Introduction
Alzheimer's disease (AD) is a common type of
neurodegenerative disease, the main clinical manifestation of which
is progressive dementia (1).
AD-associated pathological alterations include the presence of
senile plaques in the brain, neurofibrillary tangles (NFTs) and the
loss of neurons (1). As the aged
population increases, the incidence of AD continues to rise.
According to recent statistics, there are >20 million patients
with AD, and this number increases by 4.6 million annually in China
(1). In addition, for individuals
>65 years old, the risk of AD doubles every 5 years, and in
people ≥85 years old, almost half suffer from AD (1). Due to progressive decline in the
cognitive function of patients with AD, not only is patient quality
of life decreased, but the care of patients with AD is considered a
burden to families and society. AD is a major social problem
worldwide; therefore, research regarding the pathogenesis and
prevention of AD has garnered attention in recent years (1).
Sphingosine kinase 1 (SphK1) is a key enzyme in the
regulation of ceramide/sphingosine-1-phosphate (S1P). Due to their
contrasting functions, the balance between ceramide and S1P is
associated with cell death and survival (2). The balance between these two factors
is mainly regulated by SphK1, which is an enzyme that can convert
sphingomyelin into S1P (3). When
it is overexpressed, SphK1 induces the transformation of ceramide
into S1P (3). Conversely, the
downregulation of SphK1 results in accumulation of ceramide, which
is associated with anticancer therapy-induced cell death (4).
S1P is a biologically active lipid molecule, which
has recently garnered attention. The synthesis and degradation of
S1P is regulated by various enzymes, and its generation is
regulated by SphK1 (5). SphK
catalyzes the first carbon atom of sphingosine to connect with the
ethyl phosphate group; this is essential for the generation of S1P
(6). S1P has a dual role inside
and outside the cell; as a G protein-coupled receptor ligand, S1P
can regulate numerous physiological activities by activating these
receptors, including cell migration, angiogenesis, vascular
maturation, cardiac development and nerve axon functions. In
addition, S1P can be used as a second messenger to regulate
intracellular calcium ion levels for stability, cell proliferation
promotion and apoptosis inhibition (3).
S1P regulates cell death and survival, apoptosis,
calcium balance, blood vessel maturation and angiogenesis, and
participates in various biochemical processes in the central
nervous system; therefore, it has attracted wide attention
(6). Adjusting the expression of
the key enzyme SphK1, which is associated with the S1P/ceramide
balance, may therefore be considered a potential treatment for
certain neurological disorders (7). In a previous study, high expression
of SphK1 was associated with a reduction in the survival rate of
patients with primary glioblastoma multiforme with the strongest
invasive capabilities (3).
MicroRNAs (miRNAs/miRs) are non-coding
single-stranded small RNA molecules, ~22 nucleotides in length
(8,9). Mature miRNAs originate from a
precursor transcript, which has a hairpin structure containing ~70
nucleotides, by the Dicer enzyme (8). Mature miRNA molecules complementarily
bind to the untranslated region of target mRNAs, thus leading to
mRNA degradation or translational inhibition post-transcription, so
as to regulate the expression of immediate early genes (9). miRNAs exist in a wide variety of
species and are highly conserved; miRNAs serve an important role in
the regulation of gene expression and have garnered much attention
in recent years (8).
It has been reported that miRNAs serve an important
role in the central nervous system and its disorders (8). The specific expression of miRNAs in
various cell types has been reported in detail, including miR-23,
miR-26 and miR-29 in astrocytes, miR-124 and miR-128 in neurons,
and the let-7 family in hippocampal neurons (10). Cell specialization is also
associated with particular miRNAs, and it has been reported that,
in zebrafish, the specific miRNA in neural precursor cells is
miR-92b, the specific miRNAs in mature neurons are miR-124, miR-181
and miR-222, the specific miRNA in motor neurons is miR-218a, and
the specific miRNA in dendrites is miR-134 (11). Similarly, in the rat hippocampus,
neuronal cell bodies and projections contain specific miRNAs:
miR-124 and miR-26a, respectively. In addition, in the human
frontal cortex, miR-30a is highly expressed in pyramidal cells
(12). Therefore, tissue- and
cell-specific miRNA expression levels in the human central nervous
system may help further the understanding regarding their functions
(12). Ma et al revealed
that miR-125b enhances neuronal apoptosis and Tau phosphorylation
in patients with Alzheimer's e disease (13). Therefore, the present study aimed
to investigate the expression of miR-125b in patients with AD, and
to determine its potential role in AD.
Patients and methods
Patients and ethics
An initial pilot study was performed using AD
samples and healthy volunteers from the Department of Gereology,
The Third Xiangya Hospital of Central South University (Changsha,
China) from April 2014 to October 2014. A total of 24 patients with
AD (77–82 years age) were included in the present study. All
cerebrospinal fluid (CSF) samples of participants were collected by
lumbar puncture in the L3/L4 or L4/L5 interspace at a standardized
time point between 8:00 and 9:00 a.m. (14). Healthy volunteers (n=24, 22–26
years age) were designated as not exhibiting neurological disease
symptoms. The present study was approved by the Institutional
Review Board of The Third Xiangya Hospital of Central South
University; written informed consent was obtained from all of the
patients.
miRNA expression analysis
Total RNA was harvested from CSF samples using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Total RNA (5 ng) was reverse transcribed into
cDNA using a PrimeScript™ II 1st Strand cDNA Synthesis kit (Takara
Bio, Inc., Otsu, Japan). RT-quantitative polymerase chain reaction
(qPCR) was performed using an ABI 7500 instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and Terra qPCR Direct
SYBR Premix (Takara Bio, Inc.). The primer sequences were as
follows: Mouse miR-125b-5p forward, 5′-TCCCTGAGACCCTAACTTGT-3′ and
reverse, 5′-CTCGCTTCGGCAGCACACA-3′; mouse U6 forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-GTCATTGATGGCAACAATATCCACT-3′; human miR-125b-5p forward,
5′-TCCCGAGACCCTAACTTGTGA-3′; human U6 forward,
5′-CTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CAGGGGCCATGCTAAATCTTC-3′. qPCR was conducted as follows: 60 sec
at 95°C; 40 cycles at 95°C for 15 sec, 60°C for 15 sec, 72°C for 45
sec and 4°C for 1 min. Relative gene expression was determined
using the 2−∆∆Cq method (15).
Cell culture
Mouse neuroblastoma Neuro2a APPSwe/Δ9 cells were
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China) and cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal calf serum (FCS; Gibco; Thermo
Fisher Scientific, Inc.), and were seeded into 6-well plates at a
concentration of 1.5–2×105 cells/well the day prior to
transfection at 37°C in 5% CO2.
