Introduction
Endometriosis, one of the most prevalent causes of
pelvic pain, infertility and menstrual disorders, is a common
chronic gynecological disorder of women at reproductive age.
Despite intensive research efforts, there is still a lack of
in-depth knowledge regarding the molecular basis of the
disease.
MicroRNAs (miRs) are RNA transcripts 19–22
nucleotides in length. The mechanism of miRs has been extensively
studied in various pathological conditions and the expression
profiles of miRs in a number of diseases, including endometriosis,
have also been investigated. A single miR can target multiple
genes, resulting in the regulation of target mRNA expression
(1,2). Therefore, alterations in the dynamic
balance between miRs and their target mRNAs may alter the normal
physiological status of tissues and may initiate pathological
processes. Emerging data indicate that aberrant miR expression is
associated with endometriosis, possibly mediating the development
and progression of endometriosis by modulating proliferation,
apoptosis, migration, invasion and estradiol signal transduction in
endometriotic cells (3–5).
It is well known that miR-449b can alter the
expression of certain molecule associated with adhesion and
invasion. Likewise, endometrial stromal cells from endometriosis
patients also changed in these aspects. Therefore it was
hypothesised that miR-449b serves an important role in the
development of endometriosis. The present study aimed to identify
the expression and function of miR-449b, a differentially expressed
miR in ectopic and eutopic tissues. The present study was designed
to evaluate the role of miR-449b in the pathogenesis of
endometriosis. Using miR-449b-transfected endometrial stromal cells
(ESCs), the functional properties of miR-449b were observed. The
results from these experiments will help us to better understand
the miR-449b-mediated molecular mechanisms in ESCs.
Materials and methods
Tissue acquisition
The present study was approved by the ethics
committee of Obstetrics and Gynecology Hospital (Shanghai, China).
Ectopic (endometrioma; n=19), eutopic (n=19) and normal (n=35)
endometrial tissues from patients with or without endometriosis,
respectively who had undergone the laparoscopy and uterine
curettage were obtained at the Obstetrics and Gynecology Hospital,
Fudan University (Shanghai, China) from June 2017 to September
2017. None of the patients had received any hormonal treatments for
at least half a year prior to the operation. The menstrual cycle
phases of the patients were all in the proliferative phases, as
assessed by medical history and a histological evaluation of the
endometrium with the assistance of pathologists. The average age of
the patients in the normal group was 32.7±6.8 years and that of the
endometriosis group was 34.6±5.2 years. Patients consented to
tissue donation prior to surgery. Each sample was divided into two
parts for mRNA extraction and isolation.
Cell culture and treatment
ESCs from endometrium with or without endometriosis
were cultured by enzymatic digestion with collagenase as previously
described (6), the deposit was
re-suspended in DMEM/F-12 (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) that contained 10% fetal bovine serum (FBS), as
well as 100 U/ml penicillin and 100 mg/ml streptomycin (both from
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The ESCs
were purified through cell passage. After two generations, the
purity of ESCs can reach >95%, which had been determined by flow
cytometry with Alexa Fluor 488 anti-human vimentin mAb (clone:
RV202; BD Biosciences, Franklin Lakes, NJ, USA) according to the
protocol of the manufacturer (5/100 µl). Following serum starvation
for 12 h, ESCs without endometriosis (1×105 cells/well)
were treated with progesterone (P; 10−8 mol/l),
17β-estradiol (E2; 10−8 mol/l) or
E2 (10−8 mol/l) + P (10−8 mol/l)
for 24 h; vehicle controls were also assayed (treated with ethanol,
2×10−5 mol/l, E2 solution).
