Introduction
Ochratoxin A (OTA) is produced by the Penicillium
verrucosum fungus and various species of aspergillus, and among
the mycotoxins with great public health and agroeconomic importance
(1). It is a widespread
contaminant of a variety of animal and human food and is not easy
decomposed, and dietary intake of OTA may be unavoidable (2,3). As
the elimination of OTA from the body is slow, it accumulates in the
tissues and fluids of humans and animals that consume food
contaminated with this toxin (4).
Notably, reports have demonstrated that even exposure to low
concentrations of OTA in domestic and experimental animals leads to
morphological and functional alterations in renal and hepatic
tissues. In addition, it has been classified as potentially
carcinogenic, genotoxic and teratogenic to humans (group 2B
according to the International Agency for Research on Cancer
classification) (5,6).
It has been indicated that the mechanisms underlying
OTA toxicity may include the inhibition of protein synthesis,
reactive oxygen species (ROS) formation, lipid peroxidation,
altered calcium homeostasis and impaired mitochondrial oxidation
reactions (7–9). Studies have demonstrated that OTA
exhibits a dose-dependent inhibition of HepG2 human hepatoma cell
viability, presenting typical sigmoid curves (10,11).
Furthermore, OTA has been demonstrated to enhance ROS levels and
oxidative damage in certain immortalized renal and cancer cell
lines, including HK-2 human renal proximal tubular epithelial
cells, primary rat proximal tubular cells, LLC-PK1 proximal tubular
cells, HepG2 and CaCo-2 human colonic adenocarcinoma cells
(9,12–14).
In addition, it established that oxidative stress leads to the
induction of numerous cellular processes, which include apoptosis
and the arrest of growth, as well as the stimulation of certain
transcription factors. It is thought that apoptosis activation may
be among the primary cellular mechanisms underlying OTA-induced
toxicity, particularly renal toxicity (15). However, whether OTA induces
oxidative stress and apoptosis, and its potential role in chicken
primary hepatocytes, remains unknown.
Glycyrrhizin is the most important and recognized
bioactive component of licorice root. This compound has been
employed for >20 years in patients suffering from chronic
hepatitis in China and Japan (16–19).
The major components of compound ammonium glycyrrhizin (CAG) are
glycyrrhizin, glycine and methionine, and this compound is reported
to be an effective anti-inflammatory, anticancer, antihepatotoxic
and antioxidant agent (20–22).
L-arginine (L-Arg) is a semi-essential amino acid that has roles in
the synthesis of protein and creatine, and is also involved in
nitrogen balance (23). It also
functions as a scavenger of free radicals and is a substrate for
nitric oxide synthase, which means it has protective effects in
endothelial damage. Certain previous studies have demonstrated that
L-Arg exerts protective effects in certain chronic diseases
(24,25). Silymarin (Sil), a hepatoprotective
agent, is a flavonolignan that is extracted from milk thistle
(Silybum marianum) that has been employed as a natural
treatment for various liver diseases for a number of decades
(26). It has been reported that
Sil may exhibit anti-inflammatory, antioxidant and anticancer
properties, which have been associated with its potential
therapeutic effects (27).
Glucurolactone (GA) is a conventional hepatoprotective drug that is
used in epidemical hepatitis, cirrhosis of the liver and poisoning
due to food and drugs. It acts as a hepatic antidote and immune
regulator. Thus, it is reasonable to investigate the protective
effects of CAG, L-Arg, Sil and GA on OTA-induced injury in chicken
primary hepatocytes.
China faces a food shortage and available food is
frequently contaminated with mycotoxins (28), particularly OTA. As a site of
metabolism, the liver is an important target for the majority of
xenobiotics, and the effect of OTA on this organ remains uncertain.
To assess the potential for hepatoxicity following OTA exposure,
the present study performed experiments in chicken primary
hepatocytes to evaluate the potential protective effect of four
hepatoprotective agents against liver disease in chickens.
Materials and methods
Materials
OTA was purchased from Fermentek, Ltd. (Jerusalem).
Dulbecco's modified Eagle's medium (DMEM) was obtained from
Hyclone; GE Healthcare Life Sciences (Logan, UT, USA). Collagenase
(type IV), HEPES and MTT reagent were obtained from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). CAG was produced in-house (per 100
g containing 2.8 g ammonium glycyrrhizin, 2 g glycine and 2 g
methionine).
Cell culture
Hepatocytes were isolated from a male Hy-line
variety brown chicken by an improved two-step collagenase perfusion
method (29). A total of ~20
chickens were obtained from the Nanjing Tangquan Chicken Farm
(Nanjing, China) and treated at a controlled temperature (24°C)
under a 12-h light-dark cycle and fed standard laboratory chow and
water ad libitum. Chickens were housed in accordance with
the National Institutes of Nanjing Agriculture University for the
Care and Use of Laboratory Animals. The chickens were raised until
5 months old, weighing 1–1.5 kg, before experiments. The current
study was approved by the Animal Care and Use Committee of Nanjing
Agricultural University, (Nanjing, China; license number:
SYXK2017-0007). The liver was separated from the chicken after
ligating the blood vessels such as the pancreaticoduodenal veins,
mesenteric vein and inferior caval vein, which are located across
the liver. The liver was subsequently perfused with saline solution
A (33 mM/l HEPES, 127.8 mM/l NaCl, 3.15 mM/l KCl, 0.7 mM/l
Na2HPO4•12H2O, 0.6 mM/l EGTA, pH
7.4) for 30 min and saline solution B (33 mM/l HEPES, 127.8 mM/l
NaCl, 3.15 mM/l KCl, 0.7 mM/l
Na2HPO4•12H2O, 3 mM/l
CaCl2, pH 7.4) for 15 min at 37°C. Subsequently, 0.5%
collagenase IV was used to digest the liver at a flow of 20 ml/min
for 20–25 min at 37°C. Hepatocytes were separated under aseptic
conditions and cultured in DMEM containing 10% fetal bovine serum
(cat. no. 16000-044; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 0.5 mg/l bovine insulin (cat. no. I8040;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). The hepatocytes were seeded in plates, diluted to a final
concentration of 5×105 cells/ml and incubated at 37°C in
a humidified incubator with an atmosphere of 5% CO2.
OTA cytotoxicity detection by MTT
assays and alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) measurement
Hepatocytes were seeded in 96-well plates at a
density of 5×105 cells per well in 0.1 ml DMEM and were
exposed to increasing concentrations of OTA (0.25, 0.5, 1, 2 and 4
µg/ml) for 24 h. Cell viability was assayed by the MTT assay
[1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan Thiazolyl blue
formazan; cat. no. 57360-69-7; Sigma-Aldrich; Merck KGaA] (30). MTT stock solution (5 mg/ml) was
then applied to each of the wells, and the cells were incubated in
a humidified atmosphere for 4 h. The absorbance of the samples was
measured using a microtiter plate reader at a dual wavelength mode
of 490 and 655 nm. DMSO was used to dissolve the formazan. Cell
culture supernatants were collected and assayed for ALT (Reitman
Frankel assay) and AST activities using commercial kits (cat. nos.
C009-2 and C010-2; Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer's protocol.