Transfection
miR-125b (5′-GACGCAAACTTGCTGATGTT-3′ and
5′-CTGCGTTTGAACGATACAA-3′) and negative mimics
(5′-CCCCCCCCCCCCCCCC-3′ and 5′-CCCCCCCCCCCCCCCCCCC-3′) were
purchased from Sangon Biotech Co., Ltd. (Shanghai, China). In the
control group, Neuro2a APPSwe/Δ9 cells group were cultured with
DMEM at 37°C; in the negative control group, negative control
Neuro2a APPSwe/Δ9 cells were cultured with 100 ng of negative
mimics; and in the miR-125b group, Neuro2a APPSwe/Δ9 cells were
cultured with 100 ng of miR-125b. Neuro2a APPSwe/Δ9 cells were
seeded into 6-well plates (1–2×105 cells/well) were
transfected with 100 ng of miR-125b or 100 ng of negative mimics
(Sangon Biotech Co., Ltd.) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 4 h post-transfection, the
medium was replaced with fresh DMEM supplemented with 10% FCS.
Cell proliferation and apoptosis
assays
Post-transfection with miR-125b or negative mimics
for 48 h, cells were seeded into 96-well plates at
1–2×103 cells/well. Cells were treated with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
5 mg/ml) for 4 h at 37°C. Subsequently, dimethyl sulfoxide was
added to the cells and proliferation was measured using a
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA) at
492 nm.
Post-transfection with miR-125b or negative mimics
for 48 h, cells were seeded into 6-well plates at
1–2×105 cells/well at 37°C. Cells were stained with 5 µl
propidium iodide (PI) and 5 µl fluorescein isothiocyanate-labeled
Annexin V (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
according to the manufacturer's protocol. The samples were assessed
by flow cytometry within 1 h using the BD FACSCanto II system and
Image-ProPlus 6.0 software (BD Biosciences, Franklin Lakes, NJ,
USA).
ELISA
Post-transfection with miR-125b or negative mimics
for 48 h, total protein was extracted from Neuro2a cells using
radioimmunoprecipitation assay (RIPA, Beyotime Institute of
Biotechnology, Nanjing, China) buffer, and was quantified using a
bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Inc.).
Subsequently, 10 ng total proteins were incubated with reagents
from ELISA kits to detect tumor necrosis factor (TNF)-α (cat. no.
PT512; Beyotime Institute of Biotechnology), interleukin (IL)-1β
(cat. no. PI301), IL-6 (cat. no. PI326; both Beyotime Institute of
Biotechnology), IL-10 (cat. no. H009; Nanjing Jiancheng Biology
Engineering Institute, Nanjing, China), superoxide dismutase (SOD;
cat. no. S0101), malondialdehyde (MDA; cat. no. S0131; both
Beyotime Institute of Biotechnology) and Aβ (cat. no. H229; Nanjing
Jiancheng Biology Engineering Institute) peptide production,
according to the manufacturer's protocol.
Western blot analysis
Post-transfection with miR-125b or negative mimics
for 48 h, total protein was extracted from Neuro2a cells using RIPA
buffer and was quantified using a BCA assay (Thermo Fisher
Scientific, Inc.). Equal amounts of protein (50 ng) were separated
by 8–12% SDS-PAGE and were transferred to nitrocellulose membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). After blocking
with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween
(TBST) for 1 h at 37°C, membranes were incubated with the following
primary antibodies: Amyloid precursor protein (APP; cat. no.
sc-9129; 1:500), β-secretase 1 (BACE1; cat. no. sc-10748; 1:500),
Tau1 (Tau1; cat. no. sc-5587; 1:500), SphK1 (cat. no. sc-48825;
1:500), p-extracellular signal-regulated kinase (ERK; cat. no.
sc-23759-R; 1:1,000), ERK (cat. no. sc-292838; 1:500; all Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and GAPDH (cat. no.
AF1186; 1:2,000; Beyotime Institute of Biotechnology) overnight at
4°C. Membranes were then washed three times with TBST and were
incubated with anti-rabbit horseradish peroxidase-conjugated
secondary antibody (cat. no. D110058; 1:5,000; Sangon Biotech Co.,
Ltd.) for 1 h at 37°C. Membranes were developed using enhanced
chemiluminescence solution (Thermo Fisher Scientific, Inc.) and
blotting was analyzed by densitometry using Quantity One software
3.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean, and all experiments were performed in triplicate.
Statistical significance was determined using Student's t-test, or
one analysis of variance (ANOVA) or two-way ANOVA followed by Tukey
post hoc test. SPSS 17.0 software (IBM Corp., Armonk, NY, USA) was
used to analyze the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
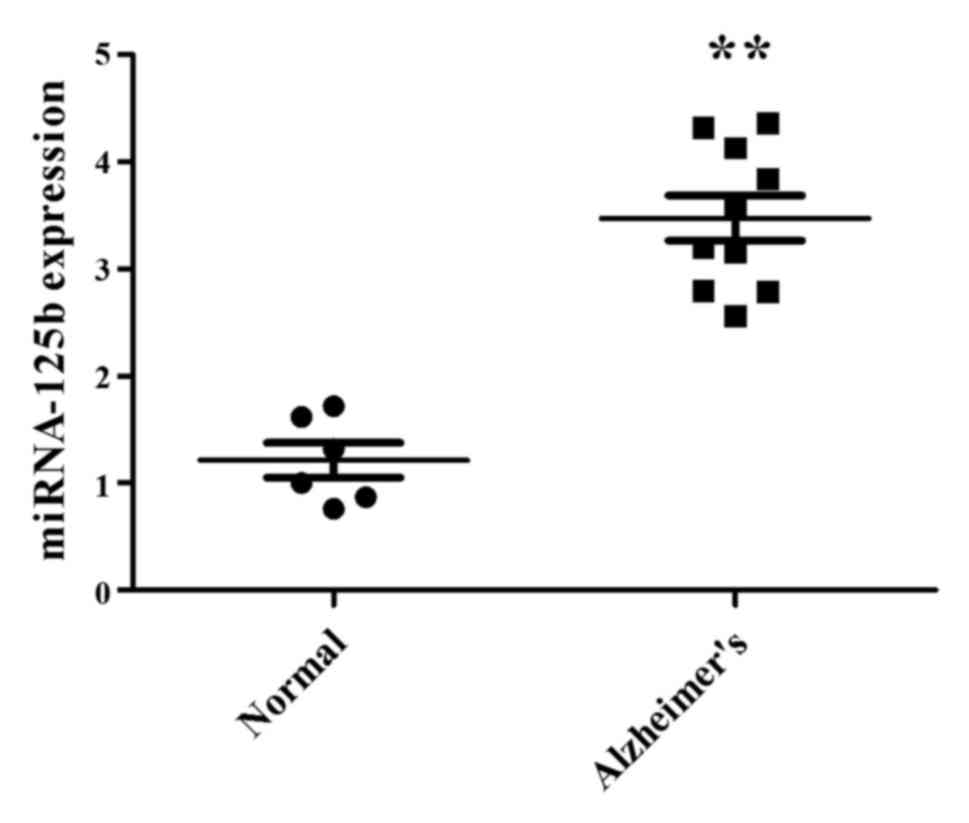
miR-125b expression in patients with
AD
The present study used CSF samples from patients
with AD to analyze miR-125b expression. In CSF samples from
patients with AD, the expression levels of miR-125b were
significantly increased compared with in samples from the normal
participants (Fig. 1). These
results indicated that miR-125b expression is altered in samples
from patients with AD and may be associated with AD.