Fluorescence-based reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The TRIzol reagent (Takara Bio, Inc., Otsu, Japan)
was used to isolate total RNA. Next, cDNA was synthesized and
amplified using the SYBR® PrimeScript™ RT Master Mix kit
(Takara Bio, Inc.) and the ABI PRISM 500 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The thermocycling conditions for
reverse transcription was as follows: 37°C 15 min, 85°C 5 sec, and
4°C for storage. The gene used for normalization was Hsa-U6 small
nuclear RNA (snRNA). The primers used were as follows:
5′-CGCGCGTGAATTACCGAAG-3′ (forward) and 5′-GTGCAGGGTCCGAGGT-3′
(reverse) for miR-449b-3p; 5′-CGCGCTATGGCACTGGTAG-3′ (forward) and
5′-GTGCAGGGTCCGAGGT-3′ (reverse) for miR-449b-5p;
5′-GCGCGTCGTGAAGCGTTC-3′ (forward) and 5′-GTGCAGGGTCCGAGGT-3′
(reverse) for Hsa-U6 snRNA. The conditions for qPCR were determined
according to the protocol of the SYBR-Green JumpStart Taq ReadyMix
kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). qPCR was
implemented on a 7300 Real-Time PCR Detection System (ABI). The
incubation condition for qPCR was as follows: Stage 1 (95°C 30
sec); stage 2 (40 cycle, 95°C 5 sec; 60°C 31 sec); stage 3 (95°C 15
sec; 60°C 1 min; 95°C 15 sec). The results were expressed as
arbitrary units defined by the 2−ΔΔCt method (7).
miR-449b lentivirus construction and
transduction
The precursor of the miR hsa-miR-449b-3p was
constructed by Genechem Co., Ltd. (Shanghai, China). In the present
study, the RNA primers used for the amplification of the target
gene were as follows:
5′-GAGGATCCCCGGGTACCGGGTGACTATTAAGATTAGAGTTCTG-3′ and
5′-CACACATTCCACAGGCTAGGACAGCAGTTGCATGTTAGC-3′, which had been
confirmed by sequencing. The control green fluorescence
protein-lentivirus, (GFP-LV) and the recombinant lentivirus
overexpressing miR-449b-3p (miR-449b-LV) were prepared and diluted
to 1.09 transfection U/ml. Preparation included the
following four steps: Target gene insertion and plasmid
construction, packaging processing, purification and amplification,
dilution and storage, which had been conducted by Genechem Co.,
Ltd. (Shanghai, China) according to the protocol of the
manufacturer (Genechem Co., Ltd.).
The ESCs of the normal group were plated in 6-well
plates (5×104 cells/well) at 37°C under 5%
CO2 overnight. The next day, following discarding the
supernatants, 0.2 ml fresh complete medium containing lentiviruses
and polybrene (8 mg/ml) was added to ESCs at 37°C under 5%
CO2 for 12 h. Then ESCs were incubated in 0.3 ml freshly
prepared polybrene-Dulbecco's modified Eagle's medium (DMEM;
HyClone, GE Healthcare Life Sciences) for another 24 h. Finally,
following discarding the supernatants and replacing with fresh
DMEM, the cells were cultured for 3 days. The efficiency of
lentivirus transduction was investigated by the detection of GFP
signals using fluorescence microscopy (IX71; Olympus Corporation,
Tokyo, Japan) at 72 h following transduction. The expression of
miR-449b-3p in stably transduced ESCs was tested by RT-qPCR. The
ESCs transduced with miR-449b-LV (miR-449b up) and GFP-LV (NC) were
cryopreserved for further functional analysis.
Measurement of cell viability by MTT
assay
The ESCs (control/untransfected cells/NC/miR-449b
up) (2.0×103 cells/well) were cultured in DMEM
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) in 96-well plates (Costar; Corning, Inc.,
Corning, NY, USA). Following incubation for 1–5 days, 10 µl MTT
(Sigma-Aldrich; Merck KGaA) solution (5 mg/ml in ddH2O)
was added to the wells. The plates were incubated at 37°C for 4 h.
Intracellular formazan crystals were dissolved by adding 100 µl
DMSO to each well. Cell proliferation was evaluated on a microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA) set to 490
nm.
Measurement of apoptosis by flow
cytometry
According to the protocol of the BD Annexin V
Staining kit (BD Biosciences, Franklin Lakes, NJ, USA), the
apoptosis assay was performed as previously described (8). Briefly, the ESCs (control/NC/miR-449b
up) were trypsinized and collected at a concentration of
1×106 cells/ml. Following incubation in
allophycocyanin-Annexin V (5 µl/test tube respectively) for ١٥ min
at room temperature in the dark, the cells were tested by flow
cytometry (Beckman Coulter, Inc., Brea, CA, USA) as soon as
possible (within 1 h). The experimental results were analyzed using
FlowJo software (X10.0.7; BD Biosciences).
