Introduction
Osteosarcoma (OS) is a highly aggressive and common
primary bone tumor that often occurs among young individuals
worldwide (1,2). Although considerable effort has been
made to develop effective methods for OS therapy, the clinical
outcome of patients with OS has not improved. In particular, the
5-year survival rate of patients with metastatic or advanced OS is
lower than 30% (3,4). Every year, OS accounts for a large
percentage of cancer-related deaths worldwide (5). Thus, understanding the underlying
mechanism and identifying new therapeutic targets for OS treatment
are necessary.
MicroRNAs (miRNAs/miRs) are a class of short RNAs
that have a length of 21–25 nucleotides and no protein-coding
ability (6). A study on their
mechanism demonstrated that miRNAs can regulate target gene
expression by associating with the 3′-untranslated region of miRNAs
and directing its degradation (7).
In recent decades, increasing evidence indicated that miRNAs are
generally involved in diverse biological processes, such as cell
proliferation, differentiation, and death (8–10).
Dysregulation of miRNAs is proven to be closely related to human
diseases, especially tumor development and progression (11). For instance, miR-589-3p regulates
cell migration and invasion in glioblastoma (12), and miR-29c-3p participates in the
regulation of colorectal cancer (CC) growth (13). In addition, miRNA-139 can inhibit
the progression of papillary thyroid carcinoma (14). An increasing number of reports have
shown that miRNAs are good biomarkers for cancer diagnosis,
prognosis, and treatment (15,16).
Therefore, fully understanding the underlying molecular mechanism
by which miRNAs regulate tumorigenesis will benefit cancer
intervention.
miR-590-5p has been reported to inhibit tumor growth
in CC by targeting YAP1 (17).
Another study showed that miR-590-5p suppresses stemness and
metastasis in breast cancer by targeting SOX2 (18). However, the function and mechanism
of miR-590-5p on OS remain to be elucidated.
In our study, we showed that miR-590-5p was lowly
expressed in OS tissues and cell lines compared with that in normal
tissues or cells. In addition, overexpression of miR-590-5p in
SAOS2 and U2OS cells inhibited cell proliferation, migration, and
invasion. We found that miR-590-5p could suppress OS cell entry
into the cell cycle. Mechanistically, we observed that miR-590-5p
directly targeted KLF5, which is a transcription factor belonging
to the Kruppel-like factor subfamily of zinc finger proteins, in
SAOS2 and U2OS cells. Finally, restoration of KLF5 in
miR-590-5p-overexpressing SAOS2 and U2OS cells rescued
proliferation, migration, and invasion. In summary, our finding
indicated that miR-590-5p suppressed OS progression by targeting
KLF5.
Materials and methods
Clinical specimens
For this study, we collected 51 OS samples and 19
adjacent normal tissues from Huai'an First People's Hospital,
Nanjing Medical University (Huai'an, China). None of the patients
had received any adjuvant chemotherapy or radiotherapy before
surgery. Matched adjacent noncancerous tissues were collected >5
cm away from the tumors and were verified by two independent
pathologists at the same time. We obtained written informed
consents that approved the usage of these sample tissues in this
study from all patients. All of the experiments were approved by
Huai'an First People's Hospital, Nanjing Medical University. The
study protocol was approved by Huai'an First People's Hospital,
Nanjing Medical University.
Cell culture and transfection
Human osteoblast cell line (hFOB1.19) and OS cell
lines including U2OS, MG63 and SAOS2 were purchased from American
Type Culture Collection. Cells were cultured in the DMEM medium
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) and anti-biotics in a humidified
containing of 5% CO2 incubator at 37°C.
miR-590-5p mimics, inhibitors and corresponding
negative controls were purchased from Genecopoeia (Guangzhou,
China) and transduced into cells with Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufactures' instruction. For KLF5
overexpression, the coding sequence of KLF5 was cloned into
pCDNA3-vector. And then pCDNA3-KLF5 was transfected into OS cells
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufactures' instruction.
Cell proliferation assay
1×104 cells/well were seeded in 96-well
plates and cultured. Cell proliferation was measured by a CCK8 cell
proliferation assay every other day. 10 µl CCK8 solution was added
and incubated for 2 h at 37°C. Then the absorbance was analyzed at
450 nm using SUNRISE Microplate Reader (Tecan Group, Ltd.,
Mannedorf, Switzerland).
Migration and invasion assay
2×103 cells/well were seeded into the
upper chamber of 24-well chambers and cultured in serum-free
medium. The lower chamber was added medium with 10% FBS. 48 h
later, migrating cells were fixed and stained with 0.5% crystal
violet. Then three randomly fields were counted with a microscope.
For invasion assay, the seeded cells were pre-coated with 500 ng/ml
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Other steps
were the same as migration assay.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNAs were isolated using TRIzol reagent from
OS samples according to the manufacturer's protocol. cDNA were
synthesized and used for analysis of mRNA transcripts using ABI
7300 qPCR system. RT-qPCR was performed with the SYBR green Premix
Ex Taq II (Takara Bio, Inc., Otsu, Japan) on an Applied Biosystems
Step One Plus Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: An initial denaturation step at 95°C for 5 min, followed
by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 10
sec. Relative expressions were calculated and normalized to
endogenous ACTB. Relative gene expression level was
calculated using the 2−∆∆Cq (19).