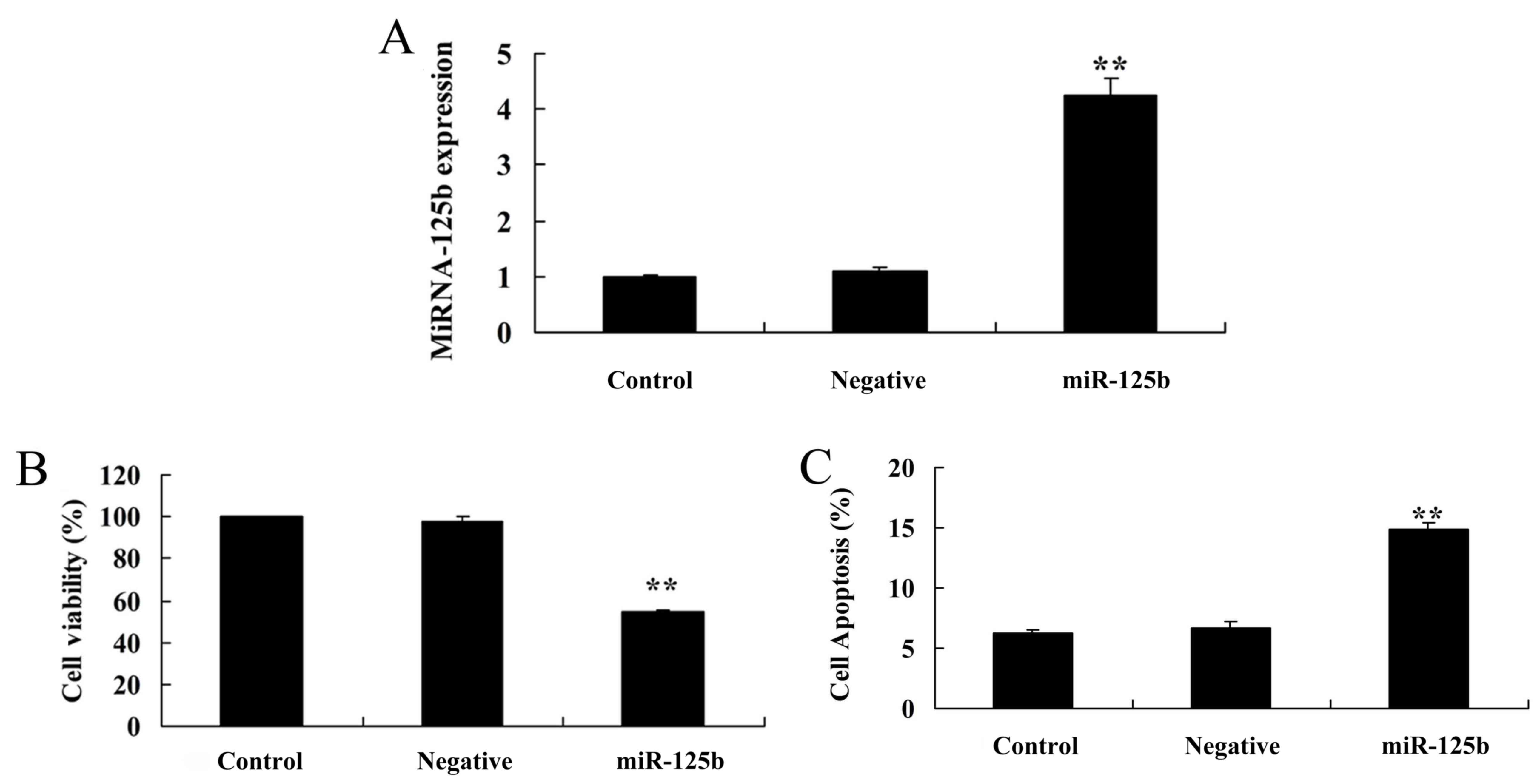
Overexpression of miR-125b inhibits
cell proliferation and induces apoptosis
Mouse neuroblastoma Neuro2a APPSwe/Δ9 cells were
used in the present study; cells were transfected with miR-125b
mimics and miR-125b overexpression was confirmed by qPCR (Fig. 2A). Post-transfection with miR-125b
mimics, cell viability of Neuro2a APPSwe/Δ9 cells was significantly
inhibited compared with the negative control group (Fig. 2B). In addition, miR-125b
over-expression significantly enhanced the apoptotic rate of
Neuro2a APPSwe/Δ9 cells compared with the negative control group
(Fig. 2C).