Measurement of angiogenesis by
capillary-like tube formation assay
The ESCs transfected with miR-449b-LV, GFP-LV and
normal ESCs (2.0×105 cells/well) were seeded in 6-well
plates. Following 24 h incubation, the supernatants were
transferred into 15 ml centrifuge tubes and centrifuged at 500 × g
at 4°C for 5 min. Then, the supernatants were collected for further
experiments. Human umbilical vein endothelial cells (HUVECs;
American Type Culture Collection, Manassas, VA, USA;
2.0×104 cells/well) were seeded on a thin layer of
Matrigel (BD Biosciences) that had been incubated at ٣٧°C for ٢ h
in 24-well plates and cultured in RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences) supplemented with 1% FBS containing the
abovementioned supernatants (normal ESCs/NC/miR-449b up) for 6 h at
37°C. Following fixation/permeabilization buffer (BD Biosciences)
was added at 4°C for 15 min in accordance with the protocol of the
manufacturer (250 µl/106 cells) and DAPI Staining
Solution at 4°C for another 10 min. The cell plates were scanned by
Cellomics (Thermo Fisher Scientific, Inc.; ArrayScan VT1) and the
data were analyzed with Cellomics software (6.1.0). Tube area, mean
tube length and mean tube nodes were calculated automatically by
the software, to measure the capillary-like structures. For rigor,
three independent experiments were performed in triplicate.
Measurement of invasiveness by
invasion (Matrigel) chamber assay
The ESCs (normal group/NC/miR-449b up;
2.5×104) were seeded on a cell culture Transwell insert
that had been coated with extracellular matrix (ECM; 8-mm pore
size, 24-well format; Costar; Corning, Inc.) in 2% FBS medium. The
complete medium (containing 10% FBS) was added into the lower
chamber. ESCs were incubated at 37°C under 5% CO2 for 24
h and then scratched from the upper chamber using a cotton swab.
Next, the invaded cells were stained on the underside of the insert
at room temperature with Giemsa staining solution. Following
rinsing with PBS, images of the undersides of the membrane were
captured using a light microscope to compare the number of invaded
cells per insert. The invaded cells were scored by randomly
counting 10 high-power fields per filter. The counting accuracy was
validated by optical density 570 nm quantification of the
methanol-solubilized dye, which was tested by a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Statistical analysis
The data represented the mean ± standard deviation
of at least 3 independent experiments. Data were analyzed using
GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). The
difference between two means was examined using a Student's t-test,
while comparison of three or more groups was determined with
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference. If P<0.05, Bonferroni's
multiple comparisons test was used for the post-hoc test.
Results
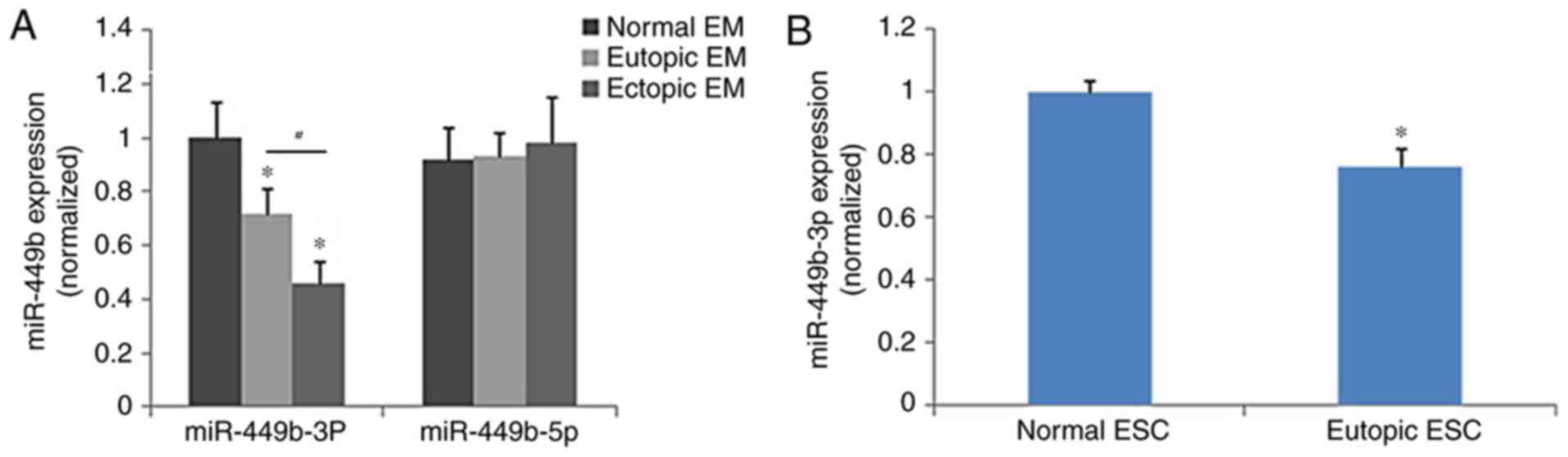
Quantification of miR-449b
expression
The eutopic endometrium expressed significantly less
miR-449b-3p compared with the control endometrium (P<0.05), and
miR-449b-3p was significantly lower in samples of ectopic
endometrium than in eutopic endometrium (P<0.05), whereas
miR-449b-5p did not demonstrate significantly different expression
among the groups (Fig. 1A). The
expression of miR-449b in ESCs isolated from eutopic and normal
endometrium was further tested as ESCs are involved in adhesion of
endometrial tissues to the peritoneal lining in the early stages of
endometriosis. The results of cell analysis were in accordance with
those of the tissue samples (P<0.05; Fig. 1B). The treatment of ESCs with
ovarian steroids (17β-estradiol and progesterone) did not regulate
the expression of miR-449b-3p in ESCs (P>0.05; data not
shown).
