Introduction
Psoriasis is a common autoimmune disease affecting
~3% of the global population (1).
Psoriasis is characterized by the abnormal proliferation of
epidermal cells and the infiltration of inflammatory cells
(2,3). Patients with psoriasis frequently
suffer from disfigurement and complications, including painful
arthritis, cardiovascular disease and metabolic syndrome (4,5).
Although the pathophysiology of psoriasis remains unclear,
increasing evidence suggests that the imbalance between
pro-inflammatory cytokines, including tumor necrosis factor-α
(TNF-α), interleukin (IL)-22 and IL-17C, and anti-inflammatory
mediators, including IL-10, contributes to the underlying disease
etiology (6). Nuclear factor-κB
(NF-κB) (7) and signal transducers
and activators of transcription (STAT) (8) are thought to be the principal
effectors that produce a large number of pro-inflammatory
cytokines, including TNF-α, IL-22, interferon-γ (IFN-γ) and IL-1.
In addition, a previous study reported that the NF-κB and STAT
signaling pathways are involved in the development and progression
of psoriasis (9). Currently, few
effective therapeutic strategies exist, in part due to the high
recurrence rate of the disease. Furthermore, the majority of the
current anti-psoriatic drugs are associated with serious side
effects.
Traditional Chinese medicine (TCM), practiced in
China for centuries, is considered to be an alternative medical
system in Western countries (10,11).
According to TCM, psoriasis is characterized by three predominant
syndromes: Blood heat, blood stasis and blood dryness (12,13).
Different prescriptions of Chinese herbal medicine (CHM) with few
side effects are routinely prescribed in China (13,14).
For example, for the past few decades, the CHM formulation
‘psoriasis 1’, which comprises 13 Chinese herbs, has successfully
treated a large number of patients with psoriasis in hospitals in
China (15). The majority of
patients exhibit an improvement in skin lesions following
application of ‘psoriasis 1’, although the underlying molecular
mechanism of action this formulation in psoriasis is unknown
(16,17).
In the present study, HaCaT cells were stimulated
with TNF-α and transfected with a lentiviral vitamin D receptor
(VDR) RNA interference (RNAi) expression vector. The effect of
‘psoriasis 1’ on psoriasis with or without Tripterygium
wilfordii polyglycoside (TWP) was investigated. The impact of
VDR inhibition on the expression levels of cytokines, and NF-кB and
STAT signaling pathway components was additionally observed. It was
demonstrated that ‘psoriasis 1’ and combined with the inhibition of
VDR decreased the concentrations of TNF-α, IFN-γ, IL-22, IL-17C,
IL-1β and IL-4, and increased the concentration of
25-hydroxyvitamin D3 (25HVD3). Furthermore, this treatment
downregulated the expression levels of NF-κB, phosphorylated
(p)-NF-κB, inhibitor of NF-κB (IKK), p-IKK, STAT3, p-STAT3, STAT4
and p-STAT4, and upregulated the expression of VDR in TNF-α-induced
HaCaT cells. It was observed that ‘psoriasis 1’ and silencing of
VDR suppressed the inflammatory response, and the activation of the
NF-κB and STAT signaling pathways. Therefore, it was hypothesized
that ‘psoriasis 1’ may alleviate psoriasis-like skin inflammation
by inhibiting the VDR-mediated nuclear NF-κB and STAT signaling
pathways.
Materials and methods
Components of the ‘psoriasis 1’
formulation
‘Psoriasis 1’ was provided by The First Affiliated
Hospital of Guangzhou University (Guangzhou, China), and was
comprised of rhizoma Smilacis glabrae (30 g), Folium
isatidis (30 g), radix isatidis (15 g), Angelica
sinensis (15 g), Hedyotis diffusa (15 g), Sichuan lovage
rhizome (12 g), plantain herb (12 g), fructus kochiae (12
g), Chinese lobelia (15 g), nidus vespae, rhizoma alismatis
(12 g), cortex dictamni (12 g) and radix glycyrrhizae
(6 g). In addition, TWP (Fujian Huitian Biological Pharmaceutical
Co., Ltd., Sanming, China; 10 mg/tablet) was used as a positive
control.
Preparation of the serum containing
‘psoriasis 1’
Specific pathogen free level Sprague-Dawley male
rats were purchased and raised at Guangzhou University of Chinese
Medicine, Guangzhou, China (license no. SCXK 20130020; animal
qualified no. 44005900002507). The rats were maintained in
environmentally controlled rooms at 20–25°C with a relative
humidity of 55% and 12–15 air changes/h, under a 12-h light-dark
cycle (artificial lighting between 8:00 am and 8:00 pm). The rats
were fed with standard laboratory food and water ad libitum.
All animal experiments were approved by the International Committee
on Laboratory Animals of Jingmen First People's Hospital (Jingmen,
China).
For the treatments, 18 SD male rats with SPF level
weighing between 220 and 260 g were randomly divided into a
negative control group (NC group, treated with an equal volume of
normal saline, n=3), low dosage Chinese medicine group (LD group;
3.09 g/kg; n=3), medium dosage Chinese medicine group (MD group;
4.64 g/kg; n=3), high dosage Chinese medicine group (HD group; 6.17
g/kg; n=3), Western medicine control group (TWP group; 0.4 mg/kg;
n=3), and a combined curative group with medium dosage and TWP
(MD+TWP group; 0.4 mg/kg, n=3). Animals were treated daily at 9:00
am by gavage, once a day for 7 consecutive days. Prior to
administration, animals were marked, fasted overnight and weighed.
Following the final drug treatment, all animals were sacrificed and
serum was collected from the rat hearts.
Cell culture
The immortalized human keratinocyte (HaCaT) and 293T
cell lines were obtained from the Chinese Academy of Sciences
(Shanghai, China), and were maintained in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified atmosphere of 5%
CO2. For construction of the cellular model of
psoriasis, HaCaT cells were treated with TNF-a (10 ng/ml) for 48
h.
RNA interference (RNAi)
Negative control (NC) and VDR siRNAs were
synthesized by Shanghai GenePharma, Co., Ltd. (Shanghai, China) and
the sequences are as follows: NC, 5′-GUACCGCACGUCAUUCGUAUC-3′
(forward), 5′-UACGAAUGACGUGCGGUACGU-3′ (reverse); VDR siRNA:
5′-CCCUUCAAUGGAGAUUGCCGCAUCA-3′ (forward),
5′-UGAUGCGGCAAUCUCCAUUGAAGGG-3′ (reverse). HaCaT cells
(2×104) were seeded into each well of a 6-well plate in
2 ml of Opti-MEM I reduced serum medium (Thermo Fisher Scientific,
Inc.) overnight at 37°C in a humidified atmosphere of 5%
CO2. Next day, cells were transfected with 50 µM NC
siRNA and 50 µM VDR siRNAs for 48 h using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol.
Lentiviral construction and
infection
LV3-NC (Lot number: E11AZ), VDR-Homo-544 (Lot
number: 160502DZ), VDR-Homo-433 (Lot number: 160510IZ) and
VDR-Homo-775 (Lot number: 160502EZ) were synthesized by Suzhou
GenePharma Co., Ltd. (Suzhou, China). Briefly, VDR full-length cDNA
was amplified by quantitative polymerase chain reaction (qPCR) from
293T cells and were inserted in to a lentiviral vector and
identified by sequencing as described in previous studies (18,19).
These lentiviral vectors were packaged in 293T cells
(1×107 in each 10-cm culture dish) by co-transfection
with packaging vectors (pCMV-VSVG, pMDLg/pRRE and pRSV-REV) to
produce the viral particles for lentiviral transduction.
