Introduction
A significant obstacle in the clinical treatment of
cancer patients is multidrug resistance (MDR) (1). Many genes are reportedly related with
MDR. Among them, the most significant are ATP-binding cassette
(ABC) genes (2). Humans have 49
ABC genes, and the high expression of these genes in cancer
patients results in decreased intracellular accumulation of
chemotherapy drugs in spite of their different chemical structures
(3). As the first identified and
typical ABC transporter, P-gp/P-gp has the MDR
characteristic of effluxing a number of commonly used
chemotherapeutic agents, e.g., Taxol, doxorubicin, vincristine,
vinblastine, colchicine, actinomycin D, and mitomycin C (4). Thus, the high expression of P-gp
plays a critical role in many kinds of cancer-chemotherapy failure,
and identifying approaches to overcoming P-gp-mediated drug
resistance is urgent.
The development of MDR reversal has progressed for
over 35 years (5). Classical
chemosensitizers including verapamil, cyclosporine A, and PSC833
can reverse P-gp-mediated MDR, but their toxicity and other side
effects in vivo limit their clinical application (6). To overcome their low efficiency and
high toxicity in cancer treatment, the proteasome inhibitor MG132
has been found to be a potent P-gp-inhibitor (7,8). To
elucidate the molecular basis underlying the reversal of
P-gp/P-gp by MG-132 in head and neck squamous cell
carcinomas (HNSCCs), we conducted an experiment on the
hypopharyngeal carcinoma cell line FaDu and the multidrug
resistance (MDR) cell line FaDu/T induced by Taxol that had been
established in our previous study.
Materials and methods
Materials
The human hypopharyngeal carcinoma cell line FaDu
and human bronchial epithelioid (HBE) cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). Media and
serum were purchased from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The primary antibodies anti-MDR1/P-gp,
anti-actin, and anti-nuclear factor-κB (NF-κB) were all purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
primary antibodies anti-p-c-Jun N-terminal kinase (JNK) and
anti-p-c-Jun were from Cell Signaling Technology. All other
reagents were from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany).
Cell culture and establishment of the
resistant cell line FaDu/T
FaDu and HBE cells were cultured as a monolayer in
Dulbecco's modified Eagle's medium containing 10% fetal calf serum,
100 U/ml penicillin, and 100 mg streptomycin at 37°C in humidified
atmosphere composed of 95% air and 5% CO2.
The methods of establishing the resistant cell line
(FaDu/T) has been described previously (9).
Cytotoxicity test
Cell viability was detected with cell counting kit-8
(CCK-8) assay kits. HBE cells were seeded in 24-well culture
plates. The plates were placed in an incubator for 24 h, and the
culture medium was changed to collect MG-132. CCK-8 assays were
performed 48 h after treatment with different concentrations of
MG-132. At the time of the CCK-8 assay, we added 10 µl of CCK-8
solution to each well of the plate, which was incubated for 2 h at
37°C. Absorbance was measured at 450 nm using a microplate reader
(BioTek, Winooski, VT, USA). Results were used to measure cell
growth.
Reverse transcription (RT)- and
semi-quantitative polymerase chain reaction (sqPCR)
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc). RT-PCR was performed using the
ExScript RT reagent kit (Takara, Dalian, China) in a final volume
of 20 µl containing 1 µg of total RNA, 4 µl of 5X ExScript buffer,
1 µl of dNTP mixture, 1 µl of oligo(dT) primer, 0.5 µl of ExScript
RTase, 0.5 µl of RNase inhibitor, and RNase-free water to a volume
of 20 µl. This reaction was performed at 42°C for 15 min and
terminated by heating at 95°C for 2 min. PCR was performed
following the manufacturer's instructions of Takara Taq™ under the
following conditions: Pre-degeneration at 95°C for 3 min,
degeneration at 95°C for 60 sec, renaturation at 58°C for 45 sec,
and elongation at 72°C for 60 sec, for a total of 25 cycles. sqPCR
was performed by running the products on a 1% agarose gel, and the
bands were quantified using ImageJ v1.48 (National Institutes of
Health, Bethesda, MD, USA). All experiments were conducted thrice.
The P-gp primers were as follows: Forward,
5′-CTGCTCAAGTTAAAGGGGCTAT-3′ and reverse,
5′-AACGGTTCGGAAGTTTTCTATT-3′. The actin primers were as follows:
Forward, 5′-GTGGGGCGCCCCAGGCACCA-3′ and reverse,
5′-CTCCTTAATGTCACGCACGATTT-3′.
Western blot analysis
Total protein was extracted using radioimmune
precipitation assay buffer (Sigma-Aldrich; Merck KGaA) and protein
lysis buffer following the manufacturer's protocols. Nuclear
proteins were solubilized and fractionated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis.
