Introduction
Triptolide (TP) is a traditional Chinese medicine,
apredominant active ingredient in Tripterygium wilfordii and
is a diterpenoid compound (1).
Numerous studies have suggested that TP can inhibit proliferation
of numerous tumor cells, such as breast cancer, gastrointestinal
cancer, prostate cancer and nervous system tumors (1–3). TP
was discovered in 1972 and the molecular mechanism of its
biological activities has been extensively studied (4). TP exhibits an immunomodulatory role,
predominantly via inhibition of nuclear factor-κB (NF-κB), and
subsequent suppression of the production of inflammatory factors
and thus the immune response of the body (5,6). In
addition, TP can induce apoptosis in numerous tumor cell types,
predominantly via inhibition of heat shock transcription factor 1,
activator protein 1 (AP-1), NF-κB and other transcriptional
regulators, thus exhibiting an antitumor effect (5,7).
Such results suggest that NF-κB may be associated with the
underlying therapeutic mechanism of TP. Furthermore, these studies
demonstrated that functional cellular tumor antigen p53 (p53) is
necessary for the performance of the various biological activities
of TP (6).
Lung cancer is the most common type of cancerous
tumor globally. Despite the possibility of early diagnosis,
chemotherapy, radiotherapy and immunotherapy, the 5-year survival
rate of lung cancer remains <15% (8). Small molecule inhibitors,
predominantly epidermal growth factor receptor-associated drugs,
including gefitinib and erlotinib, have been reported to have
significant antitumorigenic effects with fewer side effects
(9,10). Despite the wide use of these drugs,
there are significant drug resistances in patients with epidermal
growth factor receptor-negative cancer (9,11).
Thus, there is an urgent requirement to identify novel antitumor
agents that can act on irregularly functioning signaling pathways.
It has been reported that Wnt family proteins can regulate cell
development and growth via regulation of downstream proteins
(12). The most predominant
proteins associated with the Wnt signaling pathway, from the
extracellular to intracellular environment, include the Wnt protein
(predominantly encoding secretory glycoprotein), cell membrane Wnt
receptor protein, β-catenin and the anaphase promoting complex
protein (glycogen synthase-3β kinase, Axin and conductin) (13). Wnt signaling pathways are generally
expressed during embryonic development, and Wnt signaling is
subsequently downregulated or absent in mature organisms (14). However, numerous studies have
revealed that abnormal expression of Wnt signaling activates the
cell cycle, induces proliferation associated with proto-oncogenes
and promotes tumorigenesis (15,16).
Studies have also demonstrated that the expression of Wnt ligands
are upregulated in lung cancer cell lines and tissues (17,18).
Overexpression of Wnt ligands results in the ectopic activation of
β-catenin in the cytoplasm/nucleus of lung cells and the activation
of transcription of downstream target genes (predominantly encoding
a number of proto-oncogenes and cell cycle regulators) (19). Despite the cause of Wnt ligand
overexpression in lung cancer cells remaining unclear, one of the
notable associated mechanisms is the absence of the Wnt antagonist
protein (20). Wnt inhibitory
factor-1 (WIF-1) is present in normal cells via binding to Wnt
ligands to prevent over activation of the Wnt pathway (21). Studies have revealed that WIF-1 is
suppressed in lung cancer cells, thus weakening its binding to Wnt
ligands and inhibiting the sustained activation of the Wnt pathway
(22,23). In addition, it has been reported
that hypermethylation of the WIF-1 promoter is frequently present
in lung cancer specimens, with suppressed or absent expression of
WIF-1 (23). Therefore, one of the
notable causes of abnormal activation of the Wnt pathway in lung
cancer cells may be due to hypermethylation of the WIF-1 promoter
region, thus resulting in the suppressed regulation of the Wnt
ligand expression (22–24). Reversing the hypermethylation of
the WIF-1 promoter in lung cancer cells and restoring the
expression level of WIF-1 in lung cancer cells, and thus further
inhibiting the activation of the Wnt pathway, are important
therapeutic targets for the treatment of lung cancer requiring
further investigation.
The aim of the present study was to investigate the
effect of TP on the proliferation, invasion, migration and
apoptosis of human lung cancer cells, and to determine the
underlying molecular mechanism of the therapeutic effects of TP on
lung cancer cells. The results of the present study will aim to
provide a theoretical basis for further study regarding the
anticancer effect of TP.
Materials and methods
Cell culture and treatment
The lung cancer cell lines A549, H460 and NCI-H446,
and human normal bronchial epithelial cell line HBE, all purchased
from the American Type Culture Collection (Manassas, VA, USA) were
used in the present study. The cells were cultured in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS, Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany), penicillin (100 U/ml) and streptomycin (100
µg/ml) (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with a
humidified atmosphere of 5% CO2. The cells were cultured
to 70–80% confluence prior to further investigation.
Methylation-specific polymerase chain
reaction (PCR)
A549, H460, NCI-H446 and HBE cells were harvested to
detect WIF-1 methylation PCR. Following this, A549 and H460 cells
were collected and incubated with 0.03, 0.3 and 3 µM TP for 24 h.
The methylation-specific PCR protocol was performed as described
previously (25). Briefly, the
extracted DNA was treated with sodium bisulfite. This treatment
ensured that unmethylated cytosine in the CpG nucleotides was
converted to uracil, whereas the methylated cytosine remained
unaffected. The modified reaction was performed using an EZ DNA
Methylation-Gold kit (Zymo Research Corp., Irvine, CA, USA).
Subsequently, the treated DNA was amplified using
methylation-specific and non-methylation-specific primers. The
thermocycling conditions for PCR amplification: Pre-denaturations
(94°C, 5 min); 35 cycles of denaturation (94°C, 30 sec), annealing
(56°C, 30 sec) and extension (72°C, 30 sec); post-extension (72°C,
5 min). 2X PCR Master Mix (Tiangen Biotech Co., Ltd., Beijing,
China) was used in these assays. WIF-1 methylation PCR primers were
synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai,
China). The following methylation-specific primers were used:
forward, 5′-GGGCGTTTTATTGGGCGTAT-3′ and reverse,
5′-AAACCAACAATCAACGAAC-3′. The following non-methylation-specific
primers were used: forward, 5′-GGGTGTTTTATTGGGTGTAT-3′ and reverse,
5′-AAACCAACAATCAACAAAAC-3′.
Cell proliferation assay
Cell proliferation was investigated via a Cell
Counting kit-8 (CCK-8) assay. A549 and H460 cells (5×104
cells/well) were seeded in 96-well plates and incubated with 0.03,
0.3 and 3 µM TP for 6, 12 and 24 h time intervals. Subsequently, 20
µl of CCK-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
was added into the cells collected from 6, 12 and 24 h time
intervals. Following incubation for 4 h, the absorptions of cells
were determined at 450 nm using an ELISA reader (ELx800TM; BioTek
Instruments, Inc., Winooski, VT, USA).
