Introduction
Bronchial asthma is a chronic inflammatory airway
disease involving eosinophils, mast cells, neutrophils, and airway
epithelial cells (1–4), and is a common chronic respiratory
disease, which seriously endangers human health. The pathogenesis
of bronchial asthma is very complicated. Allergic airway
inflammation is the key pathogenesis of bronchial asthma (5), is the basis of airway
hyperresponsiveness and airway remodeling; the main cause of
recurrent bronchial asthma and decreased lung function index is the
persistence of airway inflammation (6). Inflammatory mediators and cytokines
construct complex network structures (7), induce airway hyperresponsiveness in
patients with bronchial asthma through multiple signaling pathways
in the body, and lead to long-term chronic inflammation, thereby
resulting in remodeling of airways and small airways (8).
Transforming growth factor (TGF)-β has a wide range
of biological activities, participates in early embryonic
development (9), cartilage and
bone formation, inflammation, interstitial fibrosis, and regulation
of immune and endocrine functions (10,11),
and tumor formation and development (12,13).
Eosinophils and bronchial epithelial cells are the major sources of
TGF-β production in the bronchial asthma airway. Local TGF-β mainly
acts as a proinflammatory cytokine to recruit eosinophils,
lymphocytes, neutrophils and mast cells to the airway, enhances the
inflammatory cell viability, and promotes their proliferation,
differentiation and degranulation, so as to play an important role
in chronic airway inflammation in bronchial asthma (14). Smads are an important intracellular
TGF-β signal transduction and regulatory molecule, and can transfer
TGF-β signals directly from the cell membrane into the nucleus.
Smads protein as a substrate, which is phosphorylated (p) by
intracellular kinase of the TGF-β receptor, can further traverse
the cell membrane; Smads protein acts as an intracellular signaling
molecule and a transcripton in genetics (15,16).
At present, the effective drugs for bronchial asthma
are glucocorticoids, but the hormone drugs cause many side effects,
so it is still urgent to find effective therapeutic agents.
Epigallocatechin gallate (EGCG) is a water-soluble component
extracted from green tea polyphenols, has high antioxidant activity
and can protect the cells and DNA from damage (17,18).
Previous studies have confirmed that EGCG has a wide range of
pharmacological effects, and has obvious preventive and therapeutic
effects on anti-inflammation (19), antivirus (20), anticancer (21), ulcerative colitis (22), and autoimmune diseases (23). In this study, mouse models of
bronchial asthma were established by OVA combined with aluminum
hydroxide gel. This study aimed to observe the effect of EGCG on
bronchial asthma and to investigate the regulatory mechanism of
TGF-β1 signaling pathway so as to provide theoretical basis for
clinical prevention and treatment of bronchial asthma and the
research and development of new drug.
Materials and methods
Animals and groups
Forty Balb/c mice, 6 weeks old, male, weighing 20–23
g, were obtained from Experimental Animal Center of China Medical
University in China [production license no. SCXK (Liao)-2013-0001,
application license no. SYXK (Liao)-2013-0007]. This study was
approved by the Animal Welfare and Ethics Committee of China
Medical University Laboratory (no. 2016099).
The mice were randomly assigned to control group
(n=8), asthma model group (AS group; n=8), dexamethasone group (DX
group; asthma + 0.5 mg/kg DX; n=8), high-dose EGCG group [HEGCG
group; asthma + 20 mg/kg/day; this dose selected came from
reference (24) EGCG; n=8],
low-dose EGCG group (LEGCG group; asthma + 10 mg/kg/day EGCG; n=8),
LY 364947 (ab141890; Abcam, Cambridge, UK) inhibitor group (LYEGCG
group; asthma + 20 mg/kg/day EGCG + 40 µg/kg/day LY 364947; n=8).
Mice were given a normal diet and water, and the following housing
conditions: Ambient temperature of 20–26°C, 40–70% relative
humidity, 12-h light/dark cycle. The mice in the control group were
daily injected with an equal volume of physiological saline via the
caudal vein. All experiment repeated three times.
Establishment of mouse models of
asthma
Except the normal control group, mice in other
groups were intraperitoneally injected with 0.1 ml antigen solution
[0.1 g/ml OVA +1 g/ml Al (OH)3]. The mice were immunized
again on day 8 of the experiment. On day 15, the mice were placed
in closed container, treated with 5% OVA saline inhalation for 20
min, for 7 days. The provocative status was observed. The mice in
the control group were given an equal volume of physiological
saline.
Sample collection
On day 23 after model establishment, mice in each
group were injected with dexamethasone and EGCG via the caudal
vein. 7 days later, venous blood was collected from mice and serum
was separated for cytometric bead array. A T-shaped incision was
made on the site of trachea, and a 22G indwelling needle was
inserted for tracheal intubation. Physiological saline 0.4 ml was
injected, repeatedly three times. Bronchoalveolar lavage fluid was
collected for eosinophil count. Mouse bronchovesicular tissue was
dissociated. Some were fixed in 10% formalin, and the other was
used for western blot assay and qRT-PCR.
Determination of airway resistance
(AWR) and lung function in mice with asthma
At 24 h of final atomization, AWR was detected using
mouse lung function instrument. The mice were anesthetized with
sodium pentobarbital (50 mg/kg). The 22G indwelling needle was
inserted for tracheal intubation. After connecting to the mouse
lung function instrument, the mice were placed on the heating plate
of a sealed incubator. A respirator was used for assisted
respiration. 12.5, 25 and 50 mg/ml of methacholine were infused
intravenously. The changes in AWR (cm H2O s/l) and lung
function index [peak expiratory flow (PEF) and the ratio of forced
expiratory volume in 0.4 sec to forced vitia capacity
(FEV0.4/FVC)] were measured. The AWR was R (baseline)
when inhaled PBS. The AWR of inhaling methacholine at different
concentrations was R (response). The maximum of the total AWR at
each concentration was taken and converted to the fold increase
during PBS provocation as the index to assess AWR according to the
following formula: Fold increase of R = [R (response) - R
(baseline)]/R (baseline).
Hematoxylin and eosin (H&E)
staining
Bronchovesicular tissue of mice was dehydrated,
permeabilized, embedded in wax, and sliced into sections. The
sections were stained with hematoxylin for 5 min, washed with PBS,
differentiated with ethanol hydrochloride for 3 sec, stained with
eosin for 2 min, and mounted with neutral resin. The changes in
bronchovesicular tissue were observed under a light microscope.
Cytometric bead array
After fully mixed Th1, Th2, Th17 and Treg
cells-related cytokines interleukin (IL)-2, IL-4, IL-6, IL-10,
IL-17a, tumor necrosis factor (TNF)-α, and IFN-γ protein
microspheres, the remaining procedures were carried out in
accordance with the kit instruction (560485, BD Biosciences,
Franklin Lakes, NJ, USA). After establishing standard curves, 50 µl
serum was added in the detected tube and microsphere assay reagent
was added at room temperature in the dark for 2 h. The samples were
centrifuged and the supernatant was discarded. The microspheres
were resuspended, and determined using flow cytometry. Results were
analyzed with FCAP Array V3 software.
