Introduction
Wear particle-induced aseptic loosening has become
one of the most important causes of arthroplasty failure, and
results in high healthcare costs and complex revision procedures
(1). Wear particles are the debris
from joint replacement implants that may induce inflammation and
bone resorption at the interface between the surface of a
prosthesis and its adjoining bone (2). These debris particles stimulate the
secretion of various proinflammatory cytokines, including tumor
necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-6 (3). Studies have demonstrated that TNF-α,
receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) and
IL-8 are present in the serum of patients with aseptic loosening
(4,5). At the bone-implant interface,
activated macrophages, multinucleated giant cells, osteoclasts and
fibroblasts are detected on the interface membranes (6). Macrophage recruitment and activation
increase the concentration of local pro-inflammatory factors and
ultimately lead to inflammation-induced osteoclastogenesis
(4). Regulation of the release of
osteoblast cytokines, including osteoprotegerin (OPG) and RANKL, is
another mechanism of wear particle-induced osteolysis (7). Therefore, the OPG-RANKL-RANK axis has
an important role in the pathophysiological process of aseptic
loosening (8). RANK is primarily
expressed on the plasma membrane of osteoclasts. RANKL activates
the NF-κB signaling pathway and subsequently induces
differentiation of osteoclasts and inhibits apoptosis by binding to
its specific receptor, RANK (9).
OPG, which is secreted by numerous cells, including osteoblasts and
mesenchymal stem cells, is a soluble competitive decoy receptor for
RANK and inhibits the NF-κB signaling pathway by decreasing the
binding of RANKL to RANK (10). In
other words, it inhibits the differentiation and activation of
osteoclasts, and induces their apoptosis. In the regulation of bone
metabolism, it is essential for the levels of OPG and RANKL to be
balanced. Therefore, osteolysis is one of the most intricate
complications prosthetic joint replacements and influences the
long-term functional recovery of patients.
The loss of estrogen is one of the physiological
characteristics of female menopause and mediates primary
osteoporosis, which is characterized by a reduction in bone density
and damage to bone structure (11). In the first few years of menopause,
the rapid decline of estrogen levels in women leads to an increase
in bone remodeling, which is manifested as increased bone formation
and bone resorption. However, the original balance of bone
metabolism later alters; bone resorption surpasses bone formation,
resulting in bone loss and eventually osteoporosis (12–14).
Estrogen deficiency during menopause has a direct effect on the
differentiation and activity of osteoblasts and osteoclasts, and it
additionally increases the secretion of inflammatory cytokines,
which may increase the activity of osteoclasts and reduce their
apoptosis (15,16). With the advancement of medical
technology, numerous chronic diseases are effectively treated.
Humans have a longer lifespan, however, consequently face the
complications of osteoporosis and possible total joint replacement
due to aging (17). A previous
study demonstrated that the cortical bone of patients with
osteoporosis is markedly thinner compared with a healthy
individual, particularly in the medial, lateral and posterior parts
of the bone (18). An osteoporotic
bone (particularly the medial and posterior parts) lacks a complete
structure and the intramedullary canal is wider compared with a
normal bone. These factors will slow bone growth in the direction
of the implant following joint replacement, thereby increasing the
risk of aseptic loosening (19).
In certain patients with severe osteoporosis, the surgical
treatment must be postponed until enough bone mass has been
restored (20). Diphosphate is a
drug that controls osteoporosis and inhibits osteoclast-mediated
bone resorption (21). A previous
study demonstrated that bisphosphonates may increase bone mass in
patients with osteoporosis and delay the development of the disease
(22). Another study revealed that
bisphosphonates reduce bone resorption at the bone-implant
interface and may prevent aseptic loosening following an
arthroplasty (23).
Strontium ranelate (SR), developed by Servier
Laboratories (Neuilly-sur-Seine, France), has been demonstrated to
be effective anti-osteoporosis therapeutic, and has the potential
to reduce the incidence of spinal and hip fracture in
postmenopausal women (24). SR has
been examined in numerous studies, which have verified its distinct
effects on bone metabolism. It has been reported to increase bone
mass and to suppress the activity of osteoclasts, thus preventing
bone loss (25). In another
previous study, SR has been observed to stimulate bone collagen
synthesis and to decrease the expression of functional osteoclast
markers, including carbonic anhydrase II and vitronectin receptor
(26).
In the present study, an ovariectomized mouse model
of long-term aseptic loosening was used to compare the effects of
SR with those of the traditional anti-osteoporosis drug alendronate
(ALN) on aseptic loosening under the conditions of osteoporosis
with estrogen deficiency.
Materials and methods
Wear particle preparation
Unmixed titanium particles (Zimmer Biomet, Warsaw,
IN, USA; ~5 µm) were used in the present study. Prior to injection,
the particles were rinsed in 70% ethanol for 48 h at room
temperature, washed twice in phosphate-buffered saline, and
subsequently autoclaved at 180°C for 6 h to remove any endotoxins.
A commercial detection kit (E-Toxate™; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to test whether the treated wear
debris contained endotoxins (27).
Animals
In the present study, 40 female C57BL/6j mice (18
months-old; Experimental Animal Center of Ningxia Medical
University, Yinchuan, China) were used, each weighing 26±2 g. All
the mice were housed in mechanically ventilated cages (4–5 mice per
cage) and maintained at 25°C constant temperature, constant
pressure, and on a 12/12 h light/dark cycle, with ad libitum
access to water and food. The experimental protocol was conducted
in accordance with the National Institutes of Health guidelines for
the care and use of laboratory animals (28) and was approved by the Ethics
Committee of the General Hospital of Ningxia Medical University
(Yinchuan, China).
Experimental groups and
treatments
The mice were randomly subdivided into four groups
(10 mice per group): Sham group; control group; SR group; and ALN
group. Ovariectomy or sham surgery was performed on the mice at 18
months of age. At 3 months after the induction of osteoporosis, all
the mice were subjected to joint prosthesis implantation into the
right lower extremity under general anesthesia induced by
intraperitoneal injection of Nembutal (0.6% pentobarbital sodium,
NeoBioscience Technology Co., Ltd., Shenzhen, China). All the
experimental methods were conducted as described previously
(29). In an aseptic environment,
the tibial plateau was exposed through the medial parapatellar
approach, and one titanium pin was implanted gently into the
proximal tibia so that the head of the pin was kept in the same
plane as the surface of the tibial plateau. The cut was washed with
normal saline containing 100 U/ml penicillin and 100 µg/ml
streptomycin, and each layer was closed separately with absorbable
string sutures. Prior to insertion of the titanium nails during the
surgical procedure, the tibia canal was injected with 10 µl
titanium suspension (4×104 particles of titanium in
normal saline). This action was followed by further 20 µl
injections of particles into the joint capsule every 2 weeks
following the operation, until the end of the experiment. Following
1 week of adaptive feeding, the SR group was orally administered SR
(Protelos®; Servier Laboratories; cat. no. S12911-2) at
625 mg/kg/day for 7 days per week. The ALN group received ALN
(Fosamax Plus; Merck & Co., Inc., Whitehouse Station, NJ, USA)
orally at 1 mg/kg/day for 7 days per week (30,31).