Subsequently, the purification was performed using
ultracentrifugation (25,000 × g for 2 h at 4°C). HaCaT cells
(1×105 cells/well) were seeded in 6-well plates and
transduced with lentiviruses supplemented with 8 mg/ml polybrene
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). G418 (Life
Technologies; Thermo Fisher Scientific, Inc.; 0.8 mg/ml) was used
to select the stable expression cell lines.
Infection [multiplicity of infection
(MOI)] detection
HaCaT cells (1×104 cells/well) were
seeded in 96-well plates at 37°C in a humidified atmosphere of 5%
CO2. The cells were transduced the following day with
lentiviruses at 37°C, and were divided into three groups: 3.75 µl
viral suspension 4×108 transducing units (TU)/ml+300 µl
completely cultured with polybrene (total MOI=50); 7.5 µl viral
suspension (4×108 TU/ml)+300 µl completely cultured with
polybrene (total MOI=100); and 15 µl viral suspension
(4×108 TU/ml)+300 µl completely cultured with polybrene
(total MOI=200). Following 24 h, the medium was replaced with 100
µl complete culture medium at 37°C. After 48 h, the cell
transfection efficiency was observed under an inverted fluorescence
microscope (Nikon Corporation, Tokyo, Japan; ×40 magnification),
and was detected by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR; see below) to determine the virus
transfection MOI.
ELISA analysis
The concentrations of TNF-α, IFN-γ, IL-22, IL-17C,
IL-1β, IL-4 and 25HVD3 in the HaCaT culture medium were determined
using ELISA kits according to the manufacturer's protocol. The
ELISA kits were IL-1β ELISA kit (EK0392), TNF-α ELISA kit (k
EK0525), IL-4 ELISA kit (EK0404), IL-17C ELISA kit (EK0789), IL-22
ELISA kit (EK0933), INF-γ ELISA kit (EK0373; all from Wuhan Boster
Biological Technology, Ltd., Wuhan, China), and 25HVD3 ELISA kit
(CSB-E08097h; Cusabio Technology LLC, Houston, TX, USA). A 96-well
plate was coated with monoclonal anti-TNF-α, anti-IFN-γ,
anti-IL-22, anti-IL-17C, anti-IL-1β, anti-IL-4 and anti-25HVD3. The
captured cytokines were detected using a secondary antibody
conjugated to horseradish peroxidase. The absorbance was determined
at 450 nm using a microtiter reader (Multiskan Go microplate
reader; Thermo Fisher Scientific, Inc.). The concentrations of
cytokines were determined by comparing the absorbance values with
those of the standards.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. First-strand cDNAs were reverse
transcribed from total RNA using a RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.). For cDNA synthesis
with random primers, the thermocycling conditions were: Incubation
for 5 min at 25°C followed by 60 min at 42°C. The expression levels
of cytokines were detected by qPCR using SYBR Premix Ex Taq (Takara
Bio, Inc., Otsu, Japan). The reaction system was performed in a
volume of 20 µl and the thermocycling conditions were as follows:
Initial denaturation at 95°C for 30 sec; 40 cycles of 95°C for 5
sec, 60°C for 34 sec. The primers for GAPDH, TNF-α, IFN-γ, IL-22,
IL-17C, IL-1β, IL-4 and 25HVD3 were obtained from Sangon, China.
The primers of the genes of interest and GAPDH (internal loading
control) are presented in Table I.
The data were analyzed using 2−∆∆Cq method (20).
 | Table I.Specific primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Specific primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Name | Sequence,
5′→3′ | Product length,
bp |
|---|
| GAPDH F |
TGTTCGTCATGGGTGTGAAC | 154 |
| GAPDH R |
ATGGCATGGACTGTGGTCAT |
|
| VDR F |
GTGGACATCGGCATGATGAAG | 181 |
| VDR R |
GGTCGTAGGTCTTATGGTGGG |
|
| IKK F |
ATGAAGAAGTTGAACCATGCCA | 110 |
| IKK R |
CCTCCAGAACAGTATTCCATTGC |
|
| NF-κB F |
ATGTGGAGATCATTGAGCAGC | 151 |
| NF-κB R |
CCTGGTCCTGTGTAGCCATT |
|
| STAT3 F |
ATCACGCCTTCTACAGACTGC | 176 |
| STAT3 R |
CATCCTGGAGATTCTCTACCACT |
|
| STAT4 F |
TGTTGGCCCAATGGATTGAAA | 119 |
| STAT4 R |
GGAAACACGACCTAACTGTTCAT |
|
Western blotting
Total proteins were extracted using 250 µl
radioimmunoprecipitation assay buffer (cat no. P0013; Beyotime
Institute of Biotechnology, Shanghai, China) at 4°C for 30 min.
Nuclear protein was extracted with NE-PER Nuclear Extraction
Reagents (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The protein concentrations were determined
by using a bicinchoninic acid (BCA) Protein Assay kit (cat. no.
23225; Thermo Fisher Scientific, Inc.). Total proteins (50 µg) were
separated by 8% SDS-PAGE and transferred to nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA). Subsequent to the
nitrocellulose membranes being blocked with 5% skimmed milk in
TBS-Tween 20 solution (100 mmol/l Tris-Cl, pH 7.5; 150 mmol/l NaCl;
and 0.1% Tween 20), the primary antibodies were incubated overnight
at 4°C, followed by incubation with horseradish
peroxidase-conjugated secondary antibodies (goat anti-mouse;
dilution, 1:2,000; cat no. SC-2005, and goat anti-rabbit; dilution,
1:2,000; cat no. SC-2004) at room temperature for 2 h (both Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The results were
detected by using an enhanced chemiluminescence (ECL) detection kit
(cat. no. 345818; EMD Millipore). The primary antibodies used in
the present study included anti-GAPDH antibody (1:2,000; cat. no.
sc-47724, Santa Cruz Biotechnology, Inc.), anti-NF-κB essential
modulator antibody (1:2,000; cat. no. 8242, Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-p-STAT3 antibody (1:100;
cat. no. sc-81523, Santa Cruz Biotechnology, Inc.), anti-STAT3
antibody (1:1,000; Abcam, Cambridge, UK; cat. no. ab5073),
anti-p-STAT4 antibody (1:100; cat. no. sc-28296, Santa Cruz
Biotechnology, Inc.), anti-STAT4 antibody (1:1,000; Abcam; cat. no.
ab68156), anti-VDR antibody (1:2,500; Abcam; cat. no. ab134826),
anti-NF-κB antibody (1:2,500; Abcam; cat. no. ab131493),
anti-p-NF-κB antibody (1:1,000; Abcam; cat. no. ab28849),
anti-p-IKK antibody (1:1,000; Abcam; cat. no. ab38515) and anti-IKK
antibody (1:1,000; cat. no. 2682; Cell Signaling Technology,
Inc.).
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation from at least three independent experiments.
One-way analysis of variance was used to analyze the differences
between the groups followed by the Dunnett test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. SPSS 18.0 (SPSS, Inc., Chicago, IL, USA)
and GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA)
software was used for data analysis.