Eosinophil and neutrophil count using
bronchoalveolar lavage fluid
Totally 10 µl lavage fluid was placed in cell count
plate to quantify the number of cells. After centrifugation, cell
sediment was resuspended with moderate amount of PBS. Cell
suspension (100 µl) was loaded. After the residual liquid was got
rid of, cells were dried in the open air, fixed in alcohol for 1
min, and received Diff-Quik staining. 400 larupcutes were counted
under a microscope. The percentages of neutrophils and eosinophils
were calculated.
Flow cytometry
Peripheral blood mononuclear cells in mice were
isolated and adjusted to a concentration of 1×106, and
coated in a 6-well plate. PMA (20 ng/ml), ionomycin (1 µg/ml) and
monensin (2 nmol/ml) were added at 37°C in 5% CO2
incubator for 5 h. Afterwards, cells were incubated with
FITC-anti-CD4+ (554843; BD Biosciences) and
PE-cy7-anti-CD25+ (552880; BD Biosciences) antibody at
room temperature in the dark for 30 min, washed with PBS, fully
mixed with 500 µl fixation/permeabilization solution at 4°C in the
dark for 45 min. After washing with 1×BD perm/wash buffer, cells
were incubated with APC-anti-IL-17a (17-7177-81; eBioscience;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
PE-anti-Foxp3+ (A18690; eBioscience; Thermo Fisher
Scientific, Inc.) at 4°C in the dark for 50 min, washed with PBS,
and resuspended with 400 µl PBS. The samples were determined using
flow cytometry and analyzed with Flow Jo V10 software.
RT-qPCR
The lung of mice was completely triturated and
treated with 1 ml TRIzol reagent (15596018; Invitrogen; Thermo
Fisher Scientific, Inc.). RNA was extracted in accordance with the
reagent instruction, reverse transcribed into first-strand DNA
(4387406; Invitrogen; Thermo Fisher Scientific, Inc.). Real-time
quantitative fluorescence PCR was performed in accordance with the
instruction of RT-PCR kit (204057; Qiagen GmbH, Hilden, Germany).
The relative gene expression data were analyzed with the
2−∆∆Cq method (25).
The primers used for real-time PCR are listed in Table I.
 | Table I.RT-qPCR using gene primers. |
Table I.
RT-qPCR using gene primers.
|
| Primer (5′→3′) |
|---|
| RORγT |
|
|
Forward |
GCACCCGCTGAGAGGGCT |
|
Reverse |
CGAACCAGCCGCAACCGA |
| Foxp3 |
|
|
Forward |
TACACTTTAGGGCTCTCA |
|
Reverse |
CCTCCTGAGAGCCTCAGG |
| GAPDH |
|
|
Forward |
AGGGTCCAGACAGCCACT |
|
Reverse |
TGAGATTGCCCTCTACAC |
Western blot assay
Mouse bronchial lung tissue was placed in RIPA
lysate containing proteinase inhibitor on the ice for 30 min, and
centrifuged. The supernatant was collected. Bicinchoninic acid
assay was applied to quantify proteins. These proteins were
subjected to electrophoresis and transferred onto the PVDF
membrane. The membrane was blocked and incubated with TGF-β1
(ab92486; Abcam), Smad2/3 (ab202445; Abcam), and p-Smad2/3
(ab63399; Abcam) antibody at 4°C overnight, and washed with TBST.
The PVDF membrane was incubated with horseradish peroxidase-labeled
secondary antibody at room temperature for 2 h. The proteins were
visualized using enhanced chemiluminescence kit and gel imaging
system. Absorbance values were analyzed using Image Tools.
Statistical analysis
All data were analyzed using SPSS 19.0 software
(SPSS, Inc., Chicago, IL, USA), and expressed as the mean ± SD.
Paired comparison was conducted using Student's t-test. Multiple
comparisons were used one-way analysis of variance with
Student-Newman-Keuls test. A value of P<0.05 was considered
statistically significant.
Results
ECGC improves provocative symptoms in
asthmatic mice
After asthma was provocated by 5% OVA atomization,