The animals were euthanized by carbon dioxide asphyxiation at 12
weeks following treatment with the drug.
Titanium prosthesis steadiness
examined by a pullout test
Following euthanasia, the tibia with the titanium
nail was removed from the body of each mouse. To expose the head of
the titanium implant, all muscles and tissues around the bone were
carefully removed. Each bone was fixed with dental cement onto a
special clamp, which was designed to align the long axis of the
implants with the long axis of the HP-100 Control electronic
universal testing machine (Yueqing Zhejiang Instrument Scientific
Co., Ltd., Zhejiang, China). With the mouse limb and the custom
fixture properly positioned, the HP-100 device pulled the pin out
of the tibia at a rate of 2 mm/min. The load values were registered
automatically by software (Edburg 1.0; Yueqing Zhejiang Scientific
Instrument Co., Ltd.).
Micro-computed tomography (CT)
scans
The tibias (with all soft tissues removed) from four
mice in each group were fixed in 4% paraformaldehyde at 4°C for 4
weeks for scanning by micro-CT in a SkyScan 1176 scanner (Bruker
microCT, Kontich, Belgium) at a resolution of 9 µm. The micro-CT
scans were acquired at 900 ms exposure time, 45 kW voltage and 550
mA current. Auto data analysis software (NRecon ver. 1.1.11; Bruker
microCT) was used to reconstruct and acquire images from the
micro-CT analyses and to evaluate the bone volume fraction (BV/TV),
trabecular thickness (Tb.Th), trabecular number (Tb.N), bone volume
(BV) and bone surface/bone volume ratio (BS/BV) of the shinbone
surrounding the titanium nail. The structure model index (SMI) is a
method intended for determining the plate- or rod-like geometry of
trabecular structures. It uses the alteration in surface area (BS,
from Isosurface) as volume increases infinitesimally to calculate
SMI=0 for plates, 3 for rods and 4 for solid spheres (32).
ELISA
This assay was performed to detect the IL-1β and
TNF-α protein expression levels in mouse serum. The quantitative
analysis was performed using mouse-specific ELISA kits (TNF-α; cat.
no. EMC102a.96; and IL-lβ; cat. no. EMC001b.96; NeoBioscience
Technology, Co., Ltd., Shenzhen, China), and the assay was
performed, according to the manufacturer's protocol.
Western blot analysis
The tissue surrounding the implant was frozen in
liquid nitrogen and ground with a chilled mortar and pestle.
Radioimmunoprecipitation assay buffer with 1 mM
phenylmethylsulfonyl fluoride (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China) was used to lyse the tissue. The protein
concentration was measured with a bicinchoninic assay kit (Nanjing
KeyGen Biotech Co., Ltd.). Subsequently, 30 µg protein mixed with
5X loading buffer was separated by Tris-glycine SDS-PAGE on a 12%
gel and transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were incubated for 1
h at room temperature in Tris-buffered saline with 0.5% Tween-20
(TBST) containing 5% nonfat dry milk, and subsequently incubated
overnight at 4°C with the following primary antibodies:
Anti-osteocalcin (OCN; cat. no. ab93876; 1:1,000),
anti-runt-related transcription factor 2 (Runx2; cat. no. ab23981;
1:1,000), anti-OPG (cat. no. ab183910; 1:1,000), anti-RANKL (cat.
no. ab9957; 1:1,000; all Abcam, Cambridge, UK), anti-β-actin (cat.
no. 4970; 1:1,000, Cell Signaling Technology, Inc., Danvers, MA,
USA) or anti-GAPDH (cat. no. 2118; 1:1,000, Cell Signaling
Technology, Inc.). Membranes were washed three times with TBST and
incubated for 1 h at room temperature with the horseradish
peroxidase-tagged secondary antibody (cat. no. PAB160009; 1:5,000;
OriGene Technologies, Inc., Beijing, China). The Enhanced
Chemiluminescent Western Blotting Detection Reagent (Nanjing KeyGen
Biotech Co., Ltd.) was used to test the bands. Quantity One
software (Ver. 4.6.7; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) served for semi quantitative analysis.
Statistical analysis
The data are presented as the mean ± standard
deviation. Each experiment was repeated three times. Differences
among the groups were evaluated by one-way analysis of variance
(ANOVA). The least-significant difference post hoc test was
conducted to distinguish the means between different groups. SPSS
19.0 (IBM Corp., Armonk, NY, USA) served as the analysis software.
P<0.05 was considered to indicate a significant difference.
Results
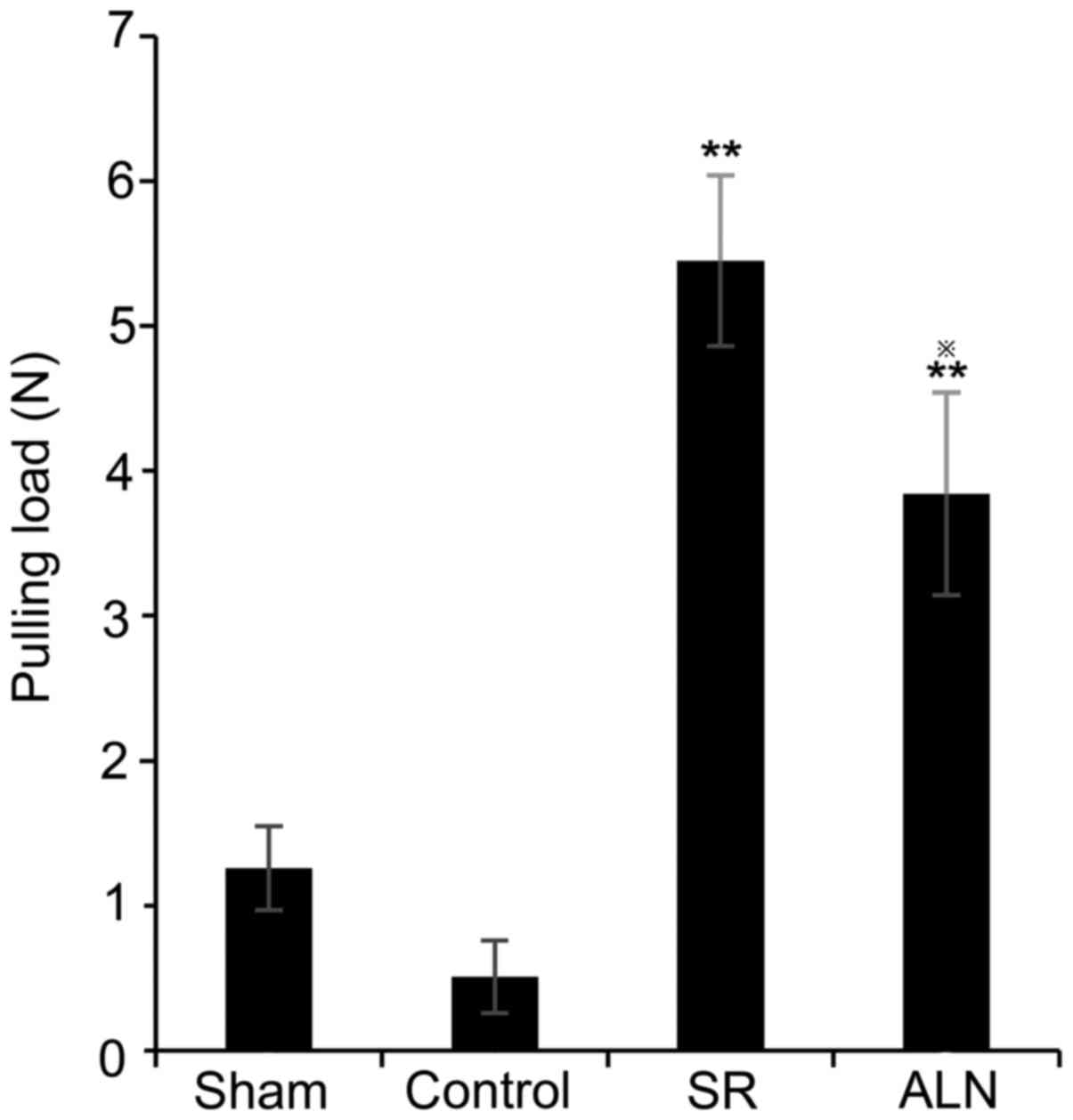
Pullout test
The special clamp was powerful enough to hold the
titanium nail during the entire pullout test. The average pulling
load was 0.51±0.25 N in the control group and 1.26±0.29 N in the
sham group (Fig. 1). There was a
significant difference in the pulling force between the SR group
(5.45±0.59 N) and the ALN group (3.84±0.7 N), the results of
one-way ANOVA demonstrated that there was a significant difference
between the SR group and the ALN group (P<0.05). Additionally,
the pulling force in the SR group and ALN group was significantly
increased compared with the control groups (Fig. 1; P<0.01).