Transwell cell migration assay
A549 cells were digested with 0.25% Trypsin-EDTA
solution, harvested and washed twice to remove serum. Cells were
resuspended in Dulbecco's Modified Eagle Medium (DMEM;
Sigma-Aldrich; Merck KGaA) and then adjusted to 2×105
/ml. Subsequently, 300 µl cell solution was added to upper chambers
(BD Biosciences, Franklin Lakes, NJ, USA) and then placed on
24-well plate for 2 h. Lower chambers were filled with 500 µl DMEM
medium containing 2.5% FBS and incubated for 24 h. Following
incubation, migratory cells were fixed with 4% formaldehyde for 15
min at room temperature and then stained with 0.1% crystal violet
dyes for 3 h at room temperature. A total of 5 fields of view were
selected randomly, observed and imaged under a light microscope
(magnification, ×400; 80i; Nikon Corporation, Tokyo, Japan).
Absorbance values at 570 nm were measured using
SpectraMax® M5/M5e (Molecular Devices, LLC, Sunnyvale,
CA, USA). The migration rate was expressed as the percentage of the
control.
Transwell cell invasion assay
Cell migration analysis was performed using an
ECM554 invasion kit (Chemicon International; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, cells from all experimental groups were collected and
resuspended in DMEM medium and then adjusted to 2×105
/ml. The upper chambers of the Transwell was placed on 24-well
plate and 300 µl serum free medium was added, and the bottom
chambers were filled with DMEM medium containing 2.5% FBS.
Following incubation for 10 min, 250 µl cell solution was used to
replaced the DMEM in the upper chambers and then underwent further
incubation for 24 h. Invaded cells were stained using 0.1% crystal
violet dye for 3 h at room temperature. A total of 5 fields of view
were selected randomly, observed and imaged under microscope
(magnification, ×400; 80i; Nikon Corporation). The invasion rate
was expressed as the percentage of the control.
Cell transfection
At 80% confluence A549 cells were transfected with
WIF1 small interfering (si)RNA sense, 5′-CCUGUCAAUAUCCAUUCCAUU-3′
and antisense, 5′-UGGAAUGGAUAUUGACAGGUU-3′; negative siRNA control
sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) via Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The medium was replaced with complete DMEM containing
10% FBS (Sigma-Aldrich; Merck KGaA) following incubation for 5 h at
37°C.
Western blot assay
A549 cells were collected and then lysed in
radioimmunoprecipitation assay lysis buffer containing 50 mM
Tris-HCl, 200 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.25%
deoxycholate, protease and phosphatase inhibitors. Total protein
concentration was determined using the bicinchoninic acid assay
(Hyclone; GE Healthcare, Chicago, IL, USA). Total protein (20–40
µg) was separated via electrophoresis using 10% SDS-PAGE gel and
the proteins were then transferred electrophoretically onto
polyvinylidene fluoride membranes. Following blocking with 5%
non-fat dry milk in TBS containing 0.1% Tween-20 (TBST) for 1 h at
room temperature, the membranes were incubated with primary
antibodies WIF1 (1:1,000; ab224335), p53 (1:1,000; ab26), MMP-9
(1:1,000; ab38898), Axin2 (1:1,000; ab109307), p-P65 (1:1,000;
ab176647), P65 (1:1,000; ab16502), β-catenin (1:5,000; ab32572) and
β-actin (1:1,000; ab8226; all Abcam, Cambridge, MA, USA) overnight
at 4°C. The membranes were then incubated with horseradish
peroxidase-conjugated secondary antibody (1:2,000 dilutions;
NA934V; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Following
washing with TBST, the blots were developed using an enhanced
chemiluminescence kit (GE Healthcare Bio-Sciences) for 2 h at room
temperature. β-actin was used as an internal control. Band
intensities were quantified by densitometry using ImageJ software
(ver 1.45; National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative PCR
assay (RT-qPCR)
A549 cells were collected and total RNA was isolated
using the RNeasy mini-kit (Qiagen GmbH, Hilden, Germany) according
to the manufacturer's protocol. The RNA was then reverse
transcribed to cDNA using NCode VILO miRNA cDNA Synthesis kit
(Invitrogen; Thermo Fisher Scientific). The temperature conditions
for RT were as follows: 30°C for 10 min, 42°C for 30 min, 99°C for
5 min, 4°C for 5 min. qPCR was performed using iQ SYBR-Green
Supermix on the iCycleriQ thermal cycler (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The thermocycling conditions for qPCR:
50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 1 min. All assays were performed in duplicate.
Gene expression was quantified using the 2−ΔΔCq method
(26). Relative mRNA expression
was normalized to β-actin expression. Primers used in this study
were as follows: WIF-1 forward, 5′-ATGAATTCCTGTCCTTGCGC-3′ and
reverse, 5′-TCCACTTCAAATGCTGCCAC-3′; β-actin forward,
5′-CCCTGGAGAAGAGCTACGAG-3′ and reverse,
5′-CGTACAGGTCTTTGCGGATG-3′.
Statistical analysis
SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was
used for data analysis. All data are expressed as mean ± standard
deviation. Results were analyzed by two-tailed and unpaired
Student's t-tests. One-way analysis of variance was used for
comparison of multiple (>2) groups and Tukey's post hoc test for
pairwise comparisons was used in this study. P<0.05 was
considered to indicate a statistically significant difference.
Results
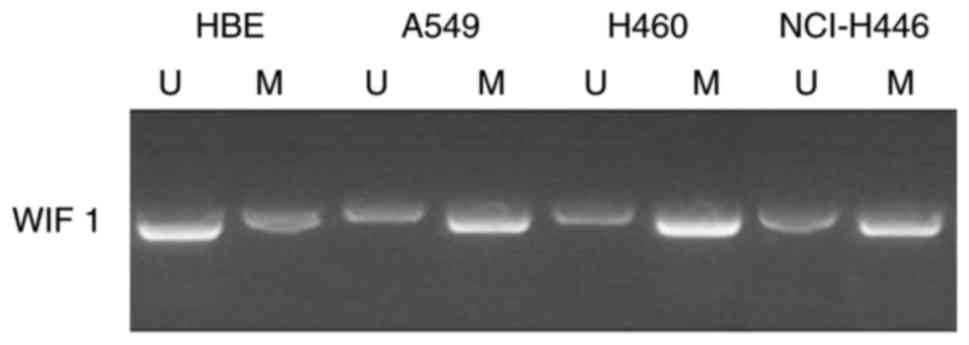
Hypermethylation of the WIF-1 promoter
is enhanced in lung cancer cell lines
To investigate whether TP has the ability to reverse
the hypermethylation of the WIF-1 promoter, the methylation status
of the WIF-1 promoter in non-small cell lung cancer A549, H460 and
NCI-H446 cell lines was determined. The results revealed that the
WIF-1 promoter was hypermethylated in A549, H460 and NCI-H446
cells, and that there was suppressed methylation in HBE cells
compared with lung cancer cell lines (Fig. 1). Due to limitations in funding,
only A549 and H460 cells were selected for subsequent analysis.