Effects of CAG, L-Arg, Sil and GA on
OTA-induced hepatocyte injury
The protective effects of CAG, L-Arg, Sil and GA on
chicken hepatocyte injury was investigated in vitro.
Hepatocytes were seeded in 96-well plates at a density of
5×105 cells per well. Different batches of cells were
then incubated with CAG, L-Arg, Sil and GA at concentrations of
0.1, 1, and 10 µg/ml for 24 h at 37°C. Following incubation, the
supernatant was discarded, and the cells were exposed to OTA
concentrations of OTA 1 µg/ml for 24 h at 37°C. Cell viability was
determined using the MTT assay. The activities of AST and ALT in
cell culture supernatants were detected using commercial kits,
according to the manufacturer's protocol (cat. nos. C009-2 and
C010-2; Nanjing Jiancheng Bioengineering Institute).
Superoxide dismutase (SOD) activity,
and glutathione (GSH) and malondialdehyde (MDA) levels
Cells were then incubated with CAG, L-Arg, Sil and
GA at concentrations of 1 µg/ml for 24 h at 37°C. Following
incubation, the supernatant was discarded, and the cells were
exposed to OTA for 24 h at a concentration that induces death of
50% of the hepatocytes at 37°C. Following treatments, hepatocytes
were washed twice with 300 µl PBS (pH 7.4). The cell supernatants
were used to measure SOD activity and the levels of GSH and MDA
using SOD (Superoxide Dismutase assay kit; WST-1 method), GSH
(reduced glutathione assay kit) and MDA (malondialdehyde assay kit;
TBA method) kits (Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer's protocol.
Flow cytometric analysis of apoptosis
by annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) staining
Hepatocytes were seeded in 96-well plates at a
density of 5×105 cells per well. Different batches of
cells were then incubated with CAG, L-Arg, Sil and GA at
concentrations of 1 µg/ml for 24 h at 37°C. Following incubation,
the supernatant was discarded, and the cells were exposed to OTA
concentrations of OTA 1 µg/ml for 24 h at a concentration that
induces death of 50% of the hepatocytes at 37°C. In order to
evaluate apoptosis, cells were treated as above and subjected to
staining with an Annexin V-FITC/PI Apoptosis Detection kit
(Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), according to
the manufacturer's protocol. The apoptosis rates were analyzed by
flow cytometry Flowjo V10.0.7; FlowJo LLC, Ashland, OR, USA).
Relative quantification of
apoptosis-associated gene expression by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Hepatocytes were seeded in 96-well plates at a
density of 5×105 cells per well. Different batches of
cells were then incubated with CAG, L-Arg, Sil and GA at
concentrations of 1 µg/ml for 24 h at 37°C. Following incubation,
the supernatant was discarded, and the cells were exposed to OTA
concentrations of OTA 1 µg/ml for 24 h at a concentration that
induces death of 50% of the hepatocytes at 37°C. Total RNA from the
six groups i) the control group, ii) OTA group, iii) OTA-treated
hepatocytes pretreated with CAG, iv) OTA-treated hepatocytes
pretreated with L-Arg, v) OTA-treated hepatocytes pretreated with
Sil and vi) OTA-treated hepatocytes pretreated with GA) was
extracted using TRIzol reagent (Thermo Fisher Scientific, Inc.).
cDNA synthesis was performed with 1 µg total RNA using a cDNA
synthesis kit (HIScipt™Q RT SuperMIX for qPCR, R123-01; Vazyme,
Piscataway, NJ, USA; 42°C for 15 min, 85°C for 2 min). The mRNA
expression levels of caspase-3, B-cell lymphoma-2 (Bcl-2),
Bcl-2-associated X (Bax) and β-actin were quantified by qPCR
(AceQ® qPCR SYBR® Green Master Mix; Vazyme;
Stage 1: Reps: 1, 95°C for 5 min; Stage 2: Reps: 40, 95°C for 10
sec, 60°C for 30 sec; Stage 3: Reps: 1, 95°C for 15 sec, 60°C for
60 sec, 95°C for 15 sec; CFX96 Real-time PCR Detection System;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primer
sequences used are listed in Table
I. The relative expression of target genes was normalized to
β-actin. Data were calculated using the 2−ΔΔCq method
where ΔΔCq=(Cq, target-Cq, actin)
treatment-(Cq, target-Cq, actin) control
(31).
 | Table I.Primer sequences of target genes for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences of target genes for
reverse transcription-quantitative polymerase chain reaction.
| Target gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| β-actin |
ATGTGGATCAGCAAGCAGGAGTA |
TTTATGCGCATTTATGGGTTTTGT |
| Caspase-3 |
CTGAAGGCTCCTGGTTTA |
TGCCACTCTGCGATTTAC |
| Bax |
GTGATGGCATGGGACATAGCTC |
TGGCGTAGACCTTGCGGATAA |
| Bcl-2 |
ATCGTCGCCTTCTTCGAGTT |
ATCCCATCCTCCGTTGTCCT |
Statistical analysis
All the experiments were repeated three times. Data
are presented as the mean ± standard deviation. Tukey's post hoc
test of one-way analysis of variance was used for statistical
comparisons. Graphs were plotted using GraphPad Prism version 5
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Cytotoxicity of OTA in primary chicken
hepatocytes
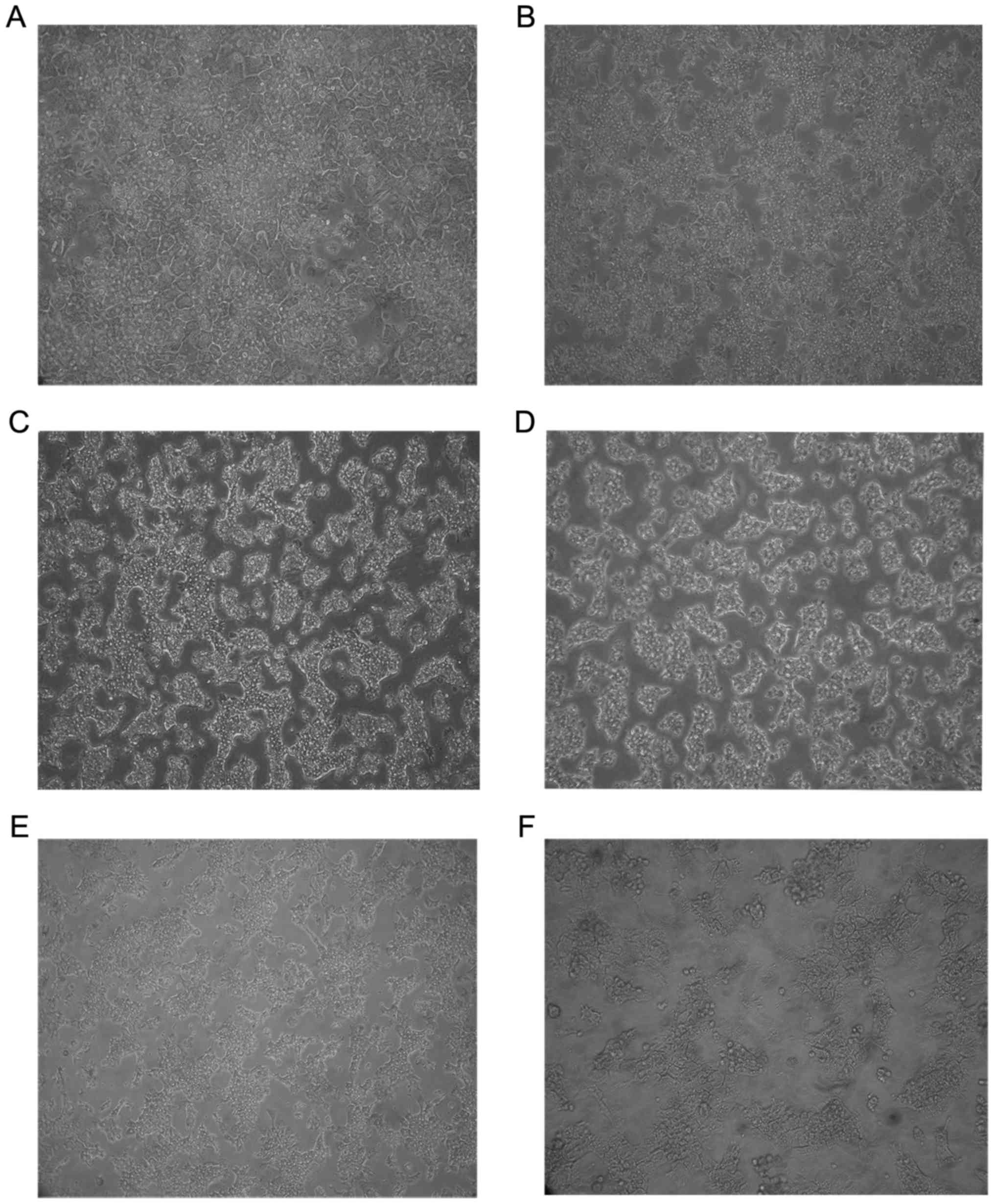
Compared with control hepatocytes (Fig. 1A), the cell number and density
decreased markedly and ruptured and necrotic hepatocytes were
present in the supernatant in hepatocytes treated with different
concentrations of OTA (Fig. 1B-F).