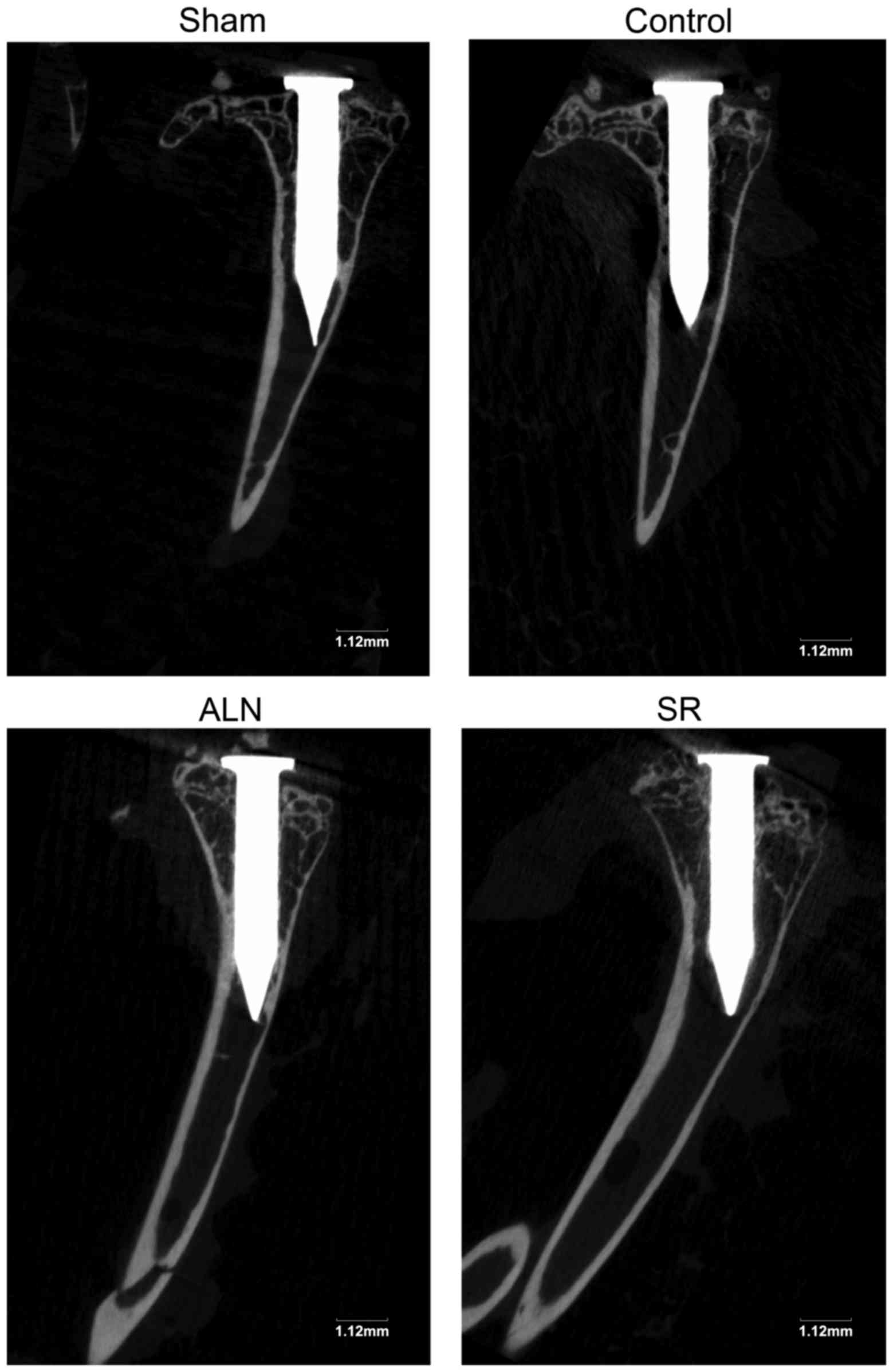
Micro-CT imaging analysis
The micro-CT scans demonstrated marked distinctions
in the bone microstructure among the four groups of mice. In
Fig. 2, although certain parts of
the surrounding bone are hidden in the shadow of the titanium pin,
osteolysis around the pin is still observed, being the most severe
in the control group.
