Introduction
Prostate cancer is a malignant tumor that occurs in
prostate tissue, which is the result of abnormal dysplasia of
prostate acinar cells. In regions such as Europe and the United
States, prostate cancer is the most common male malignancy; the
mortality rate has exceeded lung cancer. The incidence of prostate
cancer has gradually increased with the arrival of China's aging
population and the extension of life and diet changes (1). It is also known that prostate
cancer-associated fatalities frequently occur in patients with
metastatic castration-resistant prostate cancer. Although several
novel drugs for castration-resistant prostate cancer have been
approved, each of these has prolonged survival by just a few
months. Therefore, novel treatments of prostate cancer are required
to extend life expectancy even further.
Currently, immunotherapy has been reported to be an
effective treatment for cancer patients (2). Several strategies, including cancer
vaccines and immune checkpoint inhibitors, have been investigated
in clinical studies for cancer patients (3–5).
However, T cell immunotherapy of prostate cancer is still at an
early stage of clinical development. It has been reported that
several molecules are potent T cell checkpoint inhibitors which
reverse immunologic tolerance in many types of cancer, including
prostate cancer (6). Therefore,
the molecules which serve crucial roles in T cell activity during
prostate cancer may be useful for the treatment of this disease.
CXC motif chemokine ligand 9 (CXCL9) is a chemokine, which
regulates the host's response to inflammation by recruiting
leukocytes to the inflammatory environment. Chemokines serve
important roles in immune responses of the body. Chemokines are
expressed by almost all monocytes, neutrophils, eosinophils and
natural killer (NK) cells but are expressed to a lesser degree by
macrophages, B cells and T cells (7–9). It
has been reported that the absence of CXCL9 affects leukocyte
migration and tissue infiltration (10). As CXCL9 has the ability to interact
with a variety of ligands, it serves a certain biological role in
the inflammatory response. In addition, inflammation is closely
associated with the occurrence and development of tumors (11,12).
The present study aimed to investigate the effect of CXCL9 on T
cells in prostate cancer.
Materials and methods
Clinical data
Prostate cancer tissue specimens (n=37) were
obtained between July 2014 and December 2015 from Linyi People's
Hospital (Linyi, China), from patients aged between 35 and 68 years
who were diagnosed with prostate cancer without any therapy. These
tissue samples were collected with the consent of the patients. The
study was approved by the Medical Ethics Committee of the Linyi
People's Hospital. The clinical stages of the patients were
clarified according to the tumor nodes metastasis staging system.
Adenoma (n=3), Stage 0/I (n=8), II (n=10), III (n=12) and IV
(n=4).
Mice, cell and prostate cancer
models
C57BL/6 black mice (8 weeks old; male n=25; female
n=25; weight ~20–25 g), were purchased from Sun Yat-sen University
Experimental Animal Center (Guangzhou, China) and were used as
control mice. A total of four B6.Cg-Selplgtm1Fur/J mice, (8 weeks
old; male n=2; female n=2; weight ~20–25 g), which highly express
CXCL9, were purchased from Jackson Laboratory (Ben Harbor, ME,
USA). In total, 54 mice were used in this study. They were
maintained under specific pathogen-free conditions with 12-h
light/12-h dark cycles at 26–28°C and 50–65% humidity and a normal
diet (including 4% fat and 0.07% cholesterol) and water ad
libitum.
DMAB (3,2′-dimethyl 4-aminobiphenyl), was used as a
chemical carcinogenic agent. The mice were enrolled and initiation
with subcutaneous injection of DMAB (150 mg/kg b.w.) once a week
for 3 weeks to construct the prostate cancer model. The mice were
anesthetized by 10% chloral hydrate solution (3 ml/kg,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and then the blood
samples were collected. Then the mice were sacrificed by cervical
dislocation, and the prostate tissues were collected and stored.
Animal experiments were approved by the Medical Ethics Committee of
Linyi People's Hospital.
Hematoxylin-eosin (H&E)
staining
The prostate tissues of each mouse were fixed using
10% formalin at room temperature for 24 h. Subsequently, the
tissues were cut into paraffin sections (5 µm) and stained with
hematoxylin for 5 mins and eosin for 30 sec at room temperature,
mounted in a 50% glycerol solution in distilled water to prevent
autofluorescence (Mikrochem, Bratislava, Slovak Republic) and
evaluated with an Axiophot microscope (Zeiss, Oberkochen, Germany;
magnification, ×400) equipped with a CELLscan System (Scanalytics;
BD Biosciences, Franklin Lakes, NJ, US).