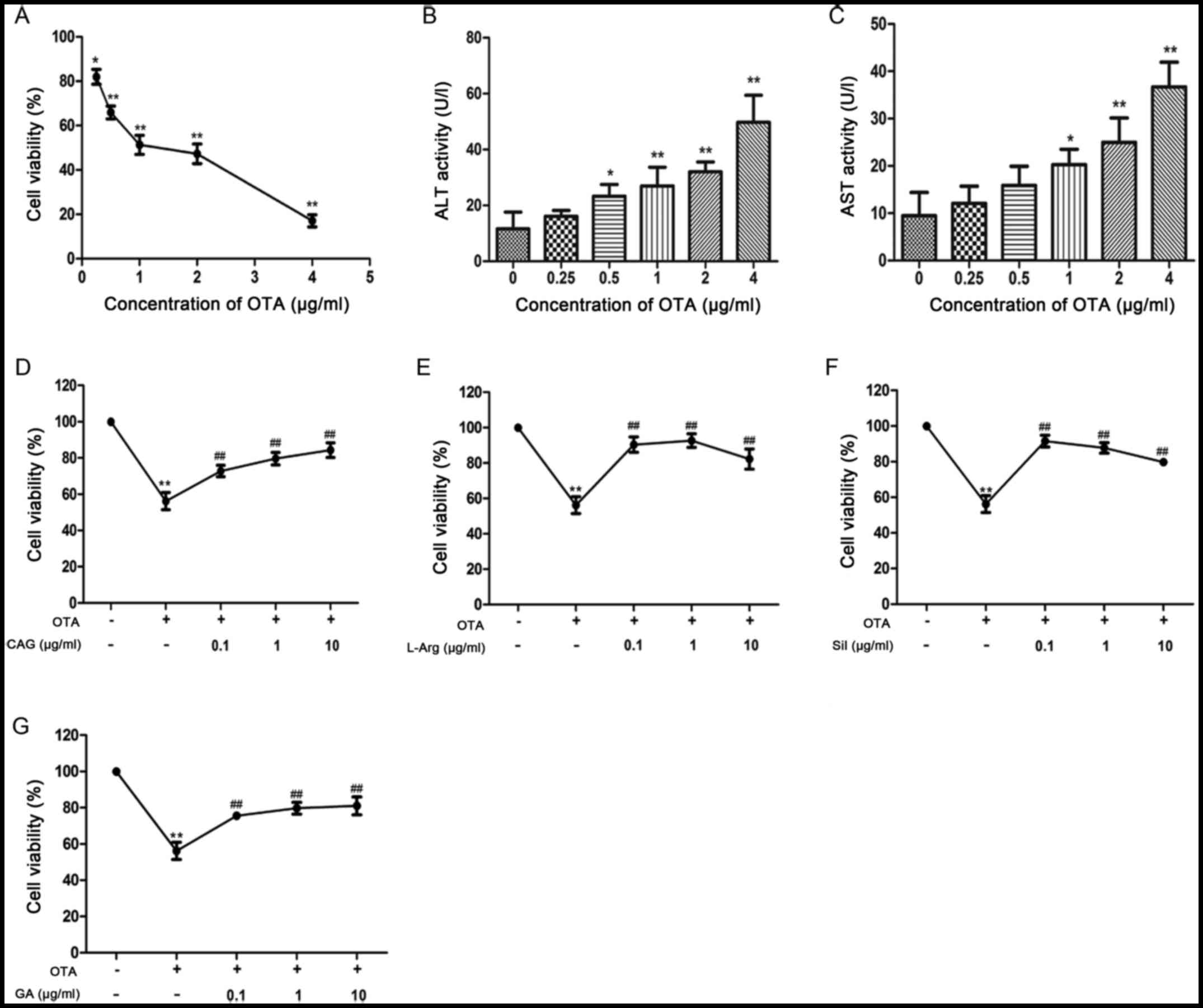
The inhibitory concentration 50 (IC50) was a standard to
determine the dose of OTA that induced liver injury. The
IC50 was estimated from Fig. 2A. Results of MTT assays revealed
that the cell viabilities were 51.33±4.27 and 47.28±4.42% when
hepatocytes were exposed to 1 and 2 µg/ml OTA, respectively
(Fig. 2A). As demonstrated in
Fig. 2B and C, the activities of
ALT and AST were dose-dependently increased compared with control
cells following treatment with different concentrations of OTA.
However, the activities of ALT and AST were significantly increased
at a dose of 1 µg/ml OTA compared with the control group
(P<0.05). These results indicated that OTA induced
hepatocellular injury and the optimum injury dose was 1 µg/ml as it
was the lowest dose to induce significant increases in ALT and
AST.
Effects of CAG, L-Arg, Sil and GA on
OTA-induced hepatocellular injury
As demonstrated in Fig.