Colony formation assay
SAOS2 and U2OS cells were trypsinized into a
single-cell suspension, and plated in each well of the 6-well plate
and cultured for 14 days. The colonies were then washed twice with
phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde,
and stained with 0.1% crystal violet. The number of colonies formed
was imaged and counted under a light microscope (Olympus
Corporation, Tokyo, Japan).
Cell cycle analysis
The cell cycle stage was determined using the Cell
Cycle Analysis kit (Beyotime Institute of Biotechnology, Shanghai,
China) following the manufacturer's instructions. Transfected cells
were seeded into 6-well plates for 48 h. Cells were then harvested
and washed three times with cold PBS and fixed in 70% ethanol in
PBS at −20°C for 2 h, followed by washing with PBS and staining the
fixed cells with 50 µg/ml propidium iodide for 30 min in the dark
at 37°C. Analyses were performed on a FACS Calibur flow cytometer
(BD Biosciences) using the ModFit software (BD Biosciences).
Western blotting
Protein from the tissues or cultured cells was
extracted on ice in radioimmunoprecipitation assay lysis buffer
containing protease inhibitor (Beyotime Institute of Biotechnology)
for 30 min. Equal amounts of protein were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
to nitrocellulose membranes (GE Healthcare, Chicago, IL, USA).
Following blocking in 5% non-fat milk in TBS, the membranes were
incubated with the following antibodies: Anti-CYCLIN D1 (no. 2922;
Cell Signaling Technology, Inc., Danvers, MA, USA), anti-CYCLIN-E1
(no. 20808; Cell Signaling Technology, Inc.), anti-p21 (no. 2947;
Cell Signaling Technology, Inc.), anti-KLF5 (no. 51586; Cell
Signaling Technology, Inc.) and anti-GAPDH (no. 5174; Cell
Signaling Technology, Inc.) overnight at 4°C. The membranes were
then washed with TBST, and incubated with a goat anti-mouse (or
rabbit IgG) conjugated with horseradish peroxidase substrate (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at room
temperature. GAPDH was used as an internal control. The protein
