine
kinase; Con, control; LDH, lactate dehydrogenase; MDA,
malondialdehyde; OGD/R, oxygen-glucose deprivation/reoxygenation;
S-H, scoparone high-dose; S-L, scoparone low-dose; S-M, scoparone
mid-dose; SOD, superoxide dismutase. |
Protective effects of scoparone on I/R
injury in rats
Following I/R injury in rats, the levels of LDH and
MDA, and SOD and CK activities were detected. I/R significantly
increased the LDH levels (4,152.51±487.31 U/l), MDA production
(10.547±0.92 mmol/ml), and CK activity (1,137.18±106.35 U/ml),
whereas SOD activity was significantly decreased (1,684.68±143.56
U/l) compared with in the sham group (Fig. 4; P<0.05). Conversely, compared
with the I/R group, scoparone was revealed to significantly
decrease the LDH levels (4,034.15±428.23, 3,502.53±412.04 U/l and
3,425.14±482.47 U/l, respectively; P<0.05), MDA production
(8.677±0.495, 7.621±0.587 and 6.805±0.647 mmol/ml, respectively;
P<0.05), and CK activity (924.478±38.841, 766.758±43.812 and
708.158±83.958 U/l, respectively; P<0.05), whereas SOD activity
was increased (1,977.341±176.902, 2,173.923±185.483 and
2,365.837±166.384 U/l, respectively; P<0.05) in a dose-dependent
manner, compared with the I/R group (Fig. 4).
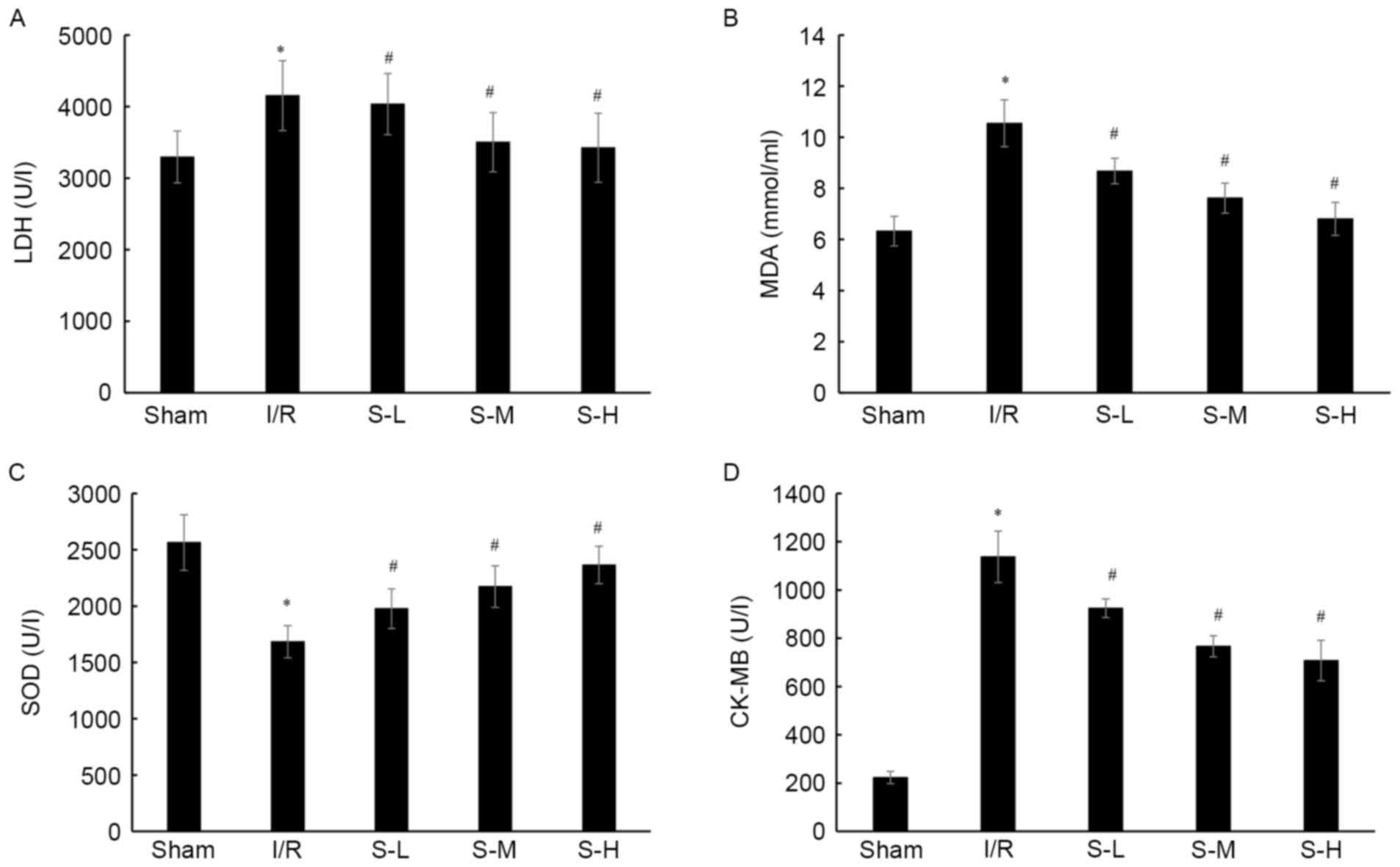 | Figure 4.Effects of scoparone on (A) LDH, (B)