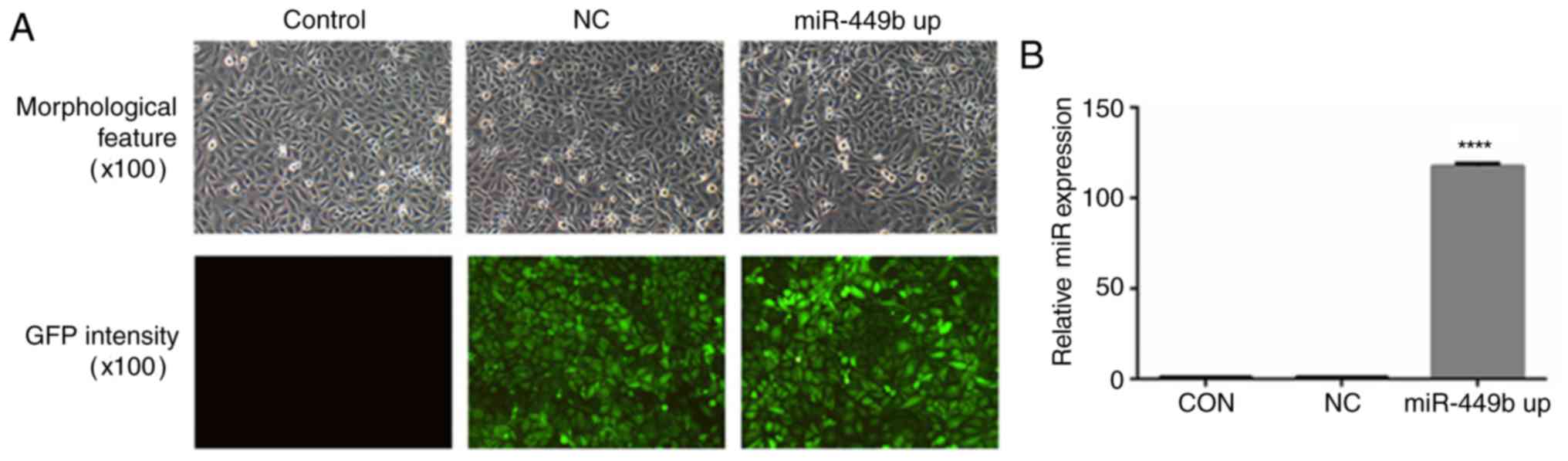
Transfection efficiency of recombinant
lentivirus
To further investigate the roles of miR-449b-3p in
ESCs, a lentiviral construct experiment for the overexpression of
miR-449b-3p was prepared. In transfected cells, >80 percent of
the cells exhibited GFP expression and maintained morphological
features similar to those of untransfected cells (Fig. 2A). The percentages were estimated
according to the intensity of green fluorescence by flow cytometry.
RT-qPCR revealed that the transfection of ESCs with miR-449b-LV
significantly increased miR-449b expression by 117-fold
(P<0.0001; Fig. 2B).
miR-449b induces proliferation, with
no effect on apoptosis of ESCs
MTT assays were conducted to assess the effect of
miR-449b-3p on cell proliferation. miR-449b-3p significantly
suppressed proliferation of ESCs from day 4–5 compared with the
negative control (P<0.05; Fig.