bands were detected with a chemiluminescent detection system
(Beyotime Institute of Biotechnology).
Tumor xenograft
Six-week old BALB/c nude mice were obtained from HFK
Biosciences. Each mouse was subcutaneously injected with
2×106 MG63 cells on the left flank region. The tumor
volumes were measured at indicative time points. After 30 days, the
weights of tumors were analyzed. Animal experiments were performed
in accordance with relevant guidelines and regulations of the
Institutional Animal Care and Use Committees at Huai'an First
People's Hospital, Nanjing Medical University.
Luciferase reporter assay
Wild type (WT) or mutant (MUT) 3′-UTR of KLF5 was
amplified and cloned into a pmiR-RB-REPORTTM Luciferase vector.
Then, the WT or MUT 3′-UTR of KLF5 as well as miR-590-5p mimic or
negative control was co-transfected into cells by Lipofectamine
2000 (Invitrogen). 48 h after co-transfection, reporter activity
was detected using a Dual-Luciferase® Reporter Assay kit
(Promega Corporation, Madison, WI, USA).
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Student's t-test and one-way
analysis of variance followed by Tukey's post hoc test were used to
analyze 2 or multiple groups, respectively, for statistical
significance. Pearson correlation coefficient analysis was used to
determine the correlations. P<0.05 was considered to indicated a
statistically significant difference.
Results
miR-590-5p was downregulated in
OS
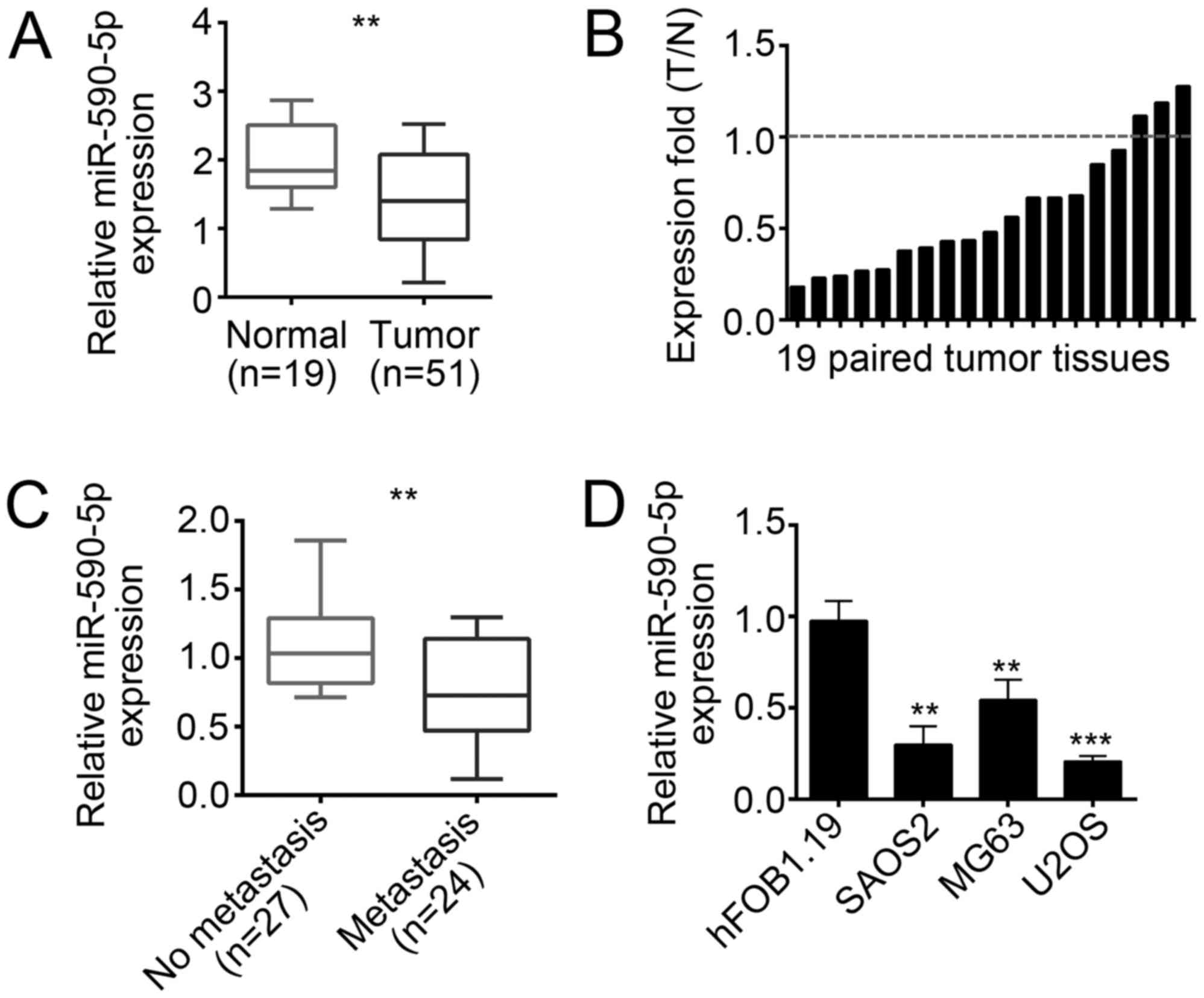
To determine the role of miR-590-5p in OS, we first
checked its expression patterns in OS tissues. We performed RT-qPCR
and found that miR-590-5p was downregulated in OS tissues
(n=51) compared with that in adjacent normal tissues
(n=19; Fig. 1A and B).
Moreover, miR-590-5p was expressed higher in non-metastatic OS
tissues than in metastatic tissues (Fig. 1C). We then analyzed the
clinicopathological characteristics of the 51 OS samples. As shown
in Table I, the expression of
miR-590-5p was inversely correlated with tumor size, metastasis,
and clinical stages. Finally, we checked the expression of