MDA, (C) SOD and (D) CK levels in rats following I/R injury. With
the exception of the sham group, the other groups underwent
ischemia for 30 min, followed by 120 min of reperfusion. Rats in
the sham and I/R groups were intravenously injected with 2 ml/kg
normal saline. Rats in the S-L, S-M and S-H groups were
intravenously injected with 25, 50 and 100 mg/kg scoparone
respectively. Data are presented as the means ± standard deviation
(n=24/group). *P<0.05 vs. the sham group; #P<0.05
vs. the I/R group. CK, creatine kinase; I/R, ischemia-reperfusion;
LDH, lactate dehydrogenase; MDA, malondialdehyde; S-H, scoparone
high-dose; S-L, scoparone low-dose; S-M, scoparone mid-dose; SOD,
superoxide dismutase. |
Effects of scoparone on I/R
injury-induced cardiac myocyte apoptosis in rats
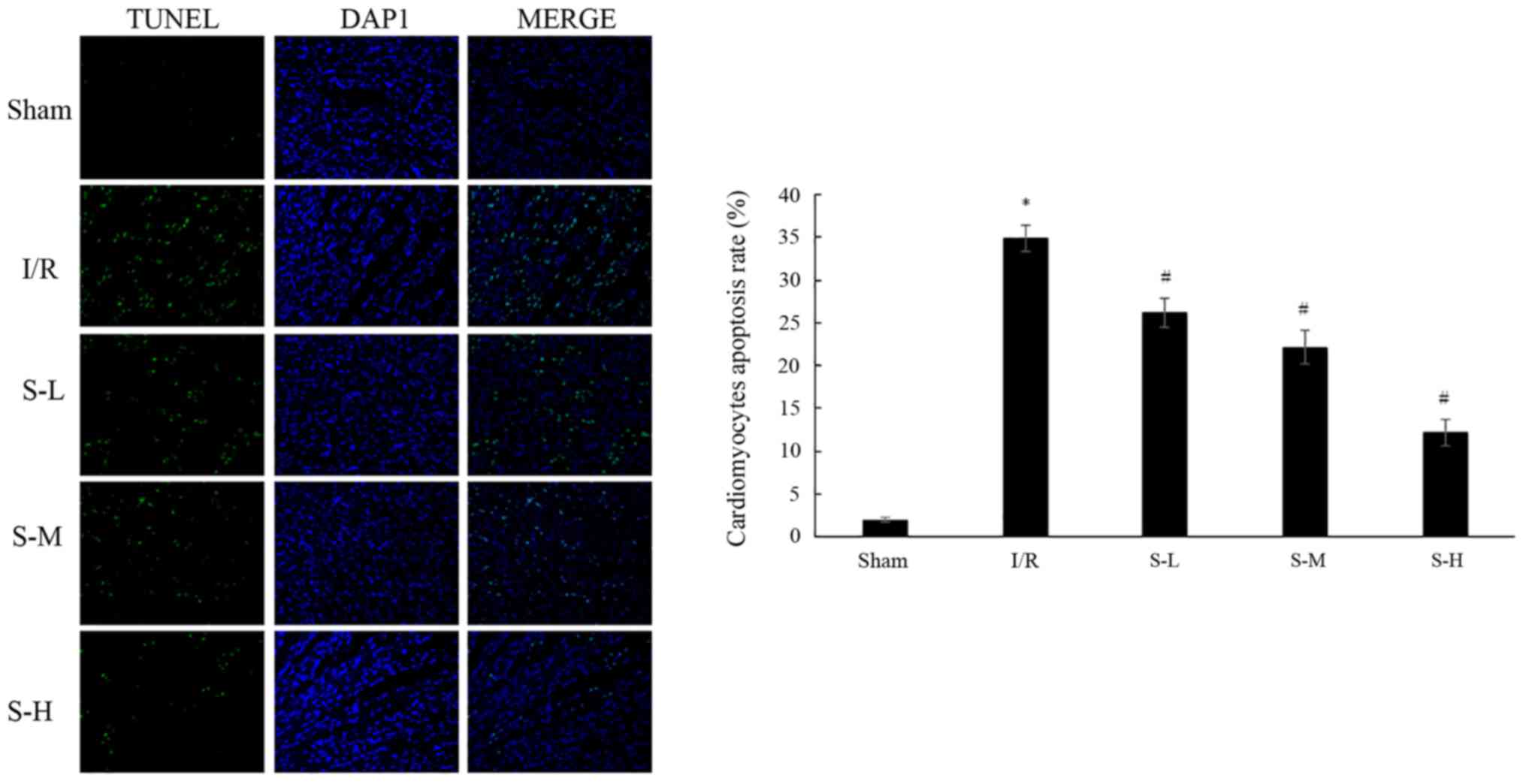
The apoptotic rate of cardiac myocytes was detected
by TUNEL staining. The results indicated that the percentage of
apoptotic cardiac myocytes was significantly increased in the I/R
group (34.95±1.53%), compared with in the sham group (Fig. 5; P<0.05). Treatment with
scoparone, at doses of 25, 50 and 100 mg/kg, resulted in a
dose-dependent reduction in the apoptotic rate of cardiac myocytes
following I/R injury, to 26.14±1.71, 22.11±1.98 and 12.13±1.54%,
respectively.