The Bradford method was used to determine the
protein concentration of the supernatant. Samples (40 µg of total
protein each) were used in western blot analysis with the first
antibodies (P-gp/P-gp 1:400, mouse antihuman; actin,
1:2,000, mouse antihuman; P-JNK 1:1,000, rabbit antihuman; and
p-c-Jun 1:200, goat antihuman). The bands of P-gp/P-gp,
P-JNK, p-c-Jun, and actin were visualized at apparent molecular
weights of 170, 46/54, 39 and 43 kDa, respectively. Relative OD
ratio was calculated with NIH software Image J by comparing to
actin from three experiments.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical calculations were performed using SPSS 16.0
software package (SPSS Inc., Chicago, IL, USA). One-way analysis of
variance with a Bonferroni post hoc test were applied to analyze
the variance, and P<0.05 was considered to indicate a
statistically significant difference.
Results
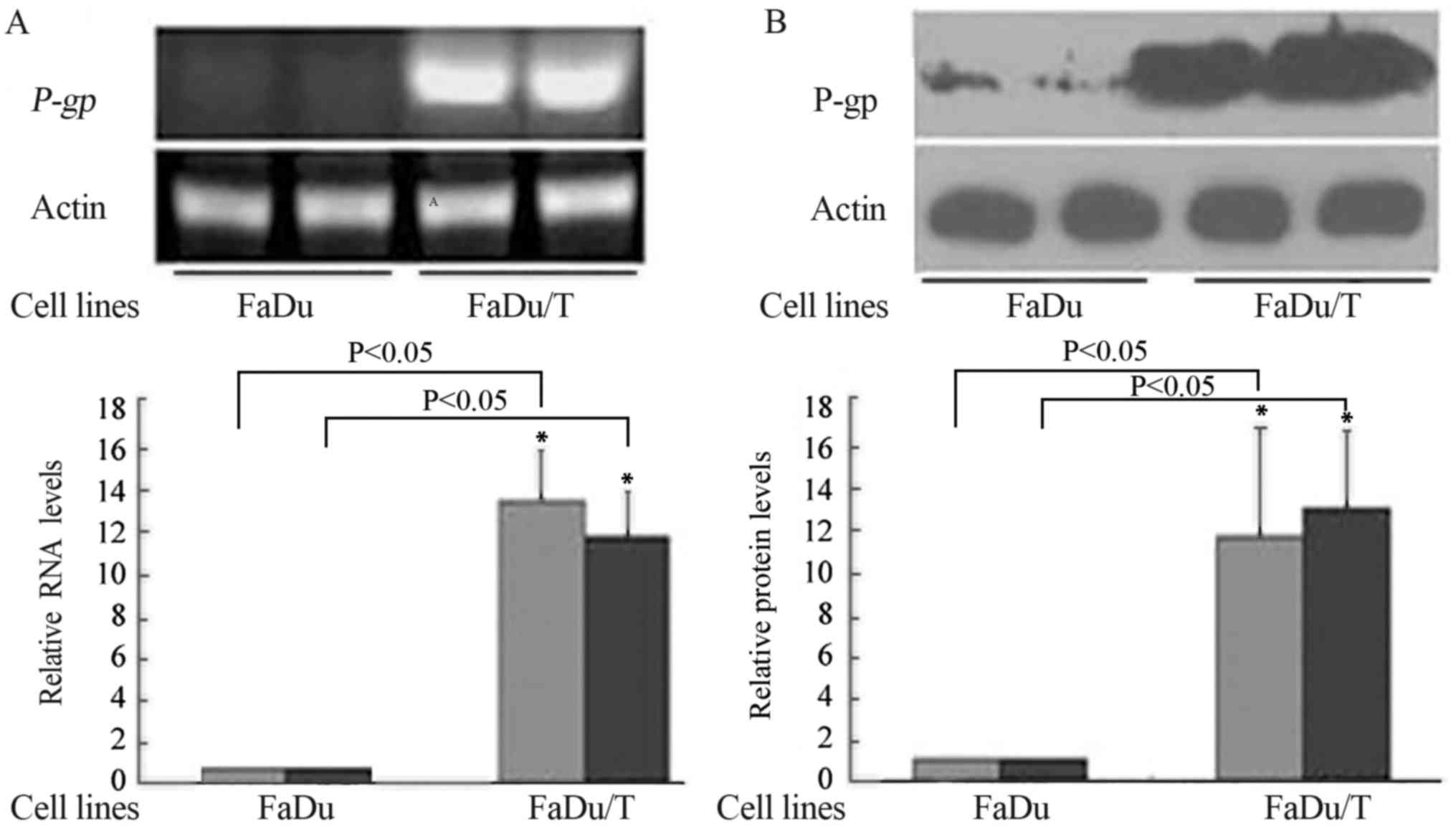
mRNA and protein levels of P-gp/P-gp
in FaDu and FaDu/T cells
Compared with FaDu, P-gp (Fig. 1A) and P-gp (Fig. 1B) were upregulated in FaDu/T cells.
ImageJ software was used to analyze the relative photodensity using
actin as a loading control. Considering a value of 1 for FaDu
groups, the relative photodensity of the FaDu/T-200 nM groups was
as follows: P-gp/actin, 14.24±2.57 and 12.42±2.23; and P-gp/actin,
11.56±5.19 and 12.49±3.60, respectively. Statistical analysis
showed significant differences between FaDu and FaDu/T cells
(P<0.05).
