Introduction
Cartilage defects are caused by various mechanisms,
such as traumatic joint damage, osteochondrosis dissecans, as well
as primary and secondary degeneration. The damage of the cartilage
surface influences the cartilage mechanics resulting in enhanced
wear rates, leading to osteoarthritis (OA). Catabolic events during
the process of OA reduce the extracellular osmolarity, which
results in decreased viscoelasticity of the tissue and inferior
biomechanical function (1).
Because of the missing intrinsic regeneration capacity, the
degeneration of the cartilage proceeds and causes severe pain for
the patient (2). No successful
therapy is currently available which can stop or reverse the
progression of OA. Current therapy approaches, including
medication, analgesics and total joint replacement provide only
palliative treatment (3).
Therefore, it is necessary to find new therapeutic approaches to
support the regeneration of hyaline cartilage.
A common repair technique for cartilage
reconstruction is the matrix-associated autologous chondrocyte
implantation (MACI). This two-step procedure includes the isolation
of autologous chondrocytes from a minor load-bearing area of
patient's hyaline articular cartilage, followed by cell expansion
in vitro and cell transfer onto a biomaterial for
transplantation into the defect. A variety of different
biomaterials (synthetic and natural) have been tested with respect
to their application for cartilage repair. Collagen as the common
protein in the cartilage tissue has been widely studied for
cartilage regeneration. In particular, collagen supports cell
attachment, proliferation, migration and differentiation (4,5).
Studies had shown that collagen type I-based biomaterials support
the chondrogenic differentiation of rat bone marrow-derived
mesenchymal stem cells (BM-MSCs) and increase production of
extracellular matrix in human chondrocytes (CH) (6,7).
However, two-dimensional (2D) environment and the
cell expansion in vitro results in de-differentiation of CHs
to a fibroblast-like cell phenotype. This is characterized by
decreased collagen type II synthesis and increased collagen type I
expression rates (8,9). The implantation of de-differentiated
CHs could result in formation of fibrocartilage which is not able
to withstand the high biomechanical loading in the knee joint
(10,11). Therefore, de-differentiated cells
have to be re-differentiated (by e.g., 3D cultures) to restore the
defect with hyaline-like tissue. In various studies, BM-MSCs are
expected to be a useful cell source for cartilage tissue
engineering due to the ease of harvest and the high differentiation
potential of BM-MSCs (12–14). Especially, the co-cultivation of
both cell types results in a robust chondrogenic differentiation
which is probably triggered by signaling via cell-cell contacts and
secreted factors generated by both cell types (15–17).
However, to create functional cartilage tissue for implantation,
specific techniques are required to facilitate adequate
chondrogenesis during in vitro cultivation (18). Besides adapting the cultivation
conditions to the physiological in vivo situation, providing
a cartilage-like oxygen environment or the use of essential growth
factors and cytokines, biophysical stimulation could be a promising
approach to achieve hyaline-like cartilage formation (1).
As in bone tissue, mechanoelectric transduction
occurs naturally within the cartilage. During weight-bearing and
joint movement, the fluid flow over fixed ionized macromolecules
within the cartilage tissue provokes strain- and
diffusion-generated electric potentials. Degeneration of the
cartilage matrix results in a loss of this fixed microenvironment,
leading to the disruption of the physiological electric field which
is important for tissue homeostasis (19).
Although therapeutic devices for electric
stimulation (ES) of bone are entering the clinical market (1), there are only few studies dealing
with ES of cartilage in vitro (19–21).
Most of the in vivo and in vitro approaches are based
on pulsed electromagnetic fields (PEMF). Data from clinical trials
suggest that PEMF are able to improve clinical scores and joint
function in osteoarthritic patients (22). In vitro, it was found that
PEMF increase the proteoglycan release of alginate-encapsulated CHs
(23) and in OA cartilage explants
(24). Moreover, Brighton et
al developed an experimental setup for capacitive coupled ES
in vitro (19–21). In contrast to PEMF, higher
frequencies up to 60 kHz are applied on cell cultures and cartilage
explants enhancing the synthesis rates of collagen type II and
aggrecan (20,21).
To investigate the influence of alternating current
(AC) on cellular differentiation process, the aim of the present
study was to develop an in vitro test setup for application
of electric fields. The electrode design is based on previously
published bone stimulation system, enabling direct coupling of AC
and application of defined electric field (25,26).
Using the in vitro test setup, we intended to analyze the
effects of ES on hyaline-like differentiation of human CHs,
mesenchymal stem cells derived from human BM-MSC as well as a
co-culture of both cell types. We assumed that ES of cartilage
cells has an impact on chondrogenic differentiation and cartilage
tissue regeneration. The evaluation of ES effects on activation of
CHs and BM-MSCs contributes to fundamental knowledge as a basis for
future applications of ES to improve cartilage healing.