Immunohistochemistry
Immunohistochemistry was performed according to a
conventional protocol (13). The
paraffin-embedded prostate tissue sections were incubated in a dry
oven at 63°C for 1 h. De-paraffinization and rehydration were then
performed. The sections were incubated with 30–50 µl anti-IL-6
(SAB3300071), anti-TGF-β (SAB4502958) and anti-PCNA (WH000511M2)
antibodies (dilution 1:100; Sigma-Aldrich; Merck KGaA) overnight at
4°C. The sections were washed with PBS buffer three times, each
time for 5 min. A total of 30–50 µl HRP conjugated IL-6 (A0192),
TGF-β (A0277) and PCNA (AF1363) secondary antibodies (1:1,000;
Beyotime, Shanghai China) was added to the tissue section and
incubated at 37°C for 1 h. The sections were washed with PBS buffer
three times, each time for 5 min. Excess liquid was dried around
the tissue and then placed flat into the moist chamber. A working
solution of 3,3′-diaminobenzidene (30–50 µl; Sigma-Aldrich; Merck
KGaA) was added to the sections and incubated at room temperature
for 1–5 min. After the color was developed, the sections were
rinsed with distilled water to stop the reaction. A positive
results was defined as the presence of staining in 10% or more of
cells. The stained tissue sections were reviewed and scored
separately by two pathologists blinded to the clinical
parameters.
Flow cytometry
Blood samples were collected from different mouse
organs, such as, orbital cavity (1 ml), caudal vein (50–100 µl),
spleen (30–50 µl) and bone marrow (80–120 µl). Then red blood cell
lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China)
was added to the blood samples, and centrifuged at 350 × g for 5
min at 4°C. The supernatant was discarded and the bottom part of
the precipitation was washed with 0.5% bovine serum albumin (BSA;
Beyotime Institute of Biotechnology) 3 times at room temperature,
each time for 5 min. Anti-CD4-FITC antibody (F1773; 1:100;
Sigma-Aldrich; Merck KGaA) was added in the dark and incubated for
30 min and centrifuged at 350 × g for 5 min at 4°C. The supernatant
was discarded and the precipitation was washed 3 times with 0.5%
BSA, the supernatant was removed and the precipitation was
re-suspended in 200 µl 0.5% BSA. The protein was detected using
FACSCanto™ II (Becton Dickinson, Franklin Lakes, NJ,
USA).
Immunofluorescent staining
The prostate tissue was fixed with 4% formaldehyde
for 15 min at room temperature, then washed 3 times with PBS before
permeabilization with 0.2% Triton X-100 (PBS) for 10 min, also at
room temperature. The paraffin-embedded tissue sections were
deparaffinized in xylene and rehydrated in a graded series of
ethanol solutions and then incubated for 20 min in 3%
H2O2 to quench the endogenous peroxidase
activity. Next, the sections were heated in target retrieval
solution (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA)
for 15 min in a microwave oven (Oriental Rotor, Tokyo, Japan) to
retrieve the antigen. Subsequently, the sections were blocked with
2% BSA at 4°C for 15 min (D3308; Beyotime Institute of
Biotechnology) and incubated with CD4+ (AF1792) and CD8+ (AF1417)
primary antibodies (1:100; Beyotime Institute of Biotechnology)
overnight at 4°C, and stained with a fluorescein-conjugated
secondary antibody anti-CD4+-FITC antibody (F1773) and
anti-CD8+-FITC antibody (F0772; 1:100; Sigma-Aldrich; Merck KGaA)
for 2 h at room temperature. Finally, images were captured with
Leica SP5 AOBS confocal microscope (Leica Microsystems GmbH,
Wetzlar, Germany), and the number of positive cells and field area
ratio were calculated with Image J software version 1.48 (National
Institutes of Health, Bethesda, MD, USA) (14).
Western blotting
Tissues were lysed in Laemmli sample buffer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with a protease inhibitor,
complete EDTA-free (Roche Diagnostics GmbH, Mannheim, Germany). The
protein lysate sample was extracted from tumor tissue of mice, and
then centrifuged at 12,000 × g for 10–15 min at 4°C. The
supernatant was collected, and the protein concentration was
measured using BCA Protein Assay Kits (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The protein sample were
electrophoresed on 10% polyacrylamide gels (Bio-Rad Laboratories)
and transferred to Immobilon-PSQ polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Then the membrane
was blocked in 2% BSA, at 4°C for 15 min with TBS containing 5%
skimmed milk and 0.1% Tween-20, and incubated with the anti-IL-6
(SAB3300071) and anti-TGF-β (SAB4502958) antibodies (Sigma-Aldrich;
Merck KGaA) at 4°C overnight. Finally, the membrane was incubated
with HRP conjugated IL-6 (A0192) and TGF-β (A0277) secondary
antibodies (1:1,000; Beyotime Institute of Biotechnology) at room
temperature for 50–90 min. The protein was visualized using an
enhanced chemiluminescence reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The proteins were semi-quantified using Image J
software version 1.48 (National Institutes of Health).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from prostate tissues of the
patients by using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Reverse transcription was performed with random
primers using the First Strand cDNA Synthesis kit (Takara
Biotechnology Co., Ltd., Dalian, China). RT-qPCR was performed with
SYBR-Green PCR Master mix (Thermo Fisher Scientific, Inc.). PCR
cycles were run on the iCycler IQ Multi-color Detection system
(Bio-Rad Laboratories, Inc.) and the cycling conditions were 95°C
for 30 sec, 1 cycle; 95°C for 5 sec and 62°C for 20 sec, 40 cycles.