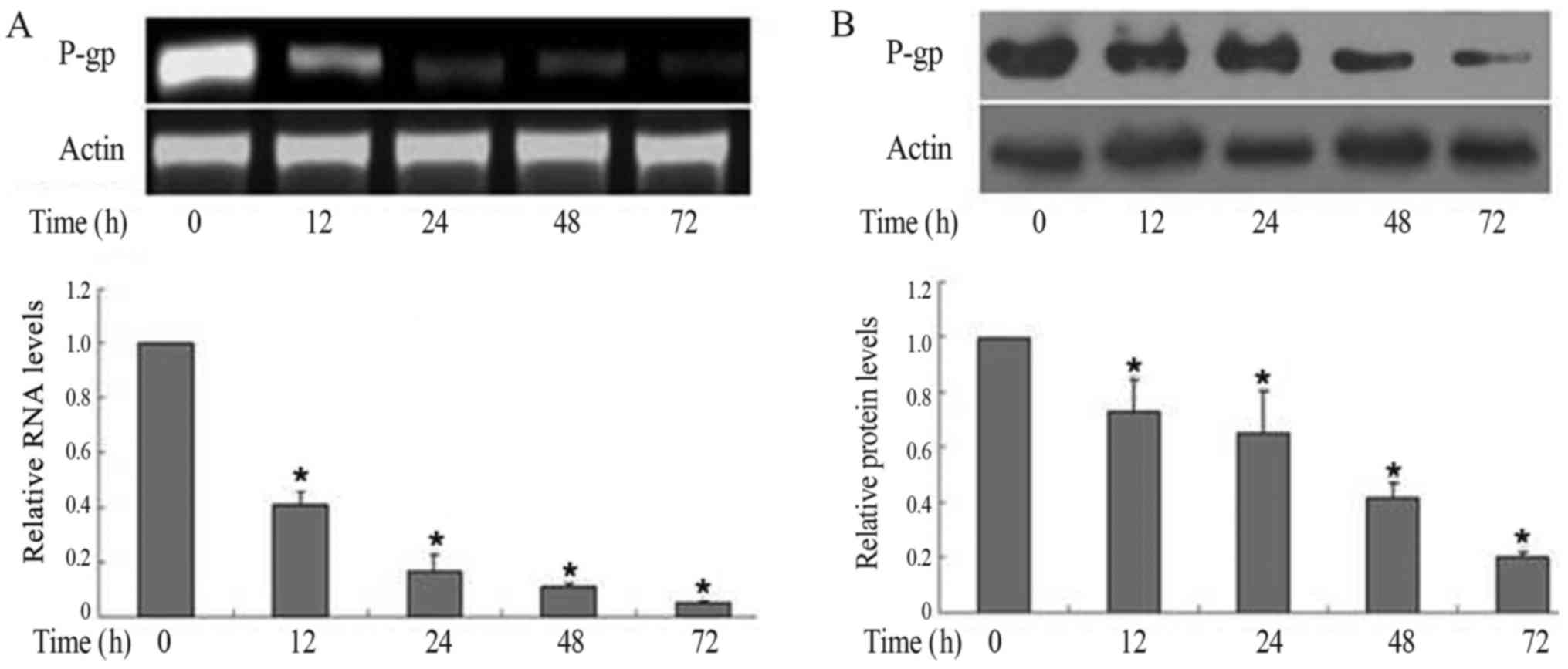
Downregulation of P-gp/P-gp by MG-132
in FaDu/T cells
To assess the capacity of MG132 in the
downregulation of P-gp, 1.5 µM MG-132 was applied in the present
research. P-gp/P-gp expression in RNA (Fig. 2A) and protein (Fig. 2B) levels both decreased in a
time-dependent manner. Considering a value of 1 for FaDu/T (0
h)/actin, the relative photodensity of P-gp/actin in FaDu/T groups
at 12, 24, 48 and 72 h was as follows: 0.41±0.05, 0.17±0.06,
0.11±0.01 and 0.05±0.006, respectively, Statistical analysis showed
significant differences between different time-points (F=252.47;
P<0.05). Meanwhile, the relative photodensity of P-gp/actin in
FaDu/T groups at 12, 24, 48 and 72 h was as follows: 0.73±0.12,
0.65±0.15, 0.42±0.05 and 0.20±0.02 respectively. Statistical
analysis showed significant differences between different times
(F=30.59; P<0.05).