Effects of scoparone on I/R
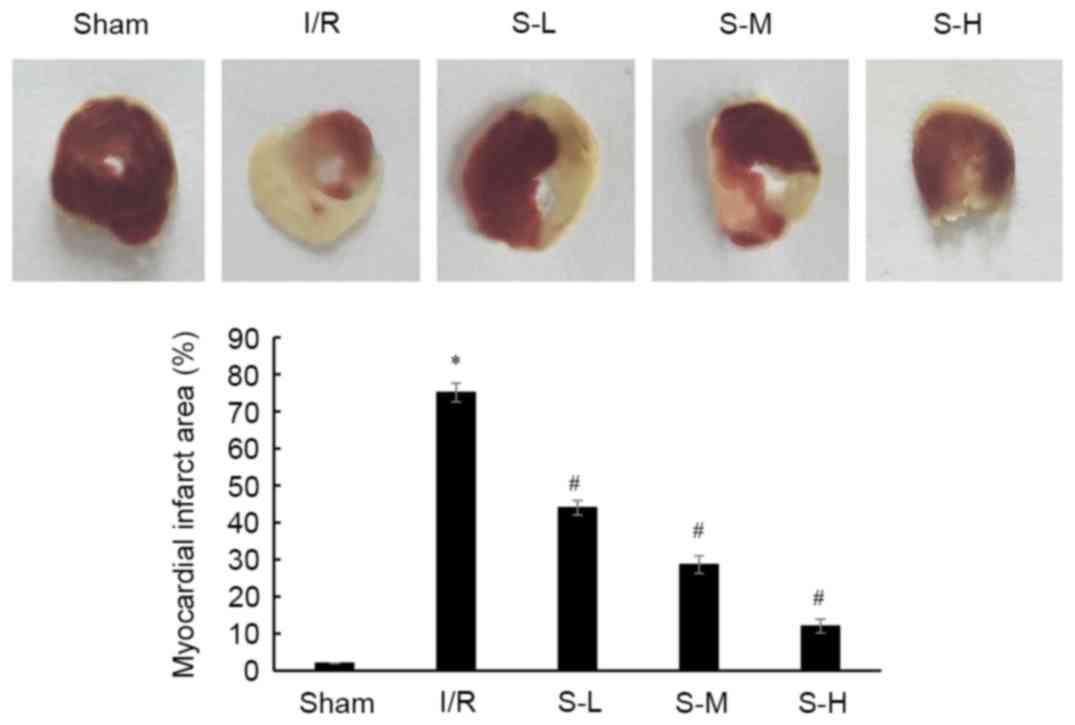
injury-induced myocardial IA in rats
TTC staining was conducted to determine the effects
of scoparone on myocardial infarct size in I/R rats (Fig. 6). In the I/R group, myocardial IA
was 85.09±2.53%, which was a significantly increased compared with
the sham group (P<0.05). Treatment of scoparone, at a dose of
25, 50 and 100 mg/kg, significantly reduced myocardial IA
(43.98±1.96, 25.64±2.36 and 9.05±1.87%, respectively) compared with
in the I/R group (P<0.05).