2D-G, the cell viability was decreased compared with the
control group when exposed to OTA for 24 h. However, the cell
viabilities increased significantly when hepatocytes were
pretreated with CAG, L-Arg, Sil and GA at concentrations of 0.1, 1
and 10 µg/ml in comparison with the OTA-only group (Fig. 2D-G). CAG and GA dose-dependently
increased cell viability (Fig. 2D and
G). However, the cell viabilities following treatment with
L-Arg and Sil at a concentration of 10 µg/ml were lower than at the
other concentrations (Fig. 2E and
F). In addition, it was demonstrated that OTA treatment
increased ALT and AST activities in the cell culture supernatant of
hepatocytes, compared with control cells (P<0.01; Table II). In comparison with the OTA
group, CAG, L-Arg, Sil and GA decreased the ALT activities
(P<0.01; Table II); however,
CAG had no significant effect on the AST activity induced by
OTA.
 | Table II.Effects of four hepatoprotective
agents on the activities of ALT and AST in cell culture
supernatants of OTA-treated hepatocytes. |
Table II.
Effects of four hepatoprotective
agents on the activities of ALT and AST in cell culture
supernatants of OTA-treated hepatocytes.
| Group | ALT (U/l) | AST (U/l) |
|---|
| Control | 14.36±2.85 | 11.53±2.29 |
| OTA (1 µg/ml) |
32.19±4.14a |
23.38±1.45a |
| CAG (µg/ml) |
|
|
|
0.1 |
21.30±4.10c | 20.47±4.73 |
| 1 |
15.15±3.25c | 18.61±3.29 |
| 10 |
14.46±5.55c | 19.23±3.04 |
| L-Arg (µg/ml) |
|
|
|
0.1 |
17.56±4.57c | 18.54±4.19 |
| 1 |
20.94±2.76c |
16.43±3.14b |
| 10 |
17.02±2.19c |
18.08±2.20b |
| Sil (µg/ml) |
|
|
|
0.1 |
11.80±3.79c |
17.68±0.36c |
| 1 |
18.40±5.76c |
11.40±0.67c |
| 10 |
21.63±4.00c |
16.81±3.63c |
| GA (µg/ml) |
|
|
|
0.1 |
19.36±5.22c |
14.28±1.19c |
| 1 |
18.49±3.06c |
11.08±1.03c |
| 10 |
14.71±2.36c |
13.32±3.19c |
Effects of CAG, L-Arg, Sil and GA on
SOD activity, and GSH and MDA levels
Treating liver cells with 1 µg/ml OTA for 24 h
reduced the SOD activity and GSH levels (Fig. 3A and B), while OTA treatment
resulted in an increase in MDA (Fig.
3C), compared with control cells. Pretreatment with CAG, L-Arg,
Sil and GA (1 µg/ml) for 24 h resulted in a significant increase in
SOD activity and GSH levels (P<0.05; Fig. 3A and B) and a decrease in MDA
levels (P<0.05; Fig. 3C),
compared with the OTA-only group. Sil exhibited the largest
significant differences compared with the OTA treatment group.
These results indicate that Sil may exhibit an enhanced
antioxidation activity compared with the other three
hepatoprotective agents employed in the current study.
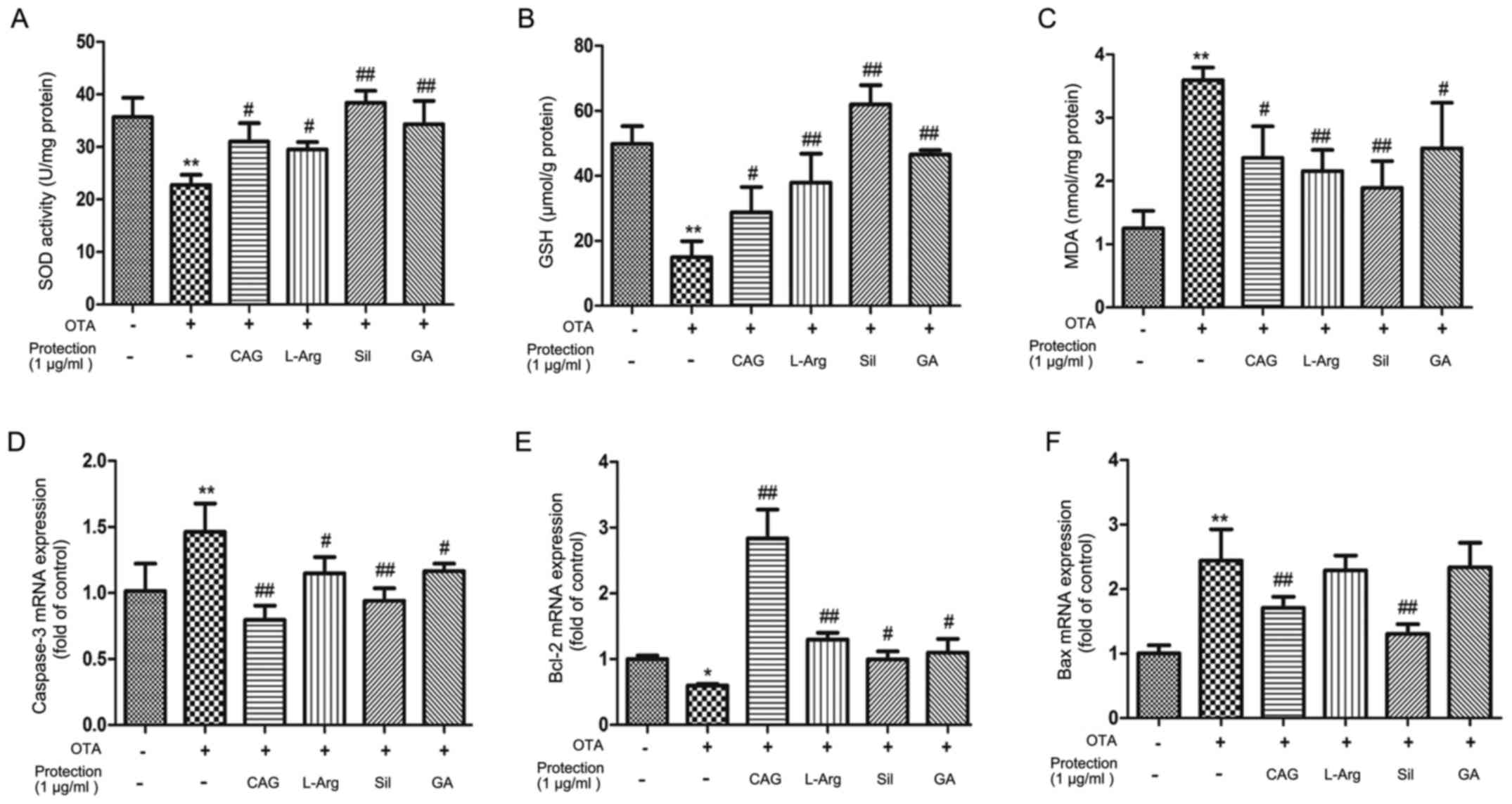 | Figure 3.The effects of CAG, L-Arg, Sil and GA
pretreatment on oxidative stress and cellular antioxidant enzymes.
The levels of (A) SOD, (B) GSH and (C) MDA were measured in
hepatocytes following treatment with 1 µg/ml OTA with or without 1
µg/ml CAG, L-Arg, Sil and GA. The effect of CAG, L-Arg, Sil and GA
on OTA-induced alterations in the mRNA expression of hepatocellular
genes associated with apoptosis, including (D) caspase-3, (E) Bcl-2
and (F) Bax. *P<0.05 and **P<0.01 vs. control group;
#P<0.05 and ##P<0.01 vs. OTA group.