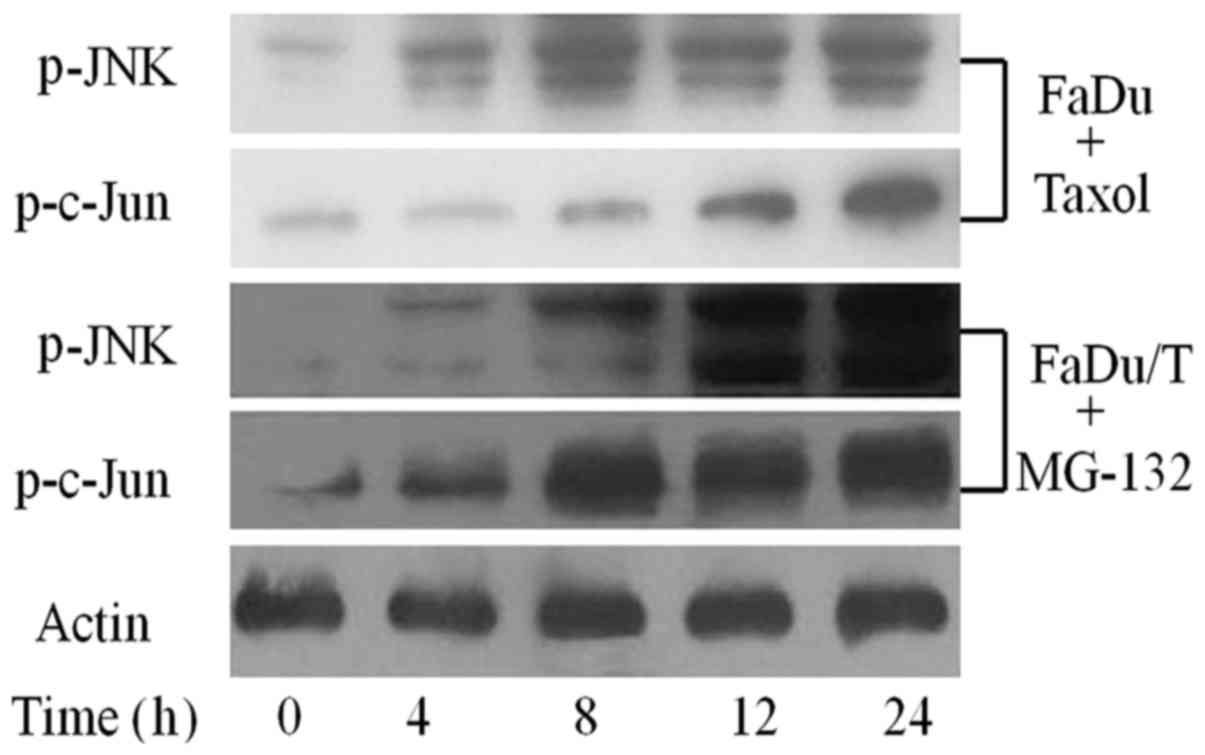
Status of JNK signaling pathway in
drug-sensitive FaDu cells and in FaDu/T cells treated with
MG-132
To examine the activation status of JNK signaling
pathway in Taxol-sensitive FaDu cells, these cells were treated
with Taxol for 48 h, and then drug-sensitive FaDu cells were
collected in a time-dependent manner. Western blot analysis showed
that the JNK signaling pathway was activated in Taxol-sensitive
FaDu cells. Furthermore, MG-132 functionally reversed the high
expression of P-gp and promoted the relative protein level of the
JNK signaling pathway phosphorylation in a time-dependent manner
when FaDu/T cells were cultured in drug- and serum-free state for
24 h (Fig. 3).
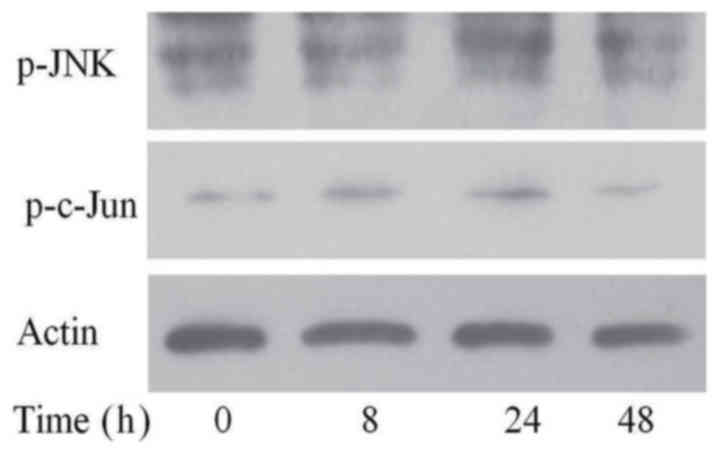
SP600125, the inhibitor of the JNK
signal pathway, inhibited the activation of this pathway
To ascertain the inhibitory effect of SP600125 on
JNK signaling, we added MG-132 to FaDu/T cells for 24 h after
adding SP600125. As shown in Fig.
4, the expression of p-JNK and p-c-Jun did not significantly
change.