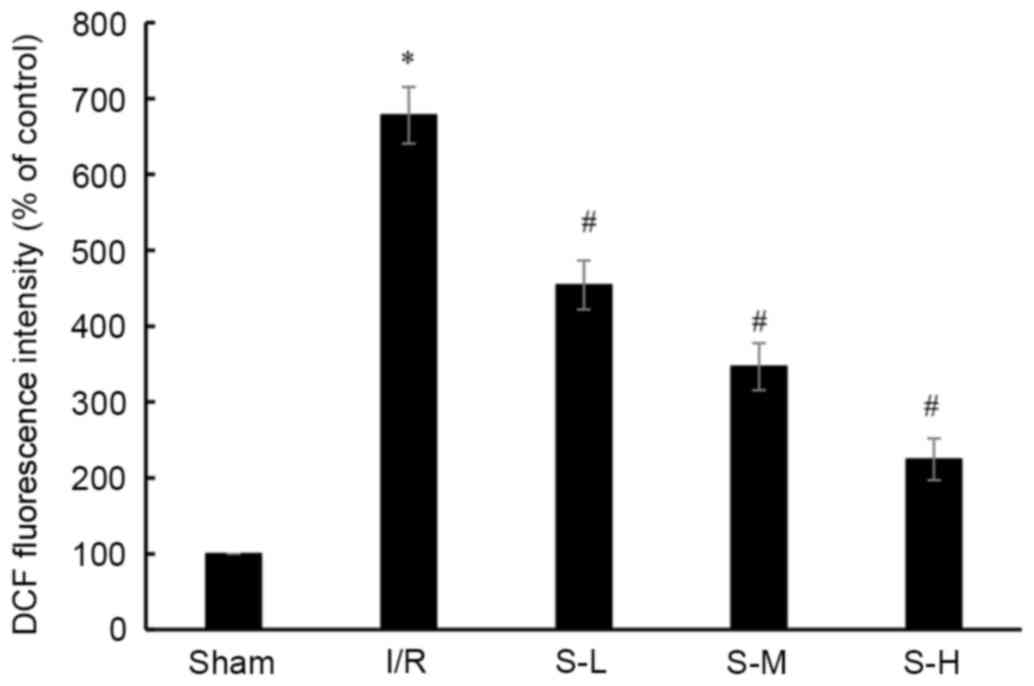
Effects of scoparone on ROS
concentration in rats following I/R injury
ROS concentration was determined following I/R using
a carboxy-H2DCFDA probe. The fluorescence intensity of
DCF represents the concentration of ROS. As shown in Fig. 7, I/R injury significantly increased
fluorescence intensity compared with in the sham group
(677.93±37.44 % of control). However, scoparone pretreatment, at a
dose of 25, 50 and 100 mg/kg, significantly decreased fluorescence
intensity (454.29±32.32, 346.64±30.90, 224.45±27.44% of control,
respectively) induced by I/R in a dose-dependent manner.
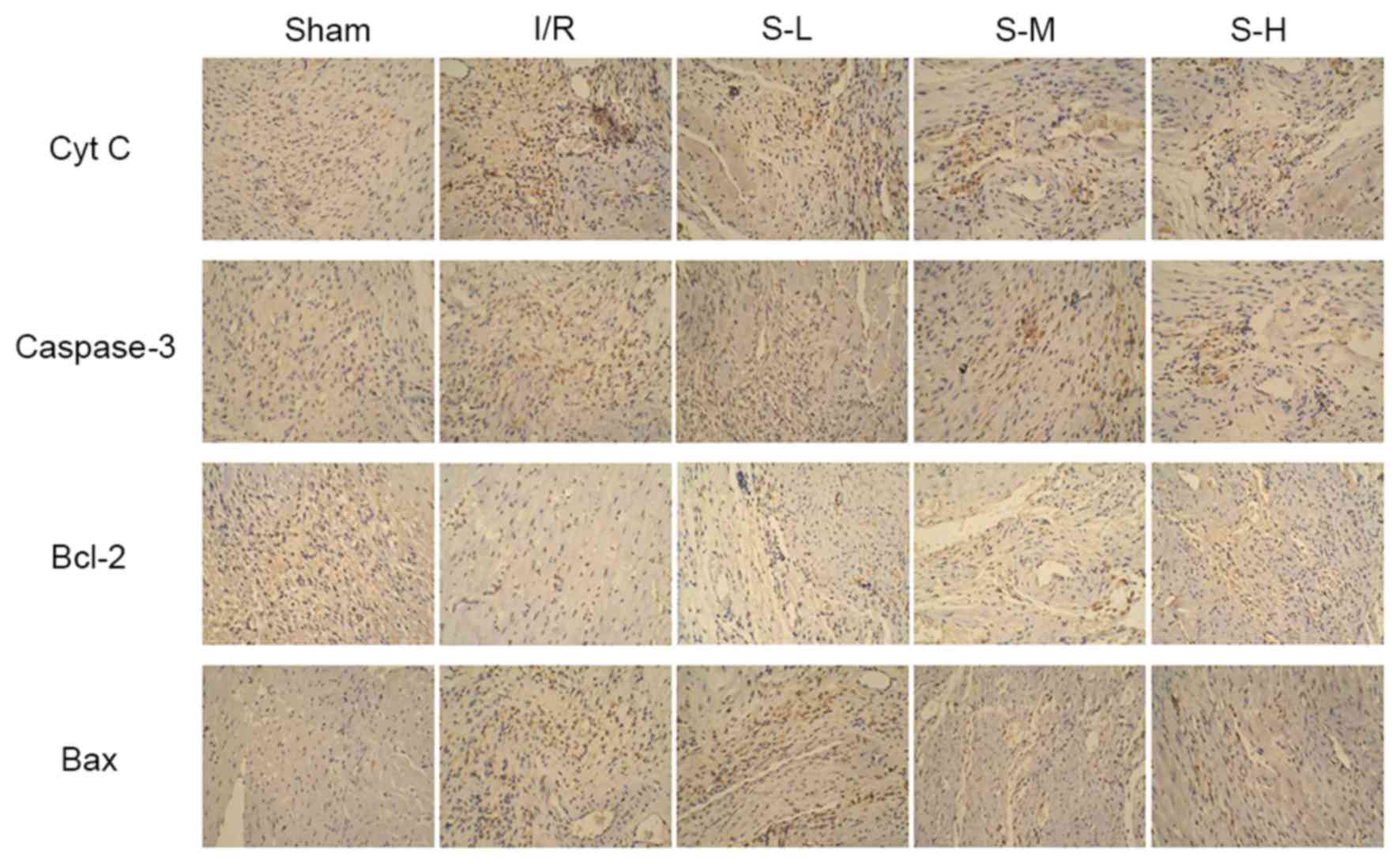
Effects of scoparone on caspase-3 and
Cyt C expression
As shown in Fig. 8,
the results of western blot analysis indicated that caspase-3 and
Cyt C expression were increased following OGD/R injury. Conversely,
scoparone, at concentrations of 100, 500, 1,000 mg/ml,
significantly and dose-dependently decreased caspase-3 and Cyt C
expression following OGD/R injury of cardiac myocytes.
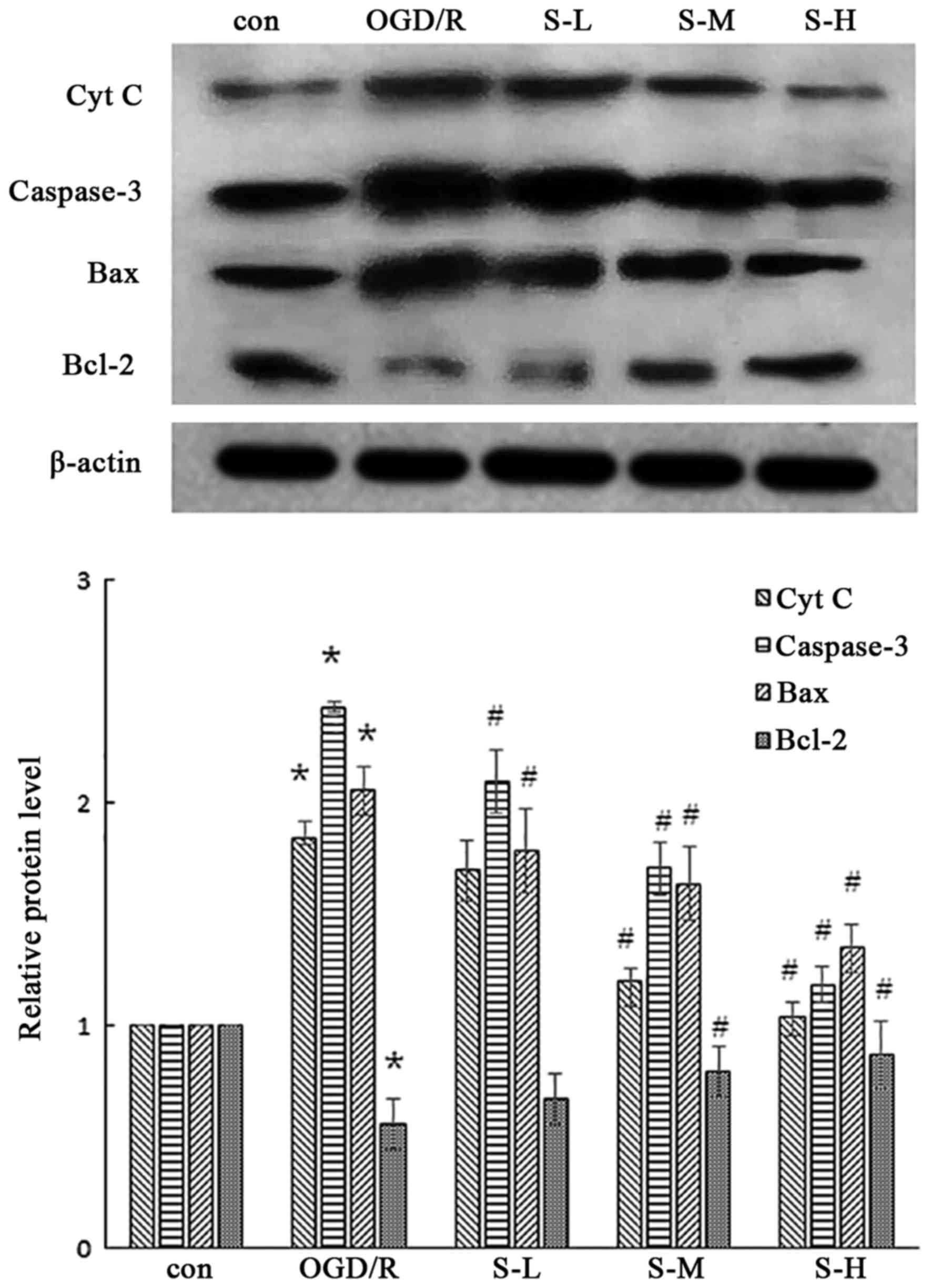 | Figure 8.Effects of scoparone on the expression
levels of caspase-3, Cyt C, Bcl-2 and Bax in cardiac myocytes
following OGD/R injury. *P<0.05 vs. the Con group;
#P<0.05 vs. the OGD/R group. Data are presented as
the means ± standard deviation (n=3). Bax, Bcl-2-associated X
protein; Bcl-2, B-cell lymphoma 2; Con, control; Cyt C, cytochrome
c; OGD/R, oxygen-glucose deprivation/reoxygenation; S-H,