The primers used for amplification were: CXCL9 F,
5′-AGGGTCGGCTGTTCCTGCATC-3′ and R, 5′-TTCACATCTGCTGAATCTGGGTTTA-3′;
GAPDH F, 5′-GCACCGTCAAGGCTGAGAAC-3′ and R,
5′-TGGTGAAGACGCCAGTGGA-3′. Relative expression level was calculated
using the 2−∆∆Cq method (15). GAPDH was employed as an endogenous
control. The data analysis was performed based on the sample
threshold cycle (Ct) value from three independent experiments.
Statistical analysis
All data were analyzed using SPSS version 19.0
statistical software (IBM Corp., Armonk, NY, USA). The results of
immunohistochemistry were analyzed by Image Pro Plus software
version 1.48 (Media Cybernetics, Inc., Rockville, MD, USA).
P<0.05 was considered to indicate a statistically significant
difference. One-way analysis of variance was conducted followed by
Bonferroni method as a post hoc test for multiple comparisons.
Kaplan-Meier survival plots were generated and comparisons were
constructed with log-rank statistics. P<0.05 was considered to
indicate a statistically significant difference.
Results
High expression of the CXCL9 gene
promotes the pathogenesis of prostate cancer mice
In order to determine whether CXCL9 overexpression
affects the pathogenesis of prostate cancer in mice, prostate
cancer was induced in C57BL/6 (C57) and B6.Cg-Selplgtm1Fur/J
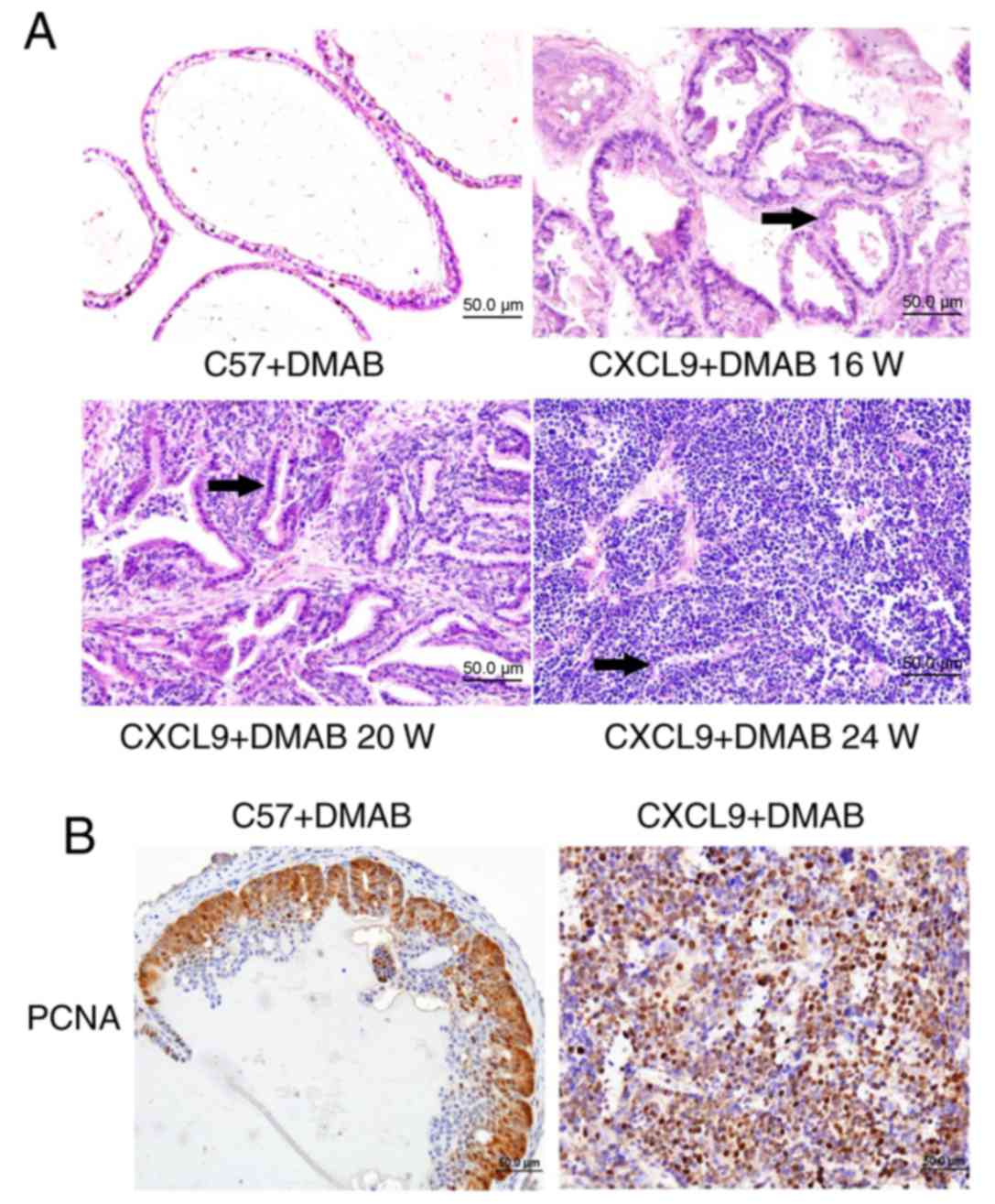
(CXCL9-overexpressed) mice. H&E staining (Fig. 1A) revealed that the prostate cancer
pathology of CXCL9-overexpressed mice was significantly greater
than C57 mice and CXCL9-overexpressed mice had developed
differentiated adenocarcinoma at 20 weeks. The nuclear staining was
significantly greater, the ducts were irregular and glandular
cavity became smaller (black arrows). At 24 weeks,
CXCL9-overexpressed mice exhibited a clear pathology of prostate
cancer with a neuroendocrine differentiation phenotype (Fig. 1A). Therefore, the mice at 24 weeks
were selected for subsequent experiments. Immunohistochemical
staining (magnification, ×400) of proliferating cell nuclear
antigen (PCNA) revealed that proliferation levels in CXCL9+DMAB
mice were increased compared with C57+DMAB mice (Fig. 1B). The aforementioned results
suggested that CXCL9 overexpression serves an important role in
promoting progression of prostate cancer and accelerates the
pathogenesis of prostate cancer.