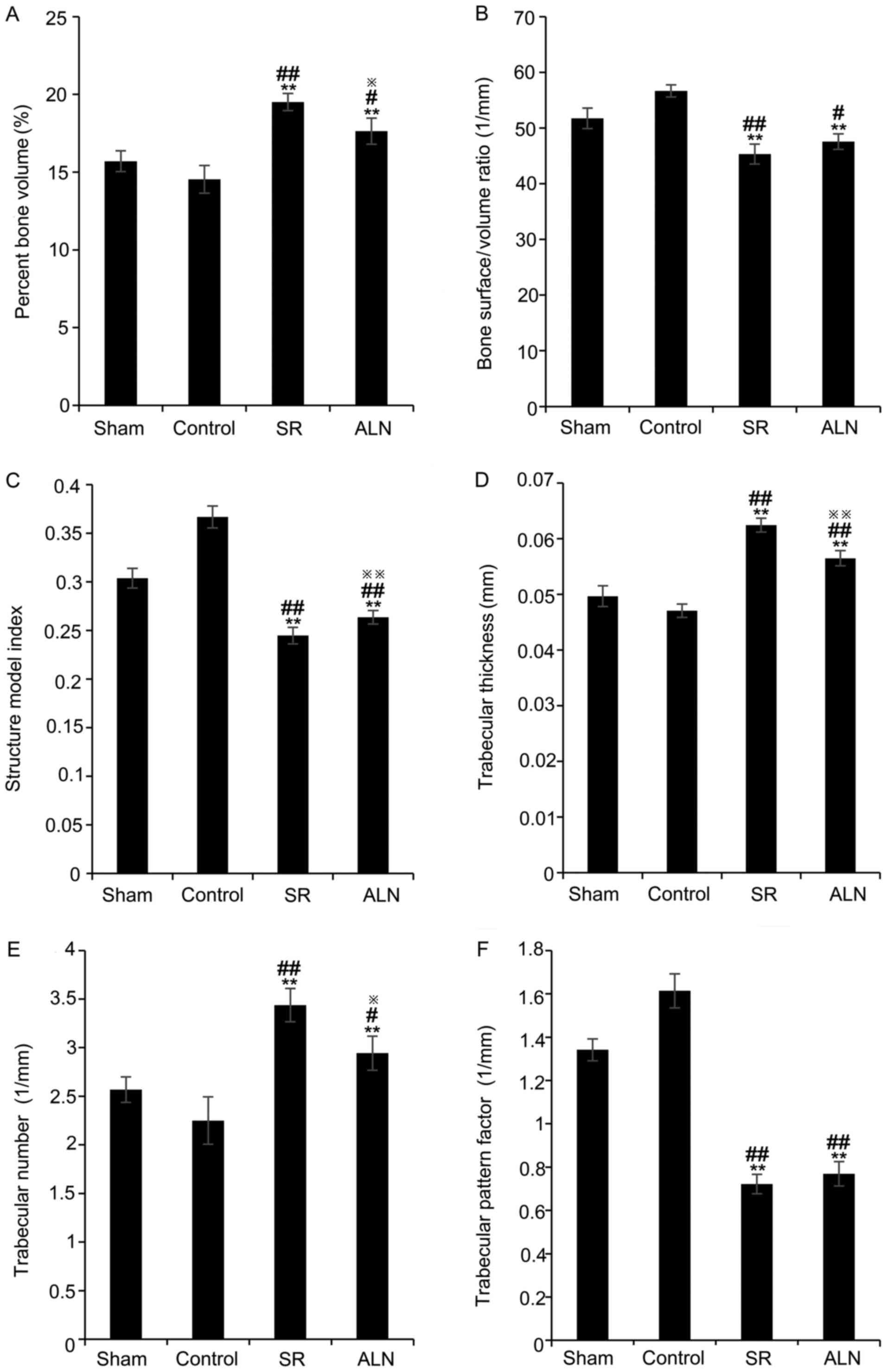
Data on the Tb.Th, Tb.N, BS/BV ratio, structure
model index (SMI), trabecular pattern factor (Tb.Pf) and BV/TV were
obtained from the micro-CT analysis of a region of interest. The
treatment of mice with SR and ALN increased the BV/TV in the two
drug-treated groups compared with the sham and control groups
(Fig. 3A; P<0.05; 15.70±0.67%
in the sham group, 14.53±0.89% in the control group, 19.50±0.55% in
the SR group and 17.63±0.84% in the ALN group); the results of
one-way ANOVA demonstrated that there was a significant difference
between the SR group and the ALN group (P<0.05). The BS/BV ratio
significantly decreased in the SR and ALN groups compared with the
sham and control groups (Fig. 3B;
P<0.05; 51.76±1.84 1/mm in the sham group, 56.69±1.09 1/mm in
the control group, 45.33±1.80 1/mm in the SR group and 47.58±1.39
1/mm in the ALN group); the results of one-way ANOVA demonstrated
that there was no significant difference between the SR group and
the ALN group (P>0.05). Additionally, the SMI significantly
decreased in the two drug-treated groups compared with the sham and
control groups (Fig. 3C;
P<0.01; 0.30±0.010 in the sham group, 0.37±0.011 in the control
group, 0.24±0.008 in the SR group, and 0.26±0.007 in the ALN
group); the results of one-way ANOVA demonstrated that there was a
significant difference between the SR group and the ALN group
(P<0.01). However, the Tb.Th significantly increased in the
drug-treated groups compared with the sham and control groups
(Fig. 3D; P<0.01; 0.05±0.001 mm
in the sham group, 0.05±0.001 mm in the control group, 0.06±0.001
mm in the SR group and 0.06±0.001 mm in the ALN group), and the
results of one-way ANOVA demonstrated that there was a significant
difference between the SR group and the ALN group (P<0.01), as
did the Tb.N (Fig. 3E; P<0.05;
2.57±0.13 1/mm in the sham group, 2.25±0.24 1/mm in the control
group, 3.44±0.17 1/mm in the SR group and 2.94±0.17 1/mm in the ALN
group); the results of one-way ANOVA demonstrated that there was a
significant difference between the SR group and the ALN group
(P<0.05). Tb.Pf significantly decreased in the SR and ALN groups
compared with the other two groups (Fig. 3F; P<0.01; 1.34±0.05 1/mm in the
sham group, 1.61±0.08 1/mm in the control group, 0.72±0.04 1/mm in
the SR group and 0.77±0.06 1/mm in the ALN group); the results of
one-way ANOVA demonstrated that there was no significant difference
between the SR group and the ALN group (P>0.05).