Results
‘Psoriasis 1’ downregulates the
expression of TNF-α, IFN-γ, IL-22, IL-17C, IL-1β and IL-4, and
upregulates the expression of 25HVD3 in TNF-α-induced psoriatic
models
TNF-α is one of the primary cytokines involved in
psoriasis-like inflammation. To investigate the effects of
‘psoriasis 1’ on psoriasis, a HaCaT cell psoriasis model was
established, wherein cells were treated with TNF-α for 1 h. As
presented in Fig. 1, the
concentrations of TNF-α, IFN-γ, IL-22, IL-17C, IL-1β and IL-4 were
increased, and the concentration of 25HVD3 was decreased in
TNF-α-induced psoriasis-like cells compared with the normal control
(NC) group. In addition, the ELISA results demonstrated that the
concentrations of TNF-α (Fig. 1A),
IFN-γ (Fig. 1C), IL-22 (Fig. 1D), IL-17C (Fig. 1E), IL-1β (Fig. 1F) and IL-4 (Fig. 1G) were downregulated, and the
concentration of 25HVD3 (Fig. 1B)
was upregulated in the LD, MD, HD, TWP and MD+TWP groups compared
with the TNF-α group. These results suggested that ‘psoriasis 1’
may inhibit the inflammatory effects observed in TNF-α-induced
psoriatic models.
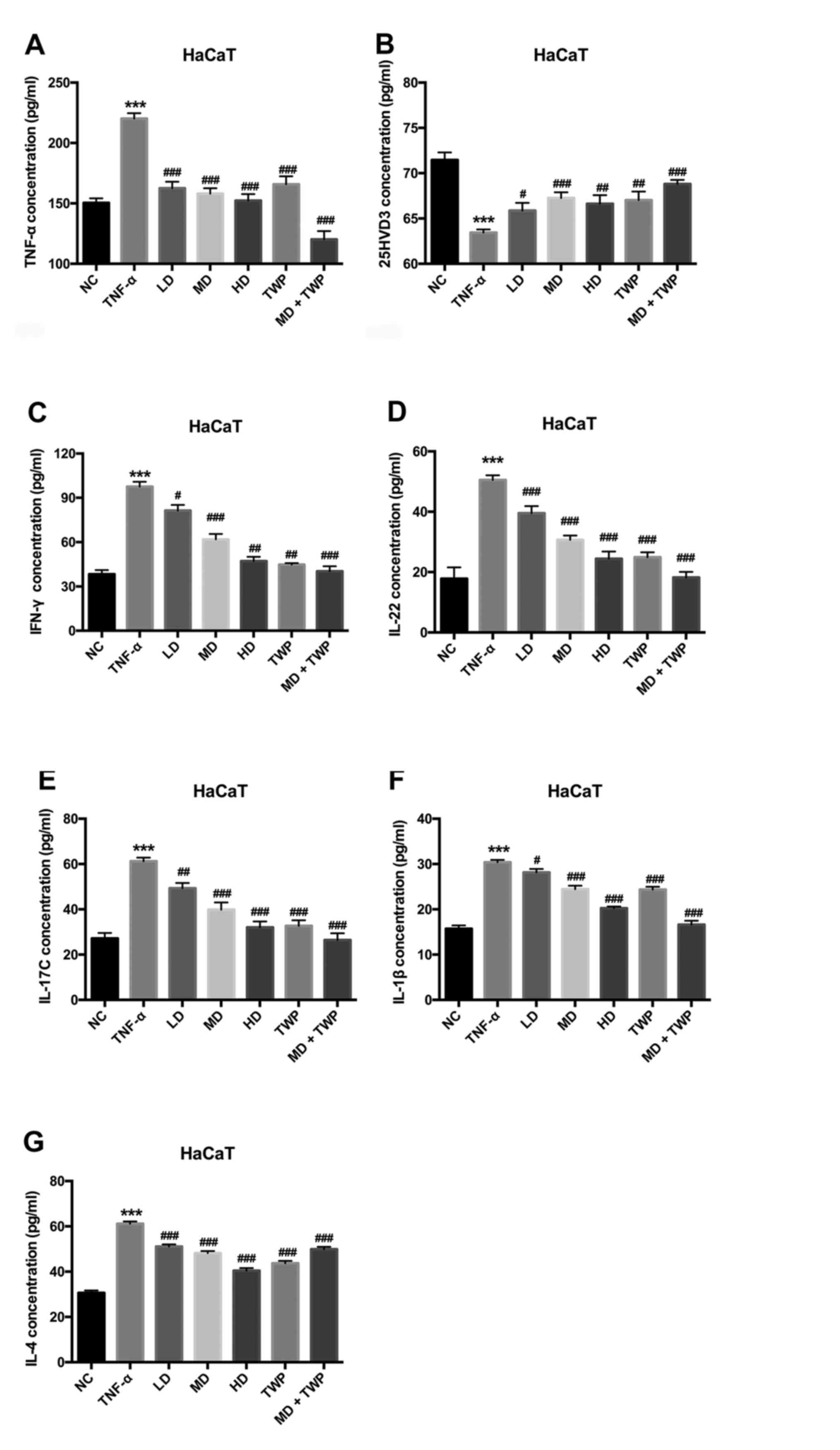 | Figure 1.‘Psoriasis 1’ downregulates the
expression of TNF-α, IFN-γ, IL-22, IL-17C, IL-1β and IL-4, and
upregulates the expression of 25HVD3 in TNF-α-induced psoriatic
models. The concentrations of (A) TNF-α, (B) 25HVD3, (C) IFN-γ, (D)
IL-22, (E) IL-17C, (F) IL-1β and (G) IL-4 were detected by ELISA.
***P<0.001 vs. NC group; #P<0.05,
##P<0.01, ###P<0.001 vs. TNF-α group.
TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; IL,
interleukin; 25HVD3, 25-hydroxyvitamin D3; LD, low dose; MD, medium
dose; HD, high dose; TWP, Tripterygium wilfordii
polyglycoside; NC, normal control. |
‘Psoriasis 1’ downregulates NF-κB,
p-NF-κB, IKK, p-IKK, STAT3, p-STAT3, STAT4 and p-STAT4 expression,
and upregulates VDR expression in TNF-α-induced psoriatic
models
The NF-κB and STAT signaling pathways regulate gene
expression by responding to certain cellular stimulants. In
addition, these two pathways are reported to be involved in the
development of psoriasis (21,22).
A model of psoriasis was established by treating HaCaT cells with
TNF-α. The effects of ‘psoriasis 1’ on the NF-κB and STAT signaling
pathways were subsequently investigated. The mRNA and protein
expression levels of IKK, VDR, STAT3, STAT4 and nuclear NF-κB were
analyzed by RT-qPCR and western blotting, respectively. As
presented in Fig. 2, the mRNA
expression levels of IKK, nuclear NF-κB, STAT3 and STAT4 were
significantly upregulated, and the mRNA expression of VDR was
significantly downregulated in TNF-α induced psoriasis-like cells
compared with the NC group. Furthermore, the results indicated that
the mRNA expression levels of IKK (Fig. 2A), nuclear NF-κB (Fig. 2C), STAT3 (Fig. 2D) and STAT4 (Fig. 2E) were significantly decreased, and
the mRNA expression level of VDR (Fig.
2B) was significantly increased in the LD, MD, HD, TWP and the
combined curative (MD+TWP) groups, compared with the TNF-α group.
These results suggested that the NF-κB and STAT signaling pathways
were activated by ‘psoriasis 1’ in the psoriasis-like cells.
Consistent with these data, western blot analysis demonstrated that
‘psoriasis 1’ downregulated the expression of NF-κB, p-NF-κB, IKK,
p-IKK, STAT3, p-STAT3, STAT4 and p-STAT4, and upregulated the
expression of VDR in TNF-α-induced psoriatic models (Fig. 2F).