3A).
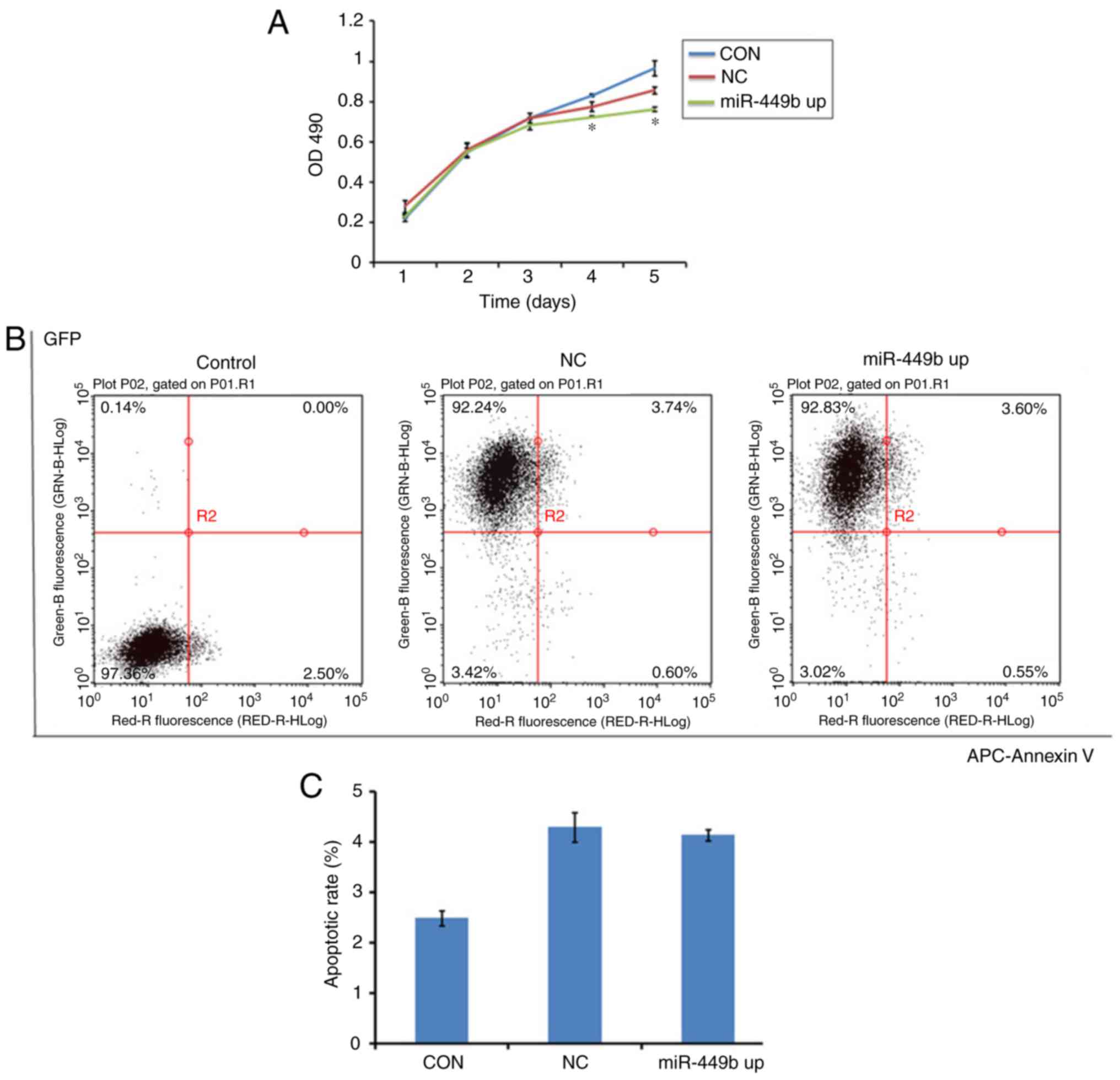 | Figure 3.Overexpression of miR-449b-3p
facilitated proliferation of ESC, with no effect on apoptosis. (A)
Proliferation of ESCs was measured by MTT assay. (B) Apoptosis of
ESCs was tested by flow cytometry with an APC-Annexin V kit.