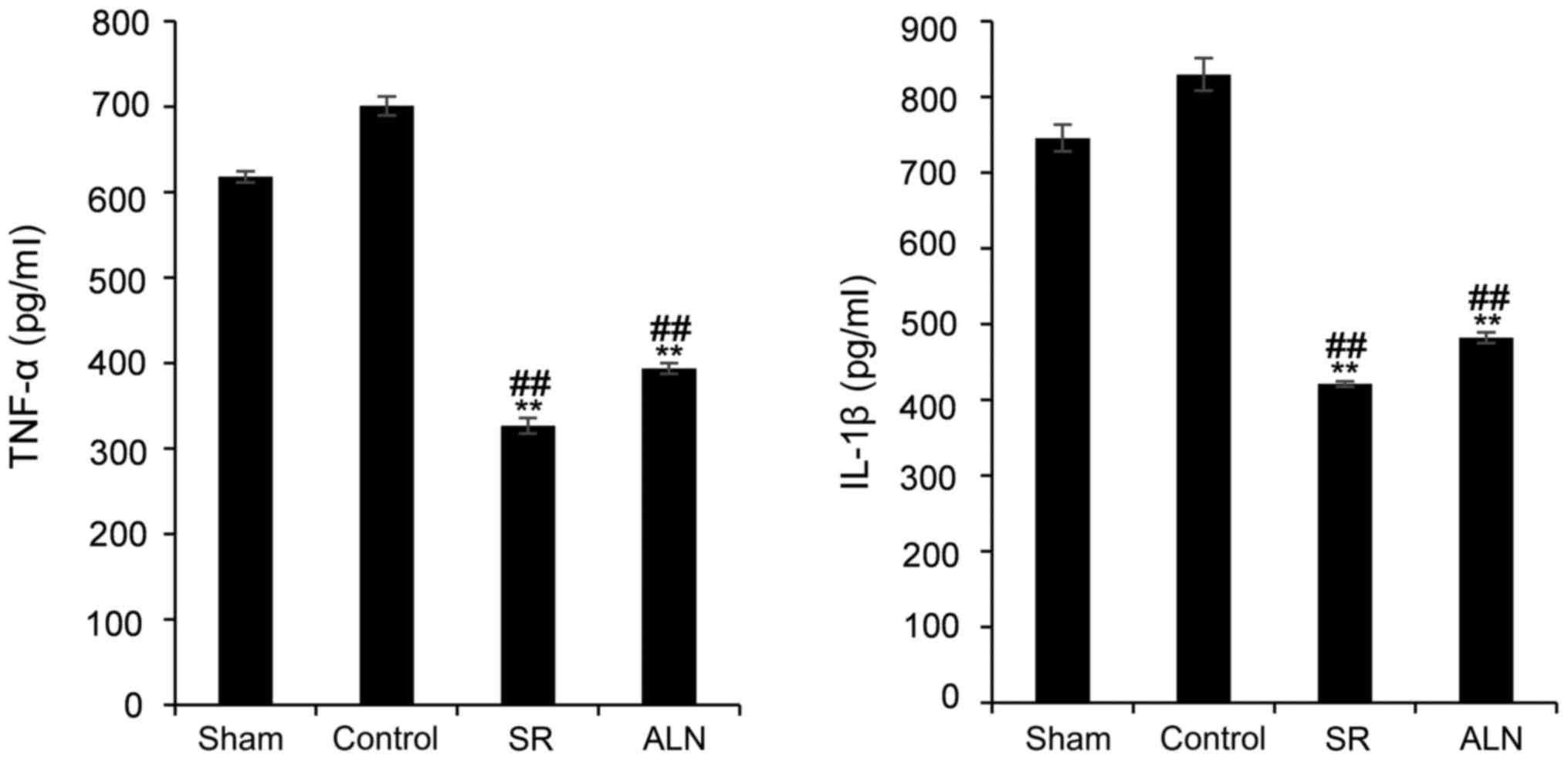
ELISA results
The serum expression levels of TNF-α in the SR and
ALN groups were significantly decreased compared with the sham and
control groups (Fig. 4; P<0.01;
618±7 pg/ml in the sham group, 701±11 pg/ml in the control group,
327±9 pg/ml in the SR group and 394±6 pg/ml in the ALN group).
Similarly, the expression level of IL-1β was significantly
decreased in the two drug-treated groups compared with the sham and
control groups (Fig. 4; P<0.01;
746±18 pg/ml in the sham group, 830±22 pg/ml in the control group,
421±4 pg/ml in the SR group and 482±7 pg/ml in the ALN group).
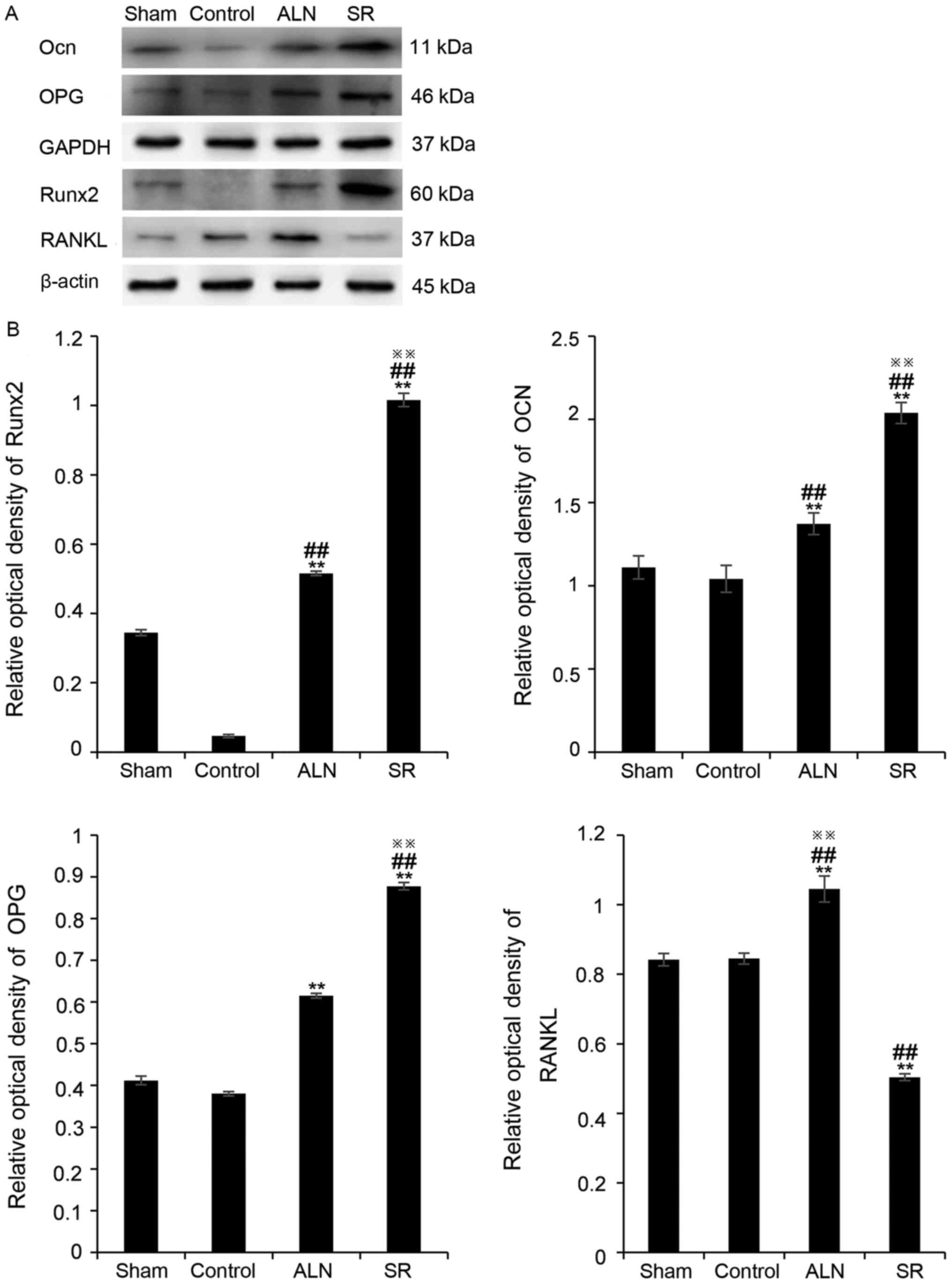
Western blot analysis
Western blotting was conducted to assess the
expression levels of the osteoblast markers Runx2 and OCN, and the
osteoblast cytokines OPG and RANKL (Fig. 5). The expression levels of Runx2
and OCN in the SR and ALN groups were significantly higher compared
with the sham and control groups (Fig.