CAG, compound ammonium glycyrrhizin; L-Arg, L-arginine; Sil,
silymarin; GA, glucurolactone; SOD, superoxide dismutase; GSH,
glutathione; MDA, malondialdehyde; OTA, ochratoxin A; Bcl-2, B-cell
lymphoma-2; Bax, Bcl-2-associated X. |
Expression of apoptosis-associated
genes
The mRNA expression levels of caspase-3 increased
following OTA treatment, compared with control cells, and decreased
following pretreatment with CAG, L-Arg, Sil and GA, compared with
the OTA-only group (Fig. 3D). The
mRNA expression levels of Bcl-2 decreased compared with control
cells following OTA treatment, and increased following pretreatment
with CAG, L-Arg, Sil and GA in OTA-treated cells (Fig. 3E). Although the mRNA expression
levels of Bax increased compared with control cells following OTA
treatment, and significantly decreased following treatment with CAG
and Sil in OTA-treated cells, Bax levels were not significantly
altered in OTA-treated cells following pretreatment with L-Arg and
GA (Fig. 3F).
Effects of CAG, L-Arg, Sil and GA on
OTA-induced apoptosis
The apoptosis rate (only the Q3 quadrant) was
determined in chicken hepatocytes by flow cytometry, and the
results demonstrated that exposure to OTA induced a significant
increase in apoptosis compared with control cells (Fig. 4A and B, and Table III; P<0.01). Following
pretreatment with CAG, L-Arg, Sil and GA, the apoptotic rate
decreased to 14.90±1.50, 18.76±1.55, 12.86±2.12 and 27.50±2.16%,
respectively (Table III).
Representative flow cytometry plots are given for CAG, L-Arg, Sil
and GA pretreatment groups in Fig.
4C-F.
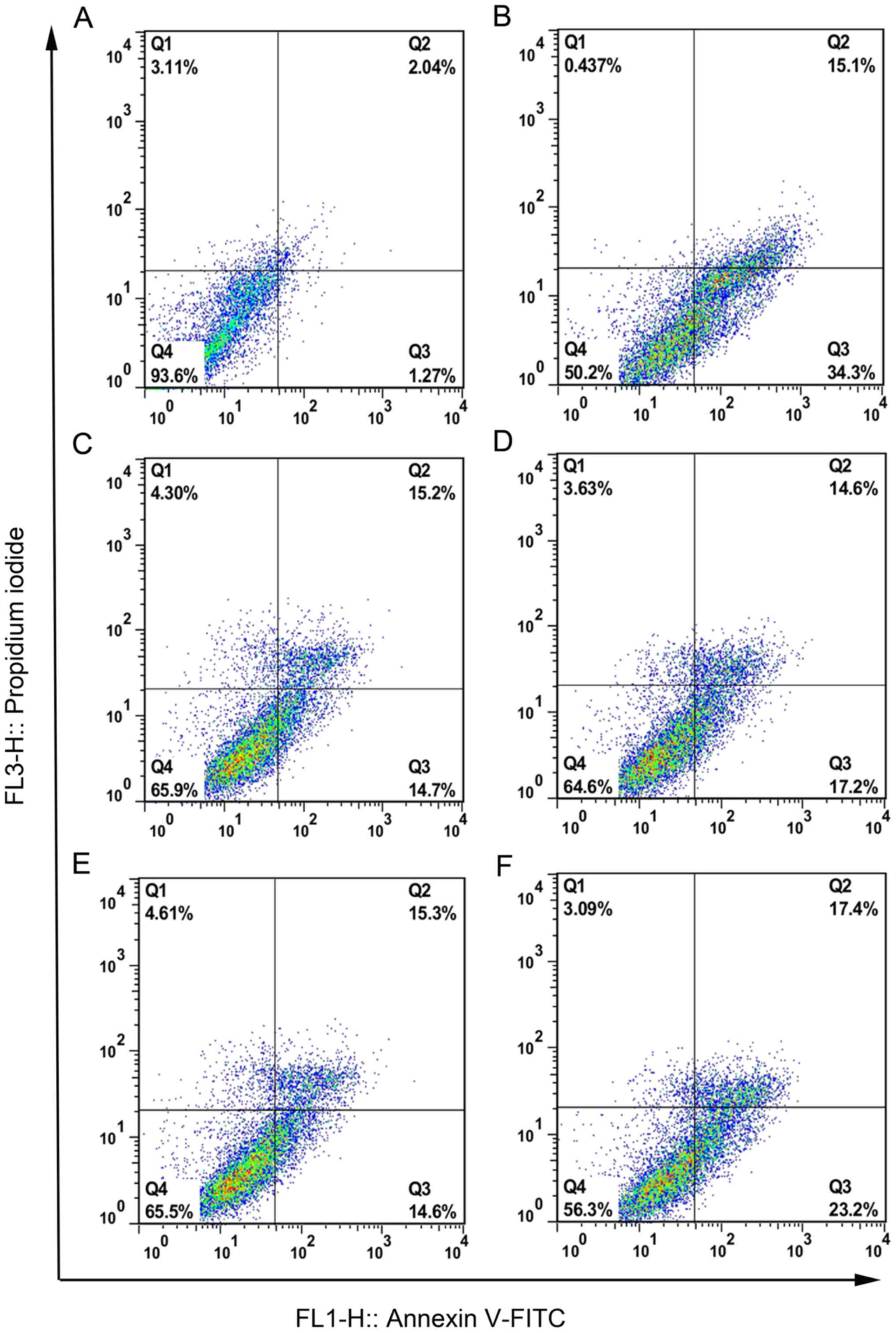 | Figure 4.The effects of CAG, L-Arg, Sil and GA
pretreatment on apoptosis in OTA-treated chicken hepatocytes.
Representative flow cytometry plots are presented for (A) control
group, (B) OTA group, (C) OTA-treated hepatocytes pretreated with
CAG, (D) OTA-treated hepatocytes pretreated with L-Arg, (E)
OTA-treated hepatocytes pretreated with Sil and (F) OTA-treated
hepatocytes pretreated with GA. Q3 indicated early apoptotic cells.
CAG, compound ammonium glycyrrhizin; L-Arg, L-arginine; Sil,
silymarin; GA, glucurolactone; OTA, ochratoxin A; FITC, fluorescein
isothiocyanate. |
 | Table III.Early apoptosis rate in different
treatment groups. |
Table III.
Early apoptosis rate in different
treatment groups.
| Group | Apoptosis rate
(%) |
|---|
| Control | 1.80±0.45 |
| OTA |
32.06±2.11a |
| CAG |
14.90±1.50c |
| L-Arg |
18.76±1.55c |
| Sil |
12.86±2.12c |
| GA |
27.50±2.16b |
Discussion
OTA is a mycotoxin contaminant of food that
primarily leads to nephrotoxicity and hepatotoxicity (3,32).
