Introduction
Cardiovascular disease is currently the global
leading cause of mortality (1). It
has previously been reported that acute myocardial infarction is a
major cause of cardiovascular disease (2). When coronary artery occlusion occurs,
in order to save the myocardial tissues, the infarct-associated
artery is promptly opened and blood supply is restored to the
ischemic myocardium. However, reperfusion causes damage to the
cardiac structure, leading to the occurrence of cardiac arrest,
decreased cardiac function and malignant cardiac arrhythmia. Such a
phenomenon is known as ischemia/reperfusion (I/R) injury (3). I/R injury is a clinicopathological
process in which varying levels of cardiomyocyte apoptosis can
occur (4). I/R injury also induces
oxidative stress (5,6); therefore, inhibition of apoptosis and
oxidative stress may reduce damage.
Recently, studies have demonstrated that traditional
Chinese medicine may be effective in the prevention and treatment
of myocardial I/R injury (7,8).
According to traditional Chinese medicine, Radix Paeoniae Rubra,
the dried root of Paeonia lactiflora Pall, is able to reduce
heat and cool blood, disperse stasis and relieve pain (9–11).
Total paeony glycoside (TPG) is a major component of Radix Paeoniae
Rubra, and the effective component of TPG is a monoterpene compound
(12). Modern pharmacological
studies have demonstrated that Radix Paeoniae Rubra has various
pharmacological functions that mainly produce effects on the
cardiovascular system (9,13,14).
In addition, it exerts antitumor and anti-endotoxin effects, as
well as other functions (9,13,14).
Furthermore, TPG has been used to treat cancer, atherosclerosis and
ischemic cerebrovascular disease in China (15,16).
Previous studies have demonstrated that TPG is capable of
preventing thrombosis, of inhibiting and scavenging oxygen free
radicals, and of suppressing cell apoptosis (15,17,18);
it also has the potential to effectively protect liver and brain
cells (15,19,20).
However, whether it has such a protective effect on myocardial I/R
injury remains unknown.
The phosphatidylinositol 3 kinase/protein kinase B
(PI3K/Akt) signal transduction pathway is a critical pathway that
serves a pivotal role in cell proliferation, apoptosis and
differentiation (21,22). Furthermore, studies have
demonstrated that the PI3K/Akt pathway is involved in I/R injury in
various organs, including liver, brain and heart (23–25).
However, whether the role of TPG in I/R injury is associated with
this pathway remains unknown.
In the present study, the rat cardiac myoblast cell
line H9C2 was selected to establish an I/R injury model in
vitro. The effects and mechanism of TPG on the I/R-induced
apoptosis and oxidative stress of H9C2 cells were subsequently
investigated.
Materials and methods
Drug preparation
TPG powder was obtained from Haoxuan Biological
Technology Co., Ltd. (Xi'an, China), which had been approved by the
State Food and Drug Administration for clinical trials. The powder
was dissolved in PBS, and the solution was filtered and sterilized
using a 0.22-µm filter membrane (EMD Millipore, Billerica, MA,
USA). Following filtration and sterilization, the solution was
diluted to 10, 20, 40, 80, 160 and 320 µg/ml using PBS.
Insulin-like growth factor (IGF)-1 was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany); the final
concentration used to activate PI3K/Akt was 100 ng/ml (26,27).
Cell culture
The H9C2 rat cardiac myoblast cell line was acquired
from Cobioer Biosciences Co., Ltd. (Nanjing, China). Cells were
maintained in complete high-glucose Dulbecco's Modified Eagle
Medium (DMEM; Beijing Solarbio Science & Technology Co, Ltd.,
Beijing, China) supplemented with 10% fetal bovine serum (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) and 1%
penicillin-streptomycin (Beijing Leagene Biotechnology Co., Ltd.,
Beijing, China) in an incubator containing 95% humidified air and
5% CO2 at 37°C.
Establishment of a myocardial I/R
injury model
H9C2 cells were selected to establish an I/R model,
according to a previous study (28). Briefly, cells were cultured at 37°C
for 48 h (95% humidified air and 5% CO2). After cell
culture, cells were incubated in serum- and glucose-free DMEM and
were maintained in a low-oxygen incubator at 37°C (95%
N2, 5% CO2 and 1% O2) for 10 h, in
order to mimic hypoxia. Subsequently, cells were cultured in
complete high-glucose DMEM and were maintained in an incubator
containing 95% humidified air and 5% CO2 at 37°C for 2
h, in order to mimic re-oxygenation (reperfusion). Cells in the
control group were cultured without any treatment at 37°C (95%
humidified air and 5% CO2).
Cell Counting kit-8 (CCK-8) assay
Cell viability was evaluated using CCK-8 (Wuhan
Merck Biotechnology Co., Ltd., Wuhan, China), according to the
manufacturer's protocol. Briefly, cells were incubated in 96-well
plates (2.5×103 cells/well) for 24 h at 37°C.
Subsequently, a portion of cells was treated with various
concentrations of TPG (10, 20, 40, 80, 160 and 320 µg/ml) for 12,
24 and 48 h at 37°C. Another portion of cells was used to establish
the I/R injury model and were treated with low, medium or high
concentrations of TPG (10, 40 and 160 µg/ml) for 12, 24 and 48 h at
37°C. Subsequently, CCK-8 reagent was added to the cells, which
were incubated for 4 h at 37°C. The optical density (OD) value was
measured at 450 nm using a light absorption microplate reader
(ELx808; BioTek Instruments, Inc., Winooski, VT, USA). The
concentrations of TPG used were adopted according to previous
studies (18,29).
Reactive oxygen species (ROS)
assay
ROS production was assessed using
2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma-Aldrich; Merck
KGaA), according to the manufacturer's protocol. DCFH-DA can be
oxidized by ROS into fluorescent DCF, and the fluorescent signal
indicates ROS production. Briefly, cells were seeded at a density
of 2.5×104 cells/well into a 96-well plate, after which
they were subjected to I/R injury and then treated with various
concentrations of TPG (10, 40 and 160 µg/ml) for 24 h at 37°C.
Cells were subsequently incubated with 25 µM DCFH-DA at 37°C for 30
min and were washed three times with PBS. ROS production was
measured using a FACSCalibur flow cytometer with Cell Quest 3.3
software (BD Biosciences, Franklin Lakes, NJ, USA).
Cell apoptosis assay
The Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit (Shanghai
Qcbio Science & Technologies Co., Ltd., Shanghai, China) was
performed to determine cell apoptosis, according to the
manufacturer's protocol. Briefly, cells were seeded at a density of
6×105 cells/well in 6-well plates, after which they were
subjected to I/R injury and then treated with 10 µg/ml TPG and 100
ng/ml IGF-1. Following cell treatment, the cells were rinsed three
times with PBS. Subsequently, the cells were stained with Annexin
V-FITC and PI solution in the dark at room temperature for 20 min.
Finally, 1X Binding Buffer was added to the cells on ice and cell
apoptosis was measured using flow cytometery.
Lactate dehydrogenase (LDH) assay
LDH activity was examined using a LDH detection kit
(Shanghai Genmed Pharmaceutical Technology Co Ltd., Shanghai,
China), according to the manufacturer's protocol. Following cell
treatment, the cells were exposed to reagent A for 5 min at room
temperature, and then to reagent B for 10 min at room temperature.
Subsequently, cells were centrifuged at 300 × g for 5 min and the
supernatant was added to 96-well plates, and fixed with reagent C
and reagent D in the dark for 30 min at room temperature.
Subsequently, reagent E was added to the mixture. The OD value was
measured at 490 nm using a light absorption microplate reader.
Malondialdehyde (MDA) assay
The MDA detection kit (Beijing Leagene Biotechnology
Co., Ltd.) was performed to detect MDA activity, according to the
manufacturer's protocol. Following cell treatment, the cells were
lysed on ice using an ATPIO-400SD ultrasonic cell breaker (Nanjing
ATPIO Instruments Manufacture Co., Ltd., Nanjing, China).