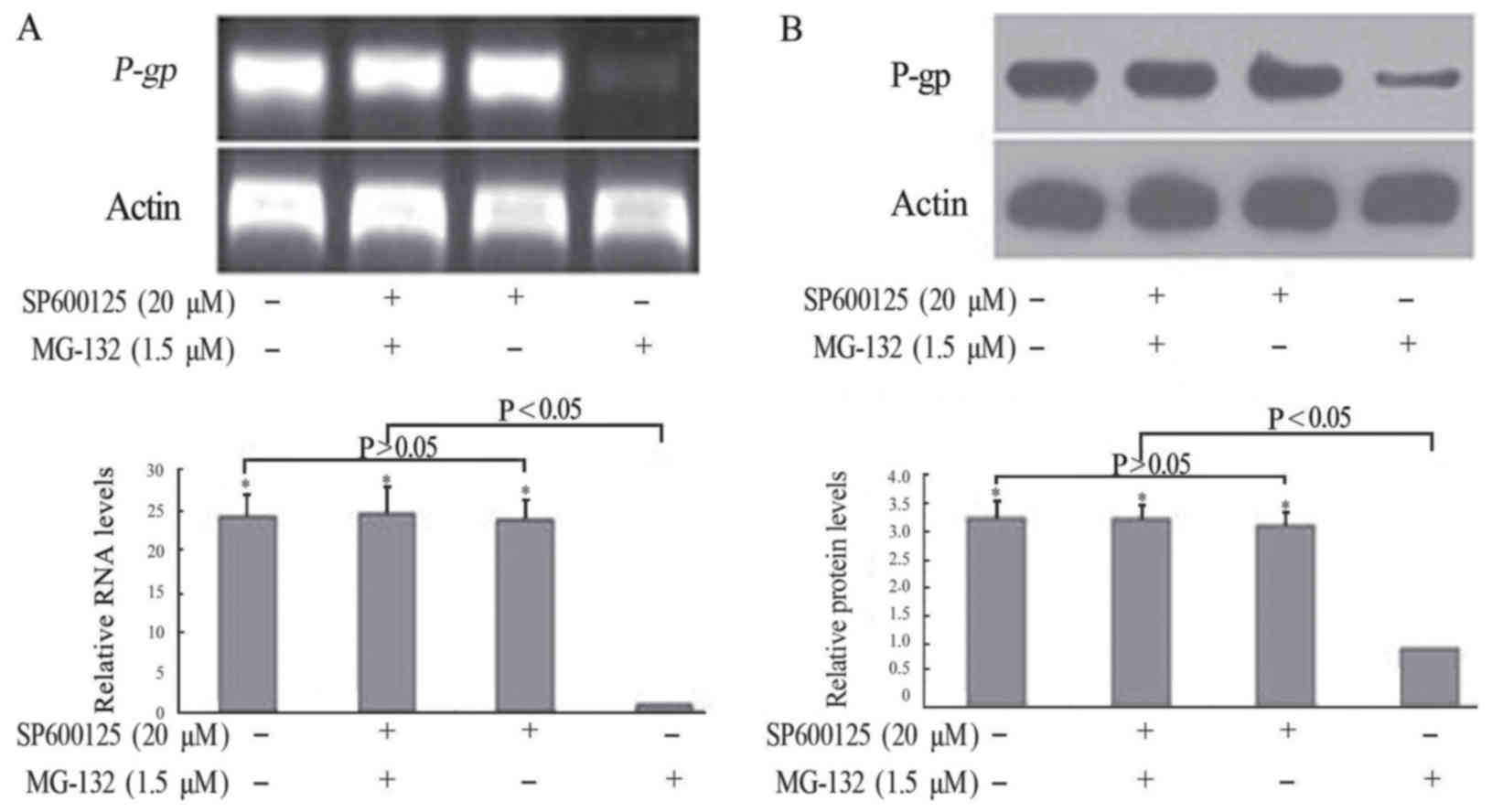
SP600125 inhibited the MG-132-induced
downregulation of P-gp/P-gp in terms of RNA and protein levels
To ascertain the mechanism of MG-132 in
downregulating P-gp, FaDu/T cells were pretreated with the JNK
signal pathway inhibitor SP600125, followed by 1.5 µM MG-132 for 72
h. As shown in Fig. 5, in the
absence of MG-132, FaDu/T cells with or without SP600125
pretreatment showed a similar expression of P-gp. By contrast,
MG-132 treatment alone induced a significantly lower expression of
P-gp, which can be reversed by pretreatment with SP600125. These
results suggested that the JNK signaling pathway was involved in
the MG-132-induced downregulation of P-gp in FaDu/T cells.
Considering a value of 1 for FaDu/T/actin, the relative
photodensity of P-gp/actin and P-gp/actin in FaDu/T cells, FaDu/T
cells treated with SP600125 and MG-132, and FaDu/T cells singly
treated single with SP600125 groups was as follows: 24.23±2.97,
24.65±3.77, 23.88±2.35; 3.24±0.36, 3.22±0.25, and 3.12±0.25,
respectively. Statistical analysis showed a significant difference
between different groups (P<0.05).