the mice presented shortness of breath, restlessness, back upright,
repeated scratching head, horripilation and slow action. The
symptoms of mice from the DX group were improved, but their
activities did not obviously increase. Their actions were still
sluggish. In the HEGCG and LEGCG groups, the respiration in mice
was smooth; sluggish action improved; the degree of pruritus and
cyanosis were relieved. H&E staining (Fig. 1) demonstrated that in the asthma
group, bronchial and alveolar tissues were disorganized and some
structures disappeared; a large number of inflammatory cells
infiltrated in the bronchial mucosa; and the arrangement of the
airway epithelium was irregular. After treatment with EGCG, the
infiltration of inflammatory cells was obviously alleviated, and
the therapeutic efficacy of high dose was more obvious than that of
low dose.
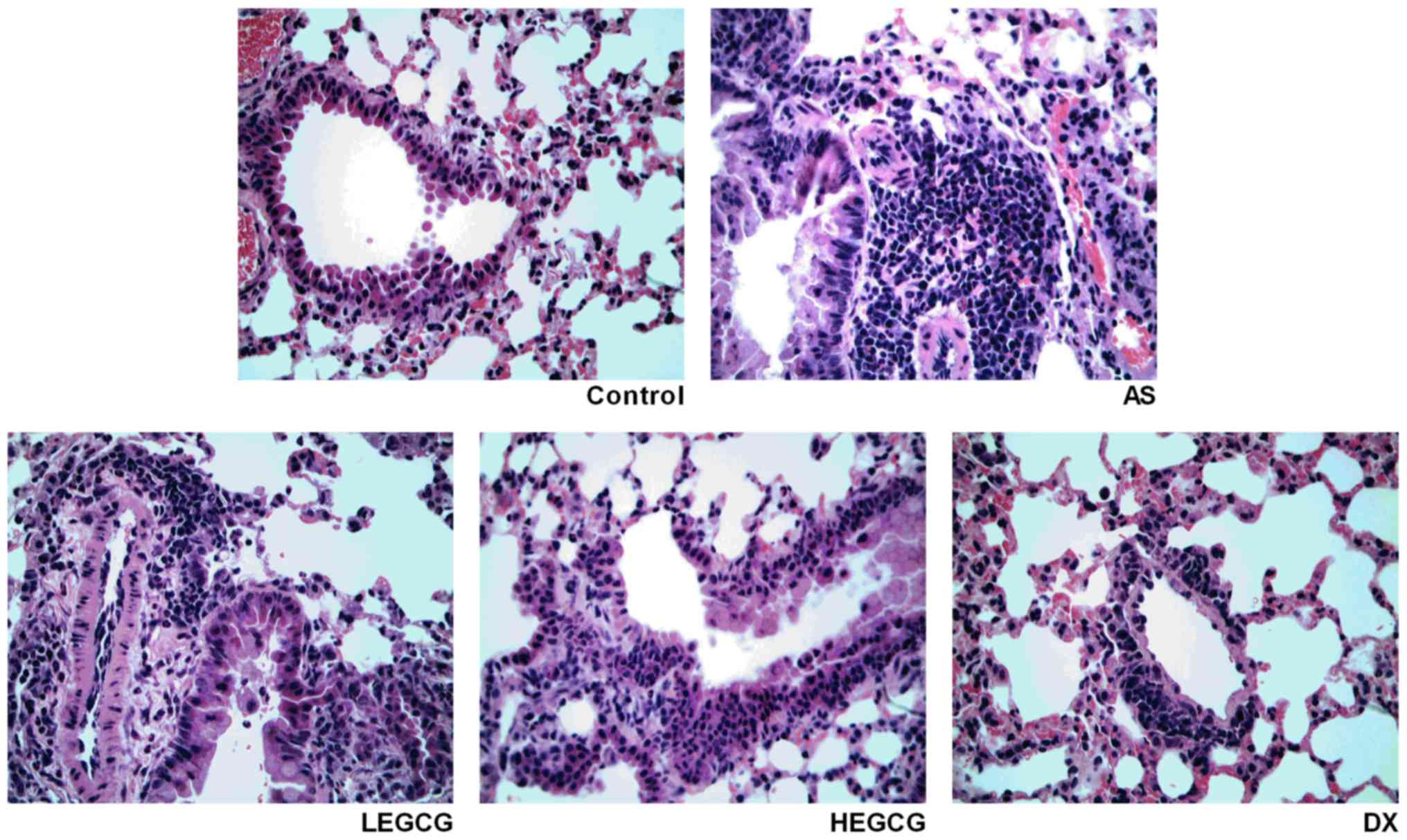 | Figure 1.Established asthmatic mice model and
treated with dexamethasone (0.5 mg/kg), high-dose EGCG (20 mg/kg)
and low-dose EGCG (10 mg/kg). On day 30 after model establishment
and treatment, mice lung tissue was collected. Lung tissue was
fixed, dehydrated, paraffin embedded, sliced. H&E staining
observed the pathology alteration (magnification, ×40). EGCG,
epigallocatechin gallate; AS, asthamatic; LEGCG, low-dose EGCG;
HEGCG, high-dose EGCG; DX, dexamethasone group; H&E,
hematoxylin and eosin. |
EGCG lessens AWR in asthmatic
mice
Lung function tests in mice showed that pulmonary
function parameters PEF, FEV0.4/FVC in asthma group were
decreased than those in control group (P<0.05 vs. control),
respiratory rate was increased (P<0.05 vs. control); LEGCG
group, HEGCG group and DX group pulmonary function parameters PEF,
FEV0.4/FVC were increased and respiratory rate was decreased
(P<0.05 vs. asthma group; Fig.
2A). After provocation with different concentrations of
methacholine, AWR was significantly increased in the asthma group,
but significantly decreased in the DX group (P<0.05 vs. control;
Fig. 2B). AWR was significantly
reduced in the HEGCG group (P<0.05 vs. asthma group; P>0.05
vs. DX group). These data indicated that EGCG 20 mg/kg/day via
caudal vein injection could remarkably mitigate AWR and improve
lung function in asthmatic mice.
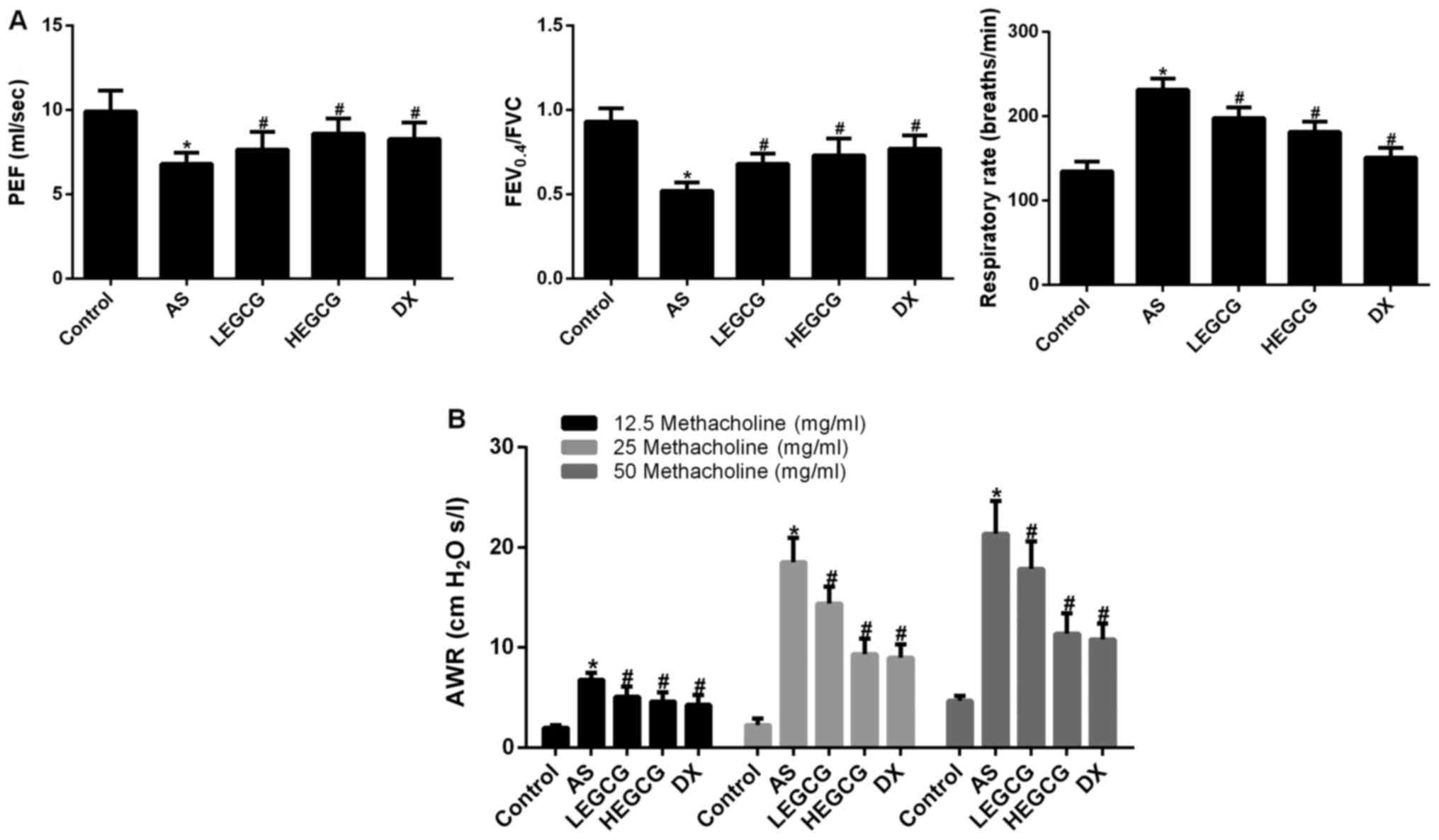 | Figure 2.After establishment the asthmatic
model, and treatment with dexamethasone (0.5 mg/kg), high-dose EGCG
(20 mg/kg) and low-dose EGCG (10 mg/kg). The lung function and AWR
was detected by mouse lung function instrument. Data collected from
control group, AS group, LEGCG group, HEGCG group and DX group. (A)
The lung function index in mice. (B) AWR was detected using
different concentrations of methacholine. *P<0.05, compared with
control group. #P<0.05, compared with AS group. EGCG,
epigallocatechin gallate; AS, asthamatic; LEGCG, low-dose EGCG;
HEGCG, high-dose EGCG; DX, dexamethasone group; AWR, airway
resistance. |
EGCG improves the number of
eosinophils and neutrophils in bronchoalveolar lavage fluid of
mice
The number of eosinophils and neutrophils was
significantly increased in the asthma group (P<0.05 vs. control)
(Fig. 3). The number of
eosinophils and neutrophils was significantly decreased in the DX
group (P<0.05 vs. asthma group). The number of eosinophils and
neutrophils was significantly diminished in the HEGCG group
(P<0.05 vs. asthma group). Although LEGCG group also can
significantly improve the number of neutrophils and eosinophils,
the effect of LEGCG group was no significant effect of HEGCG group.