scoparone high-dose; S-L, scoparone low-dose; S-M, scoparone
mid-dose. |
The effects of scoparone on caspase-3 and Cyt C
expression in I/R rats were detected by immunohistochemical
staining. As shown in Fig. 9, I/R
administration markedly increased caspase-3 and Cyt C expression
compared with in the sham group; however, scoparone inhibited
I/R-induced caspase-3 and Cyt C expression in a dose-dependent
manner.
Effects of scoparone on Bcl-2 and Bax
expression
As shown in Figs. 8
and 9, I/R injury induced an
increase in Bax expression and a decrease in Bcl-2 expression in a
cell model of OGD/R model and a rat model of I/R. Conversely,
treatment with scoparone attenuated these alterations in a
dose-dependent manner.
Discussion
Myocardial ischemia can cause tissue damage and cell
death over a certain period of time; therefore, rapid restoration
of blood perfusion is required for cardiac myocyte survival. I/R
injury in myocardial tissue is mediated by calcium overload, ROS
generation, cell apoptosis and disordered energy metabolism, which
may eventually lead to organ damage and myocardial metabolic
disorder. LDH is present in the cytoplasm of all tissues. When cell
apoptosis or necrosis occurs, the membrane structure is destroyed,
thus leading to the release of LDH from the cytoplasm to outside of
the cell. Therefore, detecting LDH levels may indirectly reflect
the extent of the damage to the cell membrane (18). CK is a cardiac-specific marker of
acute myocardial infarction and an indicator for myocardial tissue