Materials and methods
Test system for ES
To investigate the influence of electric fields on
human CHs and mesenchymal stem cells, an in vitro setup was
developed following the construction of an ES system established by
our working group (26). For
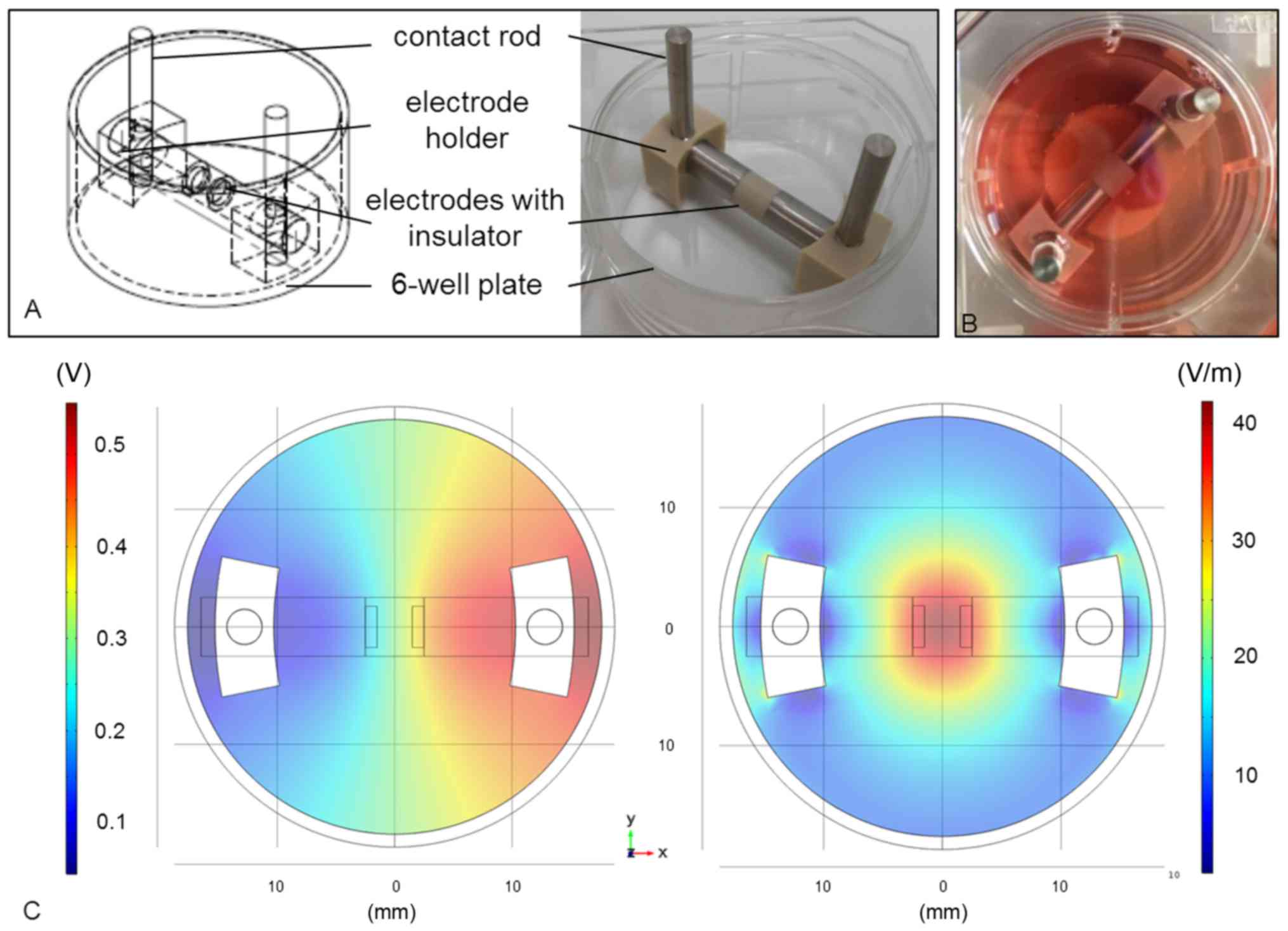
better handling, the electrode setup was adapted to the dimension
of a 6-well plate (Fig. 1A). The
cylindrical electrodes (length: 14 mm, diameter: 5 mm) were made of
pure titanium and separated by a 5 mm-long insulator made of
polyetherether ketone (PEEK). The electrode holders were made of
PEEK as well and warranted a gap of 3 mm between electrodes and
well bottom. Thus, the cells could be cultured onto a scaffold
below the electrodes (Fig. 1B).
The lid of the 6-well plate had pre-drilled holes for the contact
rods (length: 35 mm; titanium).
The electric potentials inside the stimulation
chamber were measured as VRMS at defined coordinates at
the well bottom using a DC-free sine wave (1 kHz, 0.7
VRMS). The values were used to optimize the numerical
simulation performed with the finite element method software Comsol
Multiphysics (Comsol Multiphysics 5.2; COMSOL, Stockholm, Sweden).
Based on these data, distribution of the electric potentials
(Fig. 1C, left panel) and electric
field norm (Fig. 1C, right panel)
within the stimulation chamber was calculated by Comsol
Multiphysics, indicating that the cells were stimulated by the
electric field with field strengths of 20–35 V/m.
Cell culture
For the stimulation experiments, human CHs and
mesenchymal stem cells were used. For CH isolation, human articular
knee cartilage were obtained post-mortally from human donors (n=7,
male (n=6): 51±21 years; female (n=1): 52 years) within the first
72 h after the donor's death. The patients' anamneses and cause of
death were unknown. All tissue samples macroscopically showed no
indication of OA or degeneration. The study was approved by the
Local Ethical Committee of the University of Rostock (registration
no. A2011-138). Due to forensic and legal reasons no written
approval could be obtained from the patients. However, all issued
information relating to the patients in the study are fully
anonymized. Human CHs were isolated from the human knee cartilage
under sterile conditions as previously described (27) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal calf serum (FCS), 1%
amphotericin B, 1% penicillin-streptomycin and ascorbic acid (50
µg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C under
5% CO2 and 5% O2 (hypoxia). The reduced
oxygen level was used to mimic the low oxygen partial pressure
within cartilage tissue (28).
Human CHs have to be cultured up to the 2nd passage to achieve a
sufficient cell number for the experiments. Therefore, cells in
passage three were used for electric stimulation.
Human BM-MSCs were obtained from ATCC®
(Lot-no. 63208778; LGC Standards, Wesel, Germany) and cultured in
the recommended cell culture medium (Mesenchymal Stem Cell Basal
Medium; ATCC, Wesel, Germany), supplemented with the suggested
growth kit (Mesenchymal Stem Cell Growth kit for BM-MSCs; ATCC)
under standard cell culture conditions (37°C, 5% CO2,
21% O2). As mentioned by Bentivegna et al
(29), BM-MSCs should be used for
chondrogenic differentiation in vitro between passage three
and six, because number of culture passages strictly influences
this type of differentiation capacity. Therefore, we cultured
BM-MSCs up to the 3rd passage to achieve a sufficient cell number
for all the experiments. BM-MSCs were used in passage four for the
experiments.
Cell experiments
For the ES experiments, the CHs or BM-MSCs were
seeded onto a bovine collagen-based scaffold with a diameter of 20
mm (1×105 cells/cm2). For the co-culture
experiments, the cells (CHs and BM-MSCs) were mixed at a ratio of
1:2 prior to cell seeding. The used matrix is a two-layered,
bioresorbable scaffold made of collagen type I (MedSkin Solutions
Dr. Suwelack AG, Billerbeck, Germany). Before cell seeding, the
scaffolds were stuck to the well bottom in the middle of the well
using a biocompatible silicon glue (Korasilon paste; Kurt Obermeier
GmbH & Co. KG, Bad Berleburg, Germany). The scaffolds were
placed precisely under the electrode allowing a reproducible
exposure of the cells to the electric field. After adhering for 30
min, the cell/scaffold constructs were overlaid by the cell culture
medium DMEM containing 1% ITS™
(Insulin-Transferrin-Selenium; BD Biosciences, Franklin Lakes, NJ,
USA), ascorbic acid (50 µg/ml), dexamethasone (100 nM;
Sigma-Aldrich; Merck KGaA), insulin-like growth factor-1 (IGF-1, 50
ng/ml; R&D Systems GmbH, Wiesbaden, Germany) as well as
transforming growth factor-β1 (TGF-β1; 50 ng/ml; tebu-bio GmbH,
Offenbach, Germany) and were incubated for 72 h at 37°C under 5%
CO2 either at 21% O2 (normoxia) or 5%
O2 (hypoxia) (27,30,31).