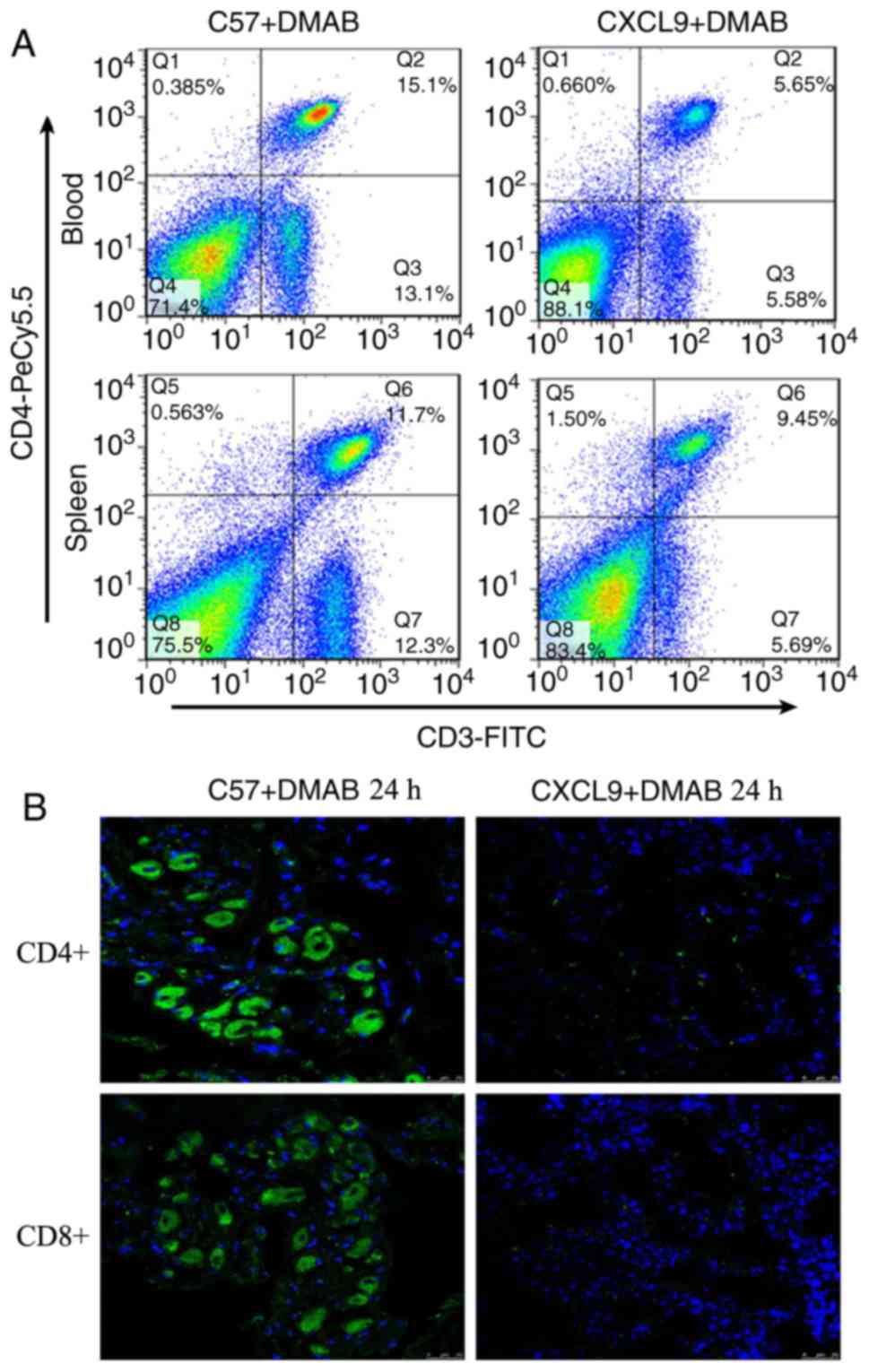
High expression of CXCL9 inhibits T
cell activation
As chemokines serve an important role in the tumor
microenvironment, it was hypothesized that CXCL9 regulates T
cell-mediated immunity. The present study examined the alterations
of T cells in peripheral blood and spleen in C57+DMAB mice and
CXCL9+DMAB mice. The results showed a reduction in the number of T
cells in peripheral blood and spleen of CXCL9+DMAB mice compared
with C57+DMAB mice (Fig. 2A) (Q4
quadrant). The infiltration number of T cells in tumor tissues was
also examined in CXCL9+DMAB mice and C57+DMAB mice. The results
suggested that high expression of CXCL9 significantly inhibited T
cell infiltration into the tumor microenvironment (Fig. 2B). Based on the above findings, it
was hypothesized that CXCL9 may regulate the progression of
prostate cancer either directly or indirectly by influencing the
number of T cells infiltrated in immune organs and the tumor
microenvironment.