OTA has a stronger toxicity compared with the other mycotoxins,
excluding aflatoxin (2). Li et
al (33) reported that OTA was
slightly more effective at reducing cell viability in HepG2 cells,
with an effective concentration 50 (EC50) of 37.30 µM,
compared with zearalenone (ZEA), which had an EC50 of
41.28 µM. In addition, OTA was demonstrated to reduce the cell
viability of KK-1 murine granular cells to a larger degree compared
with ZEA, with an EC50 ~7-fold lower compared with the
EC50 for ZEA (34). These results
indicated that OTA may exhibit a stronger effect than ZEA on cell
viability. Klarić et al (35) demonstrated that the IC50
of OTA and citrinin (CTN) in PK15 porcine kidney cells were
14.0±2.4 and 73.5±1.0 µM, respectively. Therefore, the toxicity of
OTA was much higher than CTN. Wilk-Zasadna and Minta (36) demonstrated that rat embryo midbrain
micromass cells exposed to OTA exhibited a dose-dependent reduction
in viability, and the IC50 was 2.52±0.062 µg/ml. In the
present study, the IC50 of OTA in chicken hepatocytes
was estimated to be 1 µg/ml, which was lower than in OTA-treated
rat embryo midbrain micromass cells (36). This indicates that chicken
hepatocytes may be more susceptible to OTA.
Oxidative stress is characterized by an imbalance
between pro-oxidant and antioxidant molecules, which subsequently
results in damage to cells. Various methods are employed to assess
the levels of oxidative stress, and these methods involve the
quantification of products of peroxidation or antioxidants
(37). In the present study, as an
end-product of lipid peroxidation, which is among the processes
implicated in oxidative stress-induced damage and is associated
with mycotoxin-induced cytotoxicity, MDA was selected as a measure
of oxidative stress and hepatocyte damage (38). By contrast, SOD and GSH are
reported to protect host cells against oxidative damage by
scavenging free radicals. Zheng et al (39) confirmed that OTA led to the
induction of oxidative damage in HepG2 cells, while Klarić et
al (40) demonstrated that OTA
also induced marked oxidative stress in the porcine kidney. The
present study demonstrated high levels of MDA, and decreased SOD
activity and GSH concentration, in cell lysates collected from the
OTA group, which indicated that OTA may have triggered oxidative
damage to the cell membranes of hepatocytes. This concurs with the
study by Klarić et al (40); however, a lower concentration was
used in the present study compared with this previous study.
Liver damage induced by hepatotoxins may be a result
of increased hepatocyte apoptosis, which is a form of programmed
cell death. Apoptosis facilitates the removal of damaged cells
(41). A previous study
demonstrated that OTA-induced apoptosis was a result the activation
of a mitochondrion-dependent pathway, where OTA led to increased
ROS formation and decreased mitochondrial transmembrane potential
through mitochondrial pore opening, subsequently allowing
cytochrome c release and the downstream activation of caspases
(42). El Golli Bennour et
al (43) also demonstrated
that exposure of human hepatocarcinoma cells to OTA led to the
induction of caspase-dependent apoptosis via the mitochondrial
pathway. In the present study, the apoptotic rate was determined by
flow cytometry and measuring the expression of target genes
associated with the mitochondrion-dependent apoptotic pathway by
RT-qPCR. The apoptotic rate was 32.06±2.11% when the cells were
exposed to 1 µg/ml OTA for 24 h. In addition, the mRNA expression
of caspase-3 and Bax increased, while Bcl-2 decreased, compared
with control cells. Therefore, OTA may induce chicken
hepatocellular apoptosis by activating the mitochondrion-dependent
pathway.
CAG, L-Arg, Sil and GA are commonly employed in the
clinic as hepatoprotective agents. Glycyrrhizic acid is a
triterpene saponin glycoside and the major bioactive compound of
Glyccyrhiza glabra (liquorice) plant root extract, which is
a member of the leguminosae family (44,45).
In Japan, glycyrrhizic acid has been employed in the clinic for
>20 years in patients suffering from chronic hepatitis (46). Among the 20 most numerous amino
acids in mammals, L-Arg is considered to be a semi-essential or
conditionally essential amino acid, depending on the developmental
stage and health status of each individual (47). L-Arg is the precursor in nitric
oxide synthesis (48). Sil is a
flavonoid mixture extracted from Silybum marium (49). GA is a natural compound that
functions as an essential structural component of the majority of
connective tissues (50). The
liver produces GA from glucose, which subsequently acts as an
inhibitor of the B-glucuronidase enzyme, a metabolizer of
glucuronides, which leads to increases in the blood levels of
glucuronide. Glucuronides interact with various toxic compounds,
including morphine and depot medroxyprogesterone acetate, and
convert them to water-soluble glucuronide-conjugates, which allows
them to be excreted via the urine. Yin et al (51) reported that pretreatment, and a
combination of pre- and post-treatment, of hepatocytes with
Glycyrrhiza glabra extract significantly reversed carbon
tetrachloride-induced increases in lactate dehydrogenase, glutamate
oxalate transaminase, glutamate pyruvate transaminase and MDA, and
increased levels of SOD and glutathione peroxidase that were
reduced by treatment with carbon tetrachloride. Shweta and Khanna
(52) reported that L-Arg
increased SOD and GSH levels in newborns. In the present study,
similar findings were obtained. Following pretreatment with Sil for
24 h, the cell viability increased, and the activity of ALT and AST
decreased. Kumar et al (53) demonstrated that Sil liposomes
prevented the paracetamol-induced decreases in GSH and SOD levels,
and increases in MDA, which are responsible for the toxic effect of
paracetamol. GA is commonly employed to protect the liver, however,
there are few reports concerning the underlying hepatoprotective
mechanism. The current study investigated the mechanism of GA in
protecting the liver by using chicken primary hepatocytes. The
results demonstrated that CAG, L-Arg, Sil and GA improved cell
viability and inhibited the elevation of ALT. Arg, Sil and GA also
decreased the activity of AST in supernatants, but CAG exhibit no
effect on AST activity. The cell viability increased and ALT
activity was decreased in a dose-dependent manner following
treatment with CAG and GA. L-Arg exhibited an optimum protective
effect at a lower dose. Sil had optimum function at a dose of 1
µg/ml. These observations indicated that CAG, Arg, Sil and GA may
protect the viability of chicken hepatocytes. CAG, L-Arg, Sil and
GA increased the levels of SOD and GSH, and decreased MDA levels,
with Sil exhibiting the largest effect of the four agents. These
results demonstrated that the four hepatoprotective agents employed
in the present study may exhibit anti-oxidative effects in chicken
hepatocytes.
Glycyrrhizic acid was reported to exhibit an
antiapoptotic effect via the suppression of caspase-3, which may
explain the hepatoprotective effect of glycyrrhizic acid (54). Glycyrrhizic acid has also been
demonstrated to inhibit cytochrome c release into the cytoplasm
from the mitochondria. Tuorkey (55) reported that Sil may protect
cardiomyocytes against apoptosis induced by diabetes. The
sarcoplasm of diabetic rats that received Sil treatment had an
appearance that was similar to that of non-diabetic rats. There are
few reports investigating the antiapoptotic effects of L-Arg and
GA. In the present study, cell apoptosis rates were decreased when
pretreated with CAG, L-Arg, Sil and GA, in comparison with the OTA
group. The antiapoptotic ability of the four drugs was
Sil>CAG>L-Arg>GA. These hepatoprotective drugs decreased
the mRNA expression levels of caspase-3 and increased Bcl-2
expression, but no effects of L-Arg and GA were observed on Bax
mRNA expression levels in OTA-treated cells. It was therefore
concluded that the four hepatoprotective agents used in the current
study exhibited an antiapoptotic effect in chicken hepatocytes. In
addition, CAG and GA are likely to induce their effects through the
mitochondrion-dependent pathway, while the exact hepatoprotective
mechanisms of L-Arg and GA requires further research.