miR-590-5p in OS cell lines by RT-qPCR and found that miR-590-5p
was also downregulated in SAOS2, U2OS, and MG63 cells compared with
that in hFOB1.19 cells (Fig.
1D).
 | Table I.Associations between
clinicopathological features and the expression of miR-590-5p in
osteosarcoma tissues. |
Table I.
Associations between
clinicopathological features and the expression of miR-590-5p in
osteosarcoma tissues.
| Feature | miR-590-5p low
expression (n) | miR-590-5p high
expression (n) | P-value |
|---|
| All cases | 27 | 24 |
|
| Age (years) |
|
| 1.000 |
|
<20 | 17 | 16 |
|
| ≥20 | 10 | 8 |
|
| Tumor size (cm) |
|
| 0.025 |
|
<5 | 10 | 17 |
|
| ≥5 | 17 | 7 |
|
| Metastases |
|
| 0.026 |
| No | 8 | 15 |
|
|
Yes | 19 | 9 |
|
| Clinical stage |
|
| 0.041 |
|
I/II | 13 | 19 |
|
|
III | 14 | 5 |
|
miR-590-5p overexpression inhibited
SAOS2 and U2OS cellular proliferation, migration, and invasion
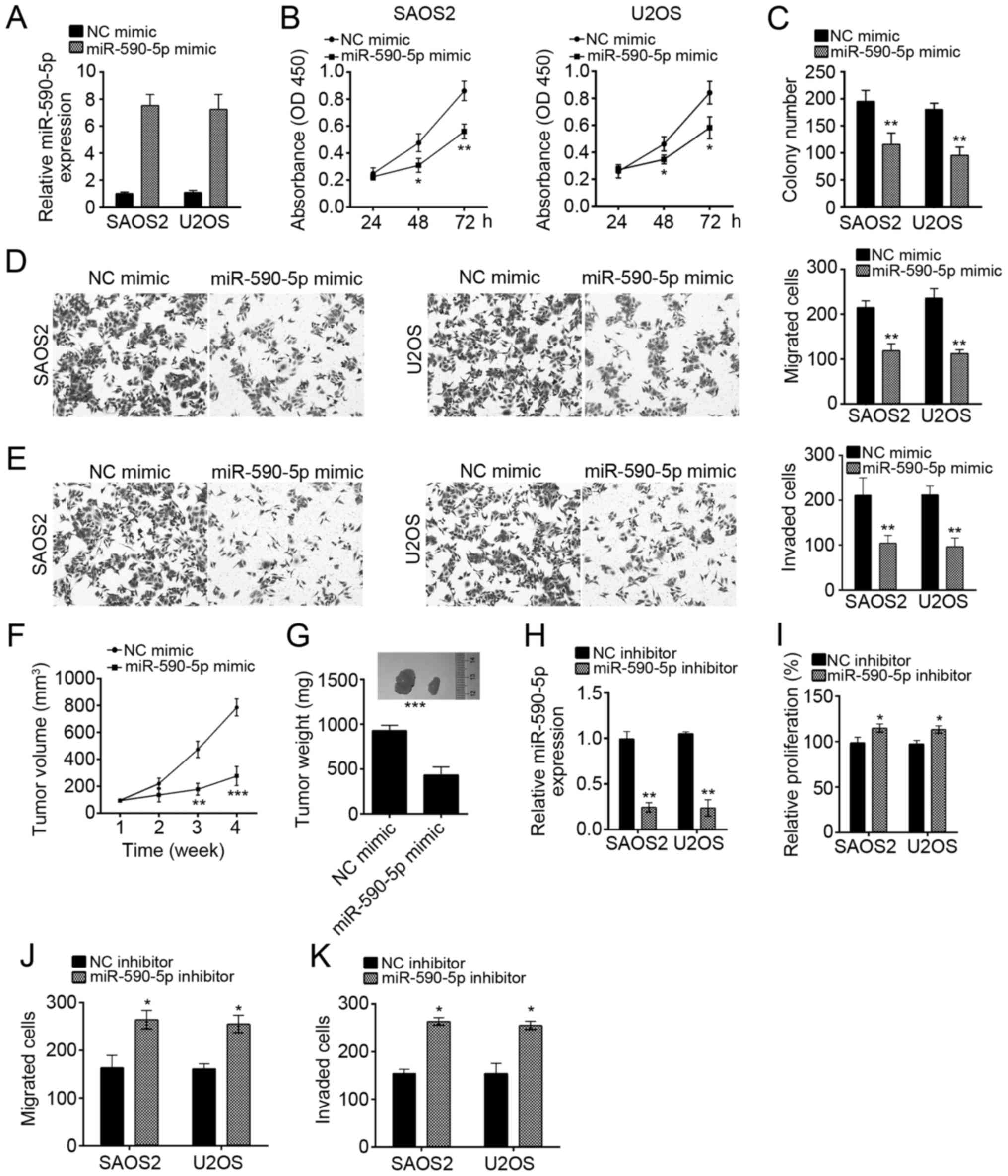
To analyze the role of miR-590-5p in OS cells, we
overexpressed miR-590-5p in SAOS2 and U2OS cells by transducing
miR-590-5p mimics (Fig. 2A). We
conducted CCK8 and colony formation assays, and the results
indicated that miR-590-5p overexpression significantly suppressed
the proliferation of SAOS2 and U2OS cells (Fig. 2B and C). Furthermore, transwell
assay revealed that miR-590-5p overexpression significantly
inhibited the abilities of migration and invasion in SAOS2 and U2OS
cells (Fig. 2D and E). To further
verify the physiological function of miR-590-5p, we performed
xenograft experiments. We found that miR-590-5p overexpression
markedly suppressed tumor growth in vivo (Fig. 2F). At the end time point of the
experiment, we measured the tumor weights and found that miR-590-5p
dramatically reduced tumor sizes (Fig.
2G).
To further confirm the effect of miR-590-5p on OS
cells, we suppressed miR-590-5p in U2OS and SAOS2 cells by
transfection with miR-590-5p inhibitors (Fig. 2H). Then we performed CCK8 and
transwell assays. Consistently, inhibition of miR-590-5p
significantly promoted the proliferation, migration and invasion of
SAOS2 and U2OS cells (Fig.
2I-K).