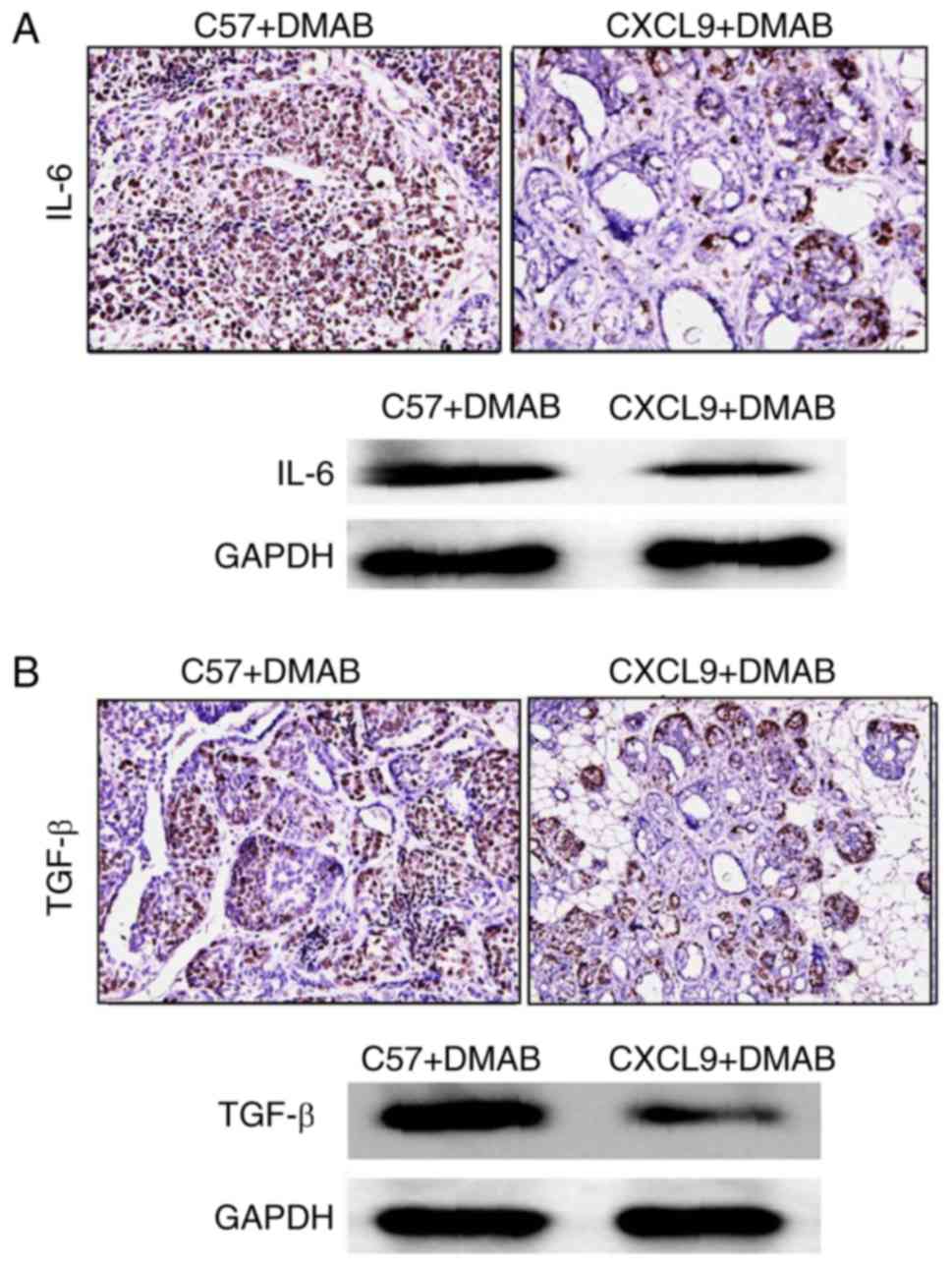
CXCL9 inhibits the secretion of IL-6
and TGF-β from T cells
In order to verify whether CXCL9 overexpression
affects the cytokines secreted in the tumor microenvironment, the
expression levels of IL-6 and TGF-β in the prostate tissues were
examined. The results demonstrated that high expression of CXCL9
downregulated the levels of IL-6 and TGF-β in tumor tissues
compared with C57+DMAB mice (Fig. 3A
and B). Therefore, it was concluded that CXCL9 reduced the
secretion of IL-6 and TGF-β via the reduction of the number of T
cells in immune organs and the tumor microenvironment, and promoted
the development of prostate cancer.