Prior to ES, the medium was replaced by the fresh cell culture
medium (mentioned above), but without the addition of the growth
factors TGF-β1 and IGF-1. AC as sinusoidal signal with a frequency
of 1 kHz and 0.7 VRMS was applied to the stimulation
chamber for seven days using a Metrix GX 305 function generator
(Metrix Electronics, Hampshire, UK). Cells were stimulated three
times per day for 45 min each, with 225 min breaks between
stimulations. During ES, cells were cultured at 37°C under 5%
CO2 and either at 21 or 5% oxygen level. For
unstimulated control, cells were similarly cultured in the presence
of the electrode system, however, without connection to the
function generator.
Cell biological tests
Metabolic activity following ES was examined by
water soluble tetrazolium (WST-1) assay (Takara Bio, Inc., Shiga,
Japan) as recommended by the manufacturer. This colorimetric assay
is based on the reduction of tetrazolium salt into formazan salt
catalyzed by intercellular dehydrogenases. The amount of generated
formazan reflects the metabolic activity of the cells.
Cell/scaffold constructs were incubated with diluted WST-reagent
(1:10 with cell culture medium) for 60 min. Afterwards, the
absorbance was measured at 450 nm (reference: 630 nm) using a
spectrophotometer (Infinite® 200 PRO; TECAN, Maennedorf,
Switzerland).
Gene expression analyses
For gene expression analyses, scaffolds were
digested by the cartilage tissue-degrading enzyme collagenase A [2%
in Hank's Balanced Salt Solution; (Gibco; Thermo Fisher Scientific,
Inc.), Roche, Mannheim, Germany] for 60 min at 37°C (32). The remaining cell suspension was
centrifuged at 180 × g and the cell pellets were resuspended in
TriReagent® (Zymo Research, Freiburg, Germany).
According to the manufacturer's protocol, the total RNA was
extracted by using the Direct-zol RNA MiniPrep kit (Zymo Research),
and the RNA concentration was determined by a spectrophotometer
(Infinite 200 PRO; TECAN). Single-stranded cDNA was synthesized
from total RNA using the High Capacity cDNA Reverse Transcription
Kit following the manufacturer's instructions (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for collagen type I (Forward:
5′-ACGAAGACATCCCACCAATC-3′, Reverse: 5′-AGATCACGTCATCGCACAAC-3′),
collagen type II (Forward: 5′-ATCCCCTTCGGAGAGTGCTG-3′, Reverse:
5′-CCTTTCTGTCCCTTTGGTCCTG-3′), aggrecan (Forward:
5′-ACAAGGTCTCACTGCCCAAC-3′, Reverse: 5′-AATGGAACACGATGCCTTTC-3′),
SOX9 (Forward: 5′-AGTACCCGCACCTGCACAAC-3′, Reverse:
5′-CGCTTCTCGCTCTCGTTCAG-3′) and alkaline phosphatase (Forward:
5′-CATTGTGACCACCACGAGAG-3′, Reverse: 5′-CCATGATCACGTCAATGTCC-3′)
were performed using the innuMIX qPCR MasterMixSyGreen (Analytik
Jena AG, Jena, Germany). The cycling conditions for amplification
were 95°C for 2 min, 40 cycles of 95°C for 5 sec and 65°C for 25
sec. The expression of all genes was normalized to the expression
of the corresponding housekeeping gene hypoxanthine guanine
phosphoribosyltransferase (HPRT, Forward:
5′-CCCTGGCGTCGTGATTAGTG-3′, Reverse: 5′-TCGAGCAAGACGTTCAGTCC-3′)
and analyzed by 2−ΔΔCq method, as previously described
(25,33).
Quantification of ECM components
After ES over seven days, supernatants were
collected and stored at −20°C. The amount of soluble pro-collagen
type I (CICP, MicroVue™ CICP EIA; QUIDEL Corporation,
San Diego, CA, USA) and pro-collagen type II (CPII; IBEX
Pharmaceuticals, Québec, Canada) within the cell supernatant was
determined using enzyme-linked immunosorbent assays (ELISA). The
quantity of the C-terminal pro-peptides of both pro-collagen types
correlate with the synthesis rate of mature collagen, because the
pro-peptides are cleaved from the collagen molecules during
incorporation into extracellular collagen fibrils (34,35).
Both ELISA assays were conducted according to manufacturer's
specifications. Absorbance was measured at a wavelength of 450 nm
for CPII and 405 nm for CICP using the Opsys MR microplate reader
(Dynex Technologies, Denkendorf, Germany).
The glycosaminoglycan (GAG) content was measured in
the sample supernatant by Blyscan™ GAG assay (Biocolor
Pharmaceuticals, Inc., Carrickfergus, UK). It quantifies the
sulphated proteoglycans and GAGs using 1,9-dimethylmethylene blue.
Samples were digested by papain (20 units/mg in 0.2 M sodium
phosphate buffer, pH 6.4; Sigma-Aldrich; Merck KGaA) overnight at
65°C. Afterwards, the assay was performed following manufacturer's
instructions and absorbance was measured at 656 nm using a
spectrophotometer (Infinite® 200 PRO; TECAN).
Additionally, the total protein content of samples
was quantified using Qubit® protein assay (Thermo Fisher
Scientific, Inc.). Afterwards, collagen or GAGs contents were
normalized to total protein amount.