Subsequently, the cell lysate was mixed with TAB solution; the
mixture was heated in boiling water for 10 min and centrifuged at
800 × g at room temperature for 5 min. Subsequently, the
precipitate was resuspended in PBS. The OD value was measured at
532 nm using a light absorption microplate reader.
Superoxide dismutase (SOD) assay
SOD activity was identified using the SOD detection
kit (Beyotime Institute of Biotechnology, Haimen, China), according
to the manufacturer's protocol. Following cell treatment, the cells
were lysed on ice using an ATPIO-400SD ultrasonic cell breaker.
Subsequently, the cell lysate was mixed with hydroxylamine working
fluid at 37°C for 30 min. Subsequently, chromogenic reagent was
added to the mixture for 16 min at room temperature. The OD value
was measured at 550 nm using a light absorption microplate
reader.
Glutathione peroxidase (GPX)
assay
GPX activity was analyzed using a GPX detection kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Following cell treatment, the cells were
lysed on ice using an ATPIO-400SD ultrasonic cell breaker.
Subsequently, cells were mixed with peroxide solution at 25°C for
10 min. The OD value at 430 nm was measured every 30 sec at 25°C
using a light absorption microplate reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). RNA (1 µg) was used to synthesize cDNA by RevertAid™ cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The PCR reaction mixture (50 µl) contained
25 µl Dream Taq Green PCR master Mix, 1 µl forward/reverse primer,
19 µl nuclease-free H2O and 4 µl cDNA. Reaction conditions were as
follows: Pre-denaturation at 96°C for 4 min, followed by 30 cycles
of denaturation at 96°C for 20 sec and annealing at 60°C for 30
sec, and final extension at 72°C for 30 sec. The primers were
purchased from Synbio Technologies, LLC (Suzhou, China) and are
listed in Table I. β-actin was
used as an internal control. The 2−ΔΔCq method was used
to compare the gene expression levels (30).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer name | Sequence
(5′-3′) | Product size
(bp) |
|---|
|
Caspase-3-forward |
TGGAATGTCAGCTCGCAATG | 224 |
|
Caspase-3-reverse |
CAGGTCCGTTCGTTCCAAAA |
|
| Bax-forward |
GAGACACCTGAGCTGACCTT | 187 |
| Bax-reverse |
CGTCTGCAAACATGTCAGCT |
|
| Bcl-2-forward |
GCCTTCTTTGAGTTCGGTGG | 221 |
| Bcl-2-reverse |
CTGAGCAGCGTCTTCAGAG |
|
| PARP1-forward |
CCAAGGCAGCAGTGAATCTC | 205 |
| PARP1-reverse |
GGGGTCCTTACTGCTGTCAT |
|
|
β-actin-forward |
TGTGTTGTCCCTGTATGCC | 232 |
|
β-actin-reverse |
AATGTCACGCACGATTTCCC |
|
Western blot analysis
Cells were lysed with enhanced
radioimmunoprecipitation assay buffer (Beijing Leagene
Biotechnology Co., Ltd.) and total protein was extracted. The
protein concentration was measured by bicinchoninic acid protein
assay (Beyotime Institute of Biotechnology). A total of 25 µg
protein was separated by 10% SDS-PAGE and bound to nitrocellulose
membranes (Shanghai Kang Lang Biological Technology Co., Ltd.,
Shanghai, China). Membranes were blocked with 5% non-fat milk at
37°C for 1 h. Subsequently, membranes were incubated with
anti-pro-caspase-3 (cat. no. AF835, 1:1,000; R&D Systems, Inc.,
Minneapolis, MN, USA), anti-cleaved caspase-3 (cat. no. MAB835;
1:1,000; R&D Systems, Inc.), anti-cleaved poly (ADP-ribose)
polymerase (PARP) 1 (cat. no. ab32561; 1:800; Abcam, Cambridge,
UK), anti-B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax; cat.
no. MA5-14003; 1:1,000), anti-Bcl-2 (cat. no. MA5-11757; 1:1,000;
both Invitrogen; Thermo Fisher Scientific, Inc.),
anti-phosphorylated (p)-Akt (cat. no. AA329), anti-Akt (cat. no.
AA326; 1:1,500; both Beyotime Institute of Biotechnology),
anti-p-PI3K (cat. no. PA5-12799; 1:1,600), anti-PI3K (cat. no.
MA5-17149; 1:1,000; both Invitrogen; Thermo Fisher Scientific,
Inc.) and anti-β-actin (cat. no. MAB8969; 1:1,000; R&D Systems,
Inc.) at 4°C overnight. Subsequently, membranes were washed 2–4
times in TBS with 2% Tween-20 and were incubated with the following
secondary antibodies: Mouse anti-rabbit immunoglobulin G (IgG)
(cat. no. 3678; 1:8,000; Cell Signaling Technologies, Inc.,
Danvers, MA, USA), rabbit anti-mouse IgG (cat. no. 58802, 1:7,000;
Cell Signaling Technologies, Inc.) and rabbit anti-goat IgG (cat.
no. ab6741; 1:8,000; Abcam) for 1.5 h at room temperature. Proteins
were detected using the iBright™ imaging system (A32749; Thermo
Fisher Scientific, Inc.).
Statistical analysis
GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA) was used to perform the data analysis. The
experimental data are presented as the means ± standard deviation.
The differences between groups were assessed by one-way analysis of
variance followed by Tukey's test. Each experiment was repeated at
least three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
TPG enhances the viability of H9C2
cells following I/R
CCK-8 was used to determine the effects of TPG on
the viability of H9C2 cells. The CCK-8 assay results demonstrated
that there was no alteration in viability when cells were treated
with TPG alone (Fig. 1A).
Additionally, when cells were impaired by I/R, cell viability was
attenuated compared with in the control group. Conversely, TPG
could significantly enhance the viability of cells inhibited by I/R
in a time- and concentration-dependent manner compared with in the
model group (P<0.05; Fig.
1B).
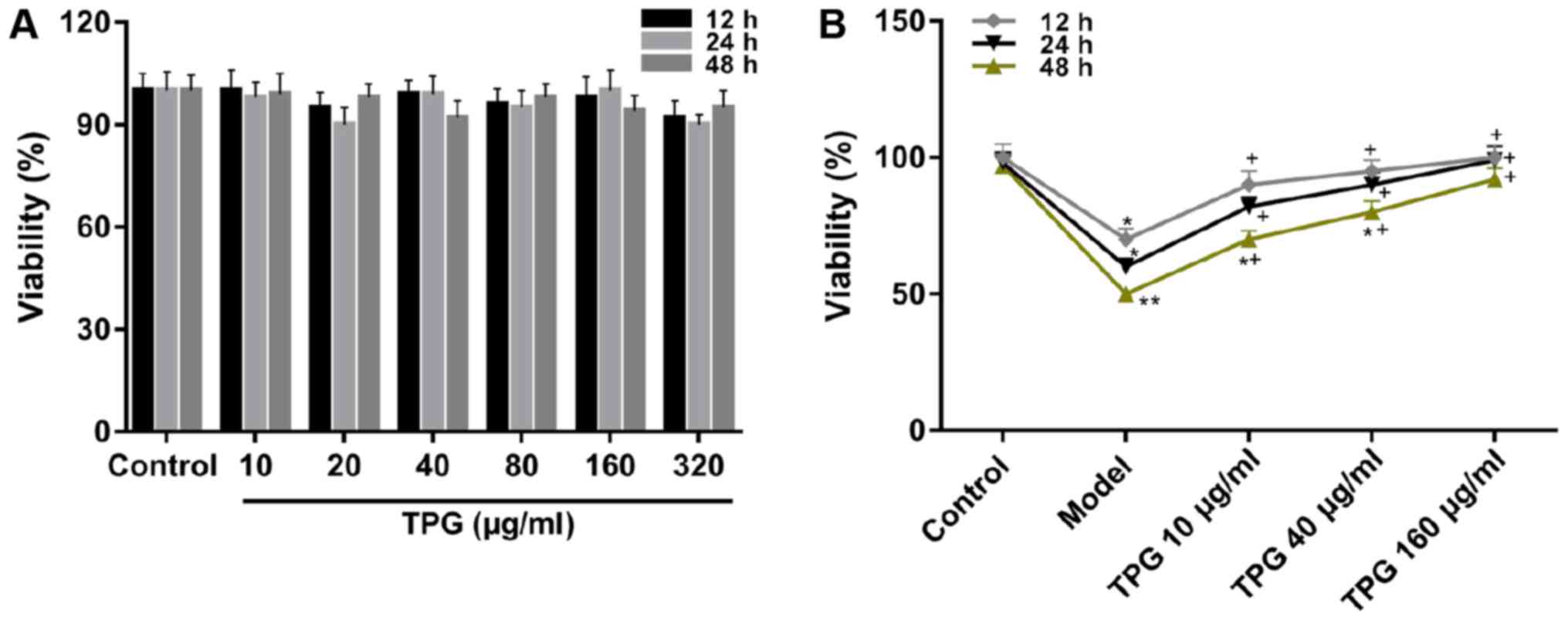 | Figure 1.TPG elevates the viability of
I/R-induced H9C2 cells. (A) Cell viability was examined using
CCK-8. H9C2 cells were treated with TPG (10, 20, 40, 80, 160 and
320 µg/ml) for 12, 24 and 48 h. (B) H9C2 cells were impaired by
I/R, and were treated with low, medium and high concentrations of
TPG (10, 40 and 160 µg/ml, respectively) for 12, 24 and 48 h.