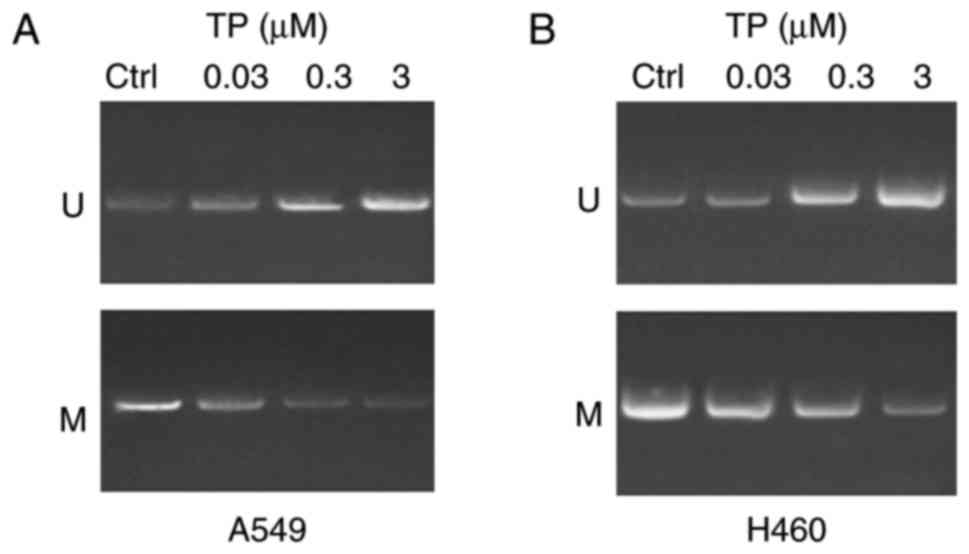
TP has a demethylation effect on the
WIF-1 promoter in A549 and H460 cells
In order to investigate whether treatment with TP
has a demethylation effect on the WIF-1 promoter in A549 and H460
cells, an in vitro intervention experiment was performed.
The results revealed that treatment with TP had a demethylation
effect on A549 cells (Fig. 2A) and
H460 cells (Fig. 2B) in a dose
dependent manner 24 h post-treatment compared with the control.
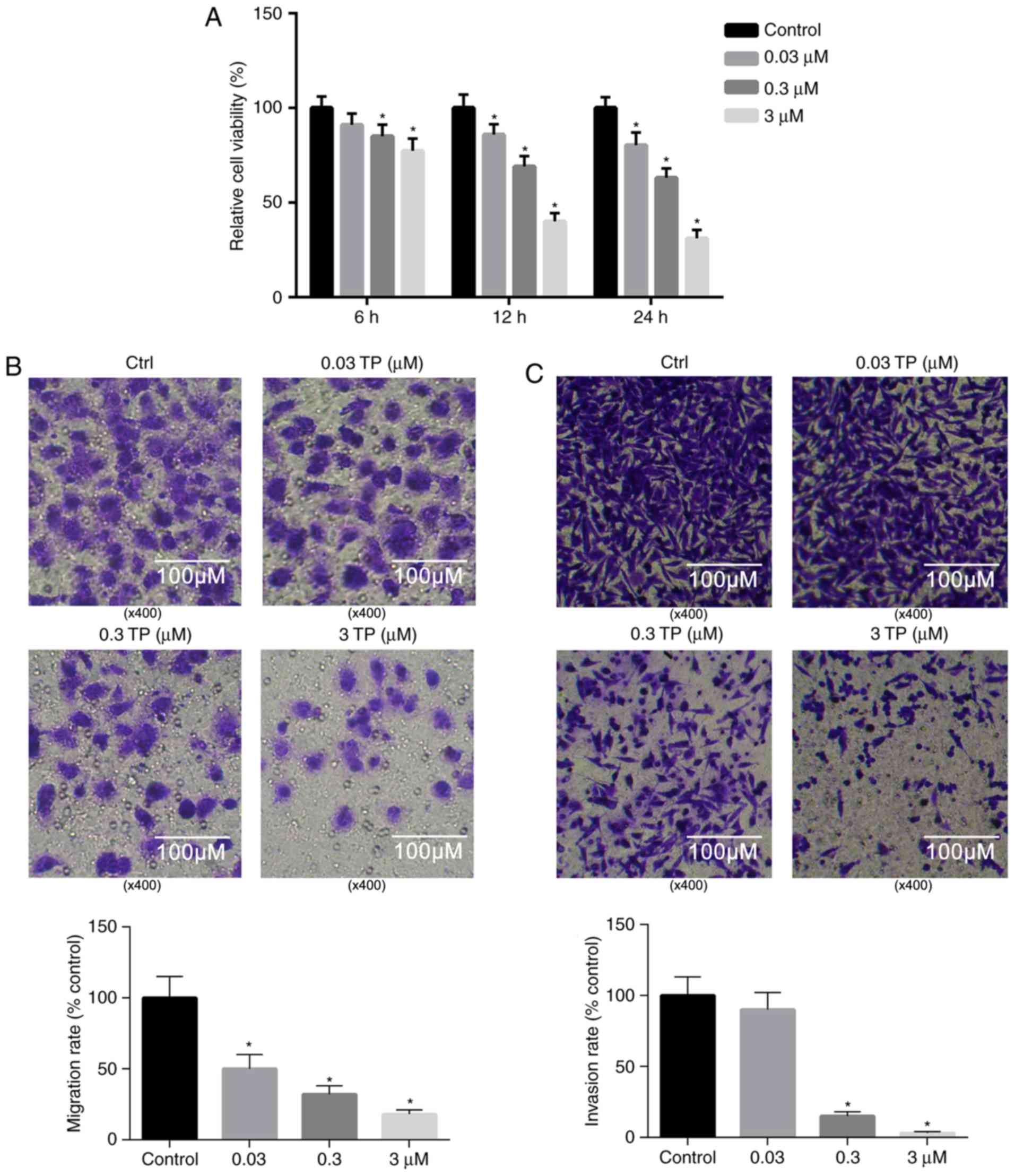
TP suppresses the proliferation of
A549 cells
Previous studies have revealed that TP has potent
tumor cytotoxicity (1–3). Therefore, the viability of A549 cells
following treatment with TP was investigated. The results presented
in Fig. 3A demonstrated that
treatment with TP significantly suppressed the viability of A549
cells in a dose- and time-dependent manner, and that the relative
cell viability reached 31.07% 24 h post-treatment with 3 µM TP
compared with the control.