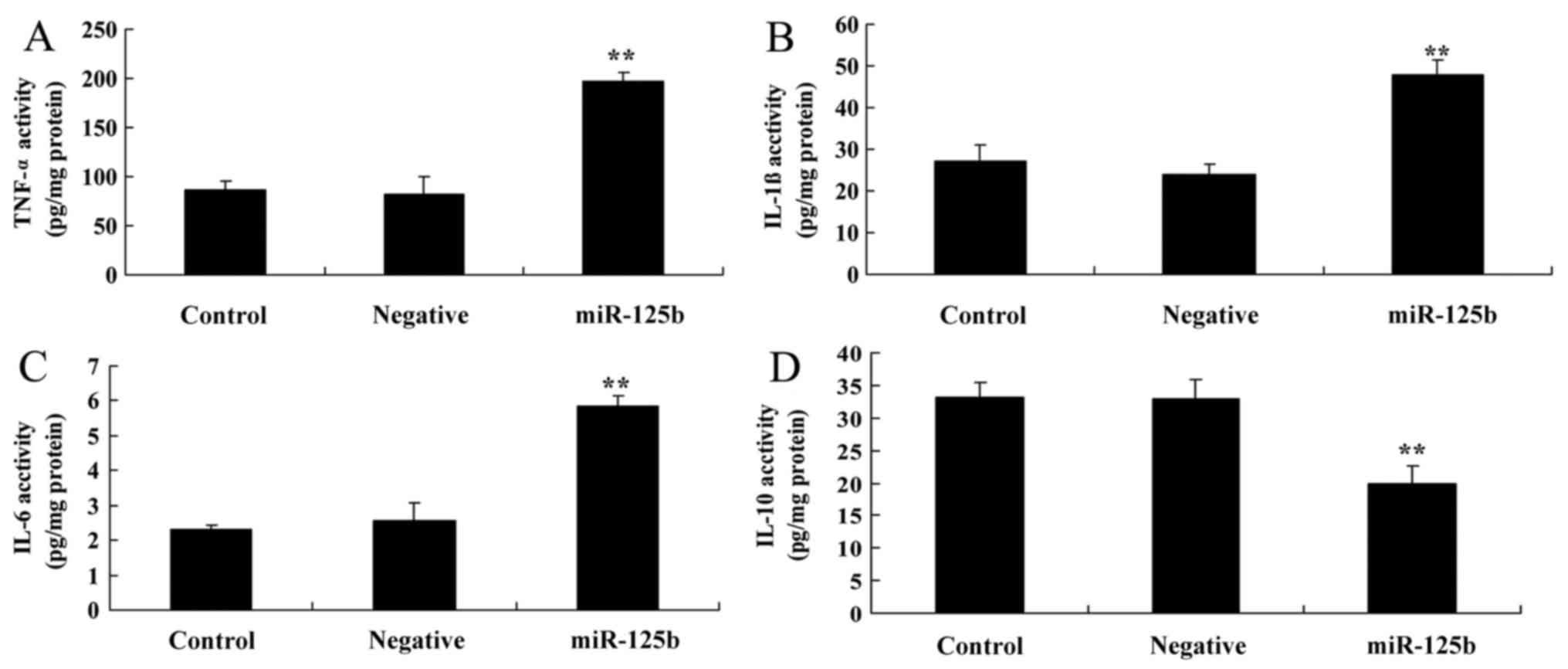
Overexpression of miR-125b enhances
the expression of inflammatory factors
The present study aimed to determine whether
overexpression of miR-125b affected the expression of inflammatory
factors. TNF-α, IL-1β, IL-6 and IL-10 activity levels were detected
in response to miR-125b overexpression. As shown in Fig. 3A-C, TNF-α, IL-1β and IL-6
activities were significantly increased in the AD in vitro
model transfected with miR-125b compared with the negative control
group. Conversely, IL-10 activity levels were significantly reduced
in the AD in vitro model, in which miR-125b was
overexpressed, compared with in the negative control group
(Fig. 3D).
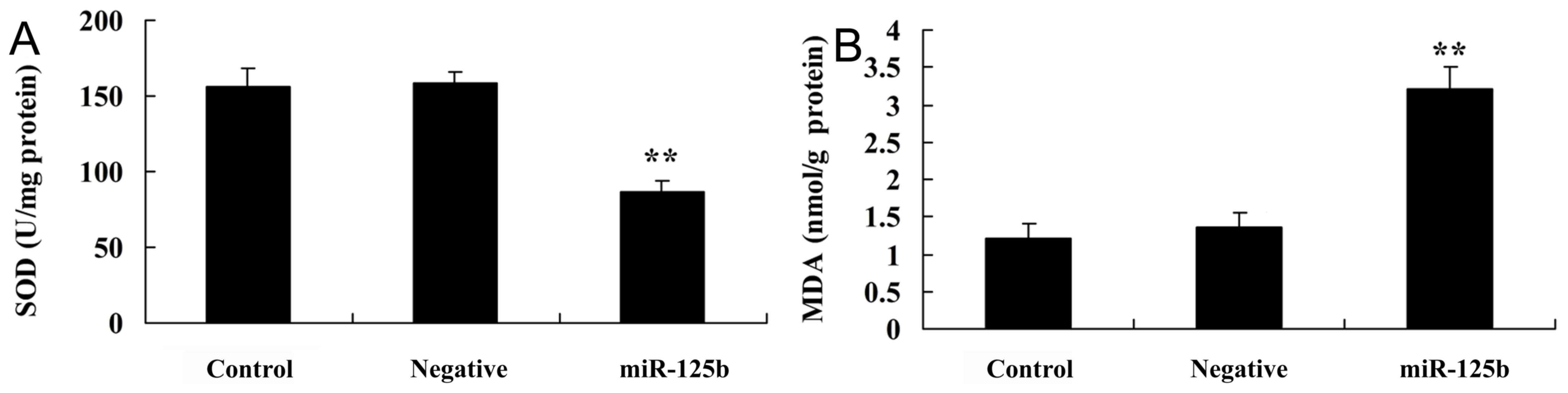
Overexpression of miR-125b enhances
oxidative stress
The present study employed miR-125b mimics to verify
oxidative stress in AD. SOD and MDA levels were detected in cells
post-transfection with miR-125b mimics. As presented in Fig. 4A, SOD levels were significantly
inhibited in the in vitro AD model group, in which miR-125b
was overexpressed, compared with in the negative control group. As
presented in Fig. 4B, MDA levels
were significantly enhanced in the in vitro AD model group,
in which miR-125b was overexpressed, compared with in the negative
control group.