*P<0.05, **P<0.01, vs. the control group;
+P<0.05, vs. the model group. I/R,
ischemia/reperfusion; TPG, total paeony glycoside. |
TPG inhibits I/R-induced apoptosis of
H9C2 cells
To investigate the effects of TPG on the apoptosis
of H9C2 cells, the Annexin V-FITC/PI apoptosis detection kit,
RT-qPCR and western blotting were performed to measure the rates of
apoptosis, and the mRNA and the protein expression levels of
apoptosis-associated factors, respectively. The results of flow
cytometry revealed that the rates of apoptosis were 4.93 and 19.00%
in the control and model groups, respectively. In the 10, 40 and
160 µg/ml TPG groups the rates of apoptosis were 9.88, 6.94 and
5.27%, respectively. Compared with in the model group, apoptosis
was reduced by TPG (P<0.05; Fig.
2). In addition, TPG markedly promoted the expression levels of
pro-caspase-3 and Bcl-2, and suppressed the expression levels of
cleaved-caspase-3, cleaved-PARP1 and Bax in a
concentration-dependent manner, compared within the model group
(P<0.05; Fig. 3).
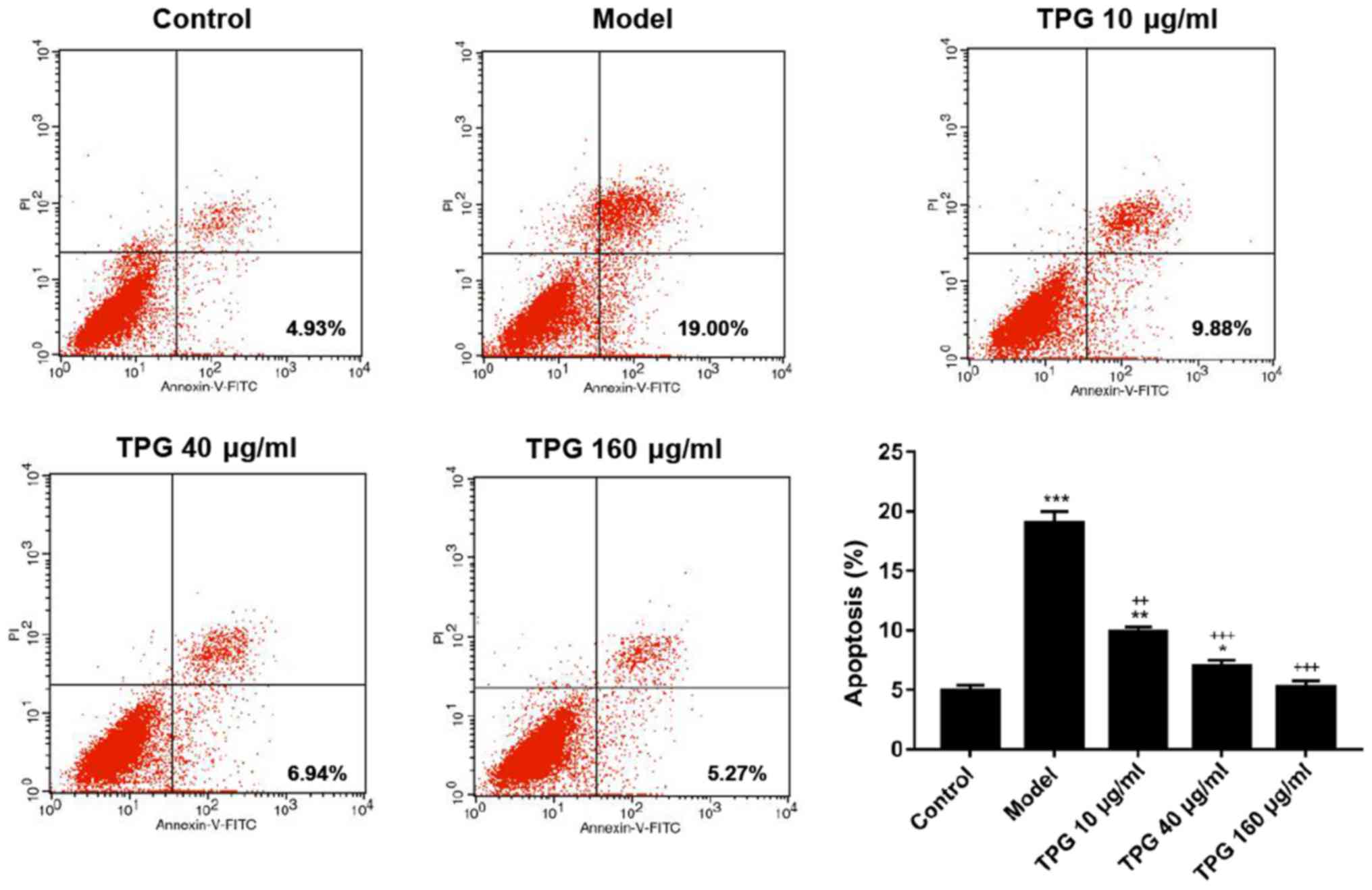 | Figure 2.TPG inhibits the apoptosis of H9C2
cells induced by I/R. H9C2 cells were subjected to I/R injury and
then treated with TPG (10, 40 and 160 µg/ml). The rate of apoptosis
was assessed using the Annexin V-FITC/PI apoptosis detection kit.