miR-590-5p overexpression arrested OS
cells in G0 phase
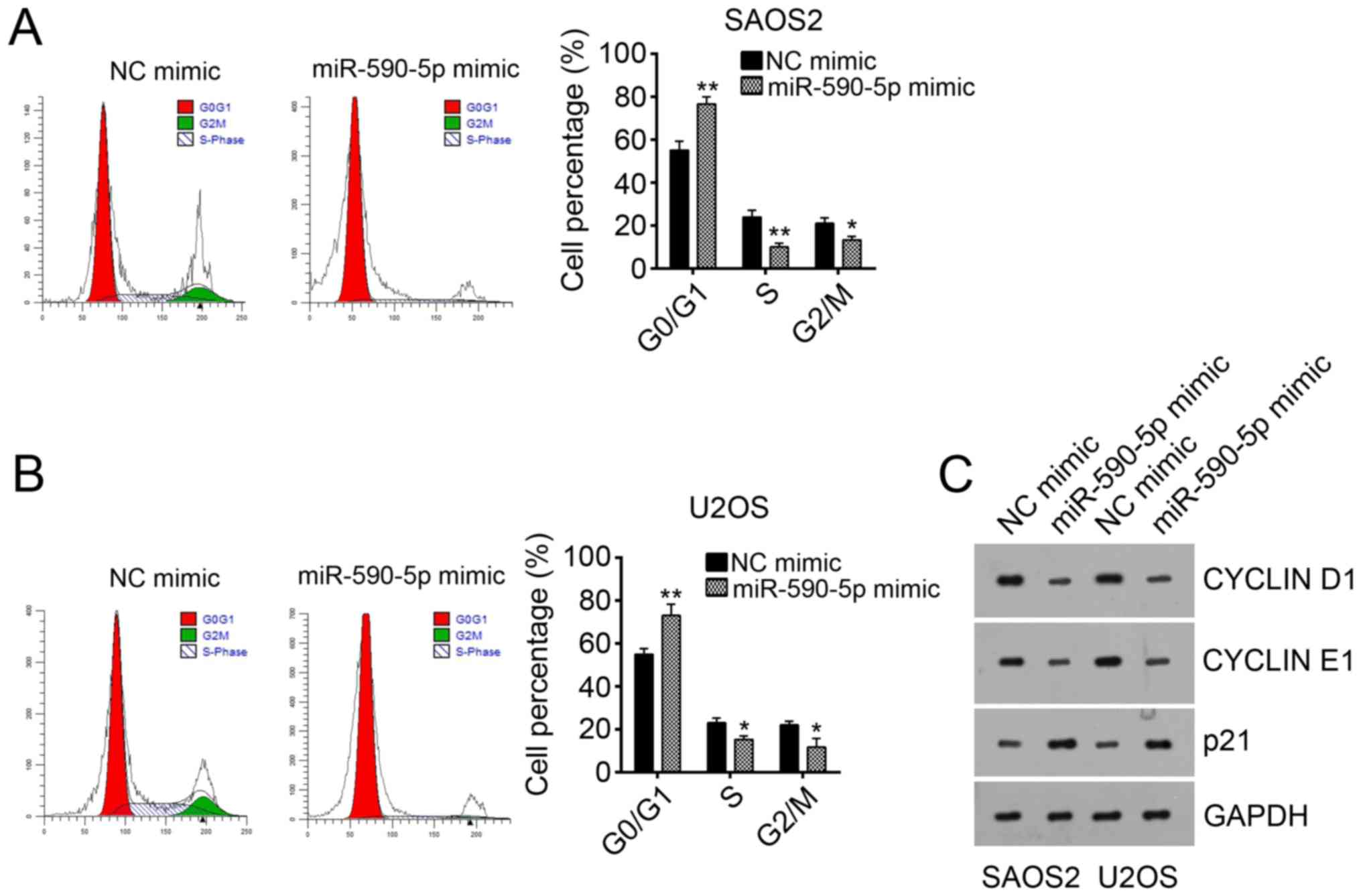
To determine the mechanism of miR-590-5p-mediated
inhibition of proliferation, we analyzed the effect of miR-590-5p
on the cell cycle. miR-590-5p overexpression significantly arrested
SAOS2 and U2OS cells in G0 phase (Fig.
3A and B). Consistently, the protein levels of CYCLIN D1 and
CYCLIN E1 were downregulated after miR-590-5p overexpression
(Fig. 3C). However, the protein
level of p21 was upregulated by miR-590-5p overexpression (Fig. 3C). Overall, miR-590-5p inhibited
cell proliferation by reducing the cell cycle.
KLF5 was a target of miR-590-5p in
OS
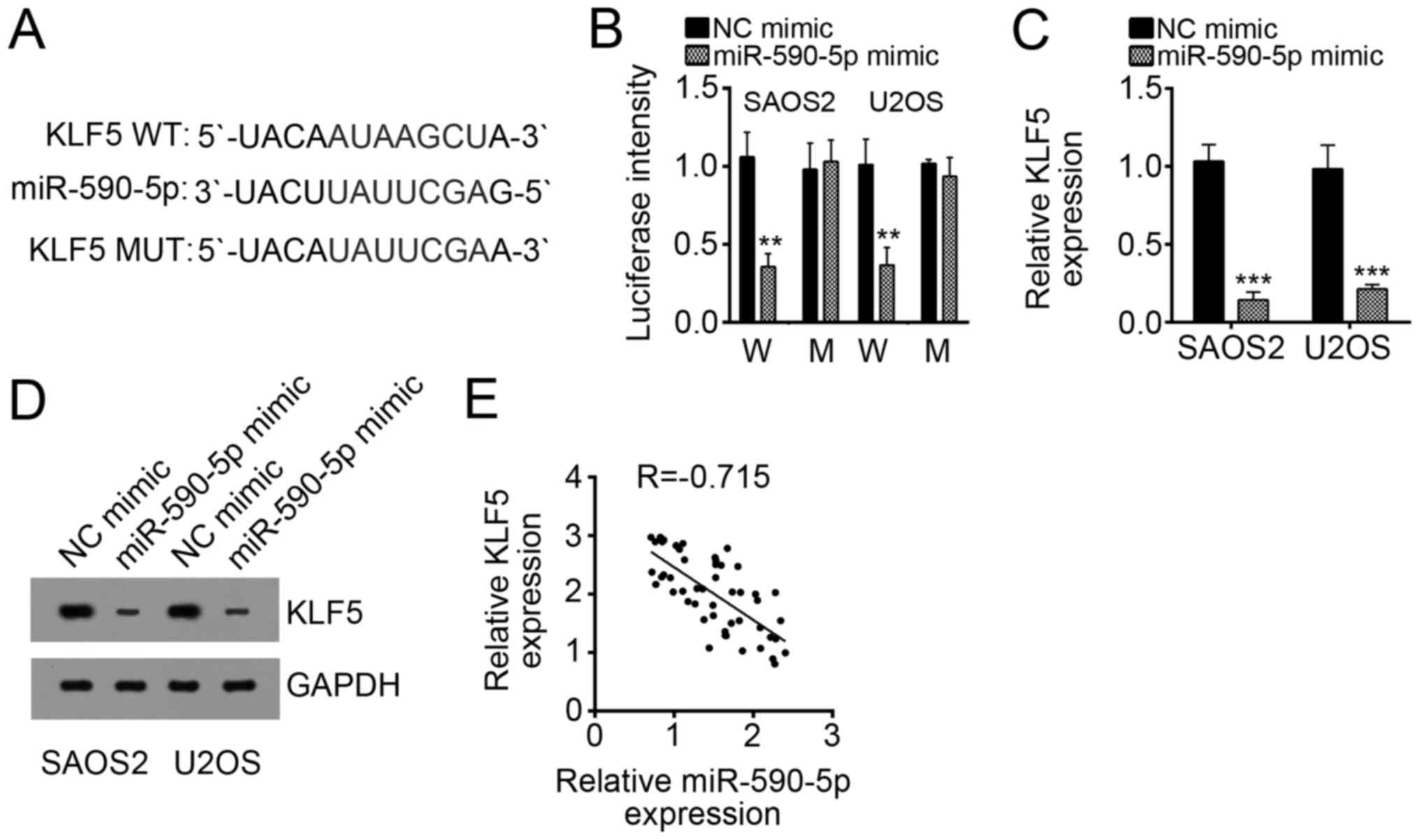
To explore the downstream target of miR-590-5p, we
performed bioinformatics analysis (http://www.targetscan.org/vert_71/). We found that
KLF5 was a potential target of miR-590-5p because of a potential
binding site of miR-590-5p in the 3′-UTR of KLF5 mRNA (Fig. 4A). To confirm this prediction, we
performed luciferase activity reporter assay. We observed that
miR-590-5p overexpression significantly suppressed luciferase
intensity in SAOS2 and U2OS cells transduced with WT 3′-UTR of KLF5