TP significantly inhibits the invasion
and migration of A549 cells
Compared with the control, the invasion and
migration of A549 cells were markedly suppressed 24 h
post-treatment with 0.03, 0.3 and 3 µM TP (Fig. 3B and C). As revealed in Fig. 3B, the migration of A549 cells was
significantly attenuated following treatment with TP in a
dosage-dependent manner. Similarly, the invasion of A549 cells was
significantly suppressed following treatment with TP (0.3 and 0.03
µM) compared with the control (Fig.
3C).
Demethylation of the WIF-1 promoter
following treatment with TP increases the expression of WIF-1
To investigate the effects of TP on the expression
levels of WIF-1in A549 cells, the mRNA and protein levels of WIF-1
were investigated. At 24 h post-treatment, the expression of WIF-1
protein in A549 cells was enhanced in a dose-dependent manner
(Fig. 4A). The expression level of
WIF-1 in cells treated with 3 µM TP was ~20-fold that of the
control. Furthermore, the WIF-1 mRNA expression level in A549 cells
was significantly increased following TP treatment in a
dose-dependent manner (Fig.
4B).
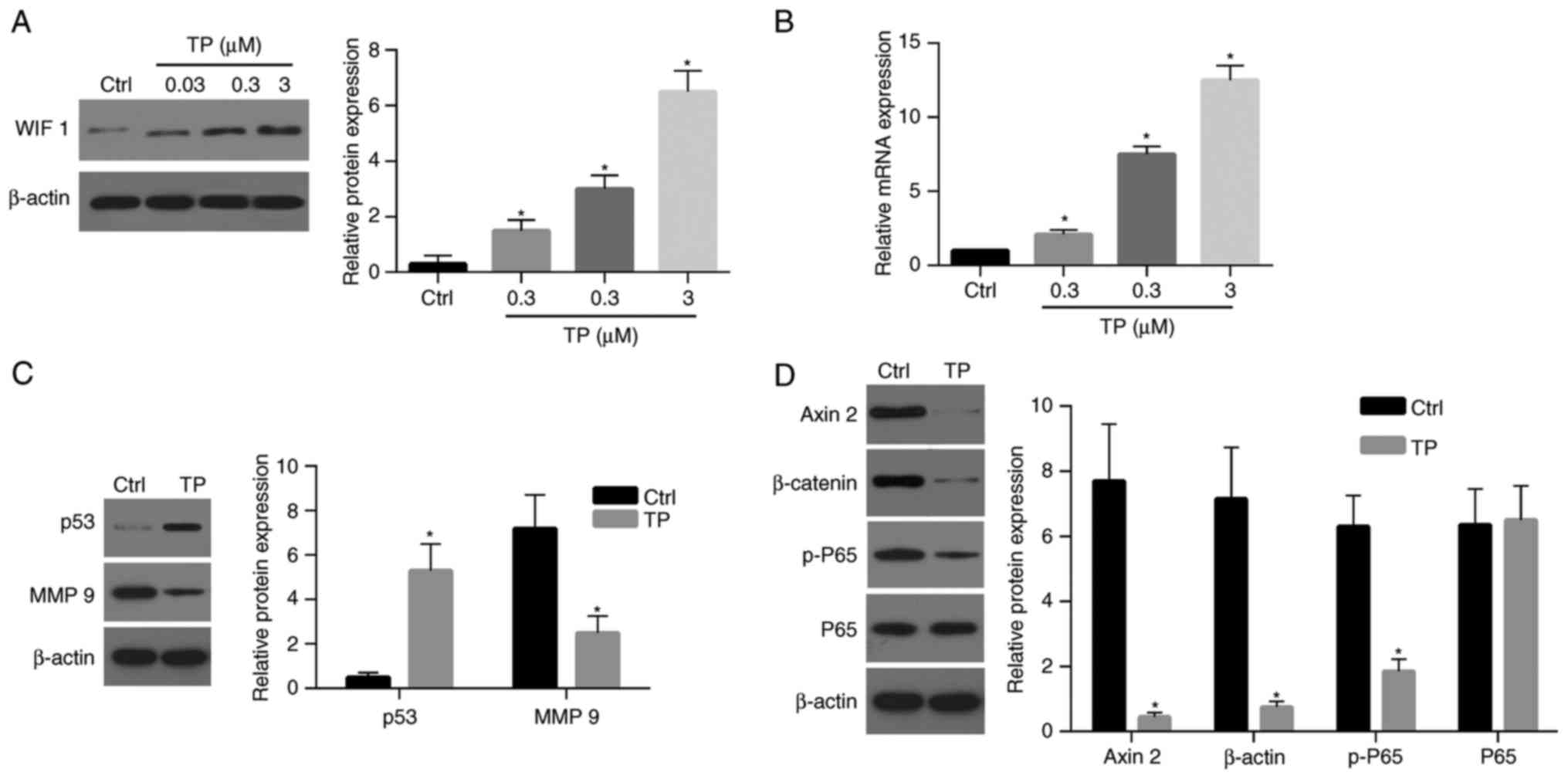 | Figure 4.TP affects the expression of numerous
genes associated with apoptosis, cell proliferation, migration and
invasion. (A) Protein and (B) mRNA expression levels of WIF-1 were
upregulated following treatment with TP in a dosage-dependent
manner. Western blot assays demonstrated that the expression level
of (C) p53 was significantly increased in cells treated with TP (3
µM) compared with the control, and the level of MMP 9 was
significantly decreased compared with the control. (D) The levels
of Axin 2, β-catenin and p-P65 in cells treated with TP (3 µM) were
significantly decreased compared with the control. TP, triptolide;
Ctrl, control; WIF 1, Wnt inhibitory factor-1; MMP-9, matrix
metalloproteinase-9; p-, phospho-; P65, NK-κB P65. *P<0.05 vs.
control. |
TP affects the expression of genes
associated with apoptosis and proliferation
The results of the present study revealed that TP is
associated with the expression of WIF-1 and the viability of A549
cells. Therefore, the present study aimed to investigate whether
increased levels of WIF-1 expression, enhanced by treatment with
TP, could inhibit the Wnt signaling pathway. Furthermore, the
present study aimed to investigate whether treatment with TP
affects the expression levels of matrix metalloproteinase-9 (MMP-9)
and p53, as well as the activation of NF-κB P65, which are
associated with cell migration, apoptosis and survival. A notable
increase regarding p53 expression was observed following treatment
with TP (3 µM) compared with control (Fig. 4C and D). By contrast, the
expression level of MMP-9 was significantly decreased in cells
treated with TP (Fig. 4C and D).
The expression levels of β-catenin and Axin2, two key molecules of
the Wnt pathway, were significantly decreased following treatment
with TP compared with the control (Fig. 4C and D). Notably, the level of
phosphorylated-P65 was also significantly decreased following
treatment with TP (Fig. 4C and D).
These results suggest that the Wnt and NF-κB signaling pathways
were strongly suppressed following treatment with TP.