These findings further suggested that EGCG 20 mg/kg could
noticeably improve the number of inflammatory cells in
bronchoalveolar lavage fluid of asthmatic mice.
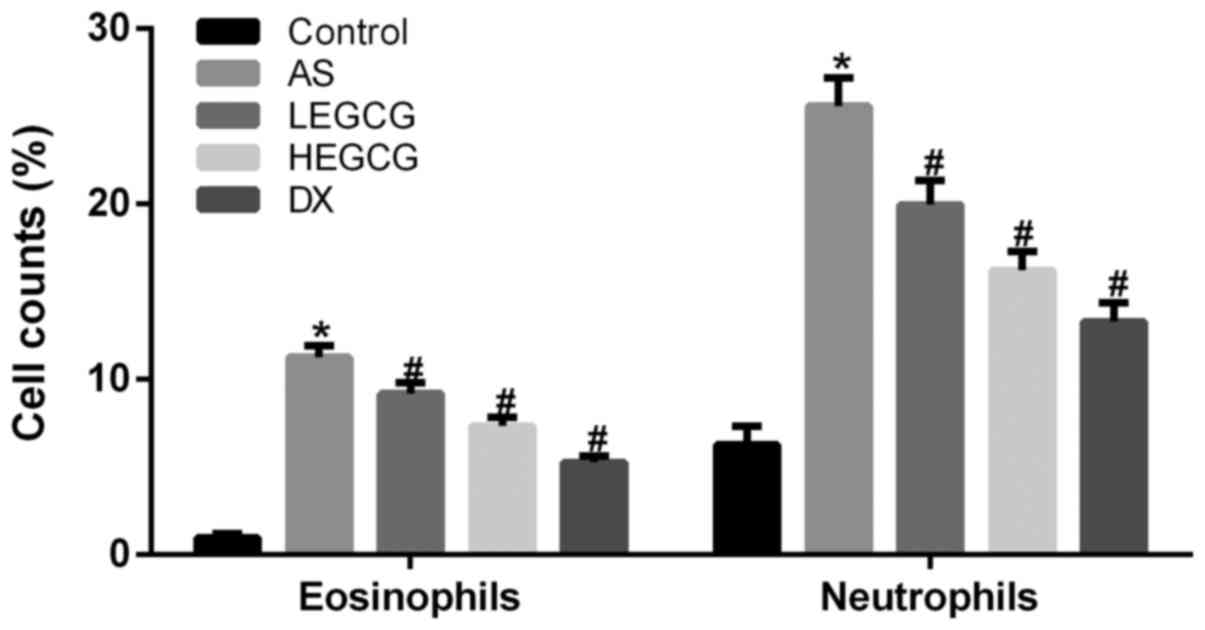 | Figure 3.Bronchoalveolar lavage fluid was
collected, the percentages of neutrophils and eosinophils were
calculated. Data collected from control group, AS group, LEGCG
group, HEGCG group and DX group. *P<0.05, compared with control
group. #P<0.05, compared with AS group. EGCG,
epigallocatechin gallate; AS, asthamatic; LEGCG, low-dose EGCG;
HEGCG, high-dose EGCG; DX, dexamethasone group. |
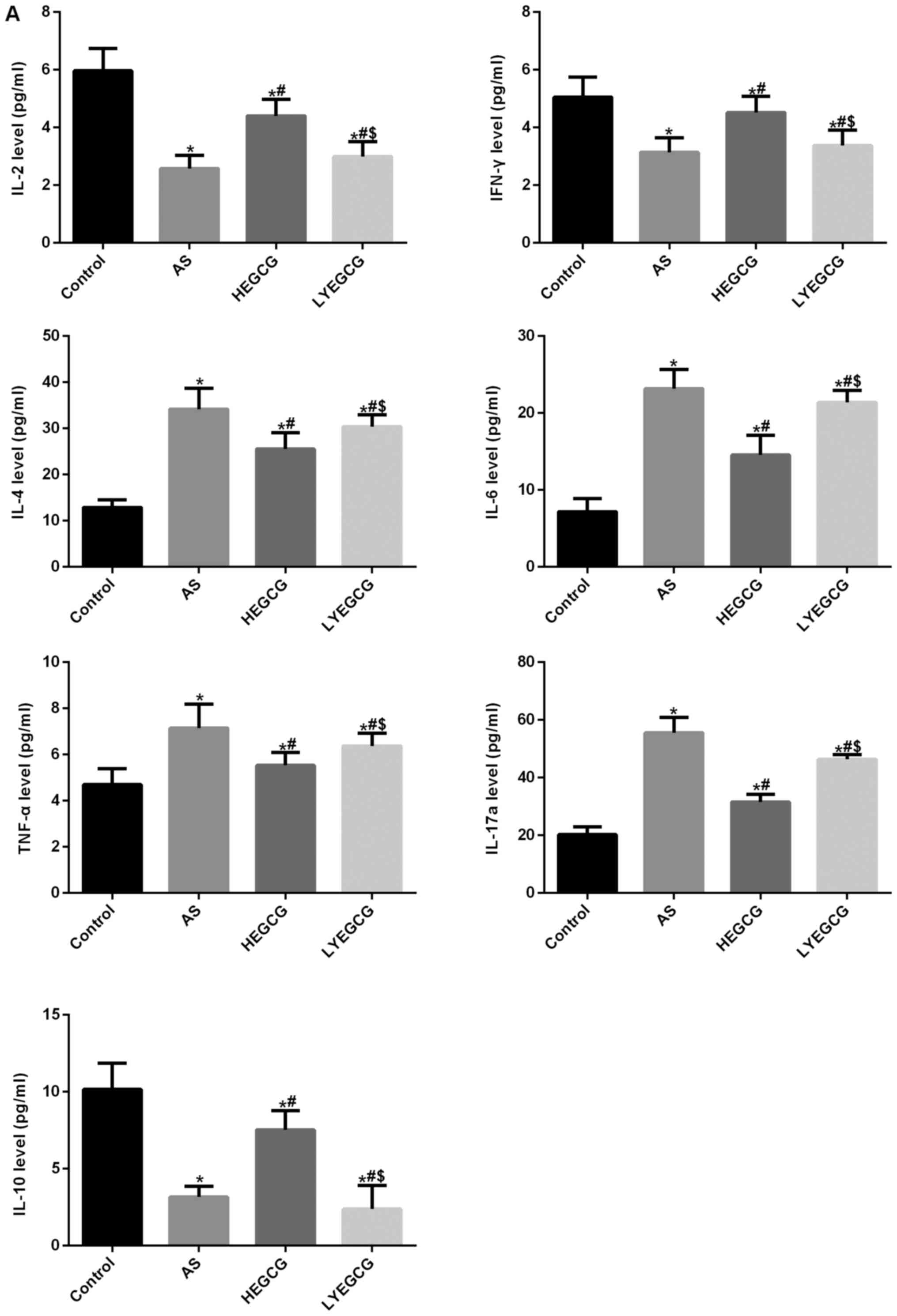
EGCG lessens inflammatory reaction in
asthmatic mice
Cytometric bead array results demonstrated that
serum IL-2 and IFN-γ levels were significantly decreased in the
asthma group (P<0.05 vs. control) (Fig. 4). IL-4, IL-6 and TNF-α levels were
significantly increased (P<0.05 vs. control). These results
verified that the imbalance of Th1/Th2 cells was found in asthmatic
mice. After treatment with EGCG, IL-2 and IFN-γ levels were
significantly increased (P<0.05 vs. asthma group); IL-4, IL-6
and TNF-α levels were significantly diminished (P<0.05 vs.