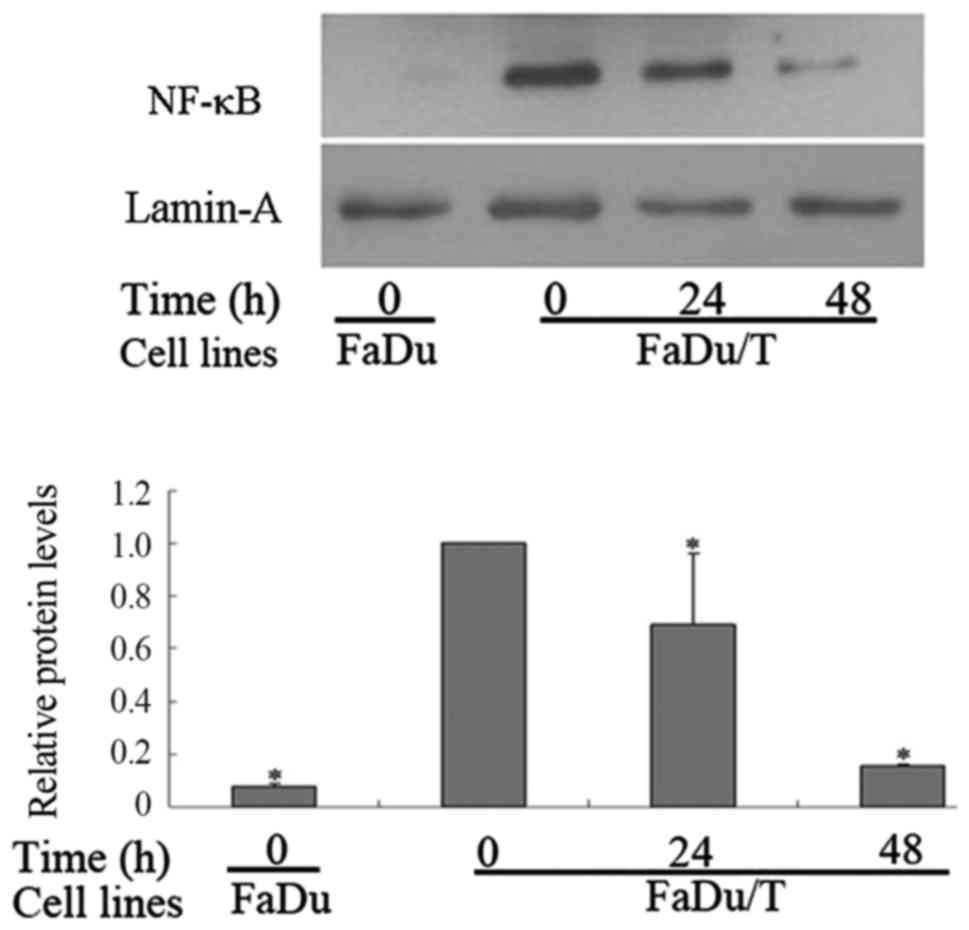
MG-132 inhibited the nuclear
translocation of NF-κB in FaDu/T cells
Compared with FaDu cells, the nuclear protein levels
of NF-κB in FaDu/T cells markedly increased when using lamin-A as a
control. Considering a value of 1 for FaDu/T (0 h)/actin, the
relative photodensity of FaDu/actin was 0.08±0.01. Statistical
analysis showed a significant difference between FaDu and FaDu/T
cells (P<0.05). However, the nuclear translocation of NF-κB was
prohibited after short-time incubation of FaDu/T cells with MG-132.
Considering a value of 1 for FaDu/T (0 h)/actin, the relative
photodensity of FaDu/T/actin (24 and 48 h) was 0.69±0.27 and
0.16±0.01, respectively (Fig. 6).
Statistical analysis showed that the difference between different
time-points was significant (P<0.05).
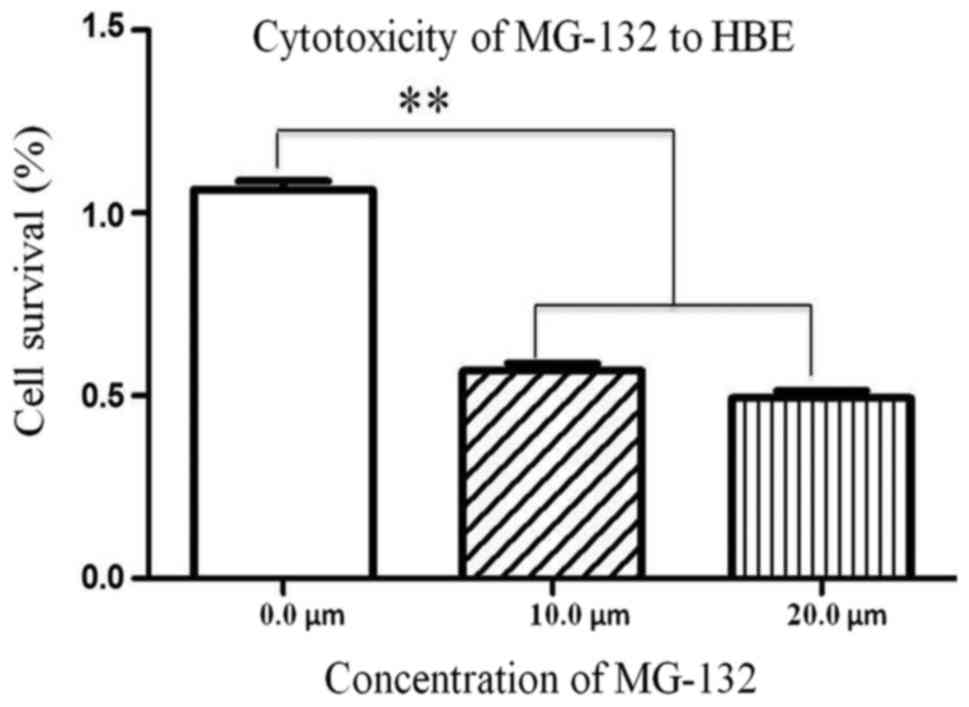
Cytotoxicity of MG-132 to HBE
cells
To evaluate the clinical value of MG-132, HBE cells
were treated with MG-132 in a concentration-dependent manner. Cell
viability was detected with CCK-8 assay kits. As shown in Fig. 7, the viability of HBE cells were
decreased significantly with increasing MG-132 concentrations.