5; P<0.01; Runx2, 0.34±0.008 in the sham group, 0.05±0.005
in the control group, 0.52±0.007 in the ALN group, and 1.02±0.019
in the SR group; the results of one-way ANOVA demonstrated that
there was a significant difference between the SR group and the ALN
group, P<0.01; OCN, 1.11±0.07 in the sham group, 1.04±0.08 in
the control group, 1.37±0.06 in the ALN group and 2.04±0.06 in the
SR group; the results of one-way ANOVA demonstrated that there was
a significant difference between the SR group and the ALN group,
P<0.01). ALN increased the expression levels of RANKL and OPG
simultaneously and decreased the OPG/RANKL ratio; however, SR
decreased the RANKL expression level, and increased the OPG
expression level and thus the OPG/RANKL ratio (Fig. 5; OPG, 0.41±0.01 in the sham group,
0.38±0.01 in the control group, 0.62±0.01 in the ALN group and
0.88±0.01 in the SR group; the results of one-way ANOVA
demonstrated that there was a significant difference between the SR
group and the ALN group, P<0.01; RANKL, 0.84±0.02 in the sham
group, 0.84±0.02 in the control group, 1.05±0.04 in the ALN group
and 0.50±0.01 in the SR group; the results of one-way ANOVA
demonstrated that there was a significant difference between the SR
group and the ALN group, P<0.01).
Discussion
Aseptic loosening is an important cause of the
failure of total joint prosthesis replacement. As the average age
of the population rises, an increasing number of postmenopausal
patients with osteoporosis require an arthroplasty. An imbalance
between bone resorption and bone formation is a common cause of
osteoporosis and aseptic loosening. Osteoporosis in patients
results in bone mass reduction, which increases the risk of aseptic
loosening. The animal experiments in the present study demonstrated
that implants fixed in the sham-operated group were more stable
compared with in ovariectomized mice. Chen et al (33) demonstrated that SR and ALN improve
the bone mass and bone quality of ovariectomized mice and promote
bone implant osseointegration. Nevertheless, to the best of our
knowledge, there are no studies confirming that SR or ALN prevent
aseptic loosening mediated by wear particles in ovariectomized
mice. The present results indicate that SR may increase osteoblast
activity, and inhibit the release of inflammatory factors,
osteoclast activity and differentiation in ovariectomized mice. SR
suppresses the aseptic loosening induced by wear particles. ALN may
additionally reduce osteolysis around the prosthesis by inhibiting
osteoclast activity. Oral administration of SR (625 mg/kg/day) was
observed to be more effective compared with ALN (1 mg/kg/week) at
reducing osteolysis in the ovariectomized mice.
Compared with the control group, the SR and ALN
groups exhibited increased BV/TV, Tb.Th and Tb.N, and lower SMI,
BS/BV and Tb.Pf around the tibial prosthesis. The present results
are consistent with those of a previous study (33). Previous studies involved young
female rats, whereas, 18-month-old mice were the subjects of the
present study; this choice may be the reason for the discrepancies
in results. The pullout test revealed that compared with the
control group, the drug-treated groups required a greater pull
force to extract the prosthesis from the tibia, as the prosthesis
and surrounding bone tissues were more solid. The SR group required
greater force in this assay compared with the ALN group. A previous
study demonstrated that the mechanisms of osteoporosis, induced by
different factors (including aging and estrogen deficiency), are
different (34). SR and ALN may
improve bone mass around a prosthesis (35); however, according to the micro-CT
data in the present study, the effect of SR is more marked.
Similar to the results of Bonnelye et al
(36), the present results suggest
that SR and ALN increase the expression of OPG in the tissue. The
difference between SR and ALN is that SR inhibited the expression
of RANKL and thus increase the OPG/RANKL ratio, in agreement with
the data of Karakan et al (25) and Huang et al (7). However, ALN promotes RANKL
expression, thus decreasing the OPG/RANKL ratio, as reported by
Faverani et al (37).
However, a specific study observed that SR has no effect on OPG and
RANKL expression in patients with osteoporosis, in contrast to the
present results (38). There are
numerous potential causes of this discrepancy, such as the
difference in the physiological environments between mice and
humans. Additionally, different tissues were collected in the
different studies. The present study measured the expression levels
of OPG and RANKL in the bone tissue around the mouse tibial
prosthesis, whereas Stuss et al (38) measured these expression levels in
human serum. The effect of SR on RANKL in their experiments was in
agreement with the results from the present study. SR and ALN may
significantly inhibit the release of proinflammatory factors; the
present results demonstrated that the serum expression levels of
TNF-α and IL-1β were significantly decreased in the SR and ALN
groups compared with the control group, in agreement with previous
studies (7,39,40,41).
TNF-α is an important factor in the regulation of osteoclast
differentiation. Raehtz et al (17) suggested that the TNF-α expression
level may affect osteoblast activity and bone formation. The
present data revealed that SR may inhibit osteoclast
differentiation and reduce bone resorption around the prosthesis by
suppressing the release of pro-inflammatory factors in
ovariectomized mice. From the detection of osteoblast markers, it
was identified that the expression levels of OCN and Runx2 in the
bone tissue around the prostheses were increased in the SR group
compared with the control group, in agreement with the results of
Bakker et al (42) and Guo
et al (43). Treatment with
ALN decreased the expression of OCN and Runx2 compared with
treatment with SR, as observed in previous studies conducted by
Chen et al (33) and Muise
et al (44). In the present
study, it was identified that there was a significant difference in
the level of runx2 between the control group and the sham group;
this may be due to suppression of osteogenic growth following
implantation of the prosthesis. Results of an in vitro study
by Kang et al (45),
examining the effects of ALN on osteoblasts, are inconsistent with
the present results; this discrepancy may be caused by the
difference in experimental materials. The study conducted by Kang
et al was an in vitro experiment, whereas the present
study observed effects in mice. The difference in the experimental
results may therefore be due to the interaction of various
biological factors in the bodies of the mice. Shimizu et al
(46) demonstrated that ALN may
inhibit osteoblast activity indirectly by increasing the
interaction between osteoclasts and osteoblasts.
Notably, previous studies have reported serious
adverse effects of SR, including Stevens-Johnson syndrome and toxic
epidermal necrolysis (47,48), although these were not observed in
the present study. Topical application of SR is a potential way to
minimize these effects (49).
Prostheses coated with SR may be able to inhibit aseptic loosening
(50,51).
In conclusion, SR and ALN have inhibitory effects on
aseptic loosening in ovariectomized mice, and SR may affect
osteogenesis and osteoclasts to inhibit aseptic loosening, in
agreement with the results of Wornham et al (52). SR has a better inhibitory effect on
aseptic loosening compared with ALN and may potentially serve as a
treatment of aseptic loosening in patients with osteoporosis.
However, certain studies (53,54)
reported that SR has serious adverse effects in practical
applications, thus raising safety concerns regarding its medicinal
use. However, in view of its excellent practical value, it is
necessary to examine possible solutions to its disadvantages.
Certain studies indicate that topical application of SR is a
potential treatment method (43)
and another study has demonstrated that a prosthesis with strontium
coating has the same inhibitory effect on aseptic loosening
(55).