To further investigate the role of WIF-1 in the
regulatory effect of TP on Wnt and NF-κB pathways, WIF-1 expression
was silenced by siRNA. As demonstrated in Fig. 5A and B, TP-induced elevations in
levels of WIF-1 protein and mRNA were clearly downregulated
compared with the control and siRNA negative control. The
expression of p53 was significantly decreased in cells treated with
TP+siRNA compared with cells treated with TP alone or TP+NC
(Fig. 5C). However, the level of
p53 in cells treated with TP+siRNA was enhanced compared with the
control. By contrast, MMP-9 expression in TP+siRNA cells was
markedly enhanced compared with cells treated with TP alone or
TP+NC, and lower than that exhibited by the control (Fig. 5C). In cells treated with TP+siRNA,
the expression levels of Axin 2 and β-catenin were recovered to a
similar level exhibited by the control, and significantly elevated
compared with cells treated with either TP alone or TP+NC (Fig. 5D). There were no significant
differences in levels of p-P65 exhibited among cells treated with
TP+siRNA, TP alone or TP+NC (Fig.
5D). These results suggest that TP not only suppresses the Wnt
pathway by increasing WIF-1 expression, but also inhibits the NF-κB
pathway (which is not regulated by WIF-1).
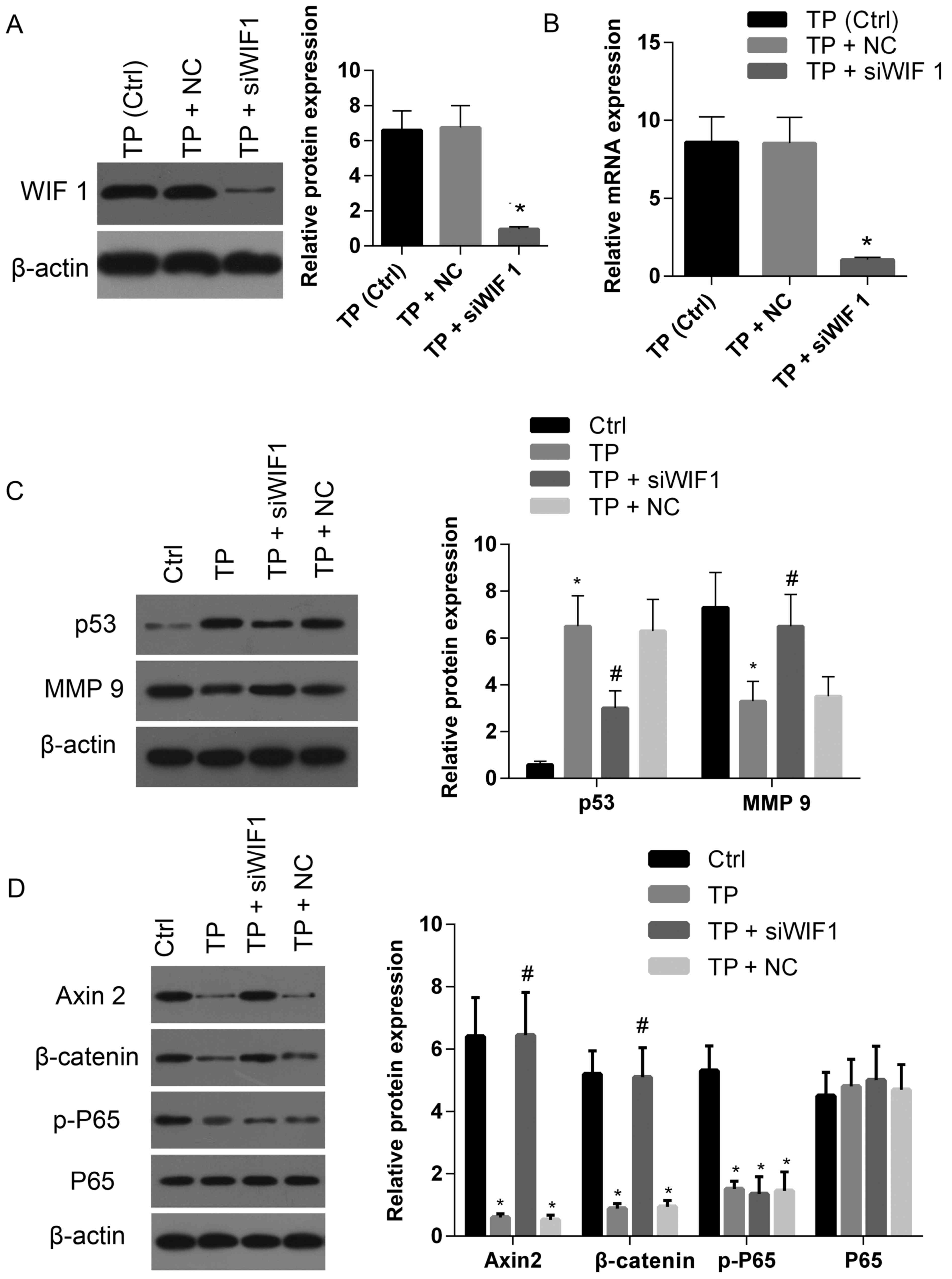 | Figure 5.Silencing WIF-1 expression partially
attenuates the effect of TP on the expression of several genes
associated with apoptosis and cell proliferation, migration and
invasion. The (A) protein and (B) mRNA expression levels of WIF-1
in cells treated with TP (3 µM) were downregulated following
treatment with siWIF 1 compared with cells treated with TP alone or
the negative control. (C) The protein levels of p53 and MMP-9 in
cells treated with TP+siWIF 1 were markedly enhanced and suppressed
compared with the control, respectively; whereas the protein levels
of p53 and MMP-9 in cells treated with TP+siWIF 1 were markedly
suppressed and enhanced compared with cells treated with TP alone.
(D) The protein levels of Axin 2 and β-catenin in cells treated
with TP+siWIF 1 were restored to the associated levels exhibited by
the control, whereas there was no significant change regarding the
level of p-P65 in cells treated with TP+siWIF 1 compared with cells
treated with TP alone or negative control (TP+NC). TP, triptolide;
Ctrl, control; NC, negative control; si, small interfering RNA; WIF
1, Wnt inhibitory factor-1; MMP 9, matrix metalloproteinase 9; p-,
phospho-; P65, NK-κB P65. *P<0.05 vs. control.
#P<0.05 TP+siWIF 1 vs. TP+NC or TP. |
Discussion
TP, a traditional Chinese medicine isolated from
Tripterygium wilfordii, has been used to treat inflammation
and autoimmune diseases (27).
Numerous studies have demonstrated that TP exhibits antitumor
effects on various cancer cell lines, including bladder, liver,
cervical and breast cancer cell lines (28,29).