*P<0.05, **P<0.01, ***P<0.001, vs. the control group;
++P<0.01, +++P<0.001, vs. the model
group. FITC, fluorescein isothiocyanate; I/R, ischemia/reperfusion;
PI, propidium iodide; TPG, total paeony glycoside. |
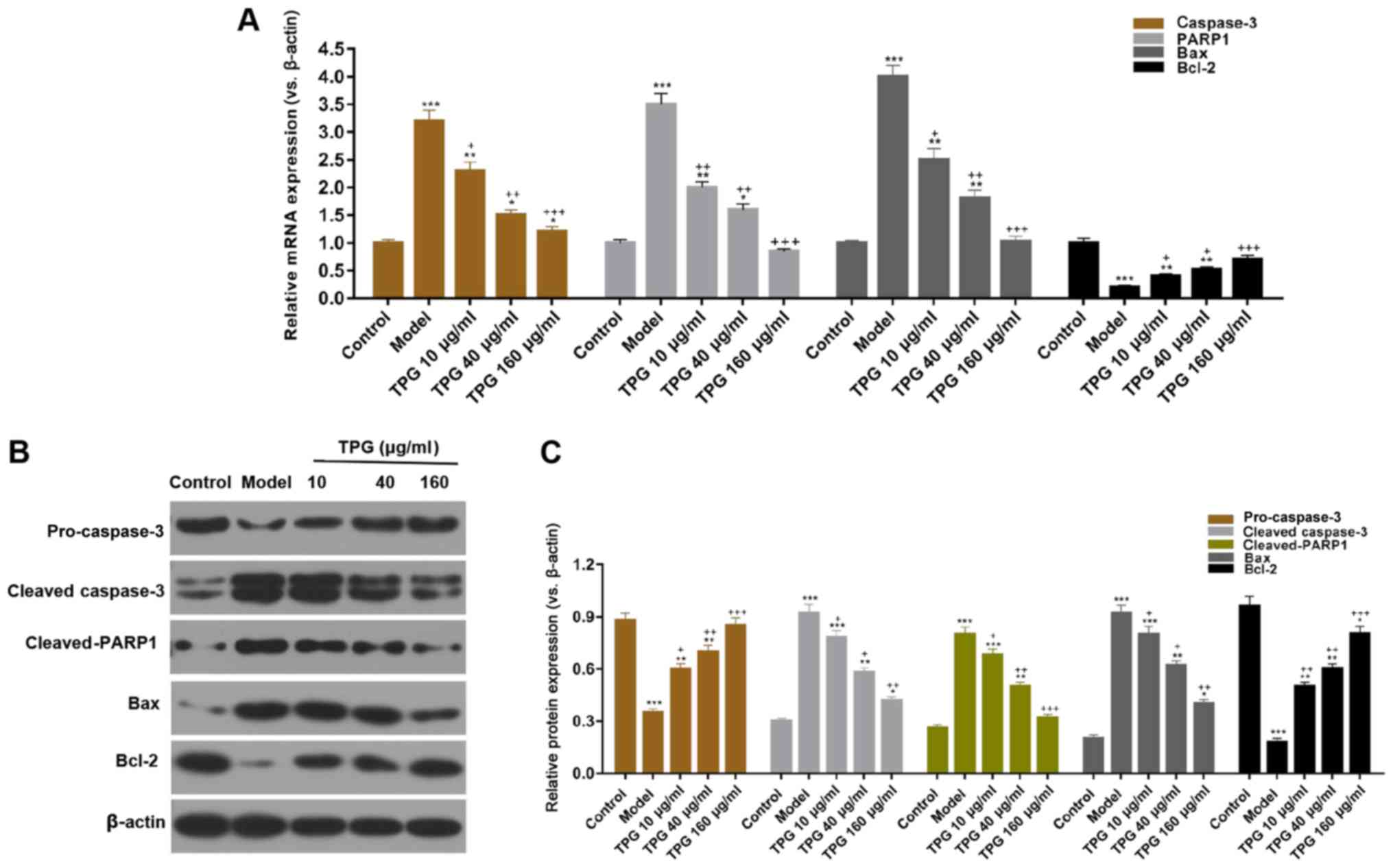 | Figure 3.TPG regulates apoptosis-associated
factors in H9C2 cells. H9C2 cells were treated with TPG (10, 40 and
160 µg/ml) for 24 h and subjected to I/R injury. (A) Relative mRNA
expression levels of caspase-3, PARP1, Bax and Bcl-2 were
investigated by reverse transcription-quantitative polymerase chain
reaction. (B) Relative protein expression levels of caspase-3,
PARP1, Bax and Bcl-2 were evaluated by western blotting and
normalized to β-actin expression. (C) Blot results were
semi-quantified. *P<0.05, **P<0.01, ***P<0.001, vs. the
control group; +P<0.05, ++P<0.01,
+++P<0.001, vs. the model group. Bax,
Bcl-2-associated X protein; Bcl-2, B-cell lymphoma; I/R,
ischemia/reperfusion; PARP, poly (ADP-ribose) polymerase; TPG,
total paeony glycoside. |
TPG suppresses I/R-induced oxidative
stress in H9C2 cells
To analyze whether TPG affects the oxidative stress
of H9C2 cells, ROS levels and the activities of LDH, MDA, SOD and
GPX were determined using various kits. The ROS assay results
demonstrated that the ROS levels according to mean fluorescence
intensity were 59.69, 195.34, 142.44, 109.22, and 75.95 in the
control, model and TPG (10, 40 and 160 µg/ml) groups, respectively.
ROS levels were increased in the model group compared with in the
control group. Conversely, the ROS levels were decreased by 52.9,
86.12 and 119.39% in the TPG (10, 40 and 160 µg/ml) groups compared
with in the model group (P<0.05; Fig. 4). Furthermore, TPG markedly reduced
the activities of LDH and MDA; however, it promoted SOD and GPX