Data illustration and statistical
analysis
Data are presented as box plots, whereby the boxes
identify the upper and lower percentile, the horizontal lines
within the boxes indicate the median and the whiskers denote
minimum and maximum values. For all analyses a minimum of three
independent donors were used. Since the data obtained were assumed
to be not normally distributed, the statistical tests were
conducted using the Mann-Whitney U test and for comparison of more
than 2 data sets Kruskal-Wallis test with Dunn-Bonferroni post hoc
tests were used (GraphPad Prism 6.0; GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
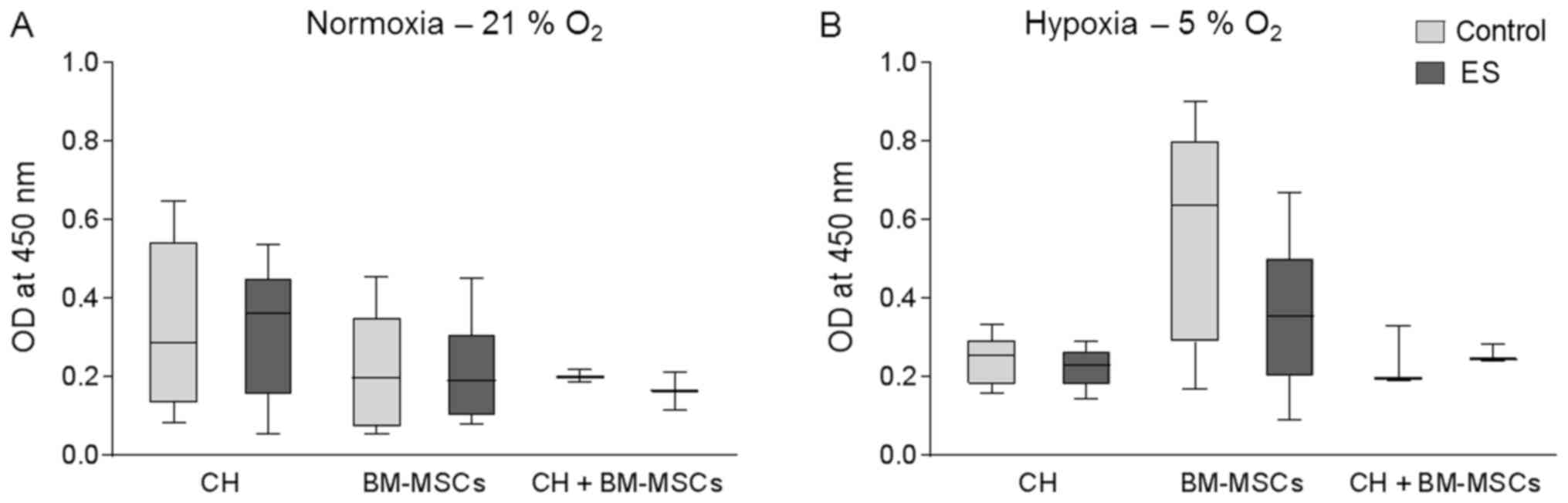
Cell viability following ES
Human CHs and BM-MSCs were cultured onto a
collagen-based 3D scaffold either separately or as co-culture
(CH+BM-MSCs). The used collagen-based scaffold was stabile over the
whole cultivation period and no sign of size reduction was
observed. Cells were exposed to an electric field for seven days
and afterwards metabolic activity was measured by WST-1 assay
(Fig. 2), whereby a high optical
density (OD) correlates with a high metabolic activity of the
cells. Under normoxia for all three groups metabolic activity of
cells exposed to electric field was not affected compared to
respective control cells (Fig.
2A). Equally, under hypoxic culture conditions metabolic
activity of CH and CH+BM-MSCs was not influenced by ES (Fig. 2B). Metabolic activity of BM-MSCs
cultured under hypoxia and stimulated by ES was non-significantly
decreased by 40% compared to unstimulated control. Nevertheless,
the metabolic activity of BM-MSCs was still higher than metabolic
activity of the other cells cultured under hypoxic condition.
Metabolic activity of human CH, BM-MSCs and
co-culture of both cell types was not significantly influenced
following ES which indicates that ES had no cytotoxic effect on
cellular metabolism.
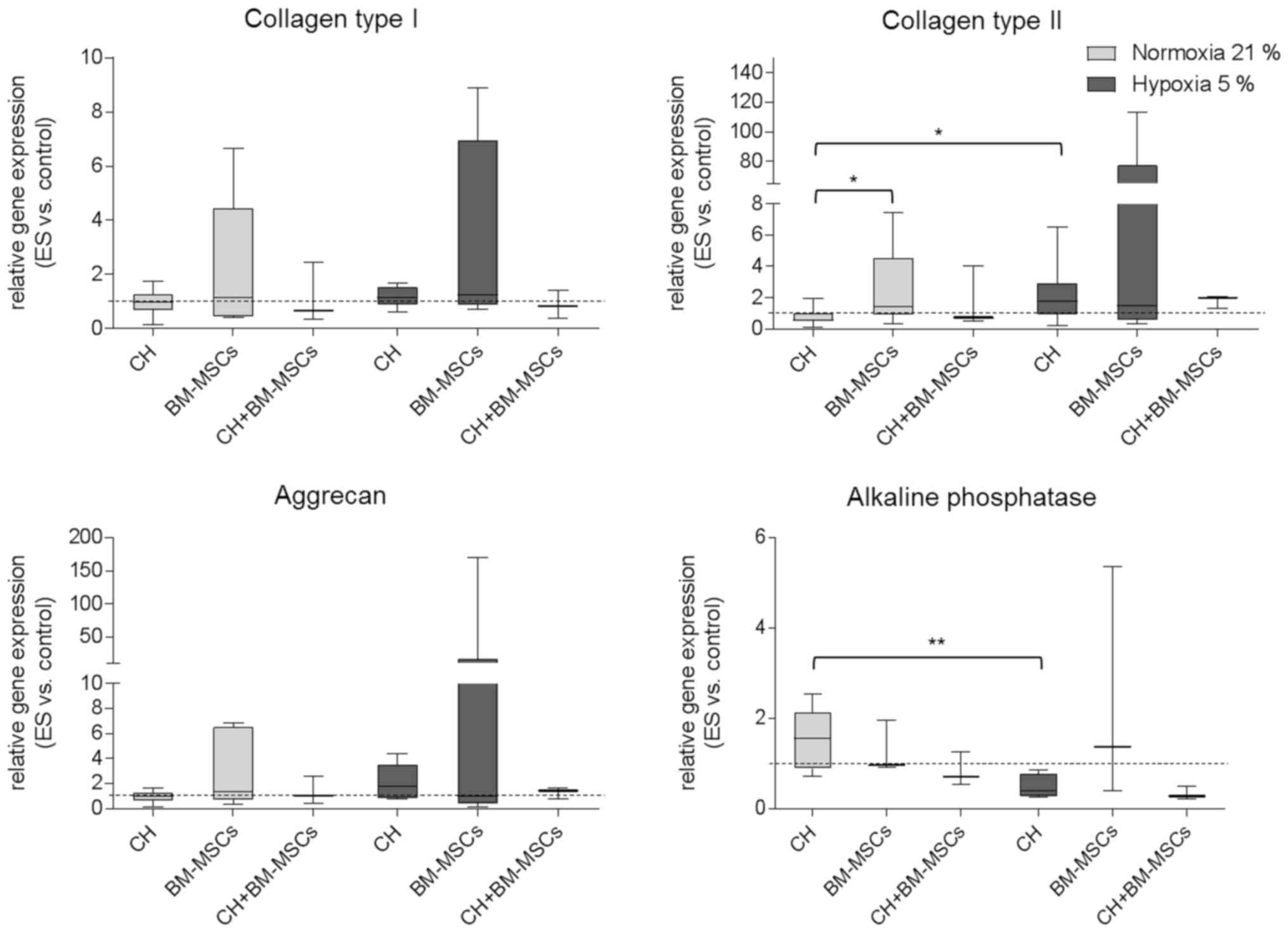
Alteration of gene expression pattern
following ES
Gene expression in human CH, BM-MSCs and CH+BM-MSCs
was analyzed after ES over seven days and presented in Fig. 3. Under hypoxia both CH and BM-MSCs
exhibited increased expression of collagen type I mRNA as a marker
of unwanted fibrocartilage compared to unstimulated control.