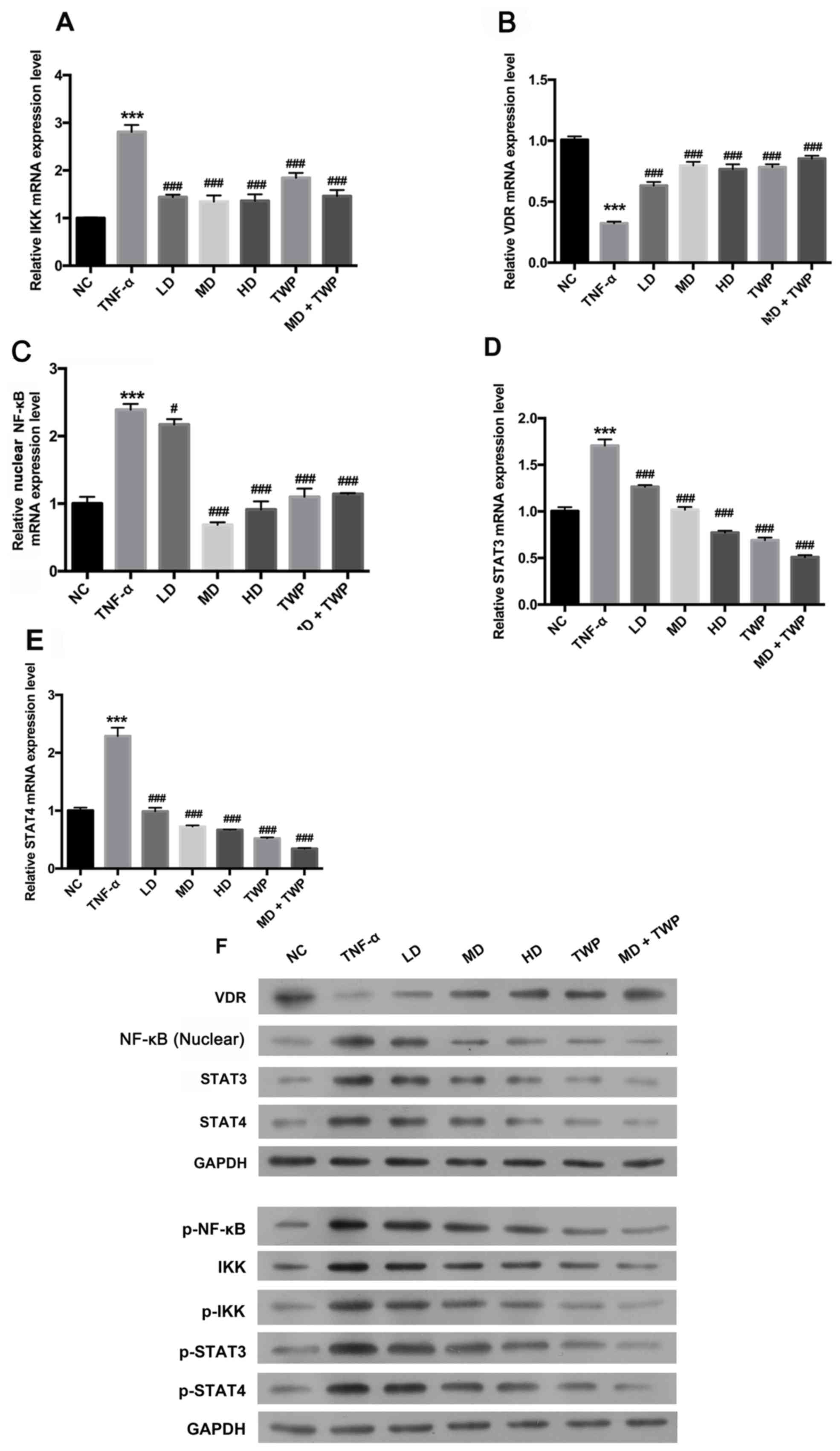 | Figure 2.‘Psoriasis 1’ downregulates NF-кB,
p-NF-кB, IKK, p-IKK, STAT3, p-STAT3, STAT4 and p-STAT4 expression,
and upregulates VDR expression in TNF-α-induced psoriatic models.
The mRNA expression levels of (A) IKK, (B) VDR, (C) nuclear NF-κB,
(D) STAT3 and (E) STAT4 were analyzed by reverse
transcription-quantitative polymerase chain reaction assay. (F)
Total proteins were extracted using radioimmunoprecipitation assay
buffer; nuclear protein was extracted using NE-PER Nuclear
Extraction Reagents. Western blotting was used to detect the
expression levels of NF-κB and p-NF-κB in the nuclei, IKK, p-IKK,
VDR, STAT3, p-STAT3, STAT4 and p-STAT4. GAPDH was used as a loading
control. ***P<0.001 vs. NC group; #P<0.05,
###P<0.001 vs. TNF-α group. TNF-α, tumor necrosis
factor-α; NF-κB, nuclear factor-κB; p, phosphorylated; IKK,
inhibitor of NF-κB; VDR, vitamin D receptor; STAT, signal
transducer and activator of transcription; NC, normal control; LD,
low dose; MD, medium dose; HD, high dose; TWP, Tripterygium
wilfordii polyglycoside. |
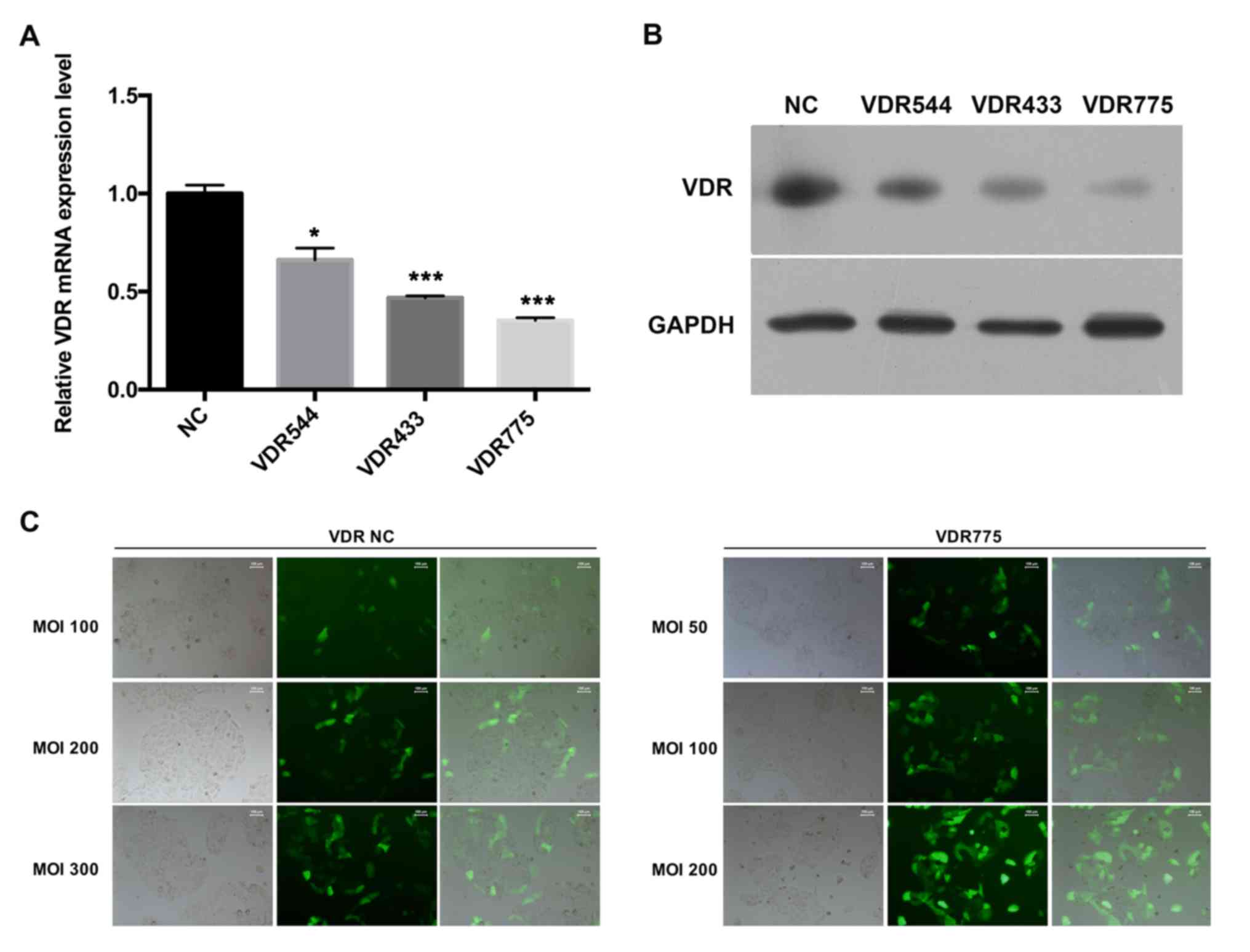
Silencing the VDR via lentiviral RNAi
expression vector in HaCaT cells
25HVD3 modulates the gene expression levels of
NF-κB, STAT3 and STAT4 by binding to the VDR (23–25).