injury. An increase in serum levels of CK indicates that the
myocardial cell biological membrane is damaged. Myocardial hypoxia
produces a large amount of ROS, which oxidize unsaturated fatty
acids, leading to damaged cell membrane structure and sarcoplasmic
reticulum calcium pump function, thus inducing extracellular
Ca2+ internal flow and calcium overload. Calcium
overload accelerates ROS generation and causes myocardial cell
damage (19). ROS also damage the
mitochondrial membrane system, oxidize Cyt C and reduce the
activity of ATP synthetase, thus resulting in mitochondrial
dysfunction, which can lead to cell apoptosis (20). MDA is formed by lipid peroxidation,
and is often used to quantify the extent of lipid peroxidation and
reflects the damage caused by ROS (14,21).
SOD is an important antioxidant, which has exhibits ROS scavenging
properties. During tissue ischemia, SOD is consumed in large
quantities, and SOD synthesis of SOD is suppressed, resulting in a
decrease in SOD levels. The serum levels of SOD are able to reflect
the ability to clear ROS.
It has previously been reported that Bcl-2 and Bax
are involved in the process of myocardial cell apoptosis (22), and the ratio of Bc1-2 to Bax may be
a critical factor for apoptosis (23). The caspase cascade also serves a
key role in apoptosis (24);
caspase-3 typically functions downstream of other caspases and
directly activates enzymes that are responsible for DNA
fragmentation in the intrinsic apoptosis pathway (25).