Numbers in quadrants indicate the percentage of cells in different
conditions. (C) Quantifications of the percentages of apoptosis are
presented. Values indicate the mean ± standard deviation, n=6.
*P<0.05, vs. NC (one-way analysis of variance). OD, optical
density; CON, control group ESCs; NC, negative control
GFP-lentivirus infected ESCs; miR-449b up, miR-449b-3p lentivirus
infected ESCs; miR, microRNA; ESCs, endometrial stromal cells; GFP,
green fluorescence protein; APC, allophycocyanin. |
To study whether miR-449b induced apoptosis in ESCs,
an Annexin V assay was performed to determine cell apoptosis in
miR-449b-overexpressing cells, the negative control group and the
control group. There was no difference between the NC and miR-449b
up group (4.3 vs. 4.14%; P>0.05; Fig. 3B and C). This suggested that
miR-449b may not directly have an apoptotic effect on ESCs.
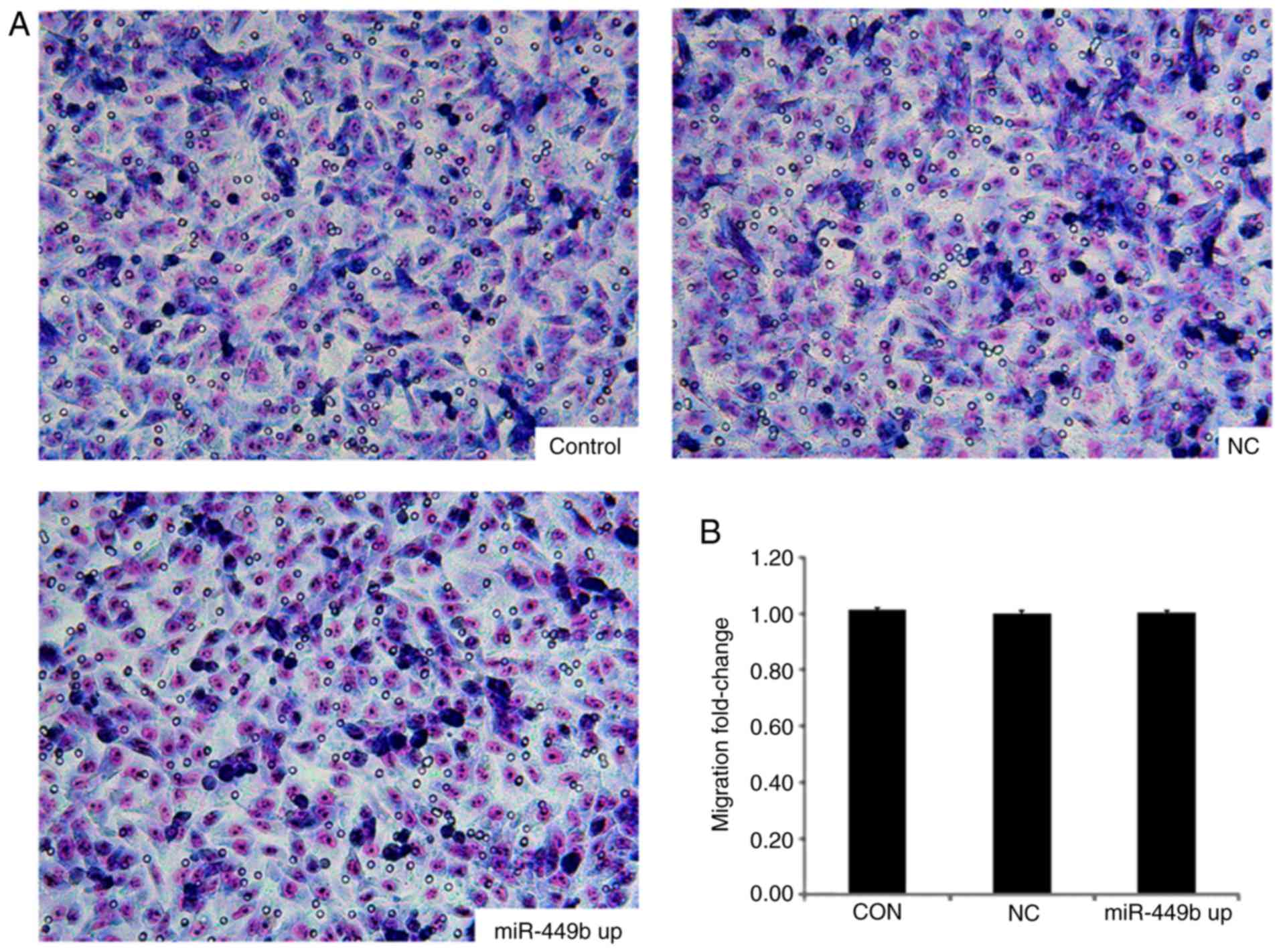
miR-449b exhibits no effect on the
invasive ability of ESCs
The effects of miR-449b-3p overexpression in ESCs
were also examined using an invasion chamber that had been coated
with ECM-Matrigel. There was no significant difference in numbers
of cells passing through the matrix between the
miR-449b-overexpressing group and the negative control group
(P>0.05; Fig. 4).