activities in a concentration-dependent manner compared with in the
model group (P<0.05; Fig.
5).
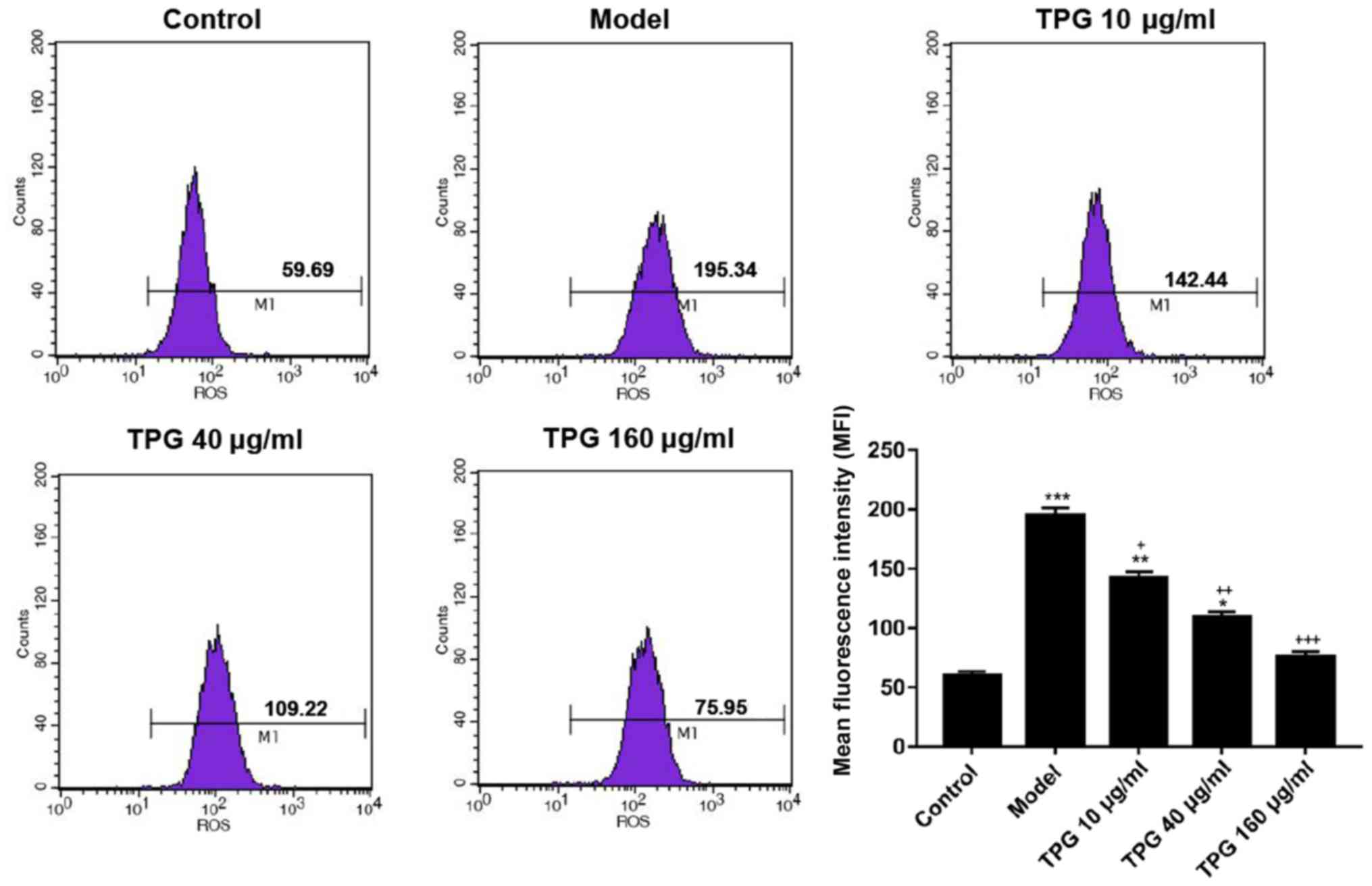 | Figure 4.TPG inhibits I/R-induced ROS
production in H9C2 cells. H9C2 cells were treated with TPG (10, 40
and 160 µg/ml) for 24 h and subjected to I/R injury. ROS production
was detected using a ROS detection kit. *P<0.05, **P<0.01,
***P<0.001, vs. the control group; +P<0.05,
++P<0.01, +++P<0.001, vs. the model
group. I/R, ischemia/reperfusion; ROS, reactive oxygen species;
TPG, total paeony glycoside. |
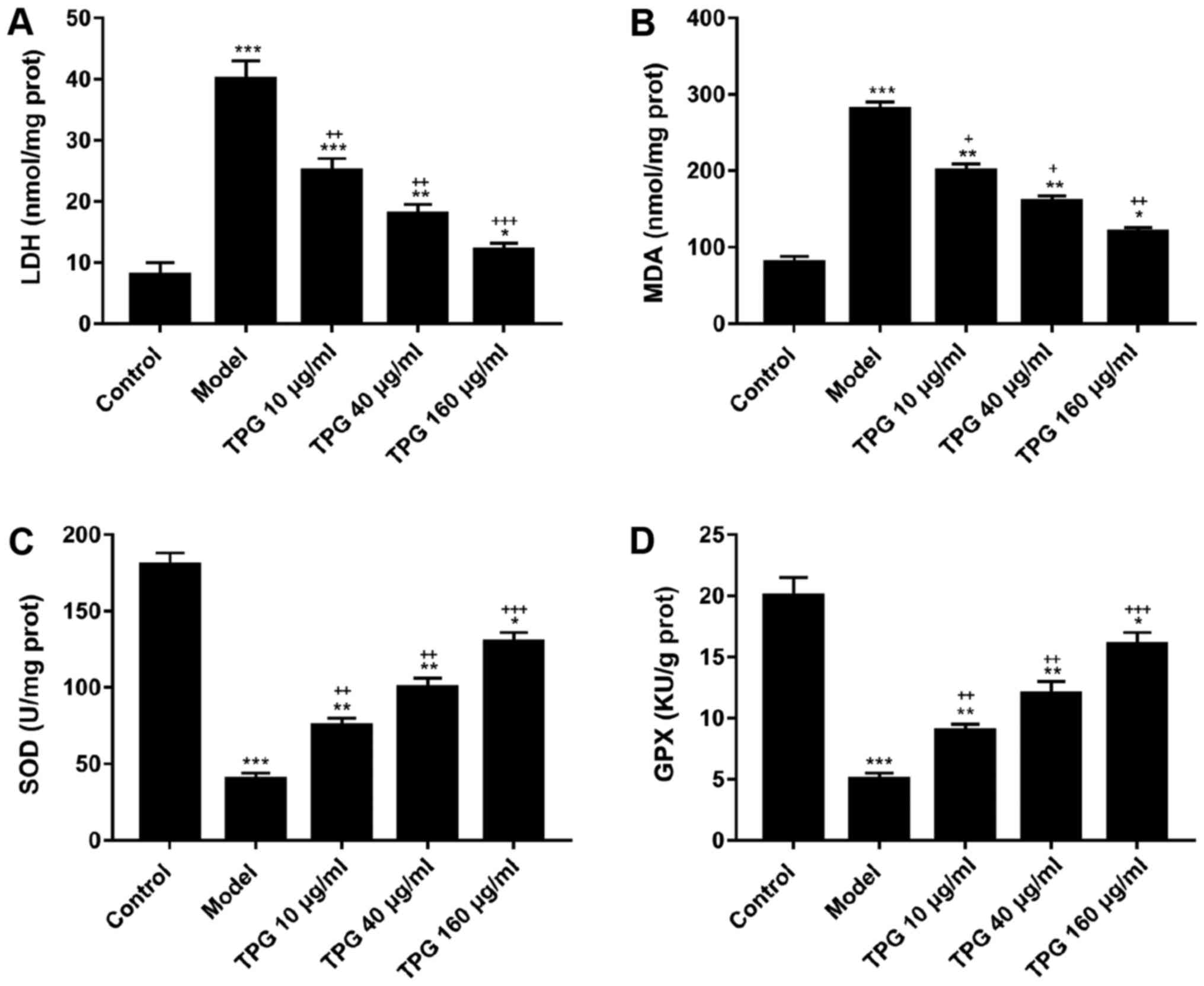 | Figure 5.TPG suppresses LDH and MDA
activities, and enhances SOD and GPX activities in I/R-induced H9C2
cells. H9C2 cells were treated with TPG (10, 40 and 160 µg/ml) for
24 h and subjected to I/R injury. (A) LDH activity, (B) MDA
activity, (C) SOD activity and (D) GPX activity were detected.
*P<0.05, **P<0.01, ***P<0.001, vs. the control group;
+P<0.05, ++P<0.01,
+++P<0.001, vs. the model group. GPX, glutathione
peroxidase; I/R, ischemia/reperfusion; LDH, lactate dehydrogenase;
MDA, malondialdehyde; SOD, superoxide dismutase; TPG, total paeony
glycoside. |
TPG suppresses the PI3K/Akt signaling
pathway in H9C2 cells following I/R
In order to further investigate the molecular
mechanism underlying the inhibitory effects of TPG on I/R-induced
apoptosis of H9C2 cells, the protein expression levels of p-PI3K,
PI3K, p-Akt and Akt were detected by western blot analysis. The
results revealed that compared with in the model group, the
expression levels of p-PI3K and p-Akt were attenuated in response
to TPG (10, 40 and 160 µg/ml; P<0.05). However, no changes were
detected in total PI3K and Akt protein expression among the various
groups (Fig. 6).