To investigate the effect of TP on the viability, invasion and
migration of lung cancer cells, human lung cancer cell lines A549
and H460 were treated with TP. The results revealed that treatment
with TP markedly suppressed the viability, invasion and migration
of lung cancer cells. Furthermore, the results demonstrated that
treatment with TP increased the expression levels of WIF-1 via
demethylation of the WIF-1 promoter. Further investigation revealed
that TP also increased the expression of p53, and decreased the
levels of MMP-9 and p-P65, which are associated with cell
apoptosis, proliferation, survival, invasion and migration
(1–4).
The Wnt signaling pathway has an important function
in the development of the embryo and is associated with the
regulation of cell proliferation, differentiation and migration
(30,31). Hypermethylation of the WIF-1
promoter is associated with excessive activation of the Wnt
signaling pathway in human lung cancer (24,32–34).
Thus, restoring normal WIF-1 expression may downregulate the Wnt
signaling pathway and inhibit the progression of lung cancer. In
human tumors, abnormal methylation of CpG islands in promoter
regions results in a suppression of the transcriptional activity of
tumor suppressor genes (35–37).
Previous studies have demonstrated that numerous tumor suppressor
genes associated with lung cancer are downregulated via methylation
of the promoter region (23,35,38).
Previous studies had also revealed that WIF-1 is silenced by
hypermethylation of the associated promoter in lung cancer cell
lines and lung cancer surgical specimens (22,38).
In addition, administration of the classic demethylation drug
5-aza-2′-deoxycytidine (DAC) demethylates the WIF-1 promoter to
restore WIF-1 expression and thus restores downregulation of the
activity of the Wnt signaling pathway (39,40).
Despite DAC exhibiting a well-established demethylation effect, its
therapeutic use is limited by its toxic effects on cells (41,42).
TP is a natural compound that exhibits low cytotoxicity and a
potential demethylation function, and thus may represent a novel
therapeutic agent for the treatment of patients with lung cancer.
The results of the present study revealed that treatment with TP
significantly suppressed the viability, invasion and migration of
A549 and H460 cells. In addition, the results demonstrated that TP
markedly suppressed the degree of methylation of the WIF-1
promoter, thus inducing upregulation of WIF-1 expression. It was
hypothesized that TP suppresses cell proliferation, invasion,
migration and apoptosis by inhibiting the Wnt signaling pathway via
upregulation of WIF-1 expression. Downregulated expression of Axin
2 and β-catenin suggested that treatment with TP suppresses the Wnt
signaling pathway. Furthermore, an increase in p53 expression and a
reduction in MMP-9 expression were observed, the results of which
were consistent with the inhibition effect of TP on cell
proliferation, migration and apoptosis.
TP had been demonstrated to exhibit significant
growth inhibitory effects on various solid tumors such as breast
cancer, gastrointestinal cancer, prostate cancer and nervous system
tumors (43–45). TP has entered Phase I clinical
trials, for the treatment of leukemia (46,47).
It has been revealed that TP exhibits an antitumor effect
predominantly via inhibition of numerous transcriptional regulators
such as NF-κB and AP-1, in which the NF-κB pathway is one of the
most important antitumor targets (5,29).
P65 is an important heterodimer of NF-κB. Inhibitor of κB (I-κB)
and NF-κB exist in the form of inactive complexes in the cytoplasm.
Following activation, the NF-κB/I-κB complex dissociates and
releases free NF-κB into the nucleus to regulate the transcription
of the target genes (48–50). Notably, the level of p-P65 was
downregulated in cells post-treatment with TP, which is consistent
with the observations of previous studies (51–53).
Recently, similar studies determined that TP not only inhibits
NF-kB nuclear translocation in human lung adenocarcinoma
paclitaxel-resistant cell line A549/Taxol, but also inhibits the
function of RNA polymerase II (54,55).
The results of the present study revealed that
knockdown of WIF-1 with siRNA resulted in a significant reduction
of WIF-1 expression. As hypothesized, compared with cells treated
with TP, the expression levels of Axin 2 and β-catenin in cells
with additional siRNA treatment were increased and recovered to
that exhibited by the control (cells without treatment), thus
suggesting that the Wnt signaling pathway was activated. Silencing
WIF-1 blocked TP-induced inhibition of the Wnt signaling pathway,
thus suggesting that TP-induced inhibition of Wnt signaling pathway
is WIF-1 dependent. Despite activation of Wnt signaling simulated
by knockdown of WIF-1 not completely recovering the expression
levels of p53, MMP-9 and p-P65 in cells treated with TP compared
with the control, the results do suggest that treatment with TP
affects p53 and NF-κB signaling pathways. Consistent with the
results of the present study, Jiang et al (6) demonstrated that TP has
anti-inflammatory and antitumor functions via suppression of cell
proliferation, induction of apoptosis and downregulation of NF-κB
and AP-1 transcriptional activity. In addition, previous studies
have demonstrated that p53 activity is important for the
anti-inflammatory, antitumor and pro-apoptotic effects of TP
treatment on human gastric cancer cells (6,56).
The present study was limited due to funding; only
A549 and H460 cells were investigated. Therefore, the findings of
the present stud require further investigation with additional cell
lines and analysis in vivo. In conclusion, the results of
the present study suggested that promoter hypermethylation of WIF-1
is responsible for the abnormal activation of Wnt signaling in lung
cancer cells. TP exhibits significant inhibition on Wnt signaling
via demethylation of the WIF-1 promoter. Silencing WIF-1 with siRNA
reversed TP-induced inhibition of Wnt signaling, thus suggesting
that TP-induced suppression of Wnt signaling is WIF-1 dependent.
Furthermore, upregulation of p53, and downregulation of MMP-9 and
p-P65, was observed in cells treated with TP. Notably, silencing
WIF-1 did not restore the levels of p53, MMP-9 and p-P65 in cells
treated with TP to those exhibited by the control, thus suggesting
that p53 and NF-κB signaling pathways also have important roles in
the antitumor effect of TP on lung cancer cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
XM and JT wrote the manuscript. XM conducted cell
culture and treatments, cell proliferation and Transwell cell
migration assays, and reverse transcription-quantitative polymerase
chain reaction and western blot analyses. YoW and ZZ performed cell
culture and treatment, cell proliferation assay, cell transfection
and Transwell cell migration assay. YY and YeW performed
methylation-specific polymerase chain reaction and reverse
transcription-quantitative polymerase chain reaction. XM and JT
made substantial contributions to the design of the study. XM and
YoW analysed data. XM and JT critically revised the manuscript for
important intellectual content. All authors read and approved the
final the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao F, Chen Y, Li R, Liu Y, Wen L and
Zhang C: Triptolide alters histone H3K9 and H3K27 methylation state
and induces G0/G1 arrest and caspase-dependent apoptosis in
multiple myeloma in vitro. Toxicology. 267:70–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borja-Cacho D, Yokoyama Y, Chugh RK,
Mujumdar NR, Dudeja V, Clawson KA, Dawra RK, Saluja AK and Vickers
SM: TRAIL and triptolide: An effective combination that induces
apoptosis in pancreatic cancer cells. J Gastrointest Surg.