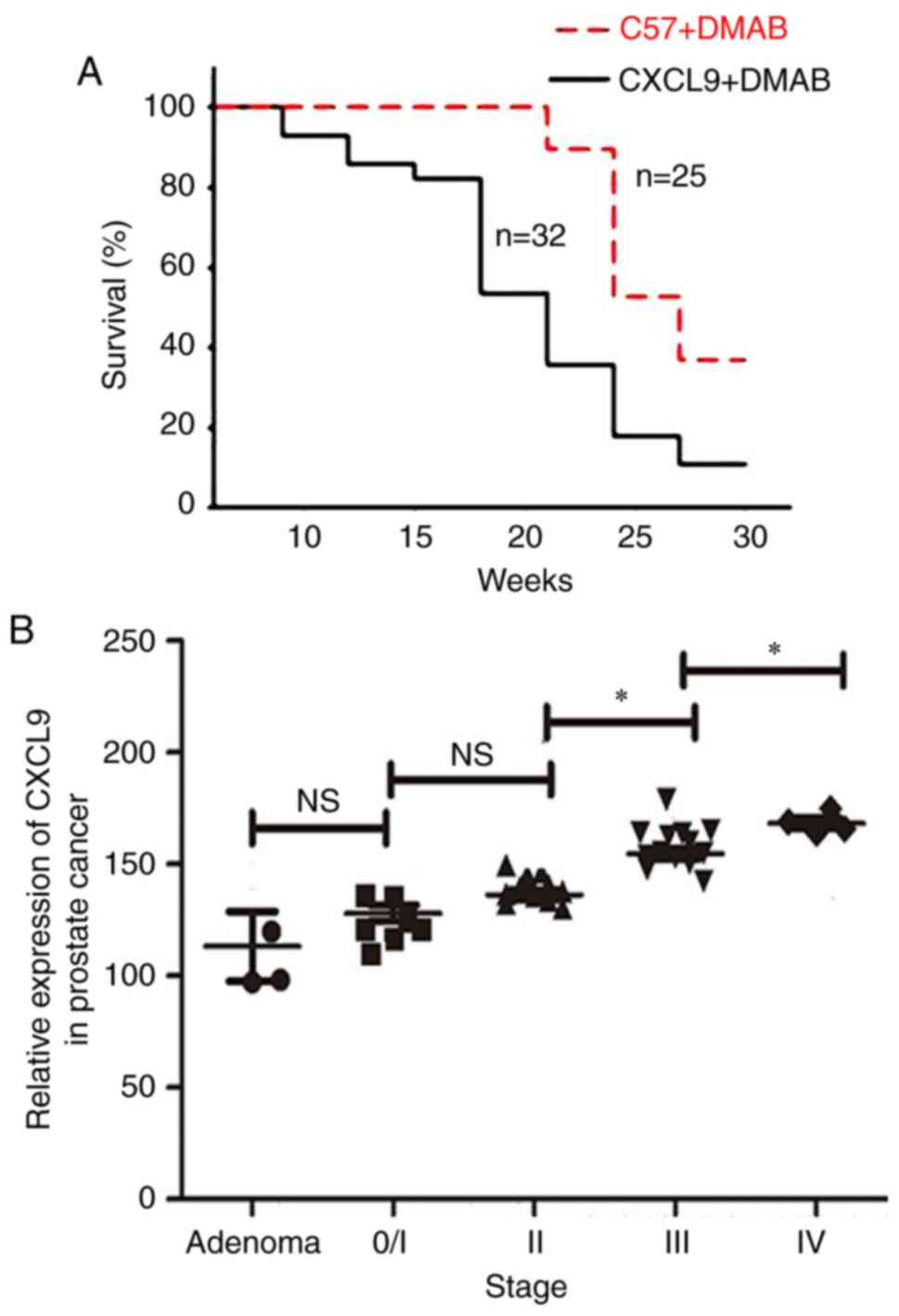
High expression of CXCL9 accelerates
the pathogenesis of prostate cancer
To address the association between CXCL9
overexpression and progression of prostate cancer, the survival
rates of C57+DMAB mice and CXCL9+DMAB mice were measured. The
results suggested that the survival rate of CXCL9+DMAB mice was
lower than C57+DMAB mice (Fig.
4A). In addition, the mRNA expression levels of CXCL9 in
clinical samples were measured. The mRNA levels of CXCL9 in
patients at stage III was greater than at stage II (P=0.024), and
the mRNA levels of CXCL9 in patients at stage IV was greater than
at stage III (P=0.012). The results suggested that the mRNA
expression levels of CXCL9 were positively associated with clinical
staging (Fig. 4B).