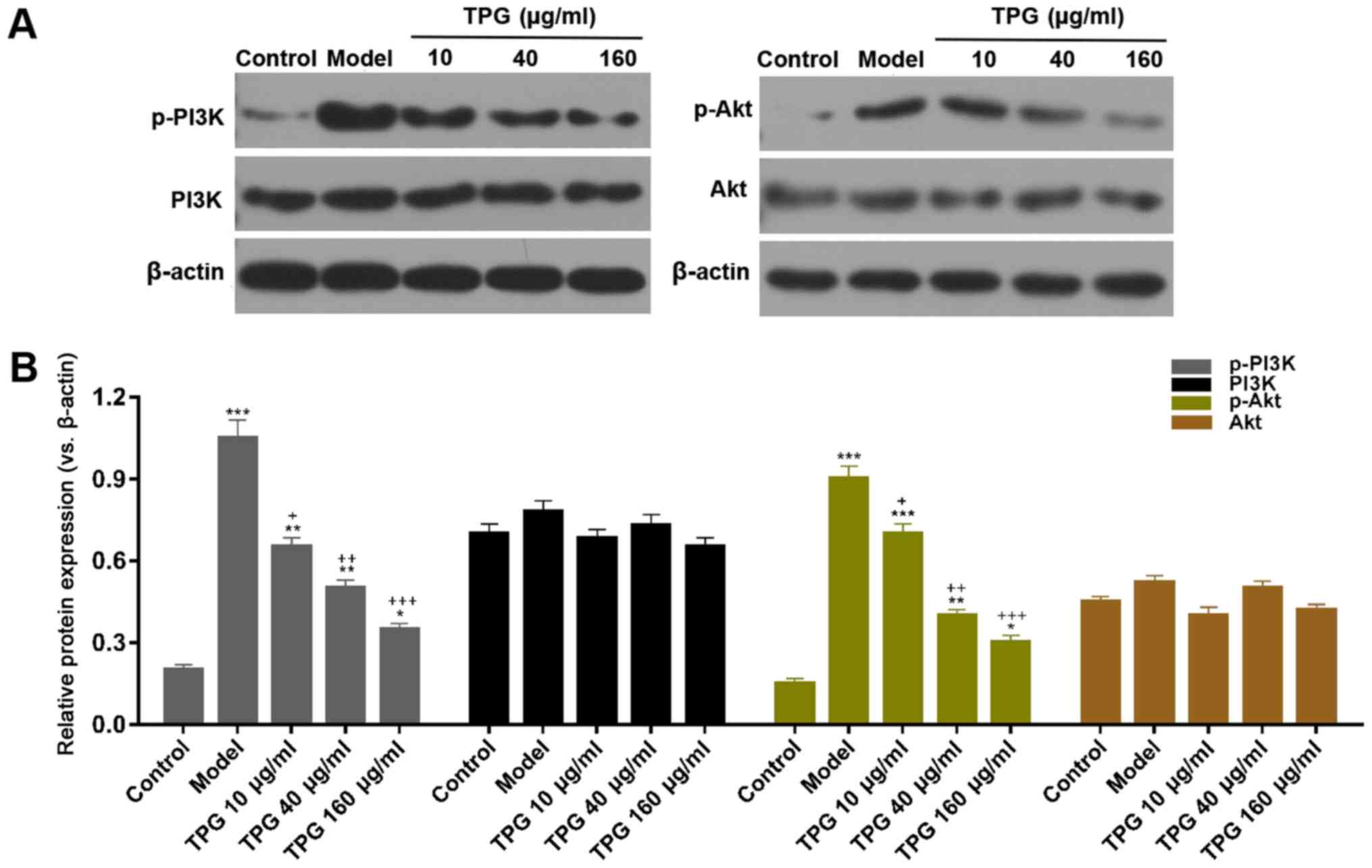 | Figure 6.TPG suppresses the PI3K/Akt signaling
pathway in I/R-induced H9C2 cells. H9C2 cells were treated with TPG
(10, 40 and 160 µg/ml) for 24 h and subjected to I/R injury. (A)
Relative protein expression levels of p-PI3K, PI3K, p-Akt and Akt
were assessed by western blotting. β-actin was used as an internal
control. (B) Blot results were semi-quantified. *P<0.05,
**P<0.01, ***P<0.001, vs. the control group;
+P<0.05, ++P<0.01,
+++P<0.001, vs. the model group. Akt, protein kinase
B; I/R, ischemia/reperfusion; p, phosphorylated; PI3K,
phosphatidylinositol 3 kinase; TPG, total paeony glycoside. |
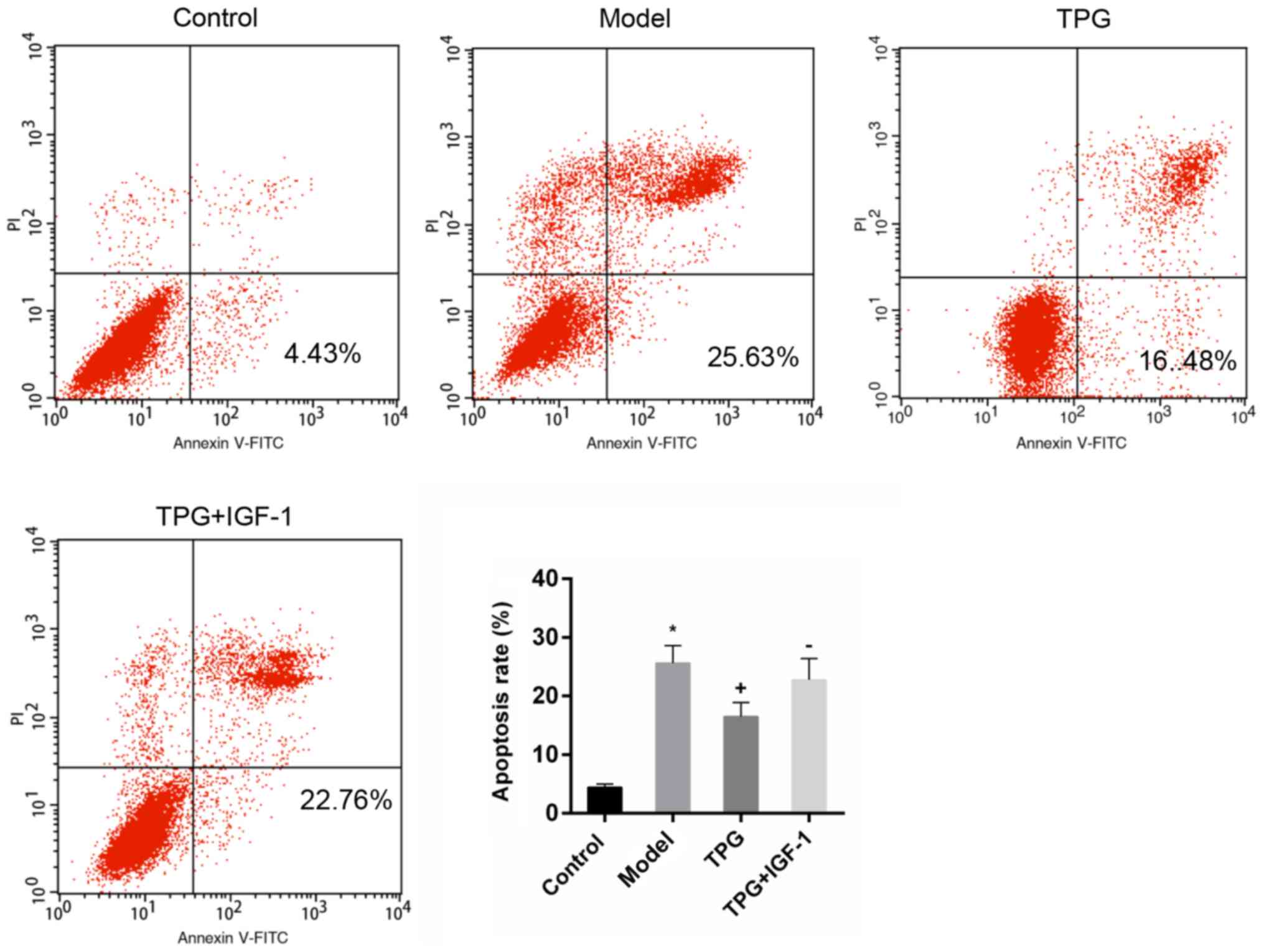
IGF-1 reverses the effects of TPG on
I/R injury
As previously described, IGF-1 is able to activate
the PI3K/Akt signaling pathway (31). In the present study, IGF-1 was used
to investigate the association of the PI3K/Akt signaling pathway
with the protective effects of TPG against I/R injury. I/R-induced
apoptosis was attenuated by TPG. Conversely, the number of
apoptotic cells in the TPG + IGF-1 group was increased compared
with in the TPG group, thus suggesting that IGF-1 treatment
reversed the effects of TPG (Fig.
7).