However, under both normoxia and hypoxia co-cultured cells
(CH+BM-MSCs) displayed a lower level of collagen type I mRNA
expression following ES. Regarding hyaline cartilage marker, the
results showed that under hypoxia ES caused increased expression of
collagen type II mRNA compared to unstimulated control. For CHs
cultured under hypoxia collagen type II mRNA expression was
significantly enhanced compared to CHs cultured under normoxia
(P=0.0473). Additionally, in CH and CH+BM-MSCs cultured under
hypoxia ES caused the highest expression level of aggrecan mRNA.
Under normoxia only the BM-MSCs displayed increased expression of
aggrecan and collagen type II mRNA following ES. Collagen type II
mRNA expression of BM-MSCs was even significantly increased
compared to collagen type II expression of CH (P=0.0188). The
expression of alkaline phosphatase mRNA as hypertrophy marker was
amplified in BM-MSCs after ES under hypoxia, however, in
co-cultured cells it did not. Cultivation of CHs under normoxia
resulted in significantly increased mRNA expression of alkaline
phosphatase (P=0.0087) compared to hypoxia.
Results demonstrated that ES tended to affect gene
expression of important chondrogenic markers, especially under
hypoxic culture conditions. However, findings were only preliminary
because differences did not reach level of significance probably
due to the wide range of the single values in each group.
Synthesis of ECM proteins following
ES
The release of collagen type I and II as well as
GAGs was measured in the supernatant to investigate the production
of ECM proteins following ES (Fig.
4). Under hypoxic culture conditions cells tended to produce
more collagen type I than under normoxia, independent of exposition
to an electric field. Especially, for unstimulated CH the collagen
type I synthesis is 1.6-fold increased (P=0.0001) after cultivation
under hypoxia compared to normoxia (Fig. 4A). Simultaneously, the decreased
oxygen level of hypoxia supported collagen type II production in CH
(Fig. 4B). For CH, the exposure to
an electric field under hypoxia resulted in a significant 1.5-fold
higher collagen type II release compared to unstimulated control
(P=0.0188) and also compared to electrically stimulated CH under
normoxia (1.4-fold, P=0.04). The same development was observed for
BM-MSCs, but not for co-culture CH+BM-MSCs. Under normoxia,
collagen type II was not upregulated.
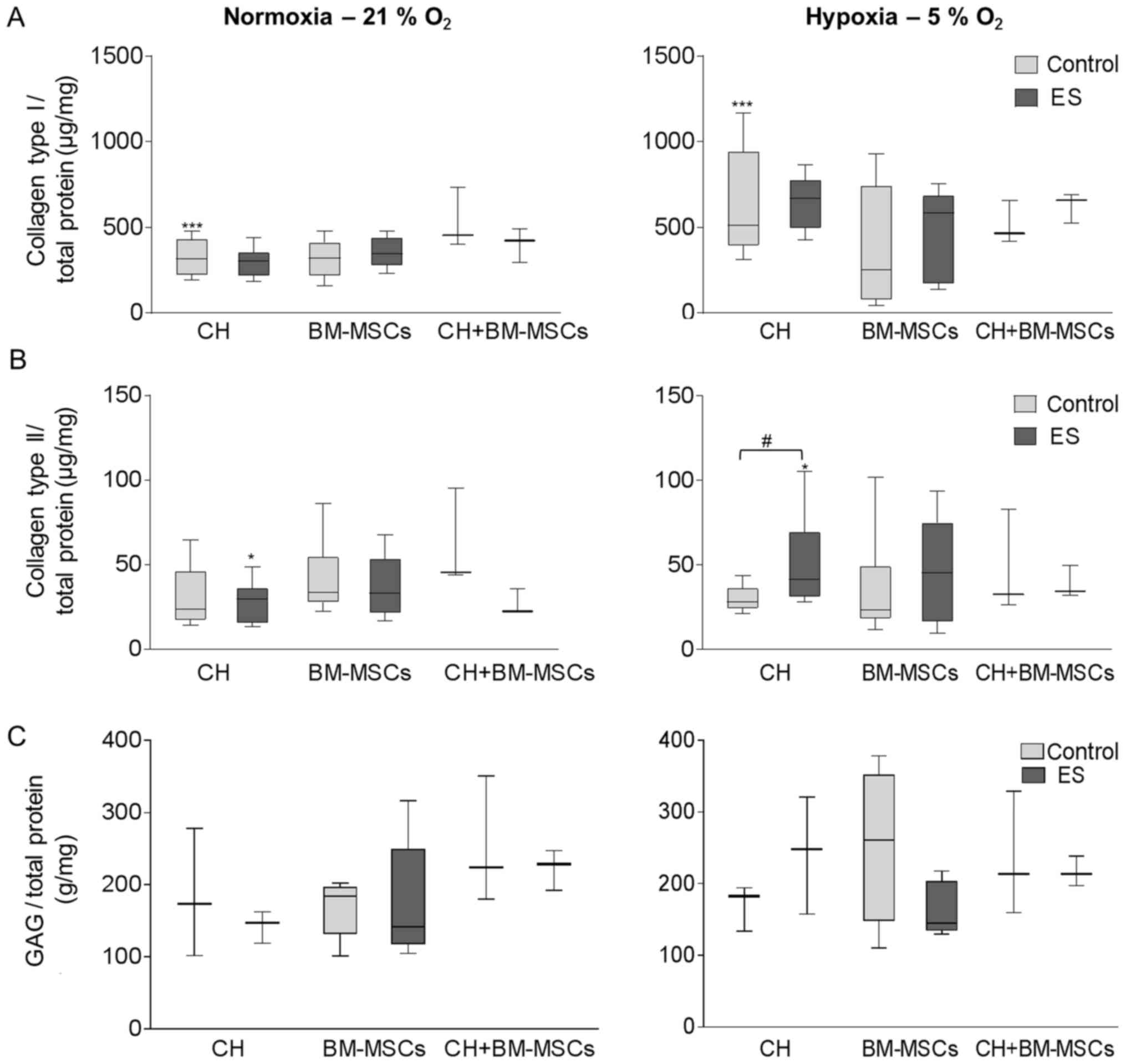 | Figure 4.ES of CH, BM-MSCs and co-culture of
the two (CH+BM-MSCs) was performed under either normoxia (left
panel) or hypoxia (right panel). Following this, the synthesis of
(A) collagen types I, (B) collagen type II and (C) GAGs was
detected in the supernatant and was related to total protein
content. Data were presented as box plots (CH, n=7; BM-MSCs, n=9;
CH+BM-MSCs, n=3). (A) ***P<0.001, as indicated for Control
normoxia vs. Control hypoxia. (B) #P<0.05, as
indicated; *P<0.05, as indicated for ES normoxia vs. ES hypoxia.