In conclusion, in vitro cell culture assays
have contributed to OTA research by investigating the biochemical
mechanisms of cytotoxicity. OTA may induce hepatotoxicity in
chicken primary hepatocytes. The results of the current study
indicate that oxidative stress and apoptosis may be implicated in
OTA-induced hepatocellular injury. OTA is likely to mediate its
effects through the mitochondrion-dependent apoptotic pathway. The
present findings also demonstrated the hepatoprotective,
antioxidant activities and antiapoptotic effects of CAG, L-Arg, Sil
and GA in OTA-treated cultured hepatocytes of chickens. The present
study demonstrates the mechanisms of OTA in chicken hepatocytes and
that CAG, L-Arg, Sil and GA administration may be used an
alternative therapy to treat or prevent acute hepatic damage.
Acknowledgements
Not applicable.
Funding
The present study was supported by the program for
National Natural Science Foundation of China (grant no.
31572569).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY designed, screened and optimized the formulation.
FW and JT performed the experiments and wrote the paper. XG and RA
did the preparation of the experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Nanjing Agricultural University (Nanjing,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coronel MB, Sanchis V, Ramos AJ and Marin
S: Review. Ochratoxin A: Presence in human plasma and intake
estimation. Food Sci Technol Int. 16:5–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Brien E and Dietrich DR: Ochratoxin A:
The continuing enigma. Crit Rev Toxicol. 35:33–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ringot D, Chango A, Schneider YJ and
Larondelle Y: Toxicokinetics and toxicodynamics of ochratoxin A, an
update. Chem Biol Interact. 159:18–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skaug MA, Helland I, Solvoll K and
Saugstad OD: Presence of ochratoxin A in human milk in relation to
dietary intake. Food Addit Contam. 18:321–327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
International Agency for Research on
Cancer: Some naturally occurring substances: Food items and
constituents, heterocyclic aromatic amines and mycotoxins, .
Apresentado em: IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans: Some Naturally Occurring Substances:
Food Items and Constituents. Lyon: 1992
|
|
6
|
Malir F, Ostry V, Pfohl-Leszkowicz A and
Novotna E: Ochratoxin A: Developmental and reproductive toxicity-an
overview. Birth Defects Res B Dev Reprod Toxicol. 98:493–502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dopp E, Müller J, Hahnel C and Schiffmann
D: Induction of genotoxic effects and modulation of the
intracellular calcium level in syrian hamster embryo (SHE)
fibroblasts caused by ochratoxin A. Food Chem Toxicol. 37:713–721.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eder S, Benesic A, Freudinger R, Engert J,
Schwerdt G, Drumm K and Gekle M: Nephritogenic ochratoxin A
interferes with mitochondrial function and pH homeostasis in
immortalized human kidney epithelial cells. Pflugers Arch.
440:521–529. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schaaf GJ, Nijmeijer SM, Maas RF,
Roestenberg P, de Groene EM and Fink-Gremmels J: The role of
oxidative stress in the ochratoxin A-mediated toxicity in proximal
tubular cells. Biochim Biophys Acta. 1588:149–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ehrlich V, Darroudi F, Uhl M, Steinkellner
H, Gann M, Majer BJ, Eisenbauer M and Knasmüller S: Genotoxic
effects of ochratoxin A in human-derived hepatoma (HepG2) cells.
Food Chem Toxicol. 40:1085–1090. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hundhausen C, Bösch-Saadatmandi C,
Augustin K, Blank R, Wolffram S and Rimbach G: Effect of vitamin E
and polyphenols on ochratoxin A-induced cytotoxicity in liver
(HepG2) cells. J Plant Physiol. 162:818–822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arbillaga L, Azqueta A, Ezpeleta O and de
Cerain López A: Oxidative DNA damage induced by ochratoxin A in the
HK-2 human kidney cell line: Evidence of the relationship with
cytotoxicity. Mutagenesis. 22:35–42. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guerra M, Galvano F, Bonsi L, Speroni E,
Costa S, Renzulli C and Cervellati R:
Cyanidin-3-O-beta-glucopyranoside, a natural free-radical scavenger
against aflatoxin B1-and ochratoxin A-induced cell damage in a
human hepatoma cell line (Hep G2) and a human colonic
adenocarcinoma cell line (CaCo-2). Br J Nutr. 94:211–220. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamp HG, Eisenbrand G, Schlatter J, Würth
K and Janzowski C: Ochratoxin A: Induction of (oxidative) DNA
damage, cytotoxicity and apoptosis in mammalian cell lines and
primary cells. Toxicology. 206:413–425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Yin S, Dong Y, Fan L and Hu H: p53
activation inhibits ochratoxin A-induced apoptosis in monkey and
human kidney epithelial cells via suppression of JNK activation.
Biochem Biophys Res Commun. 411:458–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li JY, Cao HY, Liu P, Cheng GH and Sun MY:
Glycyrrhizic acid in the treatment of liver diseases: Literature
review. Biomed Res Int. 2014:8721392014.PubMed/NCBI
|
|
17
|
Arase Y, Ikeda K, Murashima N, Chayama K,
Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M and Kumada H:
The long term efficacy of glycyrrhizin in chronic hepatitis C
patients. Cancer. 79:1494–1500. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishimoto Y, Hisatsune A, Katsuki H,
Miyata T, Yokomizo K and Isohama Y: Glycyrrhizin attenuates mucus
production by inhibition of MUC5AC mRNA expression in vivo and in
vitro. J Pharmacol Sci. 113:76–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Visavadiya NP and Narasimhacharya AV:
Hypocholesterolaemic and antioxidant effects of Glycyrrhiza
glabra (Linn) in rats. Mol Nutr Food Res. 50:1080–1086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh HM, Lee S, Park YN, Choi EJ, Choi JY,
Kim JA, Kweon JH, Han WC, Choi SC, Han JK, et al: Ammonium
glycyrrhizinate protects gastric epithelial cells from hydrogen
peroxide-induced cell death. Exp Biol Med (Maywood). 234:263–277.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Račková L, Jančinová V, Petríková M,
Drábiková K, Nosál R, Stefek M, Kostálová D, Prónayová N and
Kovácová M: Mechanism of anti-inflammatory action of liquorice
extract and glycyrrhizin. Nat Prod Res. 21:1234–1241. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yim SB, Park SE and Lee CS: Protective
effect of glycyrrhizin on 1-methyl-4-phenylpyridinium-induced
mitochondrial damage and cell death in differentiated PC12 cells. J
Pharmacol Exp Ther. 321:816–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lass A, Suessenbacher A, Wölkart G, Mayer
B and Brunner F: Functional and analytical evidence for scavenging
of oxygen radicals by L-arginine. Mol Pharmacol. 61:1081–1088.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu G, Bazer FW, Davis TA, Kim SW, Li P,
Rhoads Marc J, Satterfield Carey M, Smith SB, Spencer TE and Yin Y:
Arginine metabolism and nutrition in growth, health and disease.