To investigate whether the inhibitory effects of ‘psoriasis 1’ on
NF-κB, STAT3 and STAT4 are dependent on VDR expression, VDR
expression was knocked down using a lentiviral RNAi expression
vector, and the expression levels of VDR were detected using
RT-qPCR and western blot analyses. The results indicated that VDR
was inhibited in HaCaT cells transfected with the lentiviral RNAi
expression vector compared with the NC group, and VDR775 exhibited
the greatest knockdown effect (Fig. 3A
and B). The infectivity (MOI) of VDR775 was detected. As
observed in the control group, the cell transfection efficiency was
~10%, 20–30 and 50–60% when the MOI was 100, 200 and 300,
respectively, and the cells were in good condition. Regarding the
transfection efficiency of the VDR755 lentivirus: At an MOI of 50,
the cell transfection efficiency was ~30%; at an MOI of 100, the
cell transfection efficiency was 50–60%, and the cells grew well;
and at an MOI of 200, the cell transfection efficiency was ~80%
(the transfection efficiency was detected by RT-qPCR assay; data
not shown), although the state of the cells was poor and there was
evidence of pycnosis, which is not conducive to the selection of
stable cells. Therefore, HaCaT cells with stably silenced VDR at an
MOI of 100 were used for the subsequent experiments (Fig. 3C).
Silencing VDR decreases the expression
levels of TNF-α, IFN-γ, IL-22, IL-17C, IL-1β and IL-4, and
increases the expression of 25HVD3 in TNF-α-induced HaCaT
cells
To investigate the impact of VDR expression on
cytokine levels, cytokine levels were measured in HaCaT cells with
stably silenced VDR. The concentrations of TNF-α, 25HVD3, IFN-γ,
IL-22, IL-17C, IL-1β and IL-4 were detected by ELISA (Fig. 4). The results revealed that the
concentrations of TNF-α (Fig. 4A),
IFN-γ (Fig. 4C), IL-22 (Fig. 4D), IL-17C (Fig. 4E), IL-1β (Fig. 4F) and IL-4 (Fig. 4G) were increased, and the
concentration of 25HVD3 (Fig. 4B)
was decreased in HaCaT cells transfected with VDR RNAi compared
with NC. Therefore, it may be hypothesized that inhibiting VDR
expression decreased TNF-α, IFN-γ, IL-22, IL-17C, IL-1β and IL-4
expression levels, and increased the 25HVD3 level in TNF-α-induced
HaCaT cells.
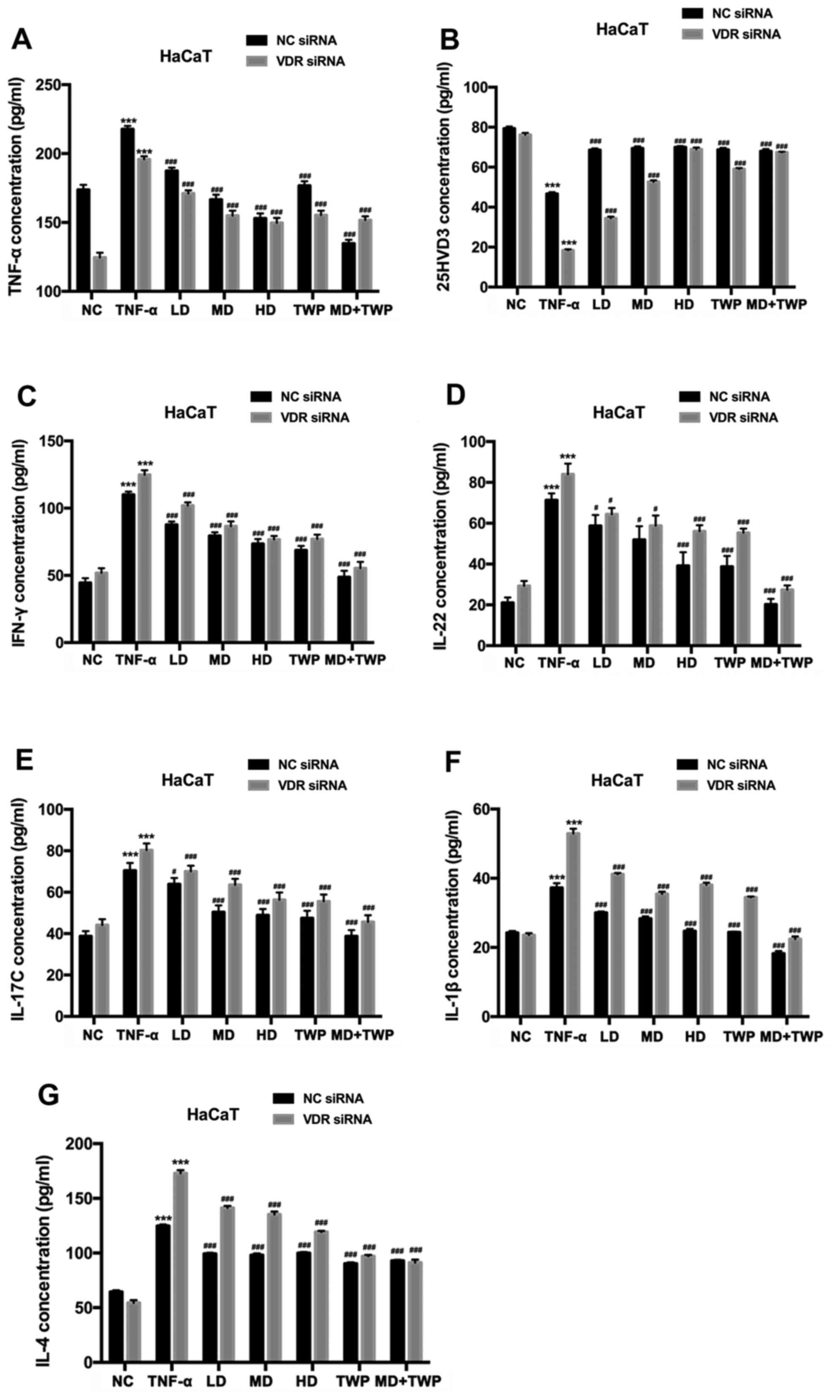 | Figure 4.Silencing VDR decreases TNF-α, IFN-γ,
IL-22, IL-17C, IL-1β and IL-4 expression levels, and increases the
25HVD3 expression level in TNF-α-induced HaCaT cells with stably
silenced VDR. The concentrations of (A) TNF-α, (B) 25HVD3, (C)
IFN-γ, (D) IL-22, (E) IL-17C, (F) IL-1β and (G) IL-4 were detected
by ELISA. ***P<0.001 vs. NC group; #P<0.05,
###P<0.001 vs. TNF-α group. VDR, vitamin D receptor;
TNF-α, tumor necrosis factor; IFN-γ, interferon-γ; IL, interleukin;
25HVD3, 25-hydroxyvitamin D3; NC, normal control; LD, low dose; MD,
medium dose; HD, high dose; TWP, Tripterygium wilfordii
polyglycoside; siRNA, small interfering RNA. |
Silencing VDR downregulates the
expression of NF-κB, p-NF-κB, IKK, p-IKK, STAT3, p-STAT3, STAT4 and
p-STAT4, and upregulates the expression of VDR in TNF-α-induced
HaCaT cells
To demonstrate the impact of VDR on the nuclear
NF-κB and STAT signaling pathways, the expression levels of IKK,
VDR, STAT3, STAT4 and NF-κB (nuclear) were measured in HaCaT cells
with stably silenced VDR using RT-qPCR and western blot analysis
(Fig. 5). The results indicated
that the mRNA expression levels of NF-кB in the nuclei (Fig. 5A), IKK (Fig. 5B), STAT3 (Fig. 5D) and STAT4 (Fig. 5E) were significantly increased, and
VDR (Fig. 5C) was significantly
decreased in HaCaT cells transfected with stably silenced VDR
compared with NC. In addition, the results of the present study
indicated that the inhibition of VDR upregulated the expression of
NF-κB, p-NF-κB, IKK, p-IKK, STAT3, p-STAT3, STAT4 and p-STAT4, and
downregulated VDR expression in TNF-α-induced HaCaT cells (Fig. 5F and G).