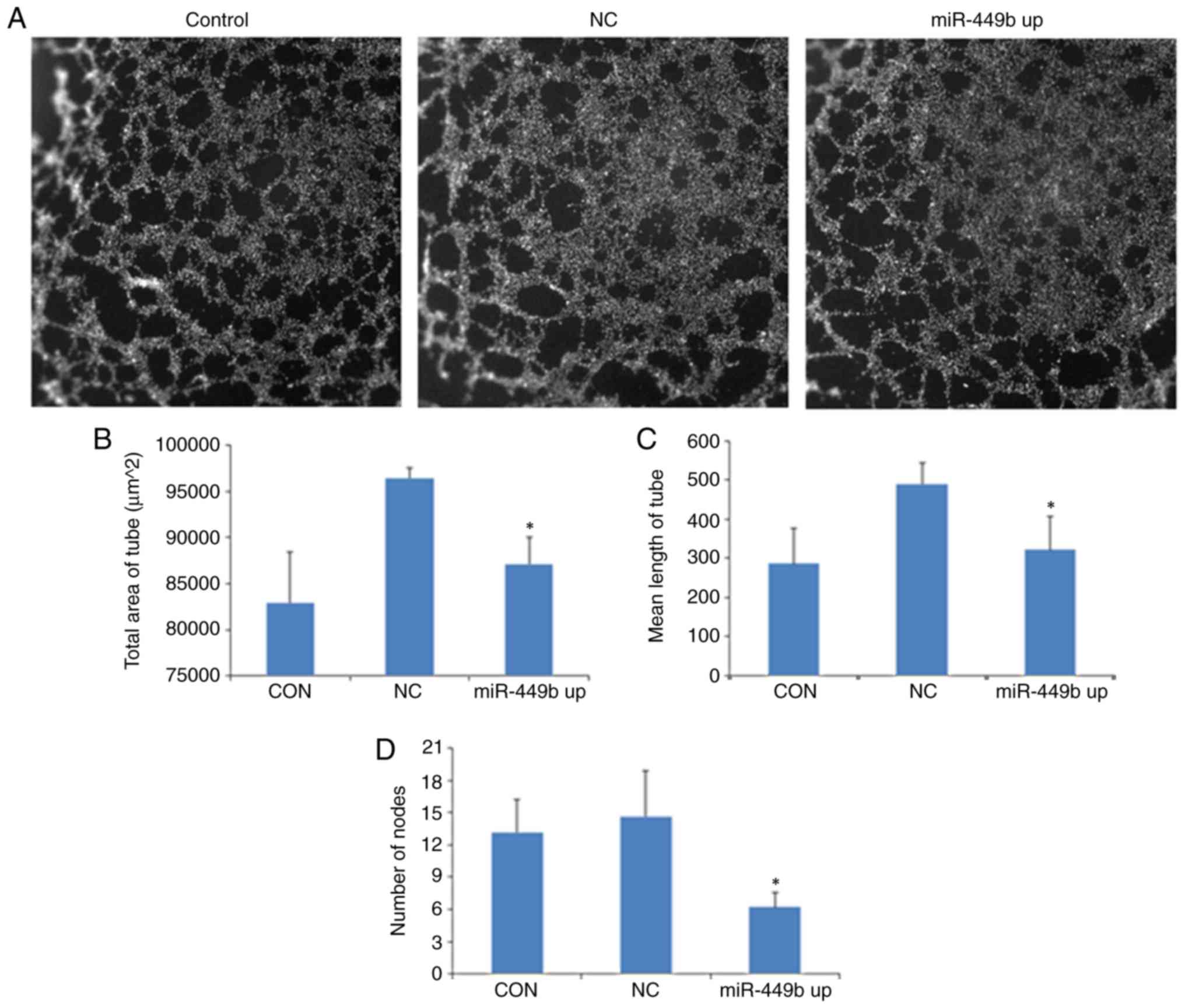
Culture supernatants from
miR-449b-3p-overexpressing ESCs enhance in vitro angiogenesis of
HUVECs
To evaluate the effect of miR-449b-3p on the
angiogenesis of HUVECs, an in vitro angiogenesis model was
established. HUVECs were seeded on a thin layer of Matrigel that
had solidified for 2 h and were incubated with the supernatants
derived from the control group ESCs, NC ESCs and
miR-449b-overexpressing ESCs, followed by a capillary-like tube
formation assay. At 6 h following seeding, there was reduced
formation of tubular structures in HUVECs treated with supernatants
from miR-449b-overexpressing ESCs compared with supernatants from
the NC group (Fig. 5A).