MG-132 exerted a cytotoxicity effect on HBE cells.
Discussion
To elucidate the molecular mechanism underlying the
downregulation of membrane protein P-gp/P-gp by MG-132, we
have previously established a multidrug-resistant cell line of FaDu
to Taxol (FaDu/T) by stepwise exposure of normal FaDu cells to
increased concentrations of Taxol for over 18 months. We find that
P-gp/P-gp (P-glycoprotein) expression increases in FaDu/T
cell lines and that the MDR of FaDu/T cells to DDP, 5-FU, Dox, and
VCR is enhanced (9). However, when
MG-132 was introduced into FaDu/T cells, in addition to decreased
P-gp, the MDR to DDP, 5-FU, and VCR also decreased. Based on the
above investigation (10), we
speculated that P-gp overexpression may be mainly responsible for
MDR in FaDu/T cells. Thus, the downregulation of P-gp by MG-132 was
crucial to the reversal of MDR. Meanwhile, to clearly determine the
mechanism of MG-132 in regulating P-gp in FaDu/T cells, we
conducted further experiments.
As a proteasome inhibitor, MG-132 has anticancer
effects through other cellular mechanisms, one of which is the
activation of the JNK signal pathway (11). As a member of the MAPK family, the
activation of the JNK signal pathway plays an important role in the
growth, differentiation, and apoptosis of cancer cells (12). Several previous studies have
suggested the existence of a negative binding site of AP-1 in the
promoter region of the P-gp gene; thus, the activation of
the JNK/c-Jun/AP-1 signal pathways can inhibit P-gp
expression in human multidrug-resistant cells (13–15).
In the present study, with the application of MG-132
in FaDu or FaDu/T cells cultured in a drug- and serum-free state,
we found decreased P-gp expression in FaDu/T cells a time-dependent
manner. We also detected that the JNK signaling pathway was
effectively reactivated in a time-dependent manner. These findings,
together with theoretical studies, led us to the hypothesis that
decreased P-gp expression in FaDu/T cell lines caused by MG-132 was
regulated by the JNK signal pathway. However, our data were
insufficient to show how AP-1 was involved in P-gp downregulation
in FaDu/T cell lines.
To further address this question, FaDu/T cells were
pretreated with SP600125 (16), a
small molecule inhibitor of the JNK signaling pathway for 24 h.
Results showed that the JNK signaling pathway was inactivated.
Compared with FaDu/T cells treated with only MG-132, P-gp
expression was not significantly decreased. All of the above
results indicated that P-gp downregulation was attributed to the
activation of the JNK signal pathway.
MG-132 is a potent inhibitor in the degradation of
IκB proteins and thus, suppresses the nuclear translocation and
activation of NF-κB. Given that the nuclear translocation of NF-κB
was closely involved in P-gp expression (17,18),
we wondered whether this pathway also existed in hypopharyngeal
cancer cells. Fig. 5 shows that
compared with FaDu cells, the nuclear translocation of NF-κB in
FaDu/T cells increased, which was reversed when FaDu/T cells was in
the presence of MG-132 for 48 h. Meanwhile, the expression of P-gp
significantly decreased, thereby providing evidence that MG132
downregulated P-gp expression also probably by suppressing NF-κB
nuclear translocation. However, FaDu/T cells were pretreated with
SP600125, a small molecule inhibitor of the JNK signaling pathway
for 24 h. Compared with FaDu/T cells treated with only MG-132, the
expression of P-gp did not significantly decrease, although MG-132
can still suppress the activation of NF-κB under this condition.
All of the above results indicated that NF-κB can upregulate P-gp
when FaDu cells were initially exposed to Taxol. However, in FaDu/T
cell lines, the downregulation of P-gp was attributed to the
activation of the JNK signal pathway, and the inactivation of NF-κB
affected only the termination of P-gp expression. These lines of
evidence suggested that the downregulation of P-gp in FaDu/T cells
was due to the activation of JNK signaling pathway. The interaction
between the function of NF-κB and the JNK signaling pathway warrant
further study.