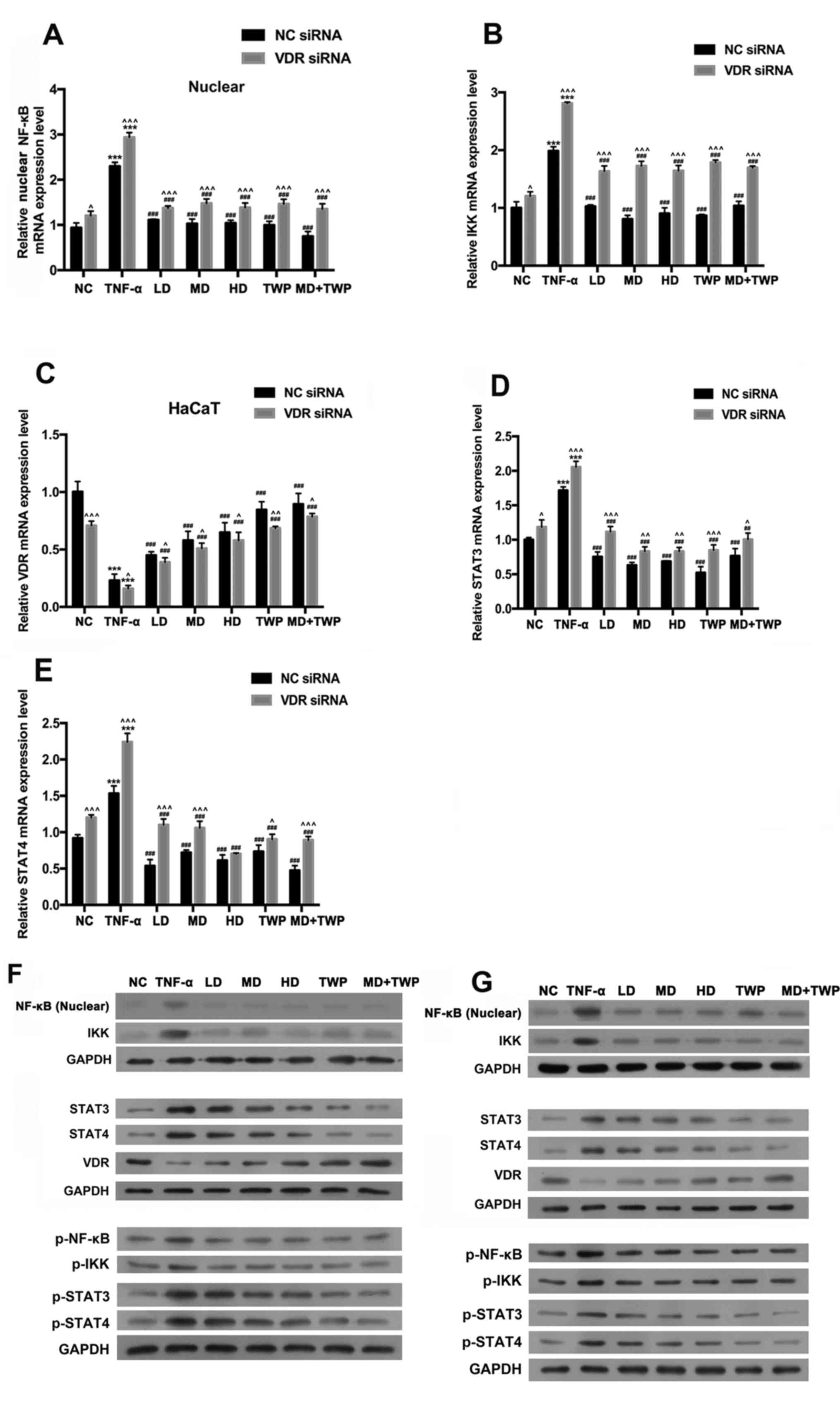 | Figure 5.Silencing of VDR upregulates NF-κB,
p-NF-κB, IKK, p-IKK, STAT3, p-STAT3, STAT4 and p-STAT4 expression
levels, and downregulates VDR expression in TNF-α-induced HaCaT
cells. The reverse transcription-quantitative polymerase chain
reaction was used to analyze the mRNA expression levels of (A)
NF-κB, (B) IKK, (C) VDR (D) STAT3 and (E) STAT4. Total proteins
were extracted using radioimmunoprecipitation assay buffer; Nuclear
protein was extracted using NE-PER Nuclear Extraction Reagents.
Western blotting was used to detect the expression levels of NF-κB
and p-NF-κB in the nuclei, and IKK, p-IKK, VDR, STAT3, p-STAT3,
STAT4 and p-STAT4, in the (F) empty vector NC group and (G) HaCaT
cells with stably silenced VDR. GAPDH was used as a loading
control. ***P<0.001 vs. NC group; ##P<0.01,
###P<0.001 vs. TNF-α group; ^P<0.05,
^^P<0.01, ^^^P<0.001 vs. respective NC
siRNA group. TNF-α, tumor necrosis factor-α; NF-κB, nuclear
factor-κB; p, phosphorylated; IKK, inhibitor of NF-κB; VDR, vitamin
D receptor; STAT, signal transducer and activator of transcription;
NC, normal control; LD, low dose; MD, medium dose; HD, high dose;
TWP, Tripterygium wilfordii polyglycoside; siRNA, small
interfering RNA. |
Discussion
Psoriasis is an inflammatory skin disease
characterized by a significant elevation in the concentration of
cytokines, including TNF-α (26,27).
The annual cost of psoriasis in China alone is estimated to be over
<1 billions of yuan, and the socioeconomic burden of psoriasis
has an important influence on the Chinese healthcare system
(28). However, because the
underlying mechanism remains undetermined, current treatments have
not yet fully met the needs of patients. TNF-α is a critical
pro-inflammatory cytokine which is produced by various cell types,
including macrophages, monocytes and activated T cells (29). It is now widely accepted that TNF-α
is a principal inducer of psoriasis and a number of drugs targeting
TNF-α are currently being assessed for the treatment of psoriasis
(30,31). TNF-α mediates the gene expression
of numerous cytokines, including IL-1, IL-6 and IL-22 (32), by activating the NF-κB and STAT
signaling pathways.