Scoparone is a major component of the shoot of
Artemisiae Scopariae Herba. In the present study, scoparone was
revealed to exert a protective effect on I/R-induced myocardial
injury.
The results of the present study demonstrated that
I/R leads to an increase in LDH levels, MDA and CK content, ROS
generation, cell apoptosis and myocardial IA, these alterations are
accompanied by reductions in cell viability and SOD activity.
However, pretreatment with scoparone prior to I/R injury,
significantly decreased LDH levels, MDA production, CK levels and
ROS generation, and increased SOD activity. These results indicated
that scoparone may reduce ROS-induced cell lipid peroxidation. In
addition, scoparone was able to increase cell viability, and
decrease cell apoptosis and myocardial IA in a dose-dependent
manner following I/R. Furthermore, there was a marked reduction in
the expression levels of Bax, caspase-3 and Cyt C, alongside a
significant increase in the expression levels of Bcl-2 in
scoparone-treated groups compared with in the I/R group. Myocardial
cell apoptosis is induced by various factors and involves two main
signaling pathways: The death receptor pathway and the
mitochondrial signaling pathway, during which the mitochondria
receive various apoptosis-stimulating signals.
Apoptosis-stimulating signal, including ROS, attack the
mitochondrial membrane, which is rich in polyunsaturated fatty
acids, thus causing mitochondrial swelling, decreases in membrane
fluidity, opening of the mitochondrial membrane permeability
transition pore and Cyt C release. Eventually, the caspase protease
cascade is activated; caspase-9 is activated first, which further
activates the downstream effector molecule caspase-3, thus leading
to cell apoptosis. The Bcl-2 family serves an important role in the
mitochondrial-dependent apoptotic pathway, and includes
proapoptotic and anti-apoptotic proteins. The Bcl-2 protein is
distributed in the outer mitochondrial membrane; Bcl-2 has an
anti-apoptotic role via inhibition of Cyt C release, thus
suppressing activation of the downstream caspase cascade. Bax is a
proapoptotic protein that belongs to the Bcl-2 family; Bax is
mainly located in the cytoplasm, and it can transfer to the outer
mitochondrial membrane in response to stimulation by apoptotic
signals. Bax can induce cell apoptosis by increasing permeability
of the mitochondrial outer membrane to promote the release of Cyt C
(26). The results of the present
study suggested that scoparone inhibited cell apoptosis by
influencing the aforementioned pathway.
In conclusion, scoparone may significantly reduce
the formation of MDA, enhance SOD activity, decrease LDH and CK
levels, and attenuate the myocardial IA. In addition, scoparone is
able to inhibit cell apoptosis, upregulate Bcl-2 expression, and
downregulate Bax, caspase-3 and Cyt C protein expression. These
findings suggested that scoparone can scavenge ROS, reduce
oxidative stress, protect mitochondria, improve myocardial
dysfunction and inhibit cell apoptosis induced by I/R via the
mitochondrial pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Key Research Program of Medical Science in Hebei Province (grant
no. ZD 20140004).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to further research
but are available from the corresponding author on reasonable
request.
Authors' contributions
CW and XG participated in the research design. CW,
YW and JM conducted experiments. CW and YW performed data analysis.
CW was a major contributor in writing the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hebei Medical University (Shijiazhuang, China).
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no competing
interests.
References
|
1
|
Okuno I, Uchida K, Nakamura M and Sakurawi
K: Studies on choleretic constituents in Artemisia
capillaris THUNB. Chem Pharm Bull (Tokyo). 36:769–775. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoon M and Kim MY: The anti-angiogenic
herbal composition Ob-X from Morus alba, Melissa officinalis and
Artemisia capillaris regulates obesity in genetically obese
ob/ob mice. Pham Biol. 49:614–619. 2011. View Article : Google Scholar
|
|
3
|
Ulicná O, Greksák M, Vancová O, Zlatos L,
Galbavý S, Bozek P and Nakano M: Hepatoprotective effect of rooibos
tea (Aspalathus linearis) on CCl4-induced liver damage in rats.
Physiol Res. 52:461–466. 2003.PubMed/NCBI
|
|
4
|
Lee HI, Seo KO, Yun KW, Kim MJ and Lee MK:
Comparative study of the hepatoprotective efficacy of Artemisia
iwayomogi and Artemisia capillaris on
ethanol-administered mice. J Food Sci. 76:T207–T211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin X, Uchiyama M, Zhang Q, Watanabe T and
Niimi M: Artemisiae capillaris herba induces prolonged
survival of fully cardiac allografts and generates regulatory cells
in mice. Transplant Proc. 44:1073–1075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwon OS, Choi JS, Islam MN, Kim YS and Kim
HP: Inhibition of 5-lipoxygenase and skin inflammation by the
aerial parts of Artemisia capillaris and its constituents.