14:252–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Li X, Sun W, Zhang L, Zhang M,
Hong B and Lv G: Triptolide triggers the apoptosis of pancreatic
cancer cells via the downregulation of Decoy receptor 3 expression.
J Cancer Res Clin Oncol. 138:1597–1605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan J: RNA polymerase-an important
molecular target of triptolide in cancer cells. Cancer Lett.
292:149–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y, Cui J, Bao X, Chan S, Young DO, Liu
D and Shen P: Triptolide attenuates oxidative stress, NF-kappaB
activation and multiple cytokine gene expression in murine
peritoneal macrophage. Int J Mol Med. 17:141–150. 2006.PubMed/NCBI
|
|
6
|
Jiang XH, Wong BC, Lin MC, Zhu GH, Kung
HF, Jiang SH, Yang D and Lam SK: Functional p53 is required for
triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB
activation in gastric cancer cells. Oncogene. 20:8009–8018. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mackenzie TN, Mujumdar N, Banerjee S,
Sangwan V, Sarver A, Vickers S, Subramanian S and Saluja AK:
Triptolide induces the expression of miR-142-3p: A negative
regulator of heat shock protein 70 and pancreatic cancer cell
proliferation. Mol Cancer Ther. 12:1266–1275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gil L and Adonis M: Genomics and
proteomics offers new hopes towards a personalized approach to lung
cancer prevention and treatment. Elec J Biotechnol. 6:401–409.
2003. View Article : Google Scholar
|
|
9
|
Lemos C, Kathmann I, Giovannetti E, Calhau
C, Jansen G and Peters GJ: Impact of cellular folate status and
epidermal growth factor receptor expression on BCRP/ABCG2-mediated
resistance to gefitinib and erlotinib. Br J Cancer. 100:1120–1127.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun JM, Won YW, Kim ST, Kim JH, Choi YL,
Lee J, Park YH, Ahn JS, Park K and Ahn MJ: The different efficacy
of gefitinib or erlotinib according to epidermal growth factor
receptor exon 19 and exon 21 mutations in Korean non-small cell
lung cancer patients. J Cancer Res Clin Oncol. 137:687–694. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rho JK, Choi YJ, Jeon BS, Choi SJ, Cheon
GJ, Woo SK, Kim HR, Kim CH, Choi CM and Lee JC: Combined treatment
with silibinin and epidermal growth factor receptor tyrosine kinase
inhibitors overcomes drug resistance caused by T790M mutation. Mol
Cancer Ther. 9:3233–3243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamulla RJ, Kane EG, Moody AE, Politi KA,
Lock NE, Foley AV and Roberts DM: Testing models of the APC tumor
suppressor/β-catenin interaction reshapes our view of the
destruction complex in Wnt signaling. Genetics. 197:1285–1302.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun J and Jin T: Both Wnt and mTOR
signaling pathways are involved in insulin-stimulated
proto-oncogene expression in intestinal cells. Cell Signal.
20:219–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang C, Ma R, Xu Y, Li N, Li Z, Yue J, Li
H, Guo Y and Qi D: Wnt2 promotes non-small cell lung cancer
progression by activating WNT/β-catenin pathway. Am J Cancer Res.
5:1032–1046. 2015.PubMed/NCBI
|
|
17
|
Benhaj K, Akcali KC and Ozturk M:
Redundant expression of canonical Wnt ligands in human breast
cancer cell lines. Oncol Rep. 15:701–707. 2006.PubMed/NCBI
|
|
18
|
Akiri G, Cherian MM, Vijayakumar S, Liu G,
Bafico A and Aaronson SA: Wnt pathway aberrations including
autocrine Wnt activation occur at high frequency in human
non-small-cell lung carcinoma. Oncogene. 28:2163–2172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uematsu K, He B, You L, Xu Z, McCormick F
and Jablons DM: Activation of the Wnt pathway in non small cell
lung cancer: Evidence of dishevelled overexpression. Oncogene.
22:7218–7221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim J, You L, Xu Z, Kuchenbecker K, Raz D,
He B and Jablons D: Wnt inhibitory factor inhibits lung cancer cell
growth. J Thorac Cardiovasc Surg. 133:733–737. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ai L, Tao Q, Zhong S, Fields CR, Kim WJ,
Lee MW, Cui Y, Brown KD and Robertson KD: Inactivation of Wnt
inhibitory factor-1 (WIF1) expression by epigenetic silencing is a
common event in breast cancer. Carcinogenesis. 27:1341–1348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao Z, Xu Z, Hung MS, Lin YC, Wang T, Gong
M, Zhi X, Jablons DM and You L: Procaine and procainamide inhibit
the Wnt canonical pathway by promoter demethylation of WIF-1 in
lung cancer cells. Oncol Rep. 22:1479–1484. 2009.PubMed/NCBI
|
|
23
|
Lee SM, Park JY and Kim DS: Wif1
hypermethylation as unfavorable prognosis of non-small cell lung
cancers with EGFR mutation. Mol Cells. 36:69–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan M, Wu J and Cai Y: Suppression of Wnt
signaling by the miR-29 family is mediated by demethylation of
WIF-1 in non-small-cell lung cancer. Biochem Biophys Res Commun.
438:673–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu YL, Yang HP, Gong L, Tang CL and Wang
HJ: Hypomethylation effects of curcumin, demethoxycurcumin and
bisdemethoxycurcumin on WIF-1 promoter in non-small cell lung
cancer cell lines. Mol Med Rep. 4:675–679. 2011.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morita T: Celastrol: A new therapeutic
potential of traditional chinese medicine. Am J Hypertens.
23:8212010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ho JN, Byun SS, Lee S, Oh JJ, Hong SK, Lee
SE and Yeon JS: Synergistic antitumor effect of triptolide and
cisplatin in cisplatin resistant human bladder cancer cells. J
Urol. 193:1016–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Jiang Z, Xiao J, Zhang Y, Lin S,
Duan W, Yao J, Liu C, Huang X, Wang T, et al: Effects of triptolide
from Tripterygium wilfordii on ERalpha and p53 expression in two
human breast cancer cell lines. Phytomedicine. 16:1006–1013. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao H, Ashihara E and Maekawa T: Targeting
the Wnt/β-catenin signaling pathway in human cancers. Expert Opin
Ther Targets. 15:873–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varela-Nallar L, Grabowski CP, Alfaro IE,
Alvarez AR and Inestrosa NC: Role of the Wnt receptor Frizzled-1 in
presynaptic differentiation and function. Neural Dev. 4:412009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mazières J, You L, He B, Xu Z, Lee AY and
Jablons DM: Wnt Inhibitory Factor 1 (WIF-1) silencing in human
non-small cell lung cancer is controlled by promoter
hypermethylation. Cancer Res. 65:430. 2005.
|
|
33
|
Liu YL, Yang HP, Zhou XD, Gong L, Tang CL
and Wang HJ: The hypomethylation agent bisdemethoxycurcumin acts on
the WIF-1 promoter, inhibits the canonical Wnt pathway and induces
apoptosis in human non-small-cell lung cancer. Curr Cancer Drug
Targets. 11:1098–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie J, Zhang Y, Hu X, Lv R, Xiao D, Jiang
L and Bao Q: Norcantharidin inhibits Wnt signal pathway via
promoter demethylation of WIF-1 in human non-small cell lung
cancer. Med Oncol. 32:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Esteller M: CpG island hypermethylation
and tumor suppressor genes: A booming present, a brighter future.