TCM has been developed and applied by the Chinese
over a long period of time, and includes acupuncture, Chinese
traumatology and CHM; CHM is considered to be the most popular type
of TCM worldwide (33,34). It has been reported that various
CHM formulations have been used for the treatment of a number of
diseases, including gastrointestinal disease, diabetes mellitus and
psoriasis (28,35,36).
In China, numerous patients with psoriasis accept treatment with
CHM, and ~2% of patients receive similar therapy in the USA
(37). Although the effects of
various CHM formulations are positive in patients with psoriasis,
the underlying mechanism of these therapies remains unknown.
For decades, ‘psoriasis 1’, a formulation
originating in CHM, has been used to effectively treat patients
with psoriasis in China (38,39).
In the present study, it was identified that the concentrations of
TNF-α, IFN-γ, IL-22, IL-17C, IL-1β and IL-4 were increased, and the
concentration of 25HVD3 was decreased in TNF-α-induced
psoriasis-like cells compared with the NC group; the concentrations
of TNF-α, IFN-γ, IL-22, IL-17C, IL-1β and IL-4 were decreased, and
the concentration of 25HVD3 was increased in the LD, MD, HD, TWP
and combined curative (MD+TWP) groups, compared with the TNF-α
group. The data indicated that TNF-α promoted inflammation, and
‘psoriasis 1’ was able to block these inflammatory effects in
TNF-α-induced psoriatic models.
Previous work demonstrated that the NF-κB and STAT
signaling pathways are involved in the gene expression of certain
cytokines during an inflammatory response. TNF-α was used to
stimulate the formation of the psoriasis cell model. TNF-α
activates the NF-κB signaling pathway, and increases p-IKK and
p-NF-кB expression levels; the activated NF-κB is transferred to
the nucleus, promotes the expression levels of STAT4 and STAT3, and
activates p-STAT3 and p-STAT4 (40–43).
Studies have demonstrated that VDR may form complexes with NF-κB
subunits which exist stably in the cytoplasm, and inhibits the
activation of the NF-κB signaling pathway (44,45).
Therefore, the expression level of VDR was reduced in the psoriasis
cell model, and silencing of VDR with small interfering RNA was
able to further activate the NF-κB signaling pathway, promoting
STAT4 and STAT3 expression. Furthermore, ‘psoriasis 1’ was able to
partially inhibit the activation of the VDR-mediated NF-κB
signaling pathway. Alternatively, STAT3, STAT4 and NF-κB may be
considered to be parallel signaling pathways, and the psoriasis
cell model and VDR intervention was able to increase inflammatory
factors, including IFN-γ, IL-22, IL-17C, IL-1β and IL-4, in
addition to activating the NF-κB/STAT signaling pathway.
The present study examined whether ‘psoriasis 1’
induced the downregulation of cytokines associated with the NF-κΒ
and STAT signalling pathways. It was identified that the expression
levels of NF-κB, p-NF-κB, IKK, p-IKK, STAT3, p-STAT3, STAT4 and
p-STAT4 were significantly upregulated, and VDR was significantly
downregulated in TNF-α-induced psoriasis-like cells compared with
the NC group, suggesting that the nuclear NF-κB and STAT signaling
pathways were activated in the psoriasis-like cell model.
Furthermore, it was demonstrated that ‘psoriasis 1’ downregulated
NF-κB, p-NF-κB, IKK, p-IKK, STAT3, p-STAT3, STAT4 and p-STAT4
expression, and upregulated VDR expression in TNF-α-induced
psoriatic models. Lentiviral VDR RNAi expression vectors were
transduced into model HaCaT cells, to eventually construct cells
with stably silenced VDR expression in vitro. The effect on
these cells of ‘psoriasis 1’ with or without TWP was investigated,
in addition to the effect of silencing VDR expression. Silencing of
VDR increased the concentrations of, IFN-γ, IL-22, IL-17C, IL-1β,
and IL-4, and decreased the concentration of TNF-α and 25HVD3. In
addition, silencing of VDR upregulated the expression levels of
NF-κB, p-NF-κB, IKK, p-IKK, STAT3, p-STAT3, STAT4 and p-STAT4, and
downregulated the expression level of VDR in TNF-α-induced HaCaT
cells. It was demonstrated that ‘psoriasis 1’ and silencing of VDR
suppressed the inflammatory reaction, and the activation of the
NF-κB and STAT signaling pathways. Therefore, it was concluded that
‘psoriasis 1’ alleviated psoriasis-like inflammation by inhibiting
the VDR-mediated nuclear NF-κB and STAT signaling pathways.
In conclusion, the results of the present study
demonstrated that ‘psoriasis 1’ suppressed the inflammatory
reaction, and the activation of the NF-κB and STAT signaling
pathways through VDR, suggesting that ‘psoriasis 1’ inhibited
psoriasis-like skin inflammation by suppressing the VDR-mediated
nuclear NF-κB and STAT signaling pathways.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81573980, 30973754
and 30672699) and the Natural Science Foundation of Hubei (grant
no. 2018CFB289).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS, YG, XY and XC designed the experiments. WS, YG,
YY, JY, ZZ, YC, YL and XP performed the experiments and conducted
data analysis.
Ethics approval and consent to
participate
All animal experiments were approved by the
International Committee on Laboratory Animals of Jingmen First
People's Hospital (Jingmen, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nickoloff BJ and Nestle FO: Recent
insights into the immunopathogenesis of psoriasis provide new
therapeutic opportunities. J Clin Invest. 113:1664–1675. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mak RK, Hundhausen C and Nestle FO:
Progress in understanding the immunopathogenesis of psoriasis.
Actas Dermosifiliogr. 100 Suppl 2:S2–S13. 2009. View Article : Google Scholar
|
|
3
|
Di Cesare A, Di Meglio P and Nestle FO:
The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J
Invest Dermatol. 129:1339–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elder JT, Bruce AT, Gudjonsson JE,
Johnston A, Stuart PE, Tejasvi T, Voorhees JJ, Abecasis GR and Nair
RP: Molecular dissection of psoriasis: Integrating genetics and
biology. J Invest Dermatol. 130:1213–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li R, Wang J, Wang X, Zhou J, Wang M, Ma H
and Xiao S: Increased βTrCP are associated with imiquimod-induced
psoriasis-like skin inflammation in mice via NF-κB signaling
pathway. Gene. 592:164–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuruta D: NF-kappaB links keratinocytes
and lymphocytes in the pathogenesis of psoriasis. Recent Pat
Inflamm Allergy Drug Discov. 3:40–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivanenkov YA, Balakin KV and Lavrovsky Y:
Small molecule inhibitors of NF-kB and JAK/STAT signal transduction
pathways as promising anti-inflammatory therapeutics. Mini Rev Med
Chem. 11:55–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan Y, Mao R and Yang J: NF-κB and STAT3
signaling pathways collaboratively link inflammation to cancer.