asthma group). EGCG could effectively improve the imbalance of
Th1/Th2 cells in asthmatic mice. We further analyzed IL-17a and
IL-10 levels. Results found that IL-17a levels were significantly
increased in the asthma group (P<0.05 vs. control). After
treatment with EGCG, IL-17a levels were significantly reduced;
IL-10 levels were significantly diminished in the asthma group
(P<0.05 vs. control). After treatment with EGCG, IL-10 levels
were significantly increased (P<0.05 vs. asthma group). These
results suggest that EGCG may improve Th17/Treg balance. These data
further confirmed that EGCG effectively improved the inflammatory
reaction in asthmatic mice.
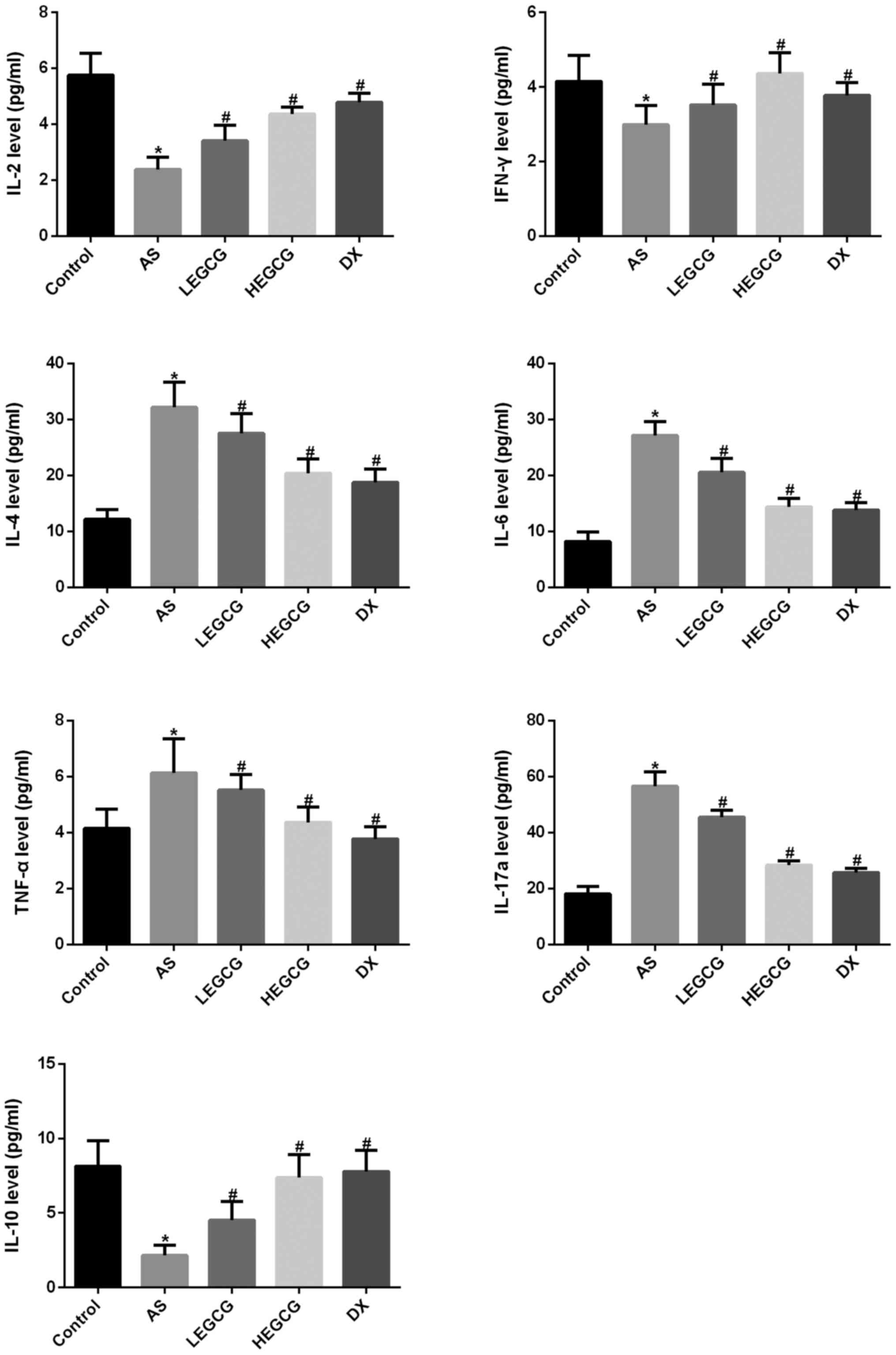 | Figure 4.Mouse 1 ml peripheral blood was
collected in each group, then 1,000 × g centrifuged 10 min, the
serum was isolated, the serum was used to detect the factors level
by Cytometric bead array. Results were analyzed with FCAP Array V3
software. *P<0.05, compared with control group.