Acknowledgements
The authors would like to thank Dr Doraemon Z.
Gbighe for guidance with experimental techniques.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460333/H0606).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TG participated in study design, performed the
experiments and data analysis, and drafted the manuscript. QJ
participated in study design, directed the execution of experiments
and revised the article critically for intellectual content. MZ, SS
and XC participated in performing the experiments. HY, SZ and HG
interpreted the results and revised the manuscript. All authors
approved the final version of the manuscript.
Ethics approval and consent to
participate
The experimental protocol in the present study was
conducted in accordance with the National Institutes of Health
Guidelines for the Care and Use of Laboratory Animals and was
approved by the Ethics Committee of the General Hospital of Ningxia
Medical University (Yinchuan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALN
|
alendronate
|
|
BV
|
bone volume
|
|
BS/BV
|
bone surface/bone volume ratio
|
|
BV/TV
|
bone volume fraction
|
|
OPG
|
osteoprotegerin
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
SR
|
strontium ranelate
|
|
Tb.N
|
trabecular number
|
|
Tb.Th
|
trabecular thickness
|
References
|
1
|
Wang ML, Sharkey PF and Tuan RS: Particle
bioreactivity and wear-mediated osteolysis. J Arthroplasty.
19:1028–1038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pioletti DP and Kottelat A: The influence
of wear particles in the expression of osteoclastogenesis factors
by osteoblasts. Biomaterials. 25:5803–5808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shao H, Shen J, Wang M, Cui J, Wang Y, Zhu
S, Zhang W, Yang H, Xu Y and Geng D: Icariin protects against
titanium particle-induced osteolysis and inflammatory response in a
mouse calvarial model. Biomaterials. 60:92–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flecher X, Rolland C, Rixrath E, Argenson
J, Robert P, Bongrand P, Wendling S and Vitte J: Local and systemic
activation of the mononuclear phagocyte system in aseptic loosening
of total hip arthroplasty. J Clin Immunol. 29:681–690. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pajarinen J, Lin T, Nabeshima A, Jämsen E,
Lu L, Nathan K, Yao Z and Goodman SB: Mesenchymal stem cells in the
aseptic loosening of total joint replacements. J Biomed Mater Res
A. 105:1195–1207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haleem-Smith H, Argintar E, Bush C,
Hampton D, Postma WF, Chen FH, Rimington T, Lamb J and Tuan RS:
Biological responses of human mesenchymal stem cells to titanium
wear debris particles. J Orthop Res. 30:853–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Li L, Yu X, Gu Z and Zhang X: The
inhibitory effect of strontium-doped calcium polyphosphate
particles on cytokines from macrophages and osteoblasts leading to
aseptic loosening in vitro. Biomed Mater. 9:0250102014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferreira E, Bortolin RH, Freire-Neto FP,
Souza K, Bezerra JF, Ururahy M, Ramos AMO, Himelfarb ST, Abreu BJ,
Didone TVN, et al: Zinc supplementation reduces rankl/opg ratio and
prevents bone architecture alterations in ovariectomized and type 1
diabetic rats. Nutr Res. 40:48–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szwarc MM, Kommagani R, Jacob AP, Dougall
WC, Ittmann MM and Lydon JP: Aberrant activation of the rank
signaling receptor induces murine salivary gland tumors. PLoS One.
10:e1284672015. View Article : Google Scholar
|
|
10
|
Neuerburg C, Wedemeyer C, Goedel J,
Schlepper R, Hilken G, Schwindenhammer B, Schilling AF, Jager M and
Kauther MD: The role of calcitonin receptor signalling in
polyethylene particle-induced osteolysis. Acta Biomater.
14:125–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Armas LA and Recker RR: Pathophysiology of
osteoporosis: New mechanistic insights. Endocrinol Metab Clin North
Am. 41:475–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riggs BL: The mechanisms of estrogen
regulation of bone resorption. J Clin Invest. 106:1203–1204. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Syed F and Khosla S: Mechanisms of sex
steroid effects on bone. Biochem Biophys Res Commun. 328:688–696.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frenkel B, Hong A, Baniwal SK, Coetzee GA,
Ohlsson C, Khalid O and Gabet Y: Regulation of adult bone turnover
by sex steroids. J Cell Physiol. 224:305–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gilbert L, He X, Farmer P, Boden S,
Kozlowski M, Rubin J and Nanes MS: Inhibition of osteoblast
differentiation by tumor necrosis factor-alpha. Endocrinology.
141:3956–3964. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manolagas SC: Birth and death of bone
cells: Basic regulatory mechanisms and implications for the
pathogenesis and treatment of osteoporosis. Endocr Rev. 21:115–137.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raehtz S, Bierhalter H, Schoenherr D,
Parameswaran N and McCabe LR: Estrogen deficiency exacerbates type
1 diabetes induced bone TNFα expression and osteoporosis in female
mice. Endocrinology. 158:2086–2101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dorr LD, Faugere MC, Mackel AM, Gruen TA,
Bognar B and Malluche HH: Structural and cellular assessment of
bone quality of proximal femur. Bone. 14:231–242. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dorr LD, Faugere MC, Mackel AM, Gruen TA,
Bognar B and Malluche HH: Structural and cellular assessment of
bone quality of proximal femur. Bone. 14:231–242. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bottai V, Dell'Osso G, Celli F, Bugelli G,
Cazzella N, Cei E, Guido G and Giannotti S: Total hip replacement
in osteoarthritis: The role of bone metabolism and its
complications. Clin Cases Miner Bone Metab. 12:247–250.
2015.PubMed/NCBI
|
|
21
|
Stepan JJ, Alenfeld F, Boivin G, Feyen JH
and Lakatos P: Mechanisms of action of antiresorptive therapies of
postmenopausal osteoporosis. Endocr Regul. 37:225–238.
2003.PubMed/NCBI
|
|
22
|
Chen BL, Xie DH, Zheng ZM, Lu W, Ning CY,
Li YQ, Li FB and Liao WM: Comparison of the effects of alendronate
sodium and calcitonin on bone-prosthesis osseointegration in
osteoporotic rats. Osteoporos Int. 22:265–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Hara LJ, Nivbrant B and Rohrl S:
Cross-linked polyethylene and bisphosphonate therapy for osteolysis
in total hip arthroplasty: A case report. J Orthop Surg (Hong
Kong). 12:114–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reginster JY, Brandi ML, Cannata-Andia J,
Cooper C, Cortet B, Feron JM, Genant H, Palacios S, Ringe JD and
Rizzoli R: The position of strontium ranelate in today's management
of osteoporosis. Osteoporos Int. 26:1667–1671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karakan NC, Akpinar A, Goze F and Poyraz
O: Investigating the effects of systemically administered strontium
ranelate on alveolar bone loss histomorphometrically and
histopathologically on experimental periodontitis in rats. J
Periodontol. 88:e24–e31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marie PJ: Optimizing bone metabolism in
osteoporosis: Insight into the pharmacologic profile of strontium
ranelate. Osteoporos Int. 14 Suppl 3:S9–S12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun S, Guo H, Zhang J, Yu B, Sun K and Jin
Q: Adenovirus-mediated expression of bone morphogenetic protein-2
activates titanium particle-induced osteoclastogenesis and this
effect occurs in spite of the suppression of tnf-alpha expression
by sirna. Int J Mol Med. 32:403–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, D.C: 2011
|
|
29
|
Yang S, Yu H, Gong W, Wu B, Mayton L,
Costello R and Wooley PH: Murine model of prosthesis failure for
the long-term study of aseptic loosening. J Orthop Res. 25:603–611.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang RC, Khan SN, Sandhu HS, Metzl JA,
Cammisa FJ, Zheng F, Sama AA and Lane JM: Alendronate inhibits
spine fusion in a rat model. Spine (Phila Pa 1976). 30:2516–2522.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geng T, Sun S, Chen X, Wang B, Guo H,
Zhang S and Jin Q: Strontium ranelate reduces the progression of
titanium particle-induced osteolysis by increasing the ratio of
osteoprotegerin to receptor activator of nuclear factor-κB ligand
in vivo. Mol Med Rep. 17:3829–3836. 2018.PubMed/NCBI
|
|
32
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Müller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen B, Li Y, Yang X, Xu H and Xie D:
Zoledronic acid enhances bone-implant osseointegration more than
alendronate and strontium ranelate in ovariectomized rats.