Discussion
Prostate cancer is a highly malignant tumor with
complex pathogenesis. Abnormal expression levels of chemokines have
been identified in prostate cancer (16). Many chemokines may have inhibitory
effects on the tumor, and certain chemokines may promote tumor
progression (17). Several
chemokines, including CXCL4 and CXCL10, have been used in
translational medicine (18). In a
previous study, an association between CXCL9 and cancer was
demonstrated; however, the effect of CXCL9 in prostate cancer has
not been reported. A previous studies revealed that CXCL9 is highly
expressed in human digestive organs, such as gastrointestinal
organs, however the expression of CXCL9 in developmental organs has
not been studied (19). In the
present study, CXCL9-overexpressed mice and C57 mice were selected
for investigation. Chemical induction was used to induce prostate
cancer in the mice, and it was demonstrated that CXCL9 may serve an
important role in promoting tumorigenesis. In addition, the effect
of inhibition or knockdown of the CXCL9 gene in tumorigenesis is
important to fully illustrate the effect of CXCL9. However, a
limitation of the present study was that these experiments were not
performed due to the difficulty of gene knock-out mouse
construction; however, this will be investigated in further
studies.
The underlying mechanism of CXCL9 in promoting the
pathogenesis of prostate cancer was investigated in the present
study. Chemokines serve an important role in the tumor
microenvironment, and it was hypothesized that CXCL9 serves an
indirect role in affecting white blood cells. The results suggested
that the T cells in the spleen were significantly reduced in
CXCL9+DMAB mice. A previous study reported that CXCL9 regulates the
homing of lymphocyte thymus and affects T cell maturation (20). In the tumor mouse model, the
function of CXCL9 in regulating T cell maturation and reducing the
number of T cells requires further investigation. IL-6 is produced
by activated T cells and has anti-tumor effects. The anti-tumor
biological activity of IL-6 primarily includes: (i) Promoting the
proliferation and killing activity of NK, and promoting NK cells to
secrete interferon and express the IL-1 receptor agonist chain;
(ii) inducing lymphocyte-activated killer cells, which may dissolve
a variety of tumor cells (21,22).
The present study demonstrated a reduction in the number of T cells
in peripheral blood and spleen in CXCL9+DMAB mice compared with
C57+DMAB mice.
TGF-β is a cytokine secreted by immune cells and has
the ability to inhibit tumor cell growth and induce tumor
degeneration (23,24). Studies have revealed that the
anti-tumor effect of TGF-β depends on its secretion by T helper 1
CD-positive cells to inhibit tumor growth and angiogenesis in
receptor-interacting serine/threonine protein kinase (RIP)-Tag2
model mice (25). A mouse model
where the RIP promoter may be controlled has been obtained from the
hybridization of RIP-Tag2 model mice and LMCV mice. It has been
demonstrated that CD8-positive T cells serve an important role in
inhibiting tumor growth (26). The
present study concluded that T cells and expression levels of IL-6
and TGF-β were reduced by increased levels of CXCL9, resulting in
enhanced growth of tumors in hybrid mice. CXCL9 overexpression
reduced the number of T cells in immune organs and the tumor
microenvironment, and reduced the secretion of IL-6 and TGF-β2, and
thereby promoted the development of prostate cancer.
It was observed that the survival rate of
CXCL9-overexpressed mice was significantly reduced compared with
C57 mice. Therefore, the targeted inhibition of CXCL9 expression
may be applied in a clinical setting. RT-qPCR detected the mRNA
expression of CXCL9 in prostate cancer patients at different
pathological stages, and the results revealed that the mRNA
expression levels of CXCL9 were positively associated with clinical
staging in clinical samples, suggesting that the patients with high
expression of CXCL9 exhibited more advanced pathological
features.
In conclusion, the present study suggested that
expression of CXCL9 in prostate cancer is associated with clinical
stage. CXCL9 may inhibit the expression of IL-6 and TGF-β in
prostate cancer model mice, however the underlying regulatory
mechanism of the development of prostate cancer requires further
investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST and YG conceived and designed the experiments;
ST, FS, YL and YG performed the experiments; ST, KW and YG analyzed
the data; ST, KW, FS and YG contributed reagents/materials/analysis
tools and ST and YG wrote the manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Medical
Ethics Committee of Linyi People's Hospital.
Patient consent for publication
These tissue samples were collected with the consent
of the patients and the study was approved by the Medical Ethics
Committee of the Linyi People's Hospital.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global Cancer Statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atherton MJ, Stephenson KB, Pol J, Wang F,
Lefebvre C, Stojdl DF, Nikota JK, Dvorkin-Gheva A, Nguyen A, Chen
L, et al: Customized viral immunotherapy for HPV-associated cancer.
Cancer Immunol Res. 5:847–859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong B, Minze LJ, Xue W and Chen W:
Molecular insights into the development of T cell-based
immunotherapy for prostate cancer. Expert Rev Clin Immunol.
10:1547–1557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumai T, Fan A, Harabuchi Y and Celis E:
Cancer immunotherapy: Moving forward with peptide T cell vaccines.
Curr Opin Immunol. 47:57–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Melssen M and Slingluff CL Jr: Vaccines
targeting helper T cells for cancer immunotherapy. Curr Opin
Immunol. 47:85–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang P, Li S, Siriwon N, Zhang X, Yang S,
Jin T, He F, Kim YJ, Mac J, Lu Z, Wang S, et al: Enhanced cancer
immunotherapy by chimeric antigen receptor-modified T Cells
engineered to secrete checkpoint inhibitors. Clin Cancer Res.