#P<0.05, compared with AS group. EGCG,
epigallocatechin gallate; AS, asthamatic; LEGCG, low-dose EGCG;
HEGCG, high-dose EGCG; DX, dexamethasone group. |
EGCG improves the proportion of
Th17/Treg cells in asthmatic mice
As we all know, Th17 cells secrete IL-17a, Treg
cells secrete IL-10, so the proportion of Th17 cells and Treg cells
in mice was determined by flow cytometry. The percentage of Th17
cells was significantly increased in the asthma group (P<0.05
vs. control; Fig. 5A), but the
percentage of Treg cells was significantly reduced (P<0.05 vs.
control) (Fig. 5B). After
treatment with EGCG, the percentage of Th17 cells was significantly
decreased (P<0.05 vs. asthma group); the percentage of Treg
cells was significantly increased (P<0.05 vs. asthma group).
After treatment with EGCG, Th17/Treg ratio was significantly
decreased (P<0.05 vs. asthma group) (Fig. 5C). The mRNA expression of Th17 cell
transcription factor RORγT and Treg cell transcription factor Foxp3
supported the results of flow cytometry (Fig. 5D). These findings verified that
EGCG could reduce the number of Th17 cells, and increase the number
of Treg cells, thereby improving the airway inflammation in
asthmatic mice.
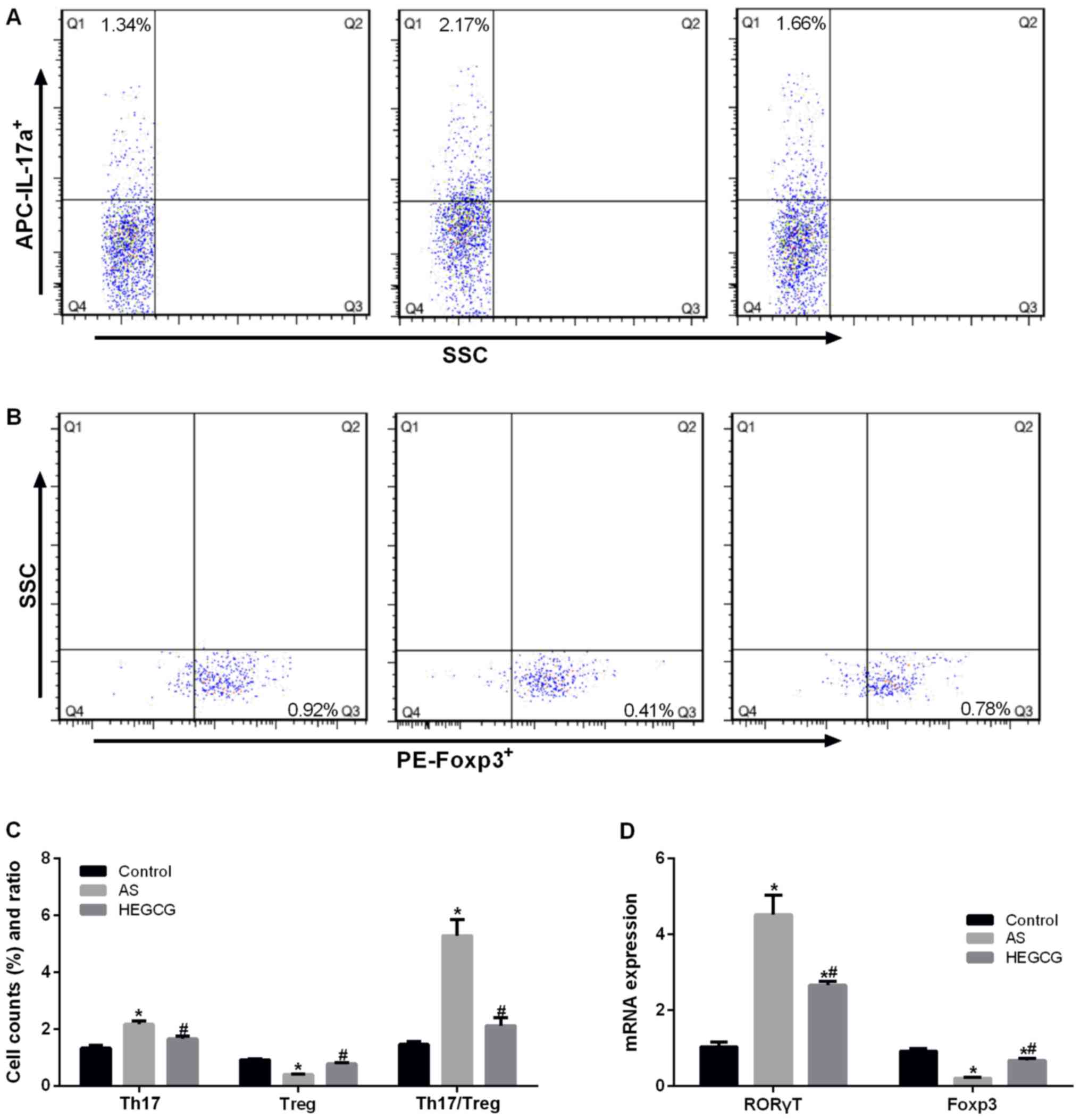 | Figure 5.PBMC were isolated from peripheral
blood, the percentage of TH17 and Treg were detected. Different
cell subsets were distinguished according to different cell labels,
i.e., the TH17 cells were CD4+ IL-17+ cells,
while Treg cells were CD4+ CD25+
Foxp3+ cells. Data collected from control group, AS
group and HEGCG group. (A) Percentage of CD4+
IL-17+ Th17 cells in CD4+ T cells gate. (B)
Percentage of CD4+ CD25+ Foxp3+
Treg cells in CD4+ CD25+ T cells gate. (C)
Bar graph of Th17 cells and Treg cells, and Th17/Treg ratio. (D)
RORγT and Foxp3, which is Th17 and Treg cells transcription factor,
were detected by RT-qPCR. *P<0.05, compared with control group.
#P<0.05, compared with AS group. EGCG,
epigallocatechin gallate; AS, asthamatic; LEGCG, low-dose EGCG;
HEGCG, high-dose EGCG; DX, dexamethasone group. |
EGCG improves inflammatory reaction in
asthmatic mice through TGF-β1 signaling pathway
To confirm the action mechanism of EGCG, we used
TGF-β1 receptor inhibitor LY 364947. Cytometric bead array results
showed that after inhibition of TGF-β1 receptor, the protective
effect of EGCG disappeared (Fig.
6A). The expression of proinflammatory cytokines such as IL-6
was significantly increased after inhibition of TGF-β1 receptor
(Fig. 6A). The expression of
anti-inflammatory factors such as IL-10 was significantly increased
after inhibition of TGF-β1 receptor (Fig. 6A). Western blot assay results
demonstrated that TGF-β1 and P-smad2/3 expression was significantly
increased in the lung of mice in the asthma group (P<0.05 vs.
control; Fig. 6B). After treatment
with EGCG, TGF-β1 and P-smad2/3 expression was significantly
reduced (P<0.05 vs. asthma group). After inhibition of TGF-β1
receptor, TGF-β1 and p-smad2/3 expression was significantly
increased (P<0.05 vs. EGCG group). These data suggested that
EGCG improved the airway inflammation in asthmatic mice through
TGF-β1 signaling pathway.
 | Figure 6.In order to further verify the
mechanism of EGCG, TGF-β1 receptor inhibitor which is LY 364947 (40
µg/kg/day) was used. The inflammatory factors and TGF-β1
pathway-related protein were detected. Data collected from control
group, AS group, HEGCG group and LYEGCG group. (A) The levels of
inflammatory factor by CBA. (B) TGF-β1 pathway-related protein
expression by western blot analysis. *P<0.05, compared with
control group. #P<0.05, compared with AS group.