mRNA (Fig. 4B). When the binding
site was mutated, miR-590-5p overexpression had no effect on
luciferase intensity (Fig. 4B),
thereby suggesting that KLF5 was a direct target of miR-590-5p.
Furthermore, overexpression of miR-590-5p remarkably decreased the
mRNA and protein levels of KLF5 in SAOS2 and U2OS cells (Fig. 4C and D). Thus, the expression of
KLF5 and miR-590-5p in OS tissues was inversely correlated
(Fig. 4E).
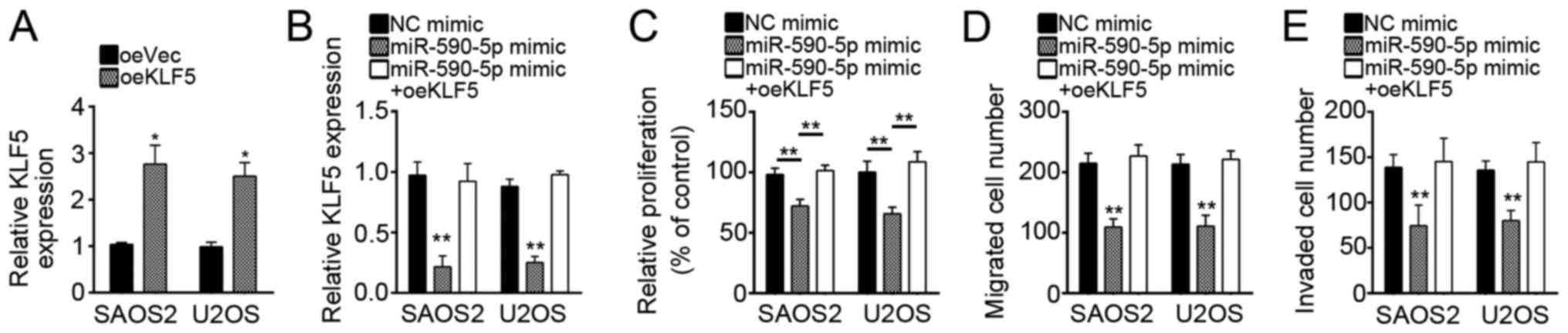
Restoration of KLF5 in
miR-590-5p-overexpressing SAOS2 and U2OS cells rescued cell
proliferation, migration, and invasion
The role of KLF5 in OS has not been previously
investigated. To define the role of KLF5 and determine whether
miR-590-5p regulates OS by targeting KLF5, we overexpressed KLF5 in
miR-590-5p-overexpressing SAOS2 and U2OS cells to restore the level
of KLF5 (Fig. 5A and B). We then
conducted CCK8 and transwell assays. We found that restoration of
KLF5 expression reversed the miR-590-5p-mediated inhibitory effect
on cell proliferation, migration, and invasion (Fig. 5C-E). Overall, our results indicated
that miR-590-5p inhibited OS cell proliferation, migration, and
invasion by targeting KLF5.