23:6982–6992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simmons G, Bertram S, Glowacka I, Steffen
I, Chaipan C, Agudelo J, Lu K, Rennekamp AJ, Hofmann H, Bates P and
Pöhlmann S: Different host cell proteases activate the
SARS-coronavirus spike-protein for cell-cell and virus-cell fusion.
Virology. 413:265–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao HM, Zhou H, Zhang F, Wilson BC, Kam W
and Hong JS: HMGB1 acts on microglia Mac1 to mediate chronic
neuroinflammation that drives progressive neurodegeneration. J
Neurosci. 31:1081–1092. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swamydas M, Ricci K, Rego SL and Dréau D:
Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor
cell invasion and the activation of matrix metalloproteinases. Cell
Adh Migr. 7:315–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuo P, Tuong ZK, Teoh SM, Frazer IH,
Mattarollo S and Leggatt GR: HPV16E7-induced hyperplasia promotes
CXCL9/10 expression and induces CXCR3+ T-cell migration
to skin. J Invest Dermatol. S0022–202X, ppi 33358-4. 2017.
|
|
11
|
Matsumoto M, Oshiumi H and Seya T:
Antiviral responses induced by the TLR3 pathway. Rev Med Virol.
21:67–77. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beider K, Abraham M and Peled A:
Chemokines and chemokine receptors in stem cell circulation. Front
Biosci. 13:6820–6833. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han K, Zhao T, Chen X, Bian N, Yang T, Ma
Q, Cai C, Fan Q, Zhou Y and Ma B: microRNA-194 suppresses
osteosarcoma cell proliferation and metastasis in vitro and in vivo
by targeting CDH2 and IGF1R. Int J Oncol. 45:1437–1449. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drouillard A, Puleo F, Bachet JB, Ouazzani
S, Calomme A, Demetter P, Verset G, Van Laethem JL and Maréchal R:
DLL4 expression is a prognostic marker and may predict gemcitabine
benefit in resected pancreatic cancer. Br J Cancer. 115:1245–1252.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arnatt CK, Adams JL, Zhang Z, Haney KM, Li
G and Zhang Y: Design, syntheses, and characterization of piperzine
based chemokine receptor CCR5 antagonists as anti prostate cancer
agents. Bioorg Med Chem Lett. 24:2319–2323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aaron M, Klein RD, Rengel RC, Clinton SK,
Kulp SK, Kashida Y, Yamaguchi M, Wang X and Chen CS:
Chemopreventive and bioenergetic signaling effects of PDK1/Akt
pathway inhibition in a transgenic mouse model of prostate cancer.
Toxicol Pathol. 35:549–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Araki S, Omori Y, Lyn D, Singh RK,
Meinbach DM, Sandman Y, Lokeshwar VB and Lokeshwar BL:
Interleukin-8 is a molecular determinant of androgen independence
and progression in prostate cancer. Cancer Res. 67:6854–6862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hol J, Wilhelmsen L and Haraldsen G: The
murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial
cytoplasmic granules but not in weibel-palade bodies. J Leukoc
Biol. 87:501–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Corbel SY, Merzaban JS, Carlow DA, Gossens
K, Duenas J, So L, Yi L and Ziltener HJ: Recruitment of adult
thymic progenitors is regulated by P-selectin and its ligand
PSGL-1. Nat Immunol. 6:626–634. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delk NA and Farach-Carson MC:
Interleukin-6: A bone marrow stromal cell paracrine signal that
induces neuroendocrine differentiation and modulates autophagy in
bone metastatic PCa cells. Autophagy. 8:650–663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rautava S, Lu L, Nanthakumar NN,
Dubert-Ferrandon A and Walker WA: TGF-β2 induces maturation of
immature human intestinal epithelial cells and inhibits
inflammatory cytokine responses induced via the NF-κB pathway. J
Pediatr Gastroenterol Nutr. 54:630–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo R, Meng Q, Guo H, Xiao L, Yang X, Cui
Y and Huang Y: TGF-β2 induces epithelial-mesenchymal transition in
cultured human lens epithelial cells through activation of the
PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 13:1105–1110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Echeverría C, Montorfano I, Tapia P,
Riedel C, Cabello-Verrugio C and Simon F: Endotoxin-induced
endothelial fibrosis is dependent on expression of transforming
growth factors β1 and β2. Infect Immun. 82:3678–3686. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen H, Sun Y, Wu C, Magyar CE, Li X,
Cheng L, Yao JL, Shen S, Osunkoya AO, Liang C and Huang J:
Pathogenesis of prostatic small cell caricinma involves the
inactivation of the P53 pathway. Endocr Relat Cancer. 19:321–331.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calzascia T, Pellegrini M, Hall H, Sabbagh
L, Ono N, Elford AR, Mak TW and Ohashi PS: TNF-alpha is critical
for antitumor but not antiviral T cell immunity in mice. J Clin
Invest. 117:3833–3845. 2007.PubMed/NCBI
|