In the present study, we presented evidence for the
first time that MG-132 functionally downregulated P-gp expression
by activating the JNK/c-Jun/AP-1 signal pathways, which promoted
the negative regulation of AP-1 to P-gp. Therefore, the effects of
MG-132 on P-gp downregulation may represent, at least in part, a
novel strategy for overcoming the P-gp-related MDR of only FaDu
cells to Taxol, and more cell lines of hypopharyngeal carcinoma to
other chemotherapy agents such as DDP, 5-FU and Afatinib are needed
to confirm the present findings in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shandong
Provincial Outstanding Young Scientist Research Award Fund of China
(grant no. BS2009YY013) and the Shandong Provincial International
Science and Technology Cooperation Project of China (grant no.
2010GHZ20202).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JM and ZL conceived and designed the research and
drafted the manuscript. XxL and XfL acquired, analyzed and
interpreted the data, and performed statistical analysis. WX
conceived and designed the study, and revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Falasca M and Linton KJ: Investigational
ABC transporter inhibitors. Expert Opin Investig Drugs. 21:657–666.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodrigues AC, Curi R, Genvigir FD, Hirata
MH and Hirata RD: The expression of efflux and uptake transporters
are regulated by statins in Caco-2 and HepG2 cells. Acta Pharmacol
Sin. 30:956–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stavrovskaya AA and Stromskaya TP:
Transport proteins of the ABC family and multidrug resistance of
tumor cells. Biochemistry (Mosc). 73:592–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharom FJ: The P-glycoprotein multidrug
transporter. Essays Biochem. 50:161–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amiri-Kordestani L, Basseville A, Kurdziel
K, Fojo AT and Bates SE: Targeting MDR in breast and lung cancer:
Discriminating its potential importance from the failure of drug
resistance reversal studies. Drug Resist Updat. 15:50–61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambudkar SV, Dey S, Hrycyna CA,
Ramachandra M, Pastan I and Gottesman MM: Biochemical, cellular,
and pharmacological aspects of the multidrug transporter. Annu Rev
Pharmacol Toxicol. 39:361–398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bang JH, Han ES, Lim I and Lee CS:
Differential response of MG132 cytotoxicity against small cell lung
cancer cells to changes in cellular GSH contents. Biochem
Pharmacol. 68:659–666. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu JH, Asai A, Chi S, Saito N, Hamada H
and Kirino T: Proteasome inhibitors induce cytochrome
c-caspase-3-like protease-mediated apoptosis in cultured cortical
neurons. J Neurosci. 20:259–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma J, Lu S, Yu L, Tian J, Li J, Wang H and
Xu W: FaDu cell characteristics induced by multidrug resistance.
Oncol Rep. 26:1189–1195. 2011.PubMed/NCBI
|
|
10
|
Ma J, Yu L, Tian J, Mu Y, Lv Z, Zou J, Li
J, Wang H and Xu W: MG132 reverse the malignant characteristics of
hypopharyngeal cancer. Mol Med Rep. 9:2587–2591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park HS, do Jun Y, Han CR, Woo HJ and Kim
YH: Proteasome inhibitor MG132-induced apoptosis via ER
stress-mediated apoptotic pathway and its potentiation by protein
tyrosine kinase p56lck in human Jurkat T cells. Biochem Pharmacol.
82:1110–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang P, Han J and Hui L: MAPK signaling
in inflammation-associated cancer development. Protein Cell.
1:218–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou J, Liu M, Aneja R, Chandra R, Lage H
and Joshi HC: Reversal of P-glycoprotein-mediated multidrug
resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer
Res. 66:445–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han L, Wang Y, Guo X, Zhou Y, Zhang J,
Wang N, Jiang J, Ma F and Wang Q: Downregulation of MDR1 gene by
cepharanthine hydrochloride is related to the activation of
c-Jun/JNK in K562/ADR cells. Biomed Res Int. 2014:1643912014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aldonza MB, Hong JY, Bae SY, Song J, Kim
WK, Oh J, Shin Y, Lee SH and Lee SK: Suppression of MAPK signaling
and reversal of mTOR-dependent MDR1-associated multidrug resistance
by 21alpha-methylmelianodiol in lung cancer cells. PLoS One.
10:e01278412015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marozin S, Altomonte J, Apfel S, Dinh PX,
De Toni EN, Rizzani A, Nüssler A, Kato N, Schmid RM, Pattnaik AK,
et al: Posttranslational modification of vesicular stomatitis virus
glycoprotein, but not JNK inhibition, is the antiviral mechanism of
SP600125. J Virol. 86:4844–4855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun J, Yeung CA, Co NN, Tsang TY, Yau E,
Luo K, Wu P, Wa JC, Fung KP, Kwok TT, et al: Clitocine reversal of
P-glycoprotein associated multi-drug resistance through
downregulation of transcription factor NF-kappaB in R-HepG2 cell
line. PLoS One. 7:e407202012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanagasabai R, Krishnamurthy K, Druhan LJ
and Ilangovan G: Forced expression of heat shock protein 27 (Hsp27)
reverses P-glycoprotein (ABCB1)-mediated drug efflux and MDR1 gene
expression in adriamycin-resistant human breast cancer cells. J
Biol Chem. 286:33289–33300. 2011. View Article : Google Scholar : PubMed/NCBI
|