Osteoporos Int. 24:2115–2121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ucer S, Iyer S, Kim H, Han L, Rutlen C,
Allison K, Thostenson JD, de Cabo R, Jilka RL, O'Brien C, et al:
The effects of aging and sex steroid deficiency on the murine
skeleton are independent and mechanistically distinct. J Bone Miner
Res. 32:560–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Muller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bonnelye E, Chabadel A, Saltel F and
Jurdic P: Dual effect of strontium ranelate: Stimulation of
osteoblast differentiation and inhibition of osteoclast formation
and resorption in vitro. Bone. 42:129–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Faverani LP, Polo TO, Ramalho-Ferreira G,
Momesso GAC, Hassumi JS, Rossi AC, Freire AR, Prado FB, Luvizuto
ER, Gruber R and Okamoto R: Raloxifene but not alendronate can
compensate the impaired osseointegration in osteoporotic rats. Clin
Oral Invest. 22:255–265. 2018. View Article : Google Scholar
|
|
38
|
Stuss M, Sewerynek E, Król I, Stępień-Kłos
W and Jędrzejczyk S: Assessment of OPG, RANKL, bone turnover
markers serum levels and BMD after treatment with strontium
ranelate and ibandronate in patients with postmenopausal
osteoporosis. Endokrynol Pol. 67:174–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Komrakova M, Weidemann A, Dullin C, Ebert
J, Tezval M, Stuermer KM and Sehmisch S: The impact of strontium
ranelate on metaphyseal bone healing in ovariectomized rats. Calcif
Tissue Int. 97:391–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gur A, Denli A, Cevik R, Nas K, Karakoc M
and Sarac AJ: The effects of alendronate and calcitonin on
cytokines in postmenopausal osteoporosis: A 6-month randomized and
controlled study. Yonsei Med J. 44:99–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu X, Zhu S, Cui J, Shao H, Zhang W, Yang
H, Xu Y, Geng D and Yu L: Strontium ranelate inhibits
titanium-particle-induced osteolysis by restraining inflammatory
osteoclastogenesis in vivo. Acta Biomater. 10:4912–4918. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bakker AD, Zandieh-Doulabi B and
Klein-Nulend J: Strontium ranelate affects signaling from
mechanically-stimulated osteocytes towards osteoclasts and
osteoblasts. Bone. 53:112–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo X, Wei S, Lu M, Shao Z, Lu J, Xia L,
Lin K and Zou D: Dose-dependent effects of strontium ranelate on
ovariectomy rat bone marrow mesenchymal stem cells and human
umbilical vein endothelial cells. Int J Biol Sci. 12:1511–1522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Muise ES, Podtelezhnikov AA, Pickarski M,
Loboda A, Tan Y, Hu G, Thomspon JR and Duong T: Effects of
long-term odanacatib treatment on bone gene expression in
ovariectomized adult rhesus monkeys: Differentiation from
alendronate. J Bone Miner Res. 31:839–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang AR, Oh YR, Kim HY, Park MJ, Joo BS,
Choi WJ, Lee JY, Jung MH, Ji YI and Choi JS: Up-regulation of
inhibitors of dna binding/differentiation gene during
alendronate-induced osteoblast differentiation. Arch Gynecol
Obstet. 285:1331–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shimizu E, Tamasi J and Partridge NC:
Alendronate affects osteoblast functions by crosstalk through
ephrinb1-ephb. J Dent Res. 91:268–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rossini M, Adami G, Adami S, Viapiana O
and Gatti D: Safety issues and adverse reactions with osteoporosis
management. Exp Opin Drug Saf. 15:321–332. 2015. View Article : Google Scholar
|
|
48
|
Guo X, Wei S, Lu M, Shao Z, Lu J, Xia L,
Lin K and Zou D: Dose-dependent effects of strontium ranelate on
ovariectomy rat bone marrow mesenchymal stem cells and human
umbilical vein endothelial cells. Int J Biol Sci. 12:1511–1522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gu Z, Huang B, Li Y, Tian M, Li L and Yu
X: Strontium-doped calcium polyphosphate/ultrahigh molecular weight
polyethylene composites: A new class of artificial joint components
with enhanced biological efficacy to aseptic loosening. Mater Sci
Eng C Mater Biol Appl. 61:526–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tian A, Zhai JJ, Peng Y, Zhang L, Teng MH,
Liao J, Sun X and Liang X: Osteoblast response to titanium surfaces
coated with strontium ranelate-loaded chitosan film. Int J Oral
Maxillofac Implants. 29:1446–1453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Newman SD, Lotfibakhshaiesh N, O'Donnell
M, Walboomers XF, Horwood N, Jansen JA, Amis AA, Cobb JP and
Stevens MM: Enhanced osseous implant fixation with
strontium-substituted bioactive glass coating. Tissue Eng Part A.
20:1850–1857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wornham DP, Hajjawi MO, Orriss IR and
Arnett TR: Strontium potently inhibits mineralisation in
bone-forming primary rat osteoblast cultures and reduces numbers of
osteoclasts in mouse marrow cultures. Osteoporos Int. 25:2477–2484.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rossini M, Adami G, Adami S, Viapiana O
and Gatti D: Safety issues and adverse reactions with osteoporosis
management. Exp Opin Drug Saf. 15:321–332. 2015. View Article : Google Scholar
|
|
54
|
Lee HY, Shen MX, Lim YL, Tay YK, Chan MM,
Pang SM, Xiao ZW, Ang SB and Ren EC: Increased risk of strontium
ranelate-related sjs/ten is associated with hla. Osteoporos Int.
27:2577–2583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tian A, Zhai JJ, Peng Y, Zhang L, Teng MH,
Liao J, Sun X and Liang X: Osteoblast response to titanium surfaces
coated with strontium ranelate-loaded chitosan film. Int J Oral
Maxillofac Implants. 29:1446–1453. 2014. View Article : Google Scholar : PubMed/NCBI
|