Discussion
Traditional Chinese medicine has been widely used to
treat various diseases in medical practice. TPG is a traditional
Chinese medicine, which possesses antitumor effects and is able to
target cardiovascular and cerebrovascular diseases (15,16).
Furthermore, previous studies have reported that TPG regulates cell
proliferation and apoptosis (17,18).
Therefore, in the present study, the effects of TPG on the
viability and apoptosis of I/R-induced H9C2 cells were examined.
The present results demonstrated that TPG alone did not affect H9C2
cell viability. However, TPG significantly promoted the viability
of H9C2 cells following I/R, and restrained apoptosis in a
dose-dependent manner, thus suggesting that TPG exerts protective
effects on an I/R injury model in vitro.
It has been reported that myocardial I/R is closely
associated with myocardial cell apoptosis (32,33).
Among the apoptosis-associated genes, Bcl-2 is an inhibitor of
apoptosis and Bax is a pro-apoptotic gene; the interaction between
these two proteins regulates apoptosis (34,35).
Furthermore, Bcl-2 can inhibit the activity of caspase-3, thus
suppressing cell apoptosis (35).
In addition, PARP1 is the most important substrate of caspase-3,
which is involved in DNA repair and gene integrity monitoring;
cleaved-PARP1 is a marker of apoptosis (36,37).
Therefore, the role of TPG in inhibiting apoptosis induced by I/R
was investigated by detecting the expression levels of these
apoptosis-associated factors. The results demonstrated that TPG
markedly upregulated the expression levels of pro-caspase-3 and
Bcl-2, whereas it downregulated cleaved-caspase-3, cleaved-PARP1
and Bax expression. Therefore, it was suggested that TPG inhibited
I/R injury-induced apoptosis in vitro.
Oxidative stress is another important factor in the
process of myocardial injury, which is characterized by the
accumulation of ROS, increased nicotinamide adenine dinucleotide
phosphate-oxidase and decreased antioxidant enzymes (38,39).
A previous study demonstrated that sodium thiosulfate conspicuously
reduces I/R damage-induced oxidative stress in rat hearts, and
decreases the ROS levels, and LDH and MDA activities; however, it
enhances SOD and GPX activities (40). Similar to previous studies, the
present data revealed that TPG markedly reduced ROS levels, and LDH
and MDA activities, whereas it increased the activities of SOD and
GPX. Therefore, the present results indicated that TPG may reduce
I/R-induced oxidative stress.
Previous studies have suggested that the PI3K/Akt
signaling pathway is involved in the apoptosis of various tumor
cells (41,42). Furthermore, it has been revealed
that certain drugs could decrease apoptosis and oxidative stress of
cells via regulation of the PI3K/Akt signaling pathway (41,43,44).
Numerous drugs and proteins exert potential protective effects on
myocardial injury via regulation of the PI3K/Akt signaling pathway
(45–47). As expected, the present study
observed that TPG significantly downregulated the phosphorylation
levels of PI3K and Akt. Furthermore, apoptosis was increased by
IGF-1, thus suggesting that the effects of TPG may be reversed by
the activation of PI3K/Akt. Therefore, it may be suggested that TPG
inhibited apoptosis and oxidative stress induced by myocardial I/R
injury via restraining PI3K/Akt signaling; however, this requires
further validation.
In conclusion, TPG facilitated cell viability, and
suppressed I/R-induced apoptosis and oxidative stress in H9C2
cells, possibly via inhibiting the PI3K/Akt signaling pathway.
Therefore, TPG may be of clinical significance in the treatment of
cardiovascular diseases; however, this requires further
investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PS and MP conceived and designed the study. JC
analyzed and interpreted the data. PS drafted the manuscript. All
authors were responsible for approving the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim Y, Keogh JB and Clifton PM: Benefits
of nut consumption on insulin resistance and cardiovascular risk
factors: Multiple potential mechanisms of actions. Nutrients.
9:pii: E1271. 2017. View Article : Google Scholar
|
|
2
|
McCarthy CP, Bhambhani V, Pomerantsev E
and Wasfy JH: In-hospital outcomes in invasively managed acute
myocardial infarction patients who receive morphine. J Interv
Cardiol. 31:150–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jennings RB, Sommers HM, Smyth GA, Flack
HA and Linn H: Myocardial necrosis induced by temporary occlusion
of a coronary artery in the dog. Arch Pathol. 70:68–78.
1960.PubMed/NCBI
|
|
4
|
Sodha NR, Clements RT, Feng J, Liu Y,
Bianchi C, Horvath EM, Szabo C and Sellke FW: The effects of
therapeutic sulfide on myocardial apoptosis in response to
ischemia-reperfusion injury. Eur J Cardiothorac Surg. 33:906–913.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorenkova N, Robinson E, Grieve DJ and
Galkin A: Conformational change of mitochondrial complex I
increases ROS sensitivity during ischemia. Antioxid Redox Signal.
19:1459–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ekeløf S, Jensen SE, Rosenberg J and
Gögenur I: Reduced oxidative stress in STEMI patients treated by
primary percutaneous coronary intervention and with antioxidant
therapy: A systematic review. Cardiovasc Drugs Ther. 28:173–181.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han J, Wang D, Ye L, Li P, Hao W, Chen X,
Ma J, Wang B, Shang J, Li D and Zheng Q: Rosmarinic acid protects
against inflammation and cardiomyocyte apoptosis during myocardial
ischemia/reperfusion injury by activating peroxisome
proliferator-activated receptor gamma. Front Pharmacol. 8:4562017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu
C and Duan J: The protective effect of Luteolin on myocardial
ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3
inflammasome pathway. Biomed Pharmacother. 91:1042–1052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang CC, Yuan W, Lin YL, Liu RS, Juan YC,
Sun WH, Tsay HJ, Huang HC, Lee YC and Liu HK: Evaluation of the in
vivo therapeutic effects of radix paeoniae rubra ethanol extract
with the hypoglycemic activities measured from multiple cell-based
assays. Evid Based Complement Alternat Med. 2016:32627902016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang YQ, Ma X, Wang J, Zhao YL, Wang JB,
Chen Z, Zhu Y, Shan LM, Wei SZ, Wang J and Xiao XH: Therapeutic
efficacy and safety of paeoniae radix rubra formulae in relieving
hyperbilirubinemia induced by viral hepatitis: A meta-analysis.
Front Pharmacol. 7:632016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie P, Cui L, Shan Y and Kang WY:
Antithrombotic effect and mechanism of radix paeoniae rubra. Biomed
Res Int. 2017:94750742017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dou ZH, Luo L and Lu D: Purification of
total paeony glycoside by macroporous resin with double indices of
albiflorin and paeoniflorin. Zhong Yao Cai. 32:1282–1284. 2009.(In
Chinese). PubMed/NCBI
|
|
13
|
Lin MY, Chiang SY, Li YZ, Chen MF, Chen
YS, Wu JY and Liu YW: Anti-tumor effect of Radix Paeoniae Rubra
extract on mice bladder tumors using intravesical therapy. Oncol
Lett. 12:904–910. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhan LY, Xia ZY, Chen C and Wang XY:
Effect of Radix Paeoniae Rubra on the expression of HO-1 and iNOS
in rats with endotoxin-induced acute lung injury. Chin J Traumatol.
9:181–186. 2006.PubMed/NCBI
|
|
15
|
Gu JF, Feng L, Yuan JR, Zhang MH and Jia
XB: Effect of different composition structures of total paeony
glycoside component and total phenolic acid component of Chuanxiong
Rhizome on human umbilical vein endothelial cells with hypoxic
injury. Zhongguo Zhong Yao Za Zhi. 40:920–926. 2015.(In Chinese).
PubMed/NCBI
|
|
16
|
Xu HY, Chen ZW and Wu YM: Antitumor
activity of total paeony glycoside against human chronic myelocytic
leukemia K562 cell lines in vitro and in vivo. Med Oncol.