Arch Pharm Res. 34:1561–1569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Habib M and Waheed I: Evaluation of
anti-nociceptive, anti-inflammatory and antipyretic activities of
Artemisia scoparia hydromethanolic extract. J
Ethnopharmacol. 145:18–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jang SI, Kim YJ, Kim HJ, Lee JC, Kim HY,
Kim YC, Yun YG, Yu HH and You YO: Scoparone inhibits PMA-induced
IL-8 and MCP-1 production through suppression of NFkappaB
activation in U937 cells. Life Sci. 78:2937–2943. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang SI, Kim YJ, Lee WY, Kwak KC, Baek SH,
Kwak GB, Yun YG, Kwon TO, Chung HT and Chai KY: Scoparone from
Artemisia capillaris inhibits the release of inflammatory
mediators in RAW 264.7 cells upon stimulation cells by
interferon-gamma Plus LPS. Arch Pharm Res. 28:203–208. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JK, Kim JY, Kim HJ, Park KG, Harris
RA, Cho WJ, Lee JT and Lee IK: Scoparone exerts anti-tumor activity
against DU145 prostate cancer cells via inhibition of STAT3
activity. PLoS One. 8:e803912013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang W, Zhang J and Moore DD: A
traditional herbal medicine enhances bilirubin clearance by
activating the nuclear receptor CAR. J Clin Invest. 113:137–143.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang HC, Weng YI, Lee CR, Jan TR, Chen YL
and Lee YT: Protection by scoparone against the alterations of
plasma lipoproteins, vascular morphology and vascular reactivity in
hyperlipidaemic diabetic rabbit. Br J Pharmacol. 110:1508–1514.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morisco C, Zebrowski D, Condorelli G,
Tsichlis P, Vatner SF and Sadoshima J: The akt-glycogen synthase
kinase 3beta pathway regulates transcription of atrial natriuretic
factor induced by beta-adrenergic receptor stimulation in cardiac
myocytes. J Biol Chem. 275:14466–14475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu L, Qiao H, Li Y and Li L: Protective
roles of puerarin and Danshensu on acute ischemic myocardial injury
in rats. Phytomedicine. 14:652–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Zhang XD, Yang J, Ding JW, Liu ZQ,
Li SG and Yang R: The cardioprotective effect of fluvastatin on
ischemic injury via down-regulation of toll-like receptor 4. Mol
Biol Rep. 38:3037–3044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao ZY, Luan P, Huang SX, Xiao SH, Zhao
J, Zhang B, Gu BB, Pi RB and Liu J: Edaravone protects HT22 neurons
from H2O2-induced apoptosis by inhibiting the
MAPK signaling pathway. CNS Neurosci Ther. 19:163–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Ma J and Liu H: Protective effect
of ischemic postconditioning against ischemia reperfusion-induced
myocardium oxidative injury in IR rats. Molecules. 17:3805–3817.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldhaber JI and Qayyum MS: Oxygen free
radicals and excitation-contraction coupling. Antioxid Redox
Signal. 2:55–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paredies G, Pelrosillo G, Pistolese M, Di
Venosa N, Federici A and Ruggiero FM: Decrease in mitoehondrial
complex 1 activity in ischemic/reperfused rat heart: In-volvement
of reactive oxygen species and eardiolipin. Cire Res. 94:53–59.
2004. View Article : Google Scholar
|
|
21
|
Zheng W, Huang LZ, Zhao L, Wang B, Xu HB,
Wang GY, Wang ZL and Zhou H: Superoxide dismutase activity and
malondialdehyde level in plasma and morphological evaluation of
acute severe hemorrhagic shock in rats. Am J Emerg Med. 26:54–58.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamm I, Schriever F and Dörken B:
Apoptosis: Implications of basic research for clinical oncology.
Lancet Oncol. 2:33–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lakhani SA, Masud A, Kuida K, Porter GA
Jr, Booth CJ, Mehal WZ, Inayat I and Flavell RA: Caspases 3 and 7:
Key mediators of mitochondrial events of apoptosis. Science.
311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Q, Li H, Wu Y, Shen W, Zeng L, Cheng H
and He L: Protective effects of muscone on ischemia-reperfusion
injury in cardiac myocytes. J Ethnopharmacol. 138:34–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song JQ and Liu YX: Effect of ramipril on
ischemia -reperfusion induced apoptosis of cardiomyocyte and
expression of apoptosis related genes. Chin Pharm Acol Bull.
22:625–628. 2006.
|