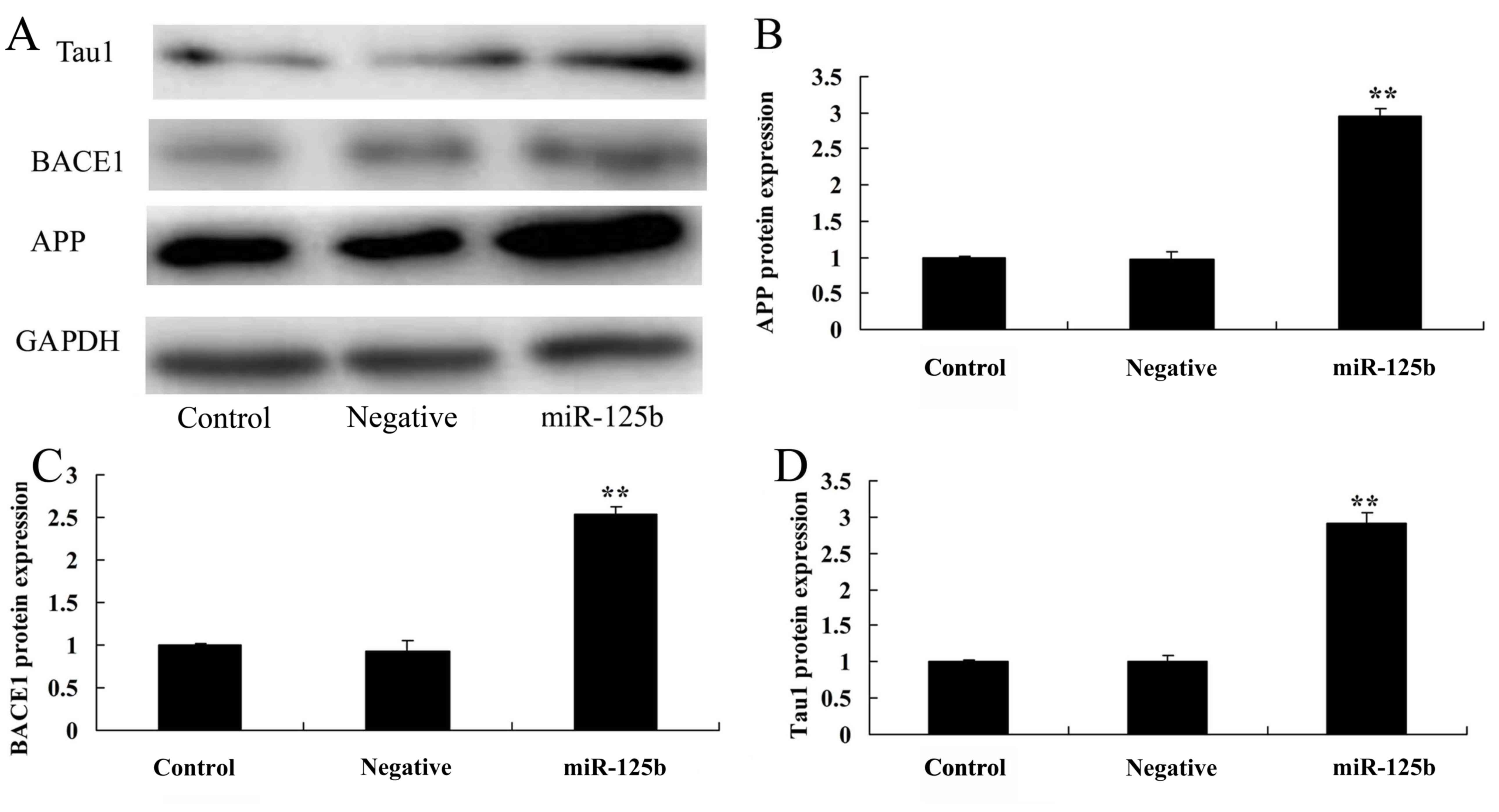
Overexpression of miR-125b promotes
APP, BACE1 and Tau1 protein levels
In order to determine whether overexpression of
miR-125b affects APP protein expression, the protein expression
levels of APP were detected by western blotting. As shown in
Fig. 5, compared with in the
negative control group, APP protein expression was significantly
increased in Neuro2a APPSwe/Δ9 cells post-transfection with
miR-125b mimics.
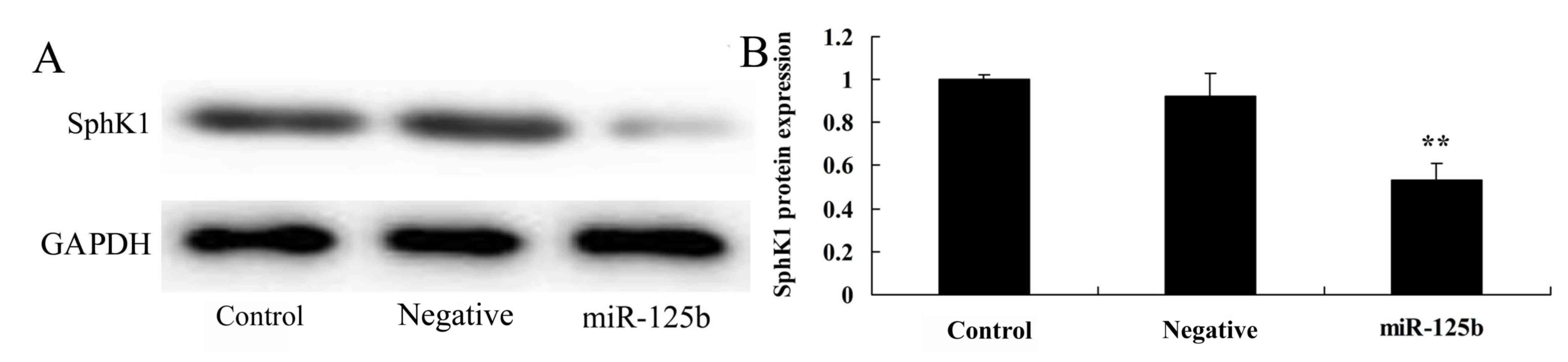
Overexpression of miR-125b suppresses
SphK1 protein expression
The present study detected SphK1 protein expression
in an AD in vitro model, in which miR-125b was
overexpressed. As shown in Fig. 6,
overexpression of miR-125b significantly inhibited SphK1 protein
expression in Neuro2a APPSwe/Δ9 cells compared with the negative
control group.