CH, human chondrocytes; BM-MSCs, bone marrow-mesenchymal stem
cells; ES, electric stimulation; GAGs, glycosaminoglycans. |
Quantifying the amount of soluble GAGs using
Blyscan™ assay, CH produced a 1.4-fold increased GAG
content following ES under hypoxic culture conditions (Fig. 4C). However, using BM-MSCs a reduced
GAG amount was detected following ES compared to unstimulated
control under both hypoxic and normoxic culture conditions.
Although the co-cultured CH+BM-MSCs showed in general a high level
of GAG synthesis compared to CH and BM-MSCs, the GAG release was
not influenced during ES.
Even if the synthesis of the undesired
fibrocartilage marker collagen type I was promoted under hypoxic
culture condition, the production of collagen type II, an important
cartilage matrix protein, and soluble GAGs were increased in CH
following ES under hypoxia which is a promising result.
Additionally, BM-MSCs showed a tendency to increase collagen type
II production following ES compared to unstimulated control,
however, differences did not reach level of significance.
Discussion
A major challenge in orthopedic surgery is the
limited healing capacity of cartilage tissue after lesion.
Cell-based treatment techniques include the isolation and
cultivation of CHs and BM-MSCs. Subsequently, cells were
transferred onto a biomaterial and implanted into the defect site.
However, during in vitro cultivation CHs de-differentiate
which could result in formation of fibrocartilage after
implantation. A major goal of research is the induction of
chondrogenesis of CHs and BM-MSCs in vitro. Besides the
application of growth factors and 3D cultivation, a new approach
using biophysical stimulation may be useful to enhance
chondrogenesis. In the present study, we have introduced an in
vitro test setup for the ES of human CHs and BM-MSCs. The
application of electric fields imitate endogenous electric signals
initiating the process of chondrogenic differentiation (1,36).
To investigate this effect, we cultured human CHs and BM-MSCs
either separately or as co-cultures onto a collagen-based scaffold.
Exposure with electric field was performed over seven days and
afterwards metabolic activity, gene expression and synthesis of
matrix proteins were analyzed.
The construction of the electrode system is based on
the design of a stimulation system providing ES on bone cells used
in previous studies (26). The
electrode system used in the present study supply AC coupled
directly to the cells to apply a defined electric field. Instead of
direct current (DC) stimulation, AC stimulation was chosen, because
DC causes additional electrochemical reactions like pH shift,
hydrogen peroxide and formation of reactive oxygen species, which
can damage exposed cells (37).
The used stimulation parameters like voltage, signal form and
stimulation period were chosen in accordance with ES stimulation of
bone cells (26). However,
frequency was selected due to findings from Brighton et al
(21) who had shown that higher
frequencies provide a stimulating effect on CHs. Therefore, we used
a frequency of 1 kHz for stimulating CHs and BM-MSCs instead of 20
Hz which had been used for ES of human osteoblasts.
The benefit of our approach was that we used a
stimulation system which was adapted to a 6-well plate format and
therefore easy to handle. Moreover, the experimental set up allowed
the incorporation of a collagen-based scaffold between well bottom
and electrode. The use of a scaffold is beneficial, because it
mimics physiological environment. This 3D cultivation supports
chondrogenic differentiation of CHs and BM-MSCs (38). The scaffold used is proven for
clinical therapy and showed high biocompatibility. Additionally,
investigation of metabolic activity using WST-1 assay detected no
cytotoxic effects of exposed electric field.
In the present study, we have found out that
exposure to an electric field increased collagen type II synthesis
significantly in human CHs compared to unstimulated cells under
hypoxic culture conditions. Other studies have also detected
enhanced collagen type II production after electric field exposure
using lower frequencies (1,36).
However, Brighton et al (21) exposed CHs to capacitively coupled
electric fields using much higher frequencies (60 kHz) resulting in
a significant upregulation of GAG production. Our results for gene
expression analyses and examination of soluble GAGs following ES
showed that human CHs tended to express more GAGs than unstimulated
controls. By applying increased frequencies for stimulation,
proteoglycan production may be enhanced which has to be evaluated
in further studies.
Using hypoxic culture conditions during cell
cultivation creates more physiological conditions due to avascular
nature of the cartilage. Jonitz-Heincke et al (28) had reported that human CHs cultured
in spheroid pellets showed superior collagen type II expression
under hypoxic culture conditions compared to normoxia. Our results
indicated a promoting effect of hypoxia on chondrogenic
differentiation of human CHs. Additionally, gene expression of
alkaline phosphatase as marker of undesired hypertrophy was
significantly decreased after cultivation under hypoxia compared to
normoxia, indicating reduced terminal differentiation of cells
which reduce quality of formed cartilage after implantation
(39).
The evaluation of extracellular matrix protein
synthesis showed that the collagen type I expression was on a very
high level compared to collagen type II and glycosaminoglykans
(GAGs). The enhanced collagen type I production was detected for
all three cell types (CHs, BM-MSCs and co-culture) with and without
application of an electric field under both normoxia and hypoxia,
indicating that the high collagen type I synthesis was probably not
caused by ES or oxygen conditions, but rather by in vitro
cultivation. High expression rates of collagen type I is
characteristic for BM-MSCs (40).
For CHs this indicates a de-differentiated cell status (41). Since we have determined enhanced
collagen type I expression levels either in control or electrical
stimulated cells we suspect that the stimulation period was too
short to initiate hyaline-like differentiation processes. Previous
studies showed that induction of re-differentiation using 3D
cultivation in the presence of important growth factors, like
fibroblast growth factor (FGF)-2, TGF-β1-3 and IGF-1 took several
weeks to achieve hyaline-like ECM (31,42).
It should also be noted that the use of collagen type I-derived
scaffolds, which are usually applied for cartilage repair, will not
reflect the physiological environment of chondrocytic cells.
MSCs play a key role for the treatment of cartilage
regeneration, and therefore, the induction of chondrogenesis in
vitro was investigated in different studies. Our findings
showed that BM-MSCs tended to increase collagen type II synthesis
following ES compared to unstimulated control. However, differences
did not reach level of significance. In the present study, the
application of growth factors occurred only for initial
pre-cultivation prior to ES to investigate the influence of
electric field on chondrogenesis. Although, the essential
importance of chondrogenic growth factors for the effect of ES on
BM-MSCs was described by Mayer-Wagner et al (43). However, Kwon et al (44) found out that BM-MSCs cultured in
micromass cultures showed an upregulation of chondrogenic
differentiation markers following ES also in the absence of growth
factors. Micromass cultures are a scaffold-free cultivation system
in which cells form dense aggregates identical to those in the
pre-cartilage environment (45,46).
Therefore, micromass cultures could be a key tool for induction of
chondrogenesis and may be superior compared to artificial collagen
type I scaffolds. For further imitating physiological in
vivo conditions, a biofunctionalized scaffold could be
integrated within the electrode system in which growth factors are
incorporated (38,47). This could enhance the biological
response of the BM-MSCs regarding ES.