Discussion
OS is the most malignant bone cancer worldwide. The
clinical outcome of OS is extremely poor because of tumor
metastasis and recurrence (20).
Therefore, research on the molecular mechanism involved in OS
development and progression, as well as screening of novel
biomarkers and therapeutic targets, is necessary. Here, we found
that miR-590-5p was downregulated in OS tissues and cell lines. We
also identified KLF5 as a target gene of miR-590-5p in OS.
miR-590-5p could suppress OS cell proliferation, migration, and
invasion by targeting KLF5.
Numerous reports have indicated that dysregulation
of miRNAs is closely associated with human cancers by targeting
specific genes (21). miRNAs can
regulate cell proliferation, apoptosis, and metastasis in various
cancers (22,23). For example, we previously reported
that miR-367 can enhance the proliferation and metastasis of OS
cells by targeting DAB2IP (24).
Cui and Shi (25) reported
that miR-187 suppresses OS growth and metastasis. Zhang and
colleagues showed that miR-375 suppresses oral squamous cell
carcinoma growth by targeting IGF-1R (26). Previous reports demonstrated that
miR-590-5p can inhibit tumor growth in CC and breast cancer
(17,18). On the contrary, another study
indicated that miR-590-5p promotes CC progression (27). Thus, the role of miR-590-5p in
cancers must be illustrated further. Here, we revealed that
miR-590-5p was lowly expressed in OS tissues and cell lines,
suggesting that miR-590-5p may have an inhibitory effect on OS
cells. The results of CCK8 and transwell assays proved that
miR-590-5p overexpression significantly inhibited cell
proliferation, migration, and invasion.
Kruppel-like factor 5 (KLF5) is a transcription
factor that belongs to the Kruppel-like factor subfamily of zinc
finger proteins. Previous research showed that KLF5 is involved in
the regulation of cell proliferation and migration in various
cancers (28). For instance,
knockdown of KLF5 suppresses gastric cancer progression (29). Another study demonstrated that KLF5
promotes the proliferation, migration, and invasion of cervical
cancer cells (28). KLF5 also
promotes the growth, migration, and invasion of triple-negative
breast cancer cells (30). Liu
et al (31) revealed that
KLF5 increases cell proliferation and invasion in human laryngeal
squamous cell carcinoma. However, the function of KLF5 in OS
remains largely unknown. Our study confirmed that KLF5 was a target
gene of miR-590-5p in OS cells. Furthermore, CCK8 and transwell
assays were conducted to reveal that KLF5 could rescue the
abilities of proliferation, migration, and invasion in
miR-590-5p-overexpressing SAOS2 and U2OS cells. The downstream
signaling regulated by KLF5 remains unclear.
Tumor metastasis to other sites, such as in the
lungs, is the main cause for OS-induced deaths (32). In our study, miR-590-5p was lowly
expressed in metastatic OS tissues compared with that in metastatic
tissues. Thus, miR-590-5p might be an ideal biomarker for OS
clinical outcomes and a potential therapeutic target for OS
treatment.
Collectively, our data demonstrated that
miR-590-5p/KLF5 axis was a novel signal that regulated OS
development and progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZS and WC conceived and designed the present study,
analyzed and interpreted the results, and wrote the manuscript. YX,
JY and WZ performed the experiments. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the protocol for the
present study was approved by the Institutional Ethics Committee of
Huai'an First People's Hospital, Nanjing Medical University
(Jiangsu, China) and all enrolled patients signed a written
informed consent document. In addition, all procedures involving
animals conformed to the national guidelines of, and were approved
by, the Animal Care Ethics Committee of Nanjing Medical
University.
Consent for publication
All patients recruited to the present study provided
written informed consent for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
3
|
Xiong Y, Wu S, Du Q, Wang A and Wang Z:
Integrated analysis of gene expression and genomic aberration data
in osteosarcoma (OS). Cancer Gene Ther. 22:524–529. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan W, Wang D, Liu Y, Tian D, Wang Y,
Zhang R, Yin L and Deng Z: miR-494 inhibits cell proliferation and
metastasis via targeting of CDK6 in osteosarcoma. Mol Med Rep.
16:8627–8634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Z, Li X, Yang Y, He Z, Qu X and Zhang
Y: Long noncoding RNAs in the progression, metastasis, and
prognosis of osteosarcoma. Cell Death Dis. 7:e23892016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ivey KN and Srivastava D: MicroRNAs as
regulators of differentiation and cell fate decisions. Cell Stem
Cell. 7:36–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao X, Li S, Li W, Wang G, Zhao W, Han J,
Diao C, Wang X and Zhang M: MicroRNA-539 suppresses tumor cell
growth by targeting the WNT8B gene in non-small cell lung cancer. J
Cell Biochem. Dec 21–2017.(Epub ahead of print).
|
|
9
|
Shentu TP, Huang TS, Cernelc-Kohan M, Chan
J, Wong SS, Espinoza CR, Tan C, Gramaglia I, van der Heyde H, Chien
S and Hagood JS: Thy-1 dependent uptake of mesenchymal stem
cell-derived extracellular vesicles blocks myofibroblastic
differentiation. Sci Rep. 7:180522017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang G, Pan J, Ye Z, Fang B, Cheng W and
Cao Z: Overexpression of miR-216b sensitizes NSCLC cells to
cisplatin-induced apoptosis by targeting c-Jun. Oncotarget.