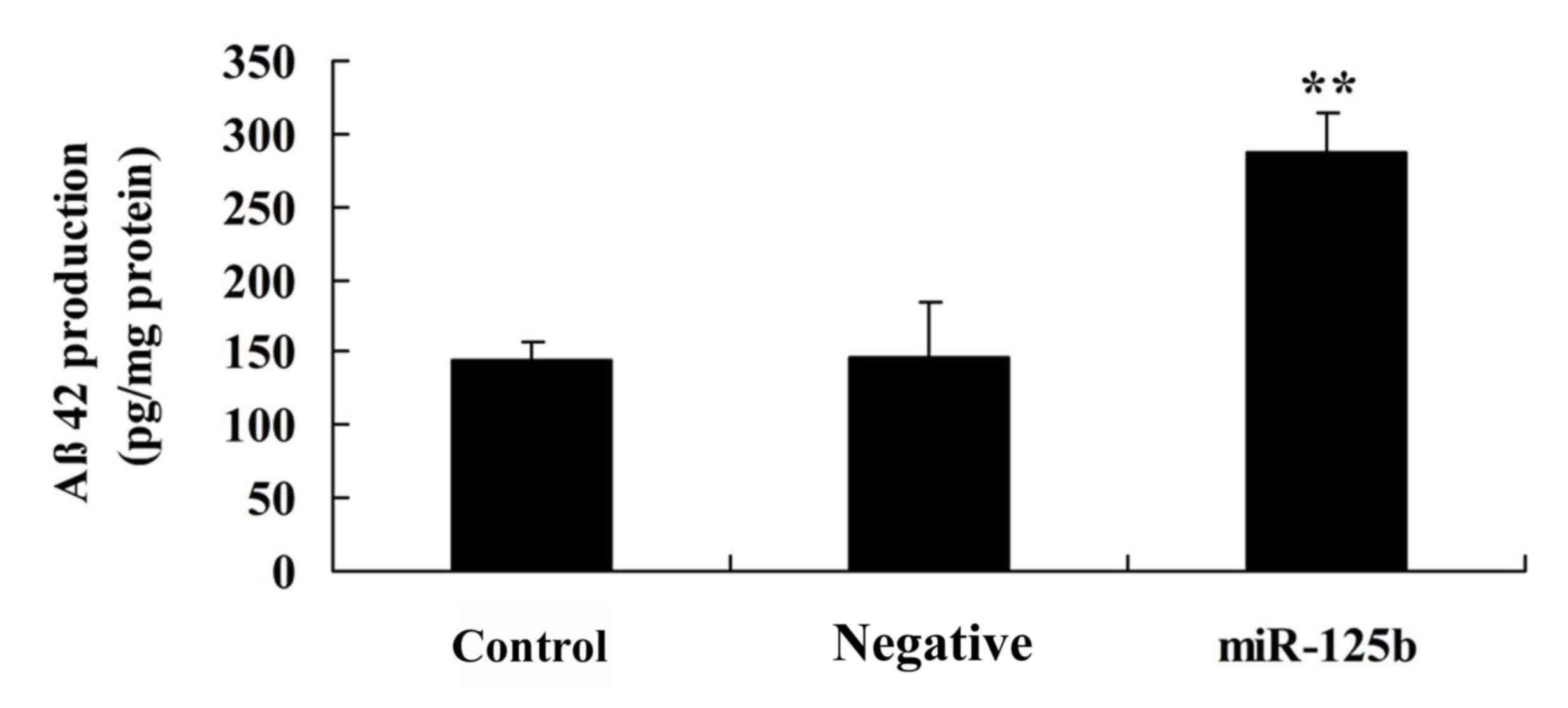
Overexpression of miR-125b promotes Aβ
peptide production
To investigate the underlying mechanism of miR-125b
in AD, an ELISA analysis was used to detect Aβ peptide production
in the in vitro AD model. Overexpression of miR-125b
significantly increased Aβ peptide production in Neuro2a APPSwe/Δ9
cells compared with in the negative control group (Fig. 7).
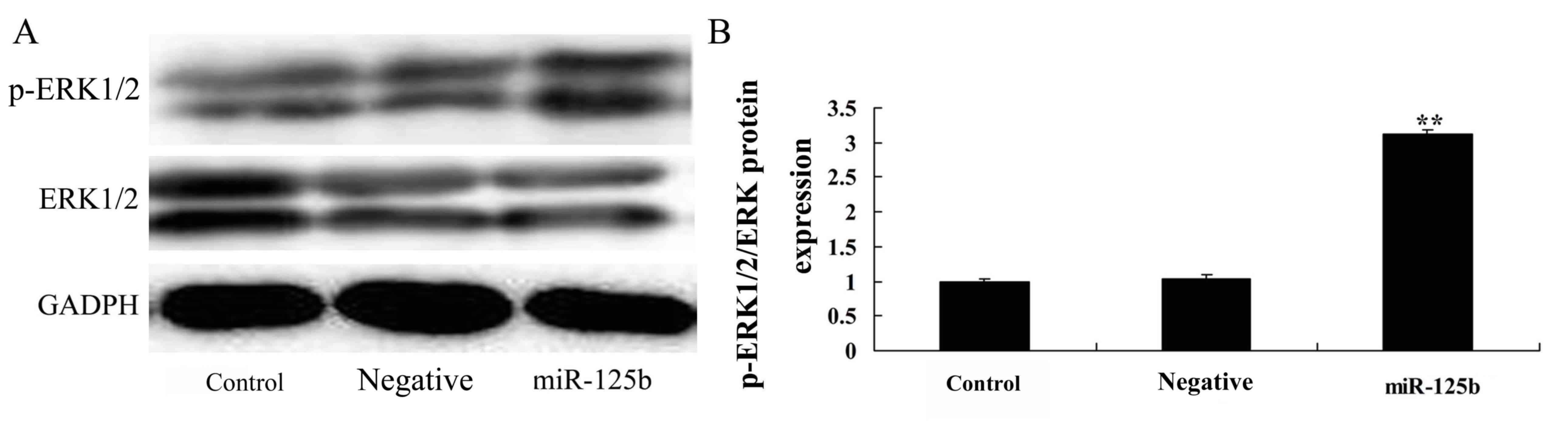
Overexpression of miR-125b promotes
p-ERK protein expression
To explore the effects of miR-125b on ERK protein
expression, western blotting was performed. The protein expression
levels of p-ERK were significantly promoted by overexpression of
miR-125b in Neuro2a APPSwe/Δ9 cells compared with in the negative
control group (Fig. 8).
Discussion
AD is a degenerative disease of the central nervous
system; >20 million people suffer from this disease globally,
among which 15% are >65 years old and 50% are >85 years old
(16). As well as progressive
memory loss and cognitive dysfunction, the main clinical symptoms
associated with AD include brain atrophy, and neuronal and synaptic
reduction, and less common symptoms include the presence of
neuritic plaques and NFTs (17).
At present, the exact etiology and pathogenesis of AD have not been
fully elucidated; therefore, an effective therapeutic strategy is
lacking. Further insights into the mechanisms underlying neuronal
degeneration and death are required, in order to identify drugs
that may delay or block these processes (17). There are numerous hypotheses
regarding the pathogenesis of AD, including Aβ aggregation,
generation of phosphorylated tau protein, genetic mutations,
oxidative stress, genetic factors and alterations in lipid
metabolism (18). The results of
the present study demonstrated that the expression of miR-125b was
markedly increased in patients with AD compared with in the normal
group. In addition, overexpression of miR-125b significantly
inhibited cell proliferation and induced apoptosis, enhanced
inflammatory factors and MDA levels, and suppressed SOD levels in
an in vitro model of AD. miR-9, miR-34a, miR-125b, miR-146a
and miR-155 have been suggested to be associated with the
neuropathology of common, age-related inflammatory
neurodegeneration of the human central nervous system (19).
Aβ is the major component of senile plaques in the
AD brain, which is generally composed of 39–43 amino acid residues,
and overexpression of Aβ42 and Aβ40 has been demonstrated to induce
AD (20). The secondary structure
of Aβ is made up of β-sheets, hence why it is known as Aβ (21). It has previously been reported that
Aβ is derived from a larger precursor protein, which is known as
APP (21). The results of the
present study suggested that overexpression of miR-125b
significantly increased Aβ peptide production in Neuro2a APPSwe/Δ9
cells.