$P<0.05, compared with HEGCG group. EGCG,
epigallocatechin gallate; AS, asthamatic; LEGCG, low-dose EGCG;
HEGCG, high-dose EGCG; DX, dexamethasone group. |
Discussion
This study established mouse models of asthma, and
found that EGCG remarkably mitigated the airway inflammation in
asthmatic mice, reduced the number of eosinophils and neutrophils
in bronchoalveolar lavage fluid, diminished the percentage of Th17
cells, and increased the number of Treg cells, thereby exerting
anti-inflammatory effect through TGF-β1 signaling pathway.
Bronchial asthma is a common and frequent chronic
inflammatory reaction, frequently occurs in autumn and has the
characteristics of recurrent and repeated attacks. Glucocorticoids
are still the best drug for the treatment of bronchial asthma; its
effect is only to relieve the symptoms associated with asthma, and
hormonal treatment is associated with many side effects (25). Therefore, finding effective
treatment remains imminent. EGCG is a water-soluble component
extracted from green tea polyphenols, and has high antioxidant
activity (26). Previous studies
demonstrated that antioxidant activity of EGCG was 30 times that of
vitamin C and vitamin E (27). It
has been found that EGCG can affect apoptosis, autophagy or (and)
block the flow of calcium ions in cells, which achieve
anti-inflammatory and anti-apoptotic effects (28–30).
The present study showed that EGCG could mitigate OVA-mediated
airway inflammation in mouse models of asthma, which was consistent
with the results of the study by Yu et al (31).
Bronchial asthma is a chronic airway inflammation
that is involved in a variety of inflammatory cells. Bronchial
asthma was considered to be a disease associated with abnormal
differentiation of CD4+ T lymphocytes (32). CD4+ T cells, including
Th1, Th2, Th17, and Treg subsets, are regulators of inflammatory
reaction of bronchial asthma. The function of these lymphocytes is
mainly achieved by secreting cytokines (33). Th17 cells are one of the effectors
of host defense, are named for their production of cytokine IL-17a,
which has specific effects. Th17 can secrete cytokines such as
IL-22, IL-17f and CCL20 at the same time, activate immune cells and
non immune cells, and participate in the invasion of extracellular
bacteria (34). Treg is a new type
of CD4+ T lymphocytes, can effectively control or
inhibit the function of related immune cells, regulate immune
response, and thus playing an important regulatory role in the
immune system (35). Results from
this study verified that EGCG could reduce the percentage of Th17
cells, increase the percentage of Treg cells, and then suppress
inflammatory reaction.
The association between TGF-β and airway remodeling
in bronchial asthma has been studied extensively, considering that
TGF-β is an important influential factor for airway remodeling
(36,37). TGF-β combines with type II receptor
to form a dimer. After conformation changes, it is recognized by
the type I receptor and combined to form trimer. Simultaneously,
type I receptor is activated by phosphorylation and activates its
substrate, amplifying the signal and sending it downstream
(38). Smads are an important
intracellular TGF-β signal transduction and regulatory molecule,
can transfer TGF-β signals directly from the cell membrane to the
nucleus. Many studies have suggested that Samd2, Smad3 and Smad7 in
Smad protein family are involved in TGF-βsignal transduction and
play an important role in the formation of airway remodeling in
bronchial asthma (39). TGF-β
antibody antagonist could adjust the expression of TGF-βsignaling
pathway by Smad2/3 expression, and then regulate airway remodeling
(40). In the present study, We
established the model of bronchial asthma and found that EGCG has
the effect of reducing airway asthma. However, the mechanism of
EGCG is unclear. We suspect that may be related to the TGF-beta1
signaling pathway, after the inhibition of TGF-β1 receptor, the
protective effect of EGCG disappeared; IL-6, TNF-α and IL-17a
levels increased; but IL-10 levels significantly decreased. These
findings further confirmed that EGCG acts through TGF-β1 signaling
pathway.
In conclusion, EGCG mitigated AWR, lessened airway
inflammation, reduced the number of eosinophils and neutrophils in
bronchoalveolar lavage fluid, suppressed the percentage of Th17
cells, and increased the percentage of Treg cells. This protective
effect is achieved via the TGF-β1 signaling pathway. This will
provide a theoretical basis for the clinical treatment of asthma
and the research and development of new drugs. This study has many
deficiencies, for example, whether EGCG plays a role through
binding to TGF-β1 receptor or by which specific mechanism EGCG
regulates TGF-β1 signaling pathway needs further study.
Acknowledgements
This study was supported by the Liaoning Natural
Fund Project (2013021017), and Shenyang Science and Technology Plan
Project (F15-139-9-35).
Competing interests
'The authors declare that they have no competing
interests.
References
|
1
|
Yu HY, Li XY, Cai ZF, Li L, Shi XZ, Song
HX and Liu XJ: Eosinophil cationic protein mRNA expression in
children with bronchial asthma. Genet Mol Res. 14:14279–14285.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sussan TE, Gajghate S, Chatterjee S,
Mandke P, McCormick S, Sudini K, Kumar S, Breysse PN, Diette GB,
Sidhaye VK and Biswal S: Nrf2 reduces allergic asthma in mice
through enhanced airway epithelial cytoprotective function. Am J
Physiol Lung Cell Mol Physiol. 309:L27–L36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erokhina IL, Voronchikhin PA, Okovityi SV
and Emel'ianova OI: Reaction of population of pulmonary mast cells
in rat bronchial asthma under the effect of beta-adrenoreceptor
antagonists. Tsitologiia. 55:472–474. 2013.(In Russian). PubMed/NCBI
|
|
4
|
Ciepiela O, Ostafin M and Demkow U:
Neutrophils in asthma-a review. Respir Physiol Neurobiol.
209:13–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang BR, Kwon HS, Kim SH, Yoon SY, Choi
JD, Hong GH, Park S, Kim TB, Moon HB and Cho YS: Interleukin-32γ
suppresses allergic airway inflammation in mouse models of asthma.
Am J Respir Cell Mol Biol. 50:1021–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adamek-Guzik T, Czerniawska-Mysik G and
Guzik T: Bronchial asthma-a chronic inflammatory disorder. Przegl
Lek. 53:12–19. 1996.(In Polish). PubMed/NCBI
|
|
7
|
Zakharyan R and Boyajyan A: Inflammatory
cytokine network in schizophrenia. World J Biol Psychiatry.
15:174–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakagome K and Nagata M: Pathogenesis of
airway inflammation in bronchial asthma. Auris Nasus Larynx.