Amino Acids. 37:153–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu G, Davis PK, Flynn NE, Knabe DA and
Davidson JT: Endogenous synthesis of arginine plays an important
role in maintaining arginine homeostasis in postweaning growing
pigs. J Nutr. 127:2342–2349. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen CH, Huang TS, Wong CH, Hong CL, Tsai
YH, Liang CC, Lu FJ and Chang WH: Synergistic anti-cancer effect of
baicalein and silymarin on human hepatoma HepG2 cells. Food Chem
Toxicol. 47:638–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jose MA, Abraham A and Narmadha M: Effect
of silymarin in diabetes mellitus patients with liver diseases. J
Pharmacol Pharmacother. 2:287–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guan S, Gong M, Yin Y, Huang R, Ruan Z,
Zhou T and Xie M: Occurrence of mycotoxins in feeds and feed
ingredients in China. J Food Agric Environ. 9:163–167. 2011.
|
|
29
|
Yu Z, Wu F, Tian J, Guo X, An R and Guo Y:
Ammonium glycyrrhizin counteracts liver injury caused by
lipopolysaccharide/amoxicillin-clavulanate potassium. Oncotarget.
8:96837–96851. 2017.PubMed/NCBI
|
|
30
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schefe JH, Lehmann KE, Buschmann IR, Unger
T and Funke-Kaiser H: Quantitative real-time RT-PCR data analysis:
Current concepts and the novel ‘gene expression's C T difference’
formula. J Mol Med (Berl). 84:901–910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Petzinger E and Ziegler K: Ochratoxin A
from a toxicological perspective. J Vet Pharmacol Ther. 23:91–98.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Zhang B, He X, Cheng WH, Xu W, Luo
Y, Liang R, Luo H and Huang K: Analysis of individual and combined
effects of ochratoxin A and zearalenone on HepG2 and KK-1 cells
with mathematical models. Toxins (Basel). 6:1177–1192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, He X, Yang X, Huang K, Luo Y, Zhu L,
Li Y and Xu W: Zinc inhibits the reproductive toxicity of
Zearalenone in immortalized murine ovarian granular KK-1 cells. Sci
Rep. 5:142772015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klarić MŠ, Želježić D, Rumora L, Peraica
M, Pepeljnjak S and Domijan AM: A potential role of calcium in
apoptosis and aberrant chromatin forms in porcine kidney PK15 cells
induced by individual and combined ochratoxin A and citrinin. Arch
Toxicol. 86:97–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wilk-Zasadna I and Minta M: Developmental
toxicity of ochratoxin A in rat embryo midbrain micromass cultures.
Int J Mol Sci. 10:37–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hassen W, Ayed-Boussema I, Oscoz AA, Ade
Lopez C and Bacha H: The role of oxidative stress in
zearalenone-mediated toxicity in Hep G2 cells: Oxidative DNA
damage, gluthatione depletion and stress proteins induction.
Toxicology. 232:294–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abid-Essefi S, Ouanes Z, Hassen W,
Baudrimont I, Creppy E and Bacha H: Cytotoxicity, inhibition of DNA
and protein syntheses and oxidative damage in cultured cells
exposed to zearalenone. Toxicol In Vitro. 18:467–474. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng J, Zhang Y, Xu W, Luo Y, Hao J, Shen
XL, Yang X, Li X and Huang K: Zinc protects HepG2 cells against the
oxidative damage and DNA damage induced by ochratoxin A. Toxicol
Appl Pharmacol. 268:123–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klarić MS, Pepeljnjak S, Domijan A-M and
Petrik J: Lipid peroxidation and glutathione levels in porcine
kidney PK15 cells after individual and combined treatment with
fumonisin B1, beauvericin and ochratoxin A. Basic Clin Pharmacol
Toxicol. 100:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lawen A: Apoptosis-an introduction.
Bioessays. 25:888–896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bouaziz C, el dein Sharaf O, Martel C, El
Golli E, Abid-Essefi S, Brenner C, Lemaire C and Bacha H: Molecular
events involved in ochratoxin A induced mitochondrial pathway of
apoptosis, modulation by Bcl-2 family members. Environ Toxicol.
26:579–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
El Golli Bennour E, Rodriguez-Enfedaque A,
Bouaziz C, Ladjimi M, Renaud F and Bacha H: Toxicities induced in
cultured human hepatocarcinoma cells exposed to ochratoxin A:
Oxidative stress and apoptosis status. J Biochem Mol Toxicol.
23:87–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ming LJ and Yin A: Therapeutic effects of
glycyrrhizic acid. Nat Prod Commun. 8:415–418. 2013.PubMed/NCBI
|
|
45
|
Lee CH, Park SW, Kim YS, Kang SS, Kim JA,
Lee SH and Lee SM: Protective mechanism of glycyrrhizin on acute
liver injury induced by carbon tetrachloride in mice. Biol Pharm
Bull. 30:1898–1904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Van Rossum T, Vulto AG, de Man RA, Brouwer
JT and Schalm SW: Review article: Glycyrrhizin as a potential
treatment for chronic hepatitis C. Aliment Pharmacol Ther.
12:199–205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tapiero H, Mathé G, Couvreur P and Tew K:
I. Arginine. Biomed Pharmacother. 56:439–445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Andrew PJ and Mayer B: Enzymatic function
of nitric oxide synthases. Cardiovasc Res. 43:521–531. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Soto C, Pérez J, García V, Uría E, Vadillo
M and Raya L: Effect of silymarin on kidneys of rats suffering from
alloxan-induced diabetes mellitus. Phytomedicine. 17:1090–1094.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stanford D and Stachulski AV: Convenient
syntheses of deoxypyranose sugars from glucuronolactone.
Tetrahedron Lett. 48:2361–2364. 2007. View Article : Google Scholar
|
|
51
|
Yin G, Cao L, Xu P, Jeney G, Nakao M and
Lu C: Hepatoprotective and antioxidant effects of Glycyrrhiza
glabra extract against carbon tetrachloride (CCl(4))-induced
hepatocyte damage in common carp (Cyprinus carpio). Fish
Physiol Biochem. 37:209–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dr S and Khanna A: The effect of antenatal
L-arginine and antioxidant supplementation on oxidative stress
marker levels in newborns. J Clin Diagn Res. 8:OC10–OC12.
2014.PubMed/NCBI
|
|
53
|
Kumar N, Rai A, Reddy ND, Raj PV, Jain P,
Deshpande P, Mathew G, Kutty NG, Udupa N and Rao CM: Silymarin
liposomes improves oral bioavailability of silybin besides
targeting hepatocytes, and immune cells. Pharmacol Rep. 66:788–798.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
El-Tahawy NF, Ali AH, Saied SR and
Abdel-Wahab Z: Effect of glycyrrhizin on
lipopolysaccharide/D-galactosamine-induced acute hepatitis in
albino rats: A histological and immunohistochemical study. Egypt J
Histol. 34:518–527. 2011. View Article : Google Scholar
|
|
55
|
Tuorkey MJ, El-Desouki NI and Kamel RA:
Cytoprotective effect of silymarin against diabetes-induced
cardiomyocyte apoptosis in diabetic rats. Biomed Environ Sci.
28:36–43. 2015.PubMed/NCBI
|