The APP gene is located in the long arm of human
chromosome 21, which is widely present in many cell membranes of
the body; in particular, APP is abundant in human neurons and
astrocytes, and is mainly located in the synapse and neuronal cell
membrane (22). However, the
function of APP is currently unclear. A previous study demonstrated
that cultured hippocampal neurons with a lack of APP exhibited
enhanced neuronal synaptic transmission (23). The present study demonstrated that
the protein expression levels of APP were significantly increased
in Neuro2a APPSwe/Δ9 cells in response to miR-125b
overexpression.
The core component of senile plaques in patients
with AD is Aβ, which is produced by the hydrolysis of APP. Although
various cells and cell lines can synthesize APP, neurons are the
main source of APP, and only brain cells are able to process APP
(24). APP is mainly hydrolyzed by
α-secretase, which hydrolyzes APP within the Aβ domain and
completely blocks Aβ generation, resulting in the generation of
APPs and C83, which is further degraded into P342 or P340 under the
role of γ-secretase; this pathway is the predominant pathway of APP
metabolism, and the release of extracellular α-soluble APP has a
neuroprotective effect (25). The
other metabolic pathway is known as the Aβ-generated pathway; APP
is initially hydrolyzed by BACE to generate βAPPs and C99, which
results in the generation of Aβ42 or Aβ40 (24). In the present study, the results
support the hypothesis that overexpression of miR-125b
significantly increases Aβ peptide production in AD brains.
Aβ formation and deposition may induce toxic effects
and mitochondrial injury, leading to an overload of
Ca2+, which can activate
Ca2+/calmodulin-dependent protein kinase II, further
leading to Tau hyperphosphorylation and inhibition of the
microtubule assembly-promoting activity of Tau (26). When microtubules cannot be properly
assembled, NFTs are generated, which eventually leads to neuronal
dysfunction and even death (27).
AD-associated dementia caused by Tau gene mutations is not
associated with amyloid deposition, even if severe NTFs appear in
the brain, thus suggesting that NFTs are generated following the
metabolic alterations associated with Aβ. Therefore, the Aβ cascade
theory has been hypothesized, which suggests that abnormal or
oversecretion of Aβ can induce other pathological alterations
associated with AD (28).
Collectively, these results suggested that miR-125b may regulate
BACE1 and Tau1 protein expression, and affect Aβ levels, resulting
in AD-associated alterations.
Taul proteins are microtubule-associated proteins,
the main functions of which are associated with microtubule
assembly, stable microtubule formation, the establishment of
cellular polarity and axonal transport maintenance in neuronal
cells (29). When Taul proteins
are excessively phosphorylated and accumulate in cells, they lose
their functions, thus resulting in damage to microtubules (30). In AD, excessively phosphorylated
Taul proteins form paired helical filaments, thus reducing their
affinity to microtubules (31). A
previous study indicated that neuronal death and cognitive
dysfunction are associated with excessively phosphorylated Taul
proteins. The results of the present study demonstrated that
miR-125b overexpression significantly promoted Tau1 protein
expression. Collectively, these results suggested that miR-125b may
regulate BACE1 and Tau1 protein expression, and affect Aβ levels,
resulting in AD-associated alterations.
Upregulation of SphK1 can significantly improve
learning and memory, and reduce the deposition of amyloid proteins
in the brains of APP/presenilin 1 (PS1) transgenic mice (32), thus indicating that high SphK1
expression may serve a protective role in APP/PS1 transgenic mice
(33). In addition, alterations in
the expression levels and the regulation of SphK1 may effectively
improve pathological alterations associated with AD, and may be
used to generate effective treatments for patients with AD
(34). In the present study, the
results suggested that overexpression of miR-125b significantly
suppressed SphK1 protein expression and enhanced the levels of
p-ERK protein in vitro. However, the present study did not
determine the effects of SphK1 inhibition on AD. In future studies
we aim to investigate the effects of SphK1 inhibitors and small
interfering RNA-SphK1.
In conclusion, the present study demonstrated that
overexpression of miR-125b significantly inhibited cell
proliferation and induced apoptosis, enhanced the expression of
inflammatory factors and oxidative stress, promoted APP and BACE1
expression, Aβ peptide production, and suppressed SphK1 protein
expression in vitro. Based on these results, it may be
hypothesized that miR-125b is associated with the pathogenesis of
AD. However, further studies are required to clarify the roles of
miR-125b and SphK1 in AD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
ML designed the experiment; YJ and QT performed the
experiments; ML and YJ analyzed the data; ML wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of The Third Xiangya Hospital of Central South
University. Written informed consent was obtained from all of the
patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cai Y, Ruan J, Yao X, Zhao L and Wang B:
MicroRNA-187 modulates epithelial-mesenchymal transition by
targeting PTRF in non-small cell lung cancer. Oncol Rep.
37:2787–2794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li L and Ma HQ: MicroRNA-216a inhibits the
growth and metastasis of oral squamous cell carcinoma by targeting
eukaryotic translation initiation factor 4B. Mol Med Rep.
12:3156–3162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Xu K, Shi L, Zhang L, Zhao Z, Xu
H, Liang F, Li H, Zhao Y, Xu X and Tian Y: Overexpression of
MicroRNA-216a suppresses proliferation, migration, and invasion of
glioma cells by targeting leucine-rich repeat-containing G
protein-coupled receptor 5. Oncol Res. 25:1317–1327. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu B, Su F, Chen M, Li Y, Qi X, Xiao J,
Li X, Liu X, Liang W, Zhang Y and Zhang J: Serum miR-21 and
miR-125b as markers predicting neoadjuvant chemotherapy response
and prognosis in stage II/III breast cancer. Hum Pathol. 64:44–52.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang X, Tang J, Liu X, Zeng L, Cheng C,
Luo Y, Li L, Qin SL, Sang Y, Deng LM and Lv XB: Downregulation of
miR-129-2 by promoter hypermethylation regulates breast cancer cell
proliferation and apoptosis. Oncol Rep. 35:2963–2969. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, You X, Wang Y, Liu Q, Liu Y, Zhang
S, Chen L, Zhang X and Ye L: The oncoprotein HBXIP enhances
angiogenesis and growth of breast cancer through modulating FGF8
and VEGF. Carcinogenesis. 35:1144–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di YF, Li DC, Shen YQ, Wang CL, Zhang DY,
Shang AQ and Hu T: MiR-146b protects cardiomyocytes injury in
myocardial ischemia/reperfusion by targeting Smad4. Am J Transl
Res. 9:656–663. 2017.PubMed/NCBI
|
|
8
|
Makhdoumi P, Roohbakhsh A and Karimi G:
MicroRNAs regulate mitochondrial apoptotic pathway in myocardial
ischemia-reperfusion-injury. Biomed Pharmacother. 84:1635–1644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akhtar N and Haqqi TM: MicroRNA-199a*
regulates the expression of cyclooxygenase-2 in human chondrocytes.