38:555–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Messler S, Kropp S, Episkopou V, Felici A,
Würthner J, Lemke R, Jerabek-Willemsen M, Willecke R, Scheu S,
Pfeffer K and Wurthner JU: The TGF-β signaling modulators
TRAP1/TGFBRAP1 and VPS39/Vam6/TLP are essential for early embryonic
development. Immunobiology. 216:343–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis MA and Sheppard D: TGF-β activation
and function in immunity. Annu Rev Immunol. 32:51–82. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu ML, Wang H, Wang ZR, Zhang YF, Chen
YQ, Zhu FH, Zhang YQ, Ma J and Li Z: TGF-β1 regulation of estrogen
production in mature rat Leydig cells. PLoS one. 8:e601972013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou F, Drabsch Y, Dekker TJ, de Vinuesa
AG, Li Y, Hawinkels LJ, Sheppard KA, Goumans MJ, Luwor RB, de Vries
CJ, et al: Nuclear receptor NR4A1 promotes breast cancer invasion
and metastasis by activating TGF-β signalling. Nat Commun.
5:33882014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Syed V: TGF-β signaling in cancer. J Cell
Biochem. 117:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al-Alawi M, Hassan T and Chotirmall SH:
Transforming growth factor β and severe asthma: A perfect storm.
Respir Med. 108:1409–1423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heldin CH and Moustakas A: Role of Smads
in TGFβ signaling. Cell Tissue Res. 347:21–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takagaki A, Otani S and Nanjo F:
Antioxidative activity of microbial metabolites of
(−)-epigallocatechin gallate produced in rat intestines. Biosci
Biotechnol Biochem. 75:582–585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson MK and Loo G: Effects of
epigallocatechin gallate and quercetin on oxidative damage to
cellular DNA. Mutat Res. 459:211–218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao Z, Han Y, Hu Y, Wu X, Wang Y, Zhang X,
Fu J, Zou X, Zhang J, Chen X, et al: Targeting HO-1 by
epigallocatechin-3-gallate reduces contrast-induced renal injury
via anti-oxidative stress and anti-inflammation pathways. PLoS One.
11:e01490322016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohno A, Kataoka S, Ishii Y, Terasaki T,
Kiso M, Okubo M, Yamaguchi K and Tateda K: Evaluation of Camellia
sinensis catechins as a swine antimicrobial feed additive that does
not cause antibiotic resistance. Microbes Environ. 28:81–86. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Min KJ and Kwon TK: Anticancer effects and
molecular mechanisms of epigallocatechin-3-gallate. Integr Med Res.
3:16–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bitzer ZT, Elias RJ, Vijay-Kumar M and
Lambert JD: (−)-Epigallocatechin-3-gallate decreases colonic
inflammation and permeability in a mouse model of colitis, but
reduces macronutrient digestion and exacerbates weight loss. Mol
Nutr Food Res. 60:2267–2274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu D, Wang J, Pae M and Meydani SN: Green
tea EGCG, T cells and T cell-mediated autoimmune diseases. Mol
Aspects Med. 33:107–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haque A, Rahman MA, Chen ZG, Saba NF,
Khuri FR, Shin DM and Amin Ruhul AR: Combination of erlotinib and
EGCG induces apoptosis of head and neck cancers through
posttranscriptional regulation of Bim and Bcl-2. Apoptosis.
20:986–995. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Funston W and Higgins B: Improving the
management of asthma in adults in primary care. Practitioner.
258:15–19. 2014.PubMed/NCBI
|
|
26
|
Chakrawarti L, Agrawal R, Dang S, Gupta S
and Gabrani R: Therapeutic effects of EGCG: A patent review. Expert
Opin Ther Pat. 26:907–916. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Intra J and Kuo SM: Physiological levels
of tea catechins increase cellular lipid antioxidant activity of
vitamin C and vitamin E in human intestinal caco-2 cells. Chem Biol
Interact. 169:91–99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamagata K, Tanaka N and Suzuki K:
Epigallocatechin 3-gallate inhibits 7-ketocholesterol-induced
monocyte-endothelial cell adhesion. Microvasc Res. 88:25–31. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inoue T, Suzuki Y and Ra C:
Epigallocatechin-3-gallate induces cytokine production in mast
cells by stimulating an extracellular superoxide-mediated calcium
influx. Biochem Pharmacol. 82:1930–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi J, Deng H, Pan H, Xu Y and Zhang M:
Epigallocatechin-3-gallate attenuates microcystin-LR induced
oxidative stress and inflammation in human umbilical vein
endothelial cells. Chemosphere. 168:25–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu NH, Pei H, Huang YP and Li YF:
(−)-Epigallocatechin-3-gallate inhibits arsenic-induced
inflammation and apoptosis through suppression of oxidative stress
in mice. Cell Physiol Biochem. 41:1788–1800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bakr SI, Mahran MZ and Soliman DA: Role of
regulatory CD4+CD25+ Foxp3 T cells in
bronchial asthma in Egyptian children. Egypt J Immunol. 20:29–38.
2013.PubMed/NCBI
|
|
33
|
Corrigan CJ and Kay AB: Asthma. Role of
T-lymphocytes and lymphokines. Br Med Bull. 48:72–84. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maddur MS, Miossec P, Kaveri SV and Bayry
J: Th17 cells: Biology, pathogenesis of autoimmune and inflammatory
diseases and therapeutic strategies. Am J Pathol. 181:8–18. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palomares O: The role of regulatory T
cells in IgE-mediated food allergy. J Investig Allergol Clin
Immunol. 23:371–382. 2013.PubMed/NCBI
|
|
36
|
Ierodiakonou D, Postma DS, Koppelman GH,
Gerritsen J, ten Hacken NH, Timens W, Boezen HM and Vonk JM: TGF-β1
polymorphisms and asthma severity, airway inflammation, and
remodeling. J Allergy Clin Immunol. 131:582–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Halwani R, Al-Muhsen S, Al-Jahdali H and
Hamid Q: Role of transforming growth factor-β in airway remodeling
in asthma. Am J Respir Cell Mol Biol. 44:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kamato D, Burch ML, Piva TJ, Rezaei HB,
Rostam MA, Xu S, Zheng W, Little PJ and Osman N: Transforming
growth factor-beta signalling: Role and consequences of Smad linker
region phosphorylation. Cell Signal. 25:2017–2024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Yang Y, Zhang X, Xu S, He S, Huang
W and Roberts MS: Compound Astragalus and Salvia
miltiorrhiza extract inhibits cell invasion by modulating
transforming growth factor-beta/Smad in HepG2 cell. J Gastroenterol
Hepatol. 25:420–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McMillan SJ, Xanthou G and Lloyd CM:
Manipulation of allergen-induced airway remodeling by treatment
with anti-TGF-beta antibody: Effect on the Smad signaling pathway.
J Immunol. 174:5774–5780. 2005. View Article : Google Scholar : PubMed/NCBI
|




