29:1137–1147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu C, Wang J and Yang J: Study on
activating blood and eliminating stasis of total paeony
glycoside(TPG). Zhong Yao Cai. 23:557–560. 2000.(In Chinese).
PubMed/NCBI
|
|
18
|
Long J, Gao M, Kong Y, Shen X, Du X, Son
YO, Shi X, Liu J and Mo X: Cardioprotective effect of total paeony
glycosides against isoprenaline-induced myocardial ischemia in
rats. Phytomedicine. 19:672–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma RQ, Chen JW, Pang JX, Lan XJ and Qiu
CH: Protective effects of total paeony glycoside against global
cerebral ischemia-reperfusion injury in gerbils. Di Yi Jun Yi Da
Xue Xue Bao. 25:471–473. 2005.(In Chinese). PubMed/NCBI
|
|
20
|
Yang J, Wang J and Liu C: Protective
effects of total paeony glycoside on cerebral ischemia mice. Zhong
Yao Cai. 24:124–126. 2001.(In Chinese). PubMed/NCBI
|
|
21
|
Parcellier A, Tintignac LA, Zhuravleva E
and Hemmings BA: PKB and the mitochondria: AKTing on apoptosis.
Cell Signal. 20:21–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pei R, Si T, Lu Y, Zhou JX and Jiang L:
Salvianolic acid A, a novel PI3K/Akt inhibitor, induces cell
apoptosis and suppresses tumor growth in acute myeloid leukemia.
Leuk Lymphoma. 59:1959–1967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Zhang L, Manaenko A, Ye Z, Liu W
and Sun X: Helium preconditioning protects mouse liver against
ischemia and reperfusion injury through the PI3K/Akt pathway. J
Hepatol. 61:1048–1055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao X, Xiang Y, Cai C, Zhou A, Zhu N and
Zeng C: Schisandrin B protects against myocardial
ischemia/reperfusion injury via the PI3K/Akt pathway in rats. Mol
Med Rep. 17:556–561. 2018.PubMed/NCBI
|
|
25
|
Zhou J, Du T, Li B, Rong Y, Verkhratsky A
and Peng L: Crosstalk between MAPK/ERK and PI3K/AKT signal pathways
during brain ischemia/reperfusion. ASN Neuro. 7:pii:
1759091415602463. 2015. View Article : Google Scholar
|
|
26
|
Macrae VE, Ahmed SF, Mushtaq T and
Farquharson C: IGF-I signalling in bone growth: Inhibitory actions
of dexamethasone and IL-1beta. Growth Horm IGF Res. 17:435–439.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Zhang Q, Zhang L, Little PJ, Xie
X, Meng Q, Ren Y, Zhou L, Gao G, Quirion R and Zheng W:
Insulin-like growth factor-1 induces the phosphorylation of PRAS40
via the PI3K/Akt signaling pathway in PC12 cells. Neurosci Lett.
516:105–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu WY, Wang WY, Ma YL, Yan H, Wang XB, Qin
YL, Su M, Chen T and Wang YP: Sodium tanshinone IIA silate inhibits
oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis
via suppression f the NF-κB/TNF-α pathway. Br J Pharmacol.
169:1058–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He LN, Yang J, He SB, Wang J and Liu C:
Protective effect of total paeony glocoside against ischemia injury
in cultured primary cortex neurons. Chin J Clin Pharmacol
Therapeut. 1:1–6. 2000.
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stitt TN, Drujan D, Clarke BA, Panaro F,
Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD and Glass DJ: The
IGF-1/PI3K/Akt pathway prevents expression of muscle
atrophy-induced ubiquitin ligases by inhibiting FOXO transcription
factors. Mol Cell. 14:395–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chua CC, Gao J, Ho YS, Xiong Y, Xu X, Chen
Z, Hamdy RC and Chua BH: Overexpression of IAP-2 attenuates
apoptosis and protects against myocardial ischemia/reperfusion
injury in transgenic mice. Biochim Biophys Acta. 1773:577–583.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yaomura T, Tsuboi N, Urahama Y, Hobo A,
Sugimoto K, Miyoshi J, Matsuguchi T, Reiji K, Matsuo S and Yuzawa
Y: Serine/threonine kinase, Cot/Tpl2, regulates renal cell
apoptosis in ischaemia/reperfusion injury. Nephrology (Carlton).
13:397–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raza A, Dikdan G, Desai KK, Shareef A,
Fernandes H, Aris V, de la Torre AN, Wilson D, Fisher A,
Soteropoulos P and Koneru B: Global gene expression profiles of
ischemic preconditioning in deceased donor liver transplantation.
Liver Transpl. 16:588–599. 2010.PubMed/NCBI
|
|
35
|
Tsujimoto Y, Finger LR, Yunis J, Nowell PC
and Croce CM: Cloning of the chromosome breakpoint of neoplastic B
cells with the t(14;18) chromosome translocation. Science.
226:1097–1099. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wesierska-Gadek J, Gueorguieva M,
Wojciechowski J and Tudzarova-Trajkovska S: In vivo activated
caspase-3 cleaves PARP-1 in rat liver after administration of the
hepatocarcinogen N-nitrosomorpholine (NNM) generating the 85 kDa
fragment. J Cell Biochem. 93:774–787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Woo M, Hakem R, Soengas MS, Duncan GS,
Shahinian A, Kägi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, et
al: Essential contribution of caspase 3/CPP32 to apoptosis and its
associated nuclear changes. Genes Dev. 12:806–819. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ansley DM and Wang B: Oxidative stress and
myocardial injury in the diabetic heart. J Pathol. 229:232–241.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elahi MM, Flatman S and Matata BM: Tracing
the origins of postoperative atrial fibrillation: The concept of
oxidative stress-mediated myocardial injury phenomenon. Eur J
Cardiovasc Prev Rehabil. 15:735–741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ravindran S, Boovarahan SR, Shanmugam K,
Vedarathinam RC and Kurian GA: Sodium thiosulfate preconditioning
ameliorates ischemia/reperfusion injury in rat hearts via reduction
of oxidative stress and apoptosis. Cardiovasc Drugs Ther.
31:511–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ni Z and Yi J: Oxymatrine induces
nasopharyngeal cancer cell death through inhibition of PI3K/AKT and
NFκB pathways. Mol Med Rep. 16:9701–9706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu H, Zou S and Xu X: The β-glucan from
Lentinus edodes suppresses cell proliferation and promotes
apoptosis in estrogen receptor positive breast cancers. Oncotarget.
8:86693–86709. 2017.PubMed/NCBI
|
|
43
|
Hui Y, Chengyong T, Cheng L, Haixia H,
Yuanda Z and Weihua Y: Resveratrol attenuates the cytotoxicity
induced by amyloid-β1–42 in PC12 cells by upregulating heme
oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem Res.
43:297–305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Xia J, Jiang N, Xian Y, Ju H, Wei Y
and Zhang X: Corin protects H2O2-induced
apoptosis through PI3K/AKT and NF-κB pathway in cardiomyocytes.
Biomed Pharmacother. 97:594–599. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ha T, Hua F, Liu X, Ma J, McMullen JR,
Shioi T, Izumo S, Kelley J, Gao X, Browder W, et al:
Lipopolysaccharide-induced myocardial protection against
ischaemia/reperfusion injury is mediated through a
PI3K/Akt-dependent mechanism. Cardiovasc Res. 78:546–553. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang L, Mo Y, Li Y, Zhong Y, He S, Zhang
Y, Tang Y, Fu S, Wang X and Chen A: Urolithin A alleviates
myocardial ischemia/reperfusion injury via PI3K/Akt pathway.
Biochem Biophys Res Commun. 486:774–780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Thokala S, Inapurapu S, Bodiga VL, Vemuri
PK and Bodiga S: Loss of ErbB2-PI3K/Akt signaling prevents zinc
pyrithione-induced cardioprotection during ischemia/reperfusion.
Biomed Pharmacother. 88:309–324. 2017. View Article : Google Scholar : PubMed/NCBI
|