Ann Rheum Dis. 71:1073–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin N, Li XY, Zhang HM, Yang Z and Su Q:
microRNA-199a-5p mediates high glucose-induced reactive oxygen
species production and apoptosis in INS-1 pancreatic β-cells by
targeting SIRT1. Eur Rev Med Pharmacol Sci. 21:1091–1098.
2017.PubMed/NCBI
|
|
11
|
Wu C, Jin B, Chen L, Zhuo D, Zhang Z, Gong
K and Mao Z: MiR-30d induces apoptosis and is regulated by the
Akt/FOXO pathway in renal cell carcinoma. Cell Signal.
25:1212–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Du N, Zhang Q, Li J, Chen X, Liu X,
Hu Y, Qin W, Shen N, Xu C, et al: MicroRNA-30d regulates
cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic
cardiomyopathy. Cell Death Dis. 5:e14792014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma X, Liu L and Meng J: MicroRNA-125b
promotes neurons cell apoptosis and Tau phosphorylation in
Alzheimer's disease. Neurosci Lett. 661:57–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Magnin E, Dumurgier J, Bouaziz-Amar E,
Bombois S, Wallon D, Gabelle A, Lehmann S, Blanc F, Bousiges O,
Hannequin D, et al: Alzheimer's disease cerebro-spinal fluid
biomarkers: A clinical research tool sometimes useful in daily
clinical practice of memory clinics for the diagnosis of complex
cases. Rev Med Interne. 38:250–255. 2017.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An G, Liang S, Sheng C, Liu Y and Yao W:
Upregulation of microRNA-205 suppresses vascular endothelial growth
factor expression-mediated PI3K/Akt signaling transduction in human
keloid fibroblasts. Exp Biol Med (Maywood). 242:275–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yue X, Wang P, Xu J, Zhu Y, Sun G, Pang Q
and Tao R: MicroRNA-205 functions as a tumor suppressor in human
glioblastoma cells by targeting VEGF-A. Oncol Rep. 27:1200–1206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niu K, Shen W, Zhang Y, Zhao Y and Lu Y:
MiR-205 promotes motility of ovarian cancer cells via targeting
ZEB1. Gene. 574:330–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guan B, Li Q, Shen L, Rao Q, Wang Y, Zhu
Y, Zhou XJ and Li XH: MicroRNA-205 directly targets Krüppel-like
factor 12 and is involved in invasion and apoptosis in basal-like
breast carcinoma. Int J Oncol. 49:720–734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JQ, Papp G, Poliska S, Póliska S,
Szabó K, Tarr T, Bálint BL, Szodoray P and Zeher M: MicroRNA
expression profiles identify disease-specific alterations in
systemic lupus erythematosus and primary Sjögren's syndrome. PLoS
One. 12:e01745852017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams AE, Choi K, Chan AL, Lee YJ,
Reeves WH, Bubb MR, Stewart CM and Cha S: Sjögren's
syndrome-associated microRNAs in CD14(+) monocytes unveils targeted
TGFβ signaling. Arthritis Res Ther. 18:952016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alevizos I and Illei GG: MicroRNAs in
Sjögren's syndrome as a prototypic autoimmune disease. Autoimmun
Rev. 9:618–621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tandon M, Gallo A, Jang SI, Illei GG and
Alevizos I: Deep sequencing of short RNAs reveals novel microRNAs
in minor salivary glands of patients with Sjögren's syndrome. Oral
Dis. 18:127–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mi L, Chen Y, Zheng X, Li Y, Zhang Q, Mo D
and Yang G: MicroRNA-139-5p suppresses 3T3-L1 preadipocyte
differentiation through notch and IRS1/PI3K/Akt insulin signaling
pathways. J Cell Biochem. 116:1195–1204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krishnan K, Steptoe AL, Martin HC,
Pattabiraman DR, Nones K, Waddell N, Mariasegaram M, Simpson PT,
Lakhani SR, Vlassov A, et al: miR-139-5p is a regulator of
metastatic pathways in breast cancer. RNA. 19:1767–1780. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mameli G, Arru G, Caggiu E, Niegowska M,
Leoni S, Madeddu G, Babudieri S, Sechi GP and Sechi LA: Natalizumab
therapy modulates miR-155, miR-26a and proinflammatory cytokine
expression in MS patients. PLoS One. 11:e01571532016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maoa R, Zou F, Yang L, Lin S, Li Y, Ma M,
Yin P, Liang X and Liu Y: The loss of MiR-139-5p promotes
colitis-associated tumorigenesis by mediating PI3K/AKT/Wnt
signaling. Int J Biochem Cell Biol. 69:153–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xin Q, Li J, Dang J, Bian X, Shan S, Yuan
J, Qian Y, Liu Z, Liu G, Yuan Q, et al: miR-155 deficiency
ameliorates autoimmune inflammation of systemic lupus erythematosus
by targeting S1pr1 in Faslpr/lpr mice. J Immunol. 194:5437–5445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Petry FR, Pelletier J, Bretteville A,
Morin F, Calon F, Hébert SS, Whittington RA and Planel E:
Specificity of anti-tau antibodies when analyzing mice models of
Alzheimer's disease: Problems and solutions. PLoS One.
9:e942512014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gratuze M, El Khoury NB, Turgeon A, Julien
C, Marcouiller F, Morin F, Whittington RA, Marette A, Calon F and
Planel E: Tau hyperphosphorylation in the brain of ob/ob mice is
due to hypothermia: Importance of thermoregulation in linking
diabetes and Alzheimer's disease. Neurobiol Dis. 98:1–8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laurents DV, Gorman PM, Guo M, Rico M,
Chakrabartty A and Bruix M: Alzheimer's Abeta40 studied by NMR at
low pH reveals that sodium 4,4-dimethyl-4-silapentane-1-sulfonate
(DSS) binds and promotes beta-ball oligomerization. J Biol Chem.
280:3675–3685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S,
Liu S, Ma S, Ma PX and Chen J: MiRNA-29b suppresses tumor growth
through simultaneously inhibiting angiogenesis and tumorigenesis by
targeting Akt3. Cancer Lett. 397:111–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Das S: Identification and targeting of
microRNAs modulating acquired chemotherapy resistance in Triple
negative breast cancer (TNBC): A better strategy to combat
chemoresistance. Med Hypotheses. 96:5–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen H, Li L, Yang S, Wang D, Zhong S,
Zhao J and Tang J: MicroRNA-29a contributes to drug-resistance of
breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling
pathway. Gene. 593:84–90. 2016. View Article : Google Scholar : PubMed/NCBI
|