Oncogene. 21:5427–5440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sandoval J, Heyn H, Moran S, Serra-Musach
J, Pujana MA, Bibikova M and Esteller M: Validation of a DNA
methylation microarray for 450,000 CpG sites in the human genome.
Epigenetics. 6:692–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Pan P, Qiao P and Liu R:
Downregulation of N-myc downstream regulated gene 1 caused by the
methylation of CpG islands of NDRG1 promoter promotes proliferation
and invasion of prostate cancer cells. Int J Oncol. 47:1001–1008.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He B, You L, Xu Z, Lee A, Reguart N,
Rossel R and Jablons D: O-003 Writ inhibitory factor-1 (WIF-1) is
silenced by promoter hypermethylation in human non-small cell lung
cancer. Lung Cancer. 49:S52005. View Article : Google Scholar
|
|
39
|
Delmas AL, Riggs BM, Pardo CE, Dyer LM,
Darst RP, Izumchenko EG, Monroe M, Hakam A, Kladde MP, Siegel EM
and Brown KD: WIF1 is a frequent target for epigenetic silencing in
squamous cell carcinoma of the cervix. Carcinogenesis.
32:1625–1633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chan SL, Cui Y, van Hasselt A, Li H,
Srivastava G, Jin H, Ng KM, Wang Y, Lee KY, Tsao GS, et al: The
tumor suppressor Wnt inhibitory factor 1 is frequently methylated
in nasopharyngeal and esophageal carcinomas. Lab Invest.
87:644–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bai W, Chen Y and Gao A: Cross talk
between poly (ADP-ribose) polymerase 1 methylation and oxidative
stress involved in the toxic effect of anatase titanium dioxide
nanoparticles. Int J Nanomedicine. 10:5561–5569. 2015.PubMed/NCBI
|
|
42
|
Hodge DR, Peng B, Cherry JC, Hurt EM, Fox
SD, Kelley JA, Munroe DJ and Farrar WL: Interleukin 6 supports the
maintenance of p53 tumor suppressor gene promoter methylation.
Cancer Res. 65:4673–4682. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai D, Musser JH and Lennox ES: Triptolide
derivatives for modulation of apoptosis and immunosuppression. US
Patent 7,847,109. Filed May 29, 2003; issued December 7. 2010.
|
|
44
|
Liang M and Fu J: Triptolide inhibits
interferon-gamma-induced programmed death-1-ligand 1 surface
expression in breast cancer cells. Cancer Lett. 270:337–341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang W, He T, Chai C, Yang Y, Zheng Y,
Zhou P, Qiao X, Zhang B, Liu Z, Wang J, et al: Triptolide inhibits
the proliferation of prostate cancer cells and down-regulates
SUMO-specific protease 1 expression. PLoS One. 7:e376932012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li L, Kanwar J, Schmitt S, Cui QC, Zhang
C, Zhao C and Dou QP: Inhibition of tumor cellular proteasome
activity by triptolide extracted from the Chinese medicinal plant
‘thunder god vine’. Anticancer Res. 31:1–10. 2011.PubMed/NCBI
|
|
47
|
Shi X, Jin Y, Cheng C, Zhang H, Zou W,
Zheng Q, Lu Z, Chen Q, Lai Y and Pan J: Triptolide inhibits Bcr-Abl
transcription and induces apoptosis in STI571-resistant chronic
myelogenous leukemia cells harboring T315I mutation. Clin Cancer
Res. 15:1686–1697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Campbell KJ, Rocha S and Perkins ND:
Active repression of antiapoptotic gene expression by RelA (p65)
NF-kappa B. Mol Cell. 13:853–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu W, Zhang G, Zhang R, Flores LG II,
Huang Q, Gelovani JG and Li C: Tumor site-specific silencing of
NF-kappaB p65 by targeted hollow gold nanosphere-mediated
photothermal transfection. Cancer Res. 70:3177–3188. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moles A, Butterworth JA, Sanchez A, Hunter
JE, Leslie J, Sellier H, Tiniakos D, Cockell SJ, Mann DA, Oakley F
and Perkins ND: A RelA (p65) Thr505 phospho-site mutation reveals
an important mechanism regulating NF-κB-dependent liver
regeneration and cancer. Oncogene. 35:4623–4632. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou Y, Hong Y and Huang H: Triptolide
attenuates inflammatory response in membranous glomerulo-nephritis
rat via downregulation of NF-κB signaling pathway. Kidney Blood
Press Res. 41:901–910. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jang BC, Lim KJ, Choi IH, Suh MH, Park JG,
Mun KC, Bae JH, Shin DH and Suh SI: Triptolide suppresses
interleukin-1beta-induced human beta-defensin-2 mRNA expression
through inhibition of transcriptional activation of NF-kappaB in
A549 cells. Int J Mol Med. 19:757–763. 2007.PubMed/NCBI
|
|
53
|
Wang X, Zhang L, Duan W, Liu B, Gong P,
Ding Y and Wu X: Anti-inflammatory effects of triptolide by
inhibiting the NF-κB signalling pathway in LPS-induced acute lung
injury in a murine model. Mol Med Rep. 10:447–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Y, Lu JJ, He L and Yu Q: Triptolide
(TPL) inhibits global transcription by inducing
proteasome-dependent degradation of RNA polymerase II (Pol II).
PLoS One. 6:e239932011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vispé S, Devries L, Créancier L, Besse J,
Bréand S, Hobson DJ, Svejstrup JQ, Annereau JP, Cussac D, Dumontet
C, et al: Triptolide is an inhibitor of RNA polymerase I and
II-dependent transcription leading predominantly to down-regulation
of short-lived mRNA. Mol Cancer Ther. 8:2780–2790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Carter BZ, Mak DH, Schober WD, Dietrich
MF, Pinilla C, Vassilev LT, Reed JC and Andreeff M: Triptolide
sensitizes AML cells to TRAIL-induced apoptosis via decrease of
XIAP and p53-mediated increase of DR5. Blood. 111:3742–3750. 2008.
View Article : Google Scholar : PubMed/NCBI
|