Different studies have described the promoting
effect of co-cultivation of CHs and BM-MSCs on chondrogenesis
(15–17). Especially, regarding results of
gene expression analyses co-cultured cells displayed enhanced
expression of collagen type II and aggrecan mRNA following ES
compared to unstimulated control and single cultivation of BM-MSCs.
However, response was inferior compared to single cultivation of
CHs. Regarding alkaline phosphatase as a hypertrophic marker,
expression levels were decreased compared to unstimulated
co-cultured cells. Our results suggest that the co-cultivation of
different cell types may be an important approach to realize a more
physiological condition and has to be investigated in further
studies.
After the initial growth factor pre-incubation over
three days, ES was performed over seven days. This stimulation
period was suitable for analyzing ES effects on CHs and BM-MSCs,
however, differences between groups were rather detected by gene
expression analyses than on protein level. Using prolonged
stimulation period differences in protein synthesis might be
detected more precisely. Furthermore, results of gene expression
showed high inter-individual ranges of single values, which
probably was a consequence of high donor variability and low sample
number impairing achievement of significance level. Additionally,
for gene expression analysis, we used the whole scaffold for RNA
isolation und subsequent reverse transcription. Consequently, the
relative gene expression rates of the investigated differentiation
marker represent mean values of the cells distributed on the whole
scaffold and exposed to the entire range of field strengths. This
could also be a reason for high range of single gene expression
values. In further studies we will analyze the cellular response
within the respective electric field norms to find possible
differences in gene expression rates depending on intensity of
electric field. However, in this first set up, the primary goal of
the study was to determine the overall effects of ES on human CHs
and mesenchymal stem cells.
The design of the stimulation chamber is appropriate
for the application of electric fields on cells cultured onto a 3D
scaffold. This indicates that the presented stimulation system is a
valuable tool to investigate the influence of ES on human CHs,
BM-MSCs and a co-culture of both. Reduction of oxygen levels during
cultivation could enhance cellular chondrogenesis. However, the
culture conditions have to be further adapted to the particular
needs of each cell type in order to optimize the ES parameters for
ES of CHs and BM-MSCs.
Acknowledgements
The authors would like to thank Mrs. Doris Hansmann
and Ms. Anika Witt (Department of Orthopaedics, Restock University
Medical Centre, Rostock, Germany) for technical support, as well as
Professor Andreas Büttner and Dr. Diana Boy (Institute of Forensic
Medicine, Rostock University Medical Centre, Rostock, Germany) for
providing the post-mortally obtained knee cartilage.
Funding
The present study was funded by the AFOR foundation
and the German Research Foundation (DFG) via the GRK Welisa.
Availability of data and materials
The data used and analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
RB, AJH, JZ, TB and BH designed the study, and TB
and JZ designed and validated the stimulation chamber used. BH, MK
and SK performed the cell experiments and analyzed the data. BH,
RB, AJH, MK and SK were involved in data interpretation, and BH and
AJH wrote the manuscript. All authors edited and reviewed the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Local Ethical
Committee of the University of Rostock (registration no.
A2011-138). As human tissues were obtained post-mortally, for
forensic and legal reasons, written approval was not obtained from
the patients, which was approved by the local ethical committee;
all information relating to the patients has been fully
anonymized.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jahr H, Matta C and Mobasheri A:
Physicochemical and biomechanical stimuli in cell-based articular
cartilage repair. Curr Rheumatol Rep. 17:222015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudert M and Wirth CJ: Knorpelregeneration
und KnorpelersatzKompendium der praktischen Medizin.
Springer-Verlag; Berlin Heidelberg: pp. 1205–1218. 2000, View Article : Google Scholar
|
|
3
|
Brady MA, Waldman SD and Ethier CR: The
application of multiple biophysical cues to engineer functional
neocartilage for treatment of osteoarthritis. Part II: Signal
transduction. Tissue Eng Part B Rev. 21:20–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernhard JC and Vunjak-Novakovic G: Should
we use cells, biomaterials, or tissue engineering for cartilage
regeneration? Stem Cell Res Ther. 7:562016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Welsch GH, Mamisch TC, Zak L, Blanke M,
Olk A, Marlovits S and Trattnig S: Evaluation of cartilage repair
tissue after matrix-associated autologous chondrocyte
transplantation using a hyaluronic-based or a collagen-based
scaffold with morphological MOCART scoring and biochemical T2
mapping: Preliminary results. Am J Sports Med. 38:934–942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasold J, Zander K, Heskamp B, Grüttner C,
Lüthen F, Tischer T, Jonitz-Heincke A and Bader R: Positive impact
of IGF-1-coupled nanoparticles on the differentiation potential of
human chondrocytes cultured on collagen scaffolds. Int J
Nanomedicine. 10:1131–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farrell E, O'Brien FJ, Doyle P, Fischer J,
Yannas I, Harley BA, O'Connell B, Prendergast PJ and Campbell VA: A
collagen-glycosaminoglycan scaffold supports adult rat mesenchymal
stem cell differentiation along osteogenic and chondrogenic routes.
Tissue Eng. 12:459–468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caldwell KL and Wang J: Cell-based
articular cartilage repair: The link between development and
regeneration. Osteoarthritis Cartilage. 23:351–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinert AF, Ghivizzani SC, Rethwilm A,
Tuan RS, Evans CH and Nöth U: Major biological obstacles for
persistent cell-based regeneration of articular cartilage.
Arthritis Res Ther. 9:2132007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ko CK, Lee EY, Jang JD, Kim SJ, Suh DS and
Chang CH: Cartilage regeneration using a fibrin and autologous
cultured chondrocytes mixture in a canine model. J Tissue Sci Eng.
3:1142012. View Article : Google Scholar
|
|
11
|
Nukavarapu SP and Dorcemus DL:
Osteochondral tissue engineering: Current strategies and
challenges. Biotechnol Adv. 31:706–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang BJ, Hu JC and Athanasiou KA:
Cell-based tissue engineering strategies used in the clinical
repair of articular cartilage. Biomaterials. 98:1–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Madeira C, Santhagunam A, Salgueiro JB and
Cabral J: Advanced cell therapies for articular cartilage
regeneration. Trends Biotechnol. 33:35–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T,
Zhang W, Liu W, Cao Y and Zhou G: In vivo ectopic chondrogenesis of
BMSCs directed by mature chondrocytes. Biomaterials. 31:9406–9414.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meretoja VV, Dahlin RL, Kasper FK and
Mikos AG: Enhanced chondrogenesis in co-cultures with articular
chondrocytes and mesenchymal stem cells. Biomaterials.