Protein Cell. 4:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tse TW: Use of common Chinese herbs in the
treatment of psoriasis. Clin Exp Dermatol. 28:469–475. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YK, Yen HR, Wong WR, Yang SH and Pang
JH: Successful treatment of pediatric psoriasis with Indigo
naturalis composite ointment. Pediatr Dermatol. 23:507–510. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang GZ, Wang JS, Wang P, Jiang CY, Deng
BX, Li P, Zhao YM, Liu WL, Qu X, Chen WW, et al: Distribution and
development of the TCM syndromes in psoriasis vulgaris. J Tradit
Chin Med. 29:195–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu C, Deng J, Li L, Wang D and Li G:
Application of metabolomics on diagnosis and treatment of patients
with psoriasis in traditional Chinese medicine. Biochim Biophys
Acta. 1844:280–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu CJ, Yu JJ and Deng JW: Disease-syndrome
combination clinical study of psoriasis: Present status,
advantages, and prospects. Chin J Integr Med. 18:166–171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu JJ, Zhang CS, Zhang AL, May B, Xue CC
and Lu C: Add-on effect of chinese herbal medicine bath to
phototherapy for psoriasis vulgaris: A systematic review. Evid
Based Complement Alternat Med. 2013:6730782013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu L, Xuan M, Yan Y, Li G, Zhou L, Wen Z
and Lu C: A statistical analysis plan for the efficiency and safety
of Chinese herbal medicine used concurrently with topical therapy
for psoriasis vulgaris. Trials. 17:4822016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tse WP, Che CT, Liu K and Lin ZX:
Evaluation of the anti-proliferative properties of selected
psoriasis-treating Chinese medicines on cultured HaCaT cells. J
Ethnopharmacol. 108:133–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang L, Lai YK, Zhang JF, Chan CY, Lu G,
Lin MC, He ML, Li JC and Kung HF: Transactivation of the TIEG1
confers growth inhibition of transforming growth
factor-β-susceptible hepatocellular carcinoma cells. World J
Gastroenterol. 18:2035–2042. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong L, Wang F, Yin X, Chen L, Li G, Lin
F, Ni W, Wu J, Jin R and Jiang L: Overexpression of S100P promotes
colorectal cancer metastasis and decreases chemosensitivity to 5-FU
in vitro. Mol Cell Biochem. 389:257–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hara-Chikuma M, Satooka H, Watanabe S,
Honda T, Miyachi Y, Watanabe T and Verkman AS: Aquaporin-3-mediated
hydrogen peroxide transport is required for NF-κB signalling in
keratinocytes and development of psoriasis. Nat Commun. 6:74542015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim BH, Lee JM, Jung YG, Kim S and Kim TY:
Phytosphingosine derivatives ameliorate skin inflammation by
inhibiting NF-κB and JAK/STAT signaling in keratinocytes and mice.
J Invest Dermatol. 134:1023–1032. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansdottir S, Monick MM, Lovan N, Powers
L, Gerke A and Hunninghake GW: Vitamin D decreases respiratory
syncytial virus induction of NF-kappaB-linked chemokines and
cytokines in airway epithelium while maintaining the antiviral
state. J Immunol. 184:965–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Q, Li H, Xie H, Fu M, Guo B, Ding Y,
Li W and Yu H: 25-Hydroxyvitamin D3 attenuates experimental
periodontitis through downregulation of TLR4 and JAK1/STAT3
signaling in diabetic mice. J Steroid Biochem Mol Biol. 135:43–50.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dhawan P, Peng X, Sutton AL, MacDonald PN,
Croniger CM, Trautwein C, Centrella M, McCarthy TL and Christakos
S: Functional cooperation between CCAAT/enhancer-binding proteins
and the vitamin D receptor in regulation of 25-hydroxyvitamin D3
24-hydroxylase. Mol Cell Biol. 25:472–487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aggarwal BB, Shishodia S, Takada Y,
Jackson-Bernitsas D, Ahn KS, Sethi G and Ichikawa H: TNF blockade:
An inflammatory issue. Ernst Schering Res Found Workshop. 161–186.
2006.PubMed/NCBI
|
|
27
|
Rozieres A, Hennino A and Nicolas JF: TNF
alpha in the physiopathology of psoriasis. Ann Dermatol Venereol.
133:174–180. 2006.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weng SW, Chen BC, Wang YC, Liu CK, Sun MF,
Chang CM, Lin JG and Yen HR: Traditional Chinese medicine use among
patients with psoriasis in Taiwan: A Nationwide population-based
study. Evid Based Complement Alternat Med. 2016:31641052016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho JW, Lee KS and Kim CW: Curcumin
attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well
as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs
as potential upstream targets. Int J Mol Med. 19:469–474.
2007.PubMed/NCBI
|
|
30
|
Cassano N, Loconsole F, Coviello C and
Vena GA: Infliximab in recalcitrant severe atopic eczema associated
with contact allergy. Int J Immunopathol Pharmacol. 19:237–240.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piérard-Franchimont C and Piérard GE:
Etanercept (Enbrel) for the treatment of moderate to severe plaque
type psoriasis. Rev Med Liege. 61:201–205. 2006.(In French).
PubMed/NCBI
|
|
32
|
Ritchlin C, Haas-Smith SA, Hicks D,
Cappuccio J, Osterland CK and Looney RJ: Patterns of cytokine
production in psoriatic synovium. J Rheumatol. 25:1544–1552.
1998.PubMed/NCBI
|
|
33
|
Teschke R, Larrey D, Melchart D and Danan
G: Traditional Chinese Medicine (TCM) and Herbal Hepatotoxicity:
RUCAM and the role of novel diagnostic biomarkers such as
MicroRNAs. Medicines (Basel). 3:pii: E18. 2016.PubMed/NCBI
|
|
34
|
Xutian S, Zhang J and Louise W: New
exploration and understanding of traditional Chinese medicine. Am J
Chin Med. 37:411–426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang CY, Lai WY, Sun MF, Lin CC, Chen BC,
Lin HJ, Chang CM, Yang CH, Huang KC and Yen HR: Prescription
patterns of traditional Chinese medicine for peptic ulcer disease
in Taiwan: A nationwide population-based study. J Ethnopharmacol.
176:311–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xuan ML, Lu CJ, Han L and Xiang Y:
Circulating levels of inflammatory cytokines in patients with
psoriasis vulgaris of different Chinese medicine syndromes. Chin J
Integr Med. 21:108–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Landis ET, Davis SA, Feldman SR and Taylor
S: Complementary and alternative medicine use in dermatology in the
United States. J Altern Complement Med. 20:392–398. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
May BH, Zhang AL, Zhou W, Lu CJ, Deng S
and Xue CC: Oral herbal medicines for psoriasis: A review of
clinical studies. Chin J Integr Med. 18:172–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu CJ, Xiang Y, Xie XL, Xuan ML and He ZH:
A randomized controlled single-blind clinical trial on 84
outpatients with psoriasis vulgaris by auricular therapy combined
with optimized Yinxieling Formula. Chin J Integr Med. 18:186–191.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Acharyya S, Villalta SA, Bakkar N,
Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S,
Sahenk Z, et al: Interplay of IKK/NF-kappaB signaling in
macrophages and myofibers promotes muscle degeneration in Duchenne
muscular dystrophy. J Clin Invest. 117:889–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Greten FR and Karin M: The IKK/NF-kappaB
activation pathway-a target for prevention and treatment of cancer.
Cancer Lett. 206:193–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma D, Zhang RN, Wen Y, Yin WN, Bai D,
Zheng GY, Li JS, Zheng B and Wen JK: 1, 25(OH)2D3-induced
interaction of vitamin D receptor with p50 subunit of NF-κB
suppresses the interaction between KLF5 and p50, contributing to
inhibition of LPS-induced macrophage proliferation. Biochem Biophys
Res Commun. 482:366–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fekrmandi F, Wang TT and White JH: The
hormone-bound vitamin D receptor enhances the FBW7-dependent
turnover of NF-κB subunits. Sci Rep. 5:130022015. View Article : Google Scholar : PubMed/NCBI
|