8:104206–104215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cesarini V, Silvestris DA, Tassinari V,
Tomaselli S, Alon S, Eisenberg E, Locatelli F and Gallo A:
ADAR2/miR-589-3p axis controls glioblastoma cell
migration/invasion. Nucleic Acids Res. 46:2045–2059. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Jin J, Tian X and Wu L:
hsa-miR-29c-3p regulates biological function of colorectal cancer
by targeting SPARC. Oncotarget. 8:104508–104524. 2017.PubMed/NCBI
|
|
14
|
Ye Y, Zhuang J, Wang G, He S, Ni J and Xia
W: MicroRNA-139 targets fibronectin 1 to inhibit papillary thyroid
carcinoma progression. Oncol Lett. 14:7799–7806. 2017.PubMed/NCBI
|
|
15
|
Romano G and Kwong LN: Diagnostic and
therapeutic applications of miRNA-based strategies to cancer
immunotherapy. Cancer Metastasis Rev. 37:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qadir MI and Faheem A: miRNA: A diagnostic
and therapeutic tool for pancreatic cancer. Crit Rev Eukaryot Gene
Expr. 27:197–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ou C, Sun Z, Li X, Li X, Ren W, Qin Z,
Zhang X, Yuan W, Wang J, Yu W, et al: miR-590-5p, a
density-sensitive microRNA, inhibits tumorigenesis by targeting
YAP1 in colorectal cancer. Cancer Lett. 399:53–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou L, Zhao LC, Jiang N, Wang XL, Zhou
XN, Luo XL and Ren J: MicroRNA miR-590-5p inhibits breast cancer
cell stemness and metastasis by targeting SOX2. Eur Rev Med
Pharmacol Sci. 21:87–94. 2017.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Zhang L, Zhang G, Li S, Duan J,
Cheng J, Ding G, Zhou C, Zhang J, Luo P, et al: Osteosarcoma
metastasis: Prospective role of ezrin. Tumour Biol. 35:5055–5059.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li SL, Gao HL, Lv XK, Hei YR, Li PZ, Zhang
JX and Lu N: MicroRNA-124 inhibits cell invasion and
epithelial-mesenchymal transition by directly repressing Snail2 in
gastric cancer. Eur Rev Med Pharmacol Sci. 21:3389–3396.
2017.PubMed/NCBI
|
|
23
|
Song YX, Sun JX, Zhao JH, Yang YC, Shi JX,
Wu ZH, Chen XW, Gao P, Miao ZF and Wang ZN: Non-coding RNAs
participate in the regulatory network of CLDN4 via ceRNA mediated
miRNA evasion. Nat Commun. 8:2892017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai W, Jiang H, Yu Y, Xu Y, Zuo W, Wang S
and Su Z: miR-367 regulation of DOC-2/DAB2 interactive protein
promotes proliferation, migration and invasion of osteosarcoma
cells. Biomed Pharmacother. 95:120–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui C and Shi X: miR-187 inhibits tumor
growth and invasion by directly targeting MAPK12 in osteosarcoma.
Exp Ther Med. 14:1045–1050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Li Y, Hou D, Shi Q, Yang S and Li
Q: MicroRNA-375 inhibits growth and enhances radiosensitivity in
oral squamous cell carcinoma by targeting insulin like growth
factor 1 receptor. Cell Physiol Biochem. 42:2105–2117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim CW, Oh ET, Kim JM, Park JS, Lee DH,
Lee JS, Kim KK and Park HJ: Hypoxia-induced microRNA-590-5p
promotes colorectal cancer progression by modulating matrix
metalloproteinase activity. Cancer Lett. 416:31–41. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma D, Chang LY, Zhao S, Zhao JJ, Xiong YJ,
Cao FY, Yuan L, Zhang Q, Wang XY, Geng ML, et al: KLF5 promotes
cervical cancer proliferation, migration and invasion in a manner
partly dependent on TNFRSF11a expression. Sci Rep. 7:156832017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang T, Chen M, Yang X, Zhang X, Zhang Z,
Sun Y, Xu B, Hua J, He Z and Song Z: Down-regulation of KLF5 in
cancer-associated fibroblasts inhibit gastric cancer cells
progression by CCL5/CCR5 axis. Cancer Biol Ther. 18:806–815. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou W, Song F, Wu Q, Liu R, Wang L, Liu
C, Peng Y, Mao S, Feng J and Chen C: miR-217 inhibits
triple-negative breast cancer cell growth, migration, and invasion
through targeting KLF5. PLoS One. 12:e01763952017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu JY, Lu JB and Xu Y: MicroRNA-153
inhibits the proliferation and invasion of human laryngeal squamous
cell carcinoma by targeting KLF5. Exp Ther Med. 11:2503–2508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji Q, Xu X, Li L, Goodman SB, Bi W, Xu M,
Xu Y, Fan Z, Maloney WJ, Ye Q and Wang Y: miR-216a inhibits
osteosarcoma cell proliferation, invasion and metastasis by
targeting CDK14. Cell Death Dis. 8:e31032017. View Article : Google Scholar : PubMed/NCBI
|