33:6362–6369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levorson EJ, Santoro M, Kasper FK and
Mikos AG: Direct and indirect co-culture of chondrocytes and
mesenchymal stem cells for the generation of polymer/extracellular
matrix hybrid constructs. Acta Biomater. 10:1824–1835. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heng BC, Cao T and Lee EH: Directing stem
cell differentiation into the chondrogenic lineage in vitro. Stem
Cells. 22:1152–1167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Wang W, Clark CC and Brighton CT:
Signal transduction in electrically stimulated articular
chondrocytes involves translocation of extracellular calcium
through voltage-gated channels. Osteoarthritis Cartilage.
17:397–405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Wang Z, Zhang G, Clark CC and
Brighton CT: Up-regulation of chondrocyte matrix genes and products
by electric fields. Clin Orthop Relat Res. 427 Suppl:S163–S173.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brighton CT, Wang W and Clark CC: The
effect of electrical fields on gene and protein expression in human
osteoarthritic cartilage explants. J Bone Joint Surg Am.
90:833–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vavken P, Arrich F, Schuhfried O and
Dorotka R: Effectiveness of pulsed electromagnetic field therapy in
the management of osteoarthritis of the knee: A meta-analysis of
randomized controlled trials. J Rehabil Med. 41:406–411. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fioravanti A, Nerucci F, Collodel G,
Markoll R and Marcolongo R: Biochemical and morphological study of
human articular chondrocytes cultivated in the presence of pulsed
signal therapy. Ann Rheum Dis. 61:1032–1033. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ongaro A, Pellati A, Masieri FF, Caruso A,
Setti S, Cadossi R, Biscione R, Massari L, Fini M and De Mattei M:
Chondroprotective effects of pulsed electromagnetic fields on human
cartilage explants. Bioelectromagnetics. 32:543–551. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hiemer B, Ziebart J, Jonitz-Heincke A,
Grunert PC, Su Y, Hansmann D and Bader R: Magnetically induced
electrostimulation of human osteoblasts results in enhanced cell
viability and osteogenic differentiation. Int J Mol Med. 38:57–64.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dauben TJ, Ziebart J, Bender T, Zaatreh S,
Kreikemeyer B and Bader R: A novel in vitro system for comparative
analyses of bone cells and bacteria under electrical stimulation.
Biomed Res Int. 2016:51786402016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jonitz A, Lochner K, Peters K, Salamon A,
Pasold J, Mueller-Hilke B, Hansmann D and Bader R: Differentiation
capacity of human chondrocytes embedded in alginate matrix. Connect
Tissue Res. 52:503–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jonitz-Heincke A, Klinder A, Boy D,
Salamon A, Hansmann D, Pasold J, Buettner A and Bader R: In vitro
analysis of the differentiation capacity of postmortally isolated
human chondrocytes influenced by different growth factors and
oxygen levels. Cartilage: 1947603517719318. 2017. View Article : Google Scholar
|
|
29
|
Bentivegna A, Roversi G, Riva G, Paoletta
L, Redaelli S, Miloso M, Tredici G and Dalprà L: The effect of
culture on human bone marrow mesenchymal stem cells: Focus on DNA
methylation profiles. Stem Cells Int. 2016:56567012016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chua KH, Aminuddin BS, Fuzina NH and
Ruszymah BH: Insulin-transferrin-selenium prevent human chondrocyte
dedifferentiation and promote the formation of high quality tissue
engineered human hyaline cartilage. Eur Cell Mater. 9:58–67. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jonitz A, Lochner K, Tischer T, Hansmann D
and Bader R: TGF-β1 and IGF-1 influence the re-differentiation
capacity of human chondrocytes in 3D pellet cultures in relation to
different oxygen concentrations. Int J Mol Med. 30:666–672. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peterkofsky B: Bacterial collagenase
methods in enzymology. 82:453–471. 1982. View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saggese G, Bertelloni S, Baroncelli GI and
Di Nero G: Serum levels of carboxyterminal propeptide of type I
procollagen in healthy children from 1st year of life to adulthood
and in metabolic bone diseases. Eur J Pediatr. 151:764–768. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parfitt AM, Simon LS, Villanueva AR and
Krane SM: Procollagen type I carboxy-terminal extension peptide in
serum as a marker of collagen biosynthesis in bone. Correlation
with Iliac bone formation rates and comparison with total alkaline
phosphatase. J Bone Miner Res. 2:427–436. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vaca-González JJ, Guevara JM, Vega JF and
Garzón-Alvarado DA: An in vitro chondrocyte electrical stimulation
framework: A methodology to calculate electric fields and modulate
proliferation, cell death and glycosaminoglycan synthesis. Cel Mol
Bioeng. 9:116–126. 2016. View Article : Google Scholar
|
|
37
|
Windisch C, Kolb W, Röhner E, Wagner M,
Roth A, Matziolis G and Wagner A: Invasive electromagnetic field
treatment in osteonecrosis of the femoral head: A prospective
cohort study. Open Orthop J. 8:125–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vinatier C, Mrugala D, Jorgensen C,
Guicheux J and Noël D: Cartilage engineering: A crucial combination
of cells, biomaterials and biofactors. Trends Biotechnol.
27:307–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen S, Fu P, Cong R, Wu H and Pei M:
Strategies to minimize hypertrophy in cartilage engineering and
regeneration. Genes Dis. 2:76–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lefebvre V and Smits P: Transcriptional
control of chondrocyte fate and differentiation. Birth Defects Res
C Embryo Today. 75:200–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sliogeryte K, Botto L, Lee DA and Knight
MM: Chondrocyte dedifferentiation increases cell stiffness by
strengthening membrane-actin adhesion. Osteoarthritis Cartilage.
24:912–920. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Haleem AM and Chu CR: Advances in tissue
engineering techniques for articular cartilage repair. Oper Tech
Orthop. 20:76–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mayer-Wagner S, Passberger A, Sievers B,
Aigner J, Summer B, Schiergens TS, Jansson V and Müller PE: Effects
of low frequency electromagnetic fields on the chondrogenic
differentiation of human mesenchymal stem cells.
Bioelectromagnetics. 32:283–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kwon HJ, Lee GS and Chun H: Electrical
stimulation drives chondrogenesis of mesenchymal stem cells in the
absence of exogenous growth factors. Sci Rep. 6:393022016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoon HH, Bhang SH, Shin JY, Shin J and Kim
BS: Enhanced cartilage formation via three-dimensional cell
engineering of human adipose-derived stem cells. Tissue Eng Part A.
18:1949–1956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nydegger UE: Progress in complement
research: 1984. Year Immunol. 171–174. 1985.PubMed/NCBI
|
|
47
|
Chen FH, Rousche KT and Tuan RS:
Technology insight: Adult stem cells in cartilage regeneration and
tissue engineering. Nat Clin Pract Rheumatol. 2:373–382. 2006.
View Article : Google Scholar : PubMed/NCBI
|