Quantitative analysis revealed that compared with GFP-LV group,
supernatants from the miR-449b-overexpressing ESCs inhibited the
tube area (96437.33±1043.03 vs. 87077.00±2940.59 µm2;
P=0.007), mean tube length (490.25±53.12 vs. 322.14±85.08 µm;
P=0.044) and mean tube node (14.61±4.23 vs. 6.21±1.33; P=0.03;
Fig. 5B-D).
Discussion
miR-449b has been implicated in several malignant,
inflammatory and premature ovarian insufficiencies (9–11);
however, its association with the pathogenesis of endometriosis has
not previously been well described. It is well established that
ectopic endometrium may have a better capacity to survive outside
the uterine cavity because of its different functions compared with
those of normal endometrium in women without endometriosis. Both
genetic and acquired molecular abnormalities may alter the ectopic
viability of the endometrium, potentially rendering certain women
susceptible to endometriosis. The findings of the current study
indicated that miR-449b-3p was in ectopic and eutopic tissues, in
accordance with the results of a previous study (12).
The cellular composition of ectopic tissues is
heterogeneous and contains cells from surrounding ovarian tissue,
inflammatory cells, endometrial stromal and epithelial cells in
variable proportions. In fact, ectopic tissues may contain only a
small fraction of endometrium-specific cells. Therefore, the
heterogeneity of endometriotic lesion biopsies presents a real
challenge in the study of endometriosis, as the molecular signature
of endometrial cells in lesions could be masked by the surrounding
tissue, leading to inconsistent or wrongly interpreted results
(13). To overcome this issue, the
differences in miR-449b levels were examined, focusing on the
isolation and analysis of ESC. In the present study, it was
demonstrated that miR-449b-3p expression in eutopic ESCs was
decreased compared with the control group.
The expression levels of miRs generated either from
−5p or −3p arms of the precursor may vary not only among various
tissues/cells but also in various states of health and disease
(14). miRs can act as regulators
of the steroid hormone response in the female reproductive tract
(15); conversely, a number miRs
may also be affected by hormone levels (16). To confirm whether ovarian steroids
have regulatory effects on miR-449b, miR-449b-3p expression in ESCs
was measured following treatment with 17β-estradiol and
progesterone by qPCR. However, in the present study, estrogen and
progesterone have no effect on expression of miR-449b-3p.
The attachment and invasion of endometrium fragments
is considered to be necessary for the formation of endometriosis.
Simultaneously, the establishment of a blood supply and a
suboptimal immune response provide favorable conditions for the
development of endometriosis. Therefore, a number of relevant
functional effects of ESCs were analyzed using in vitro
assays.
In previous studies, miR-449b was upregulated in
prostate cancer and T cells of patients with systemic lupus
erythematosus, while it was downregulated in thyroid carcinoma and
ovarian cancer, suggesting that its roles can vary according to the
cellular context (17–20). It is involved in a number of
cellular functions, including cell cycle control and cell
differentiation (21). The
induction of miR-449 expression can lead to cell cycle stagnation
and apoptosis by inhibiting cyclin dependent kinase and cell
division cycle 25A. It can also protect against the proliferation
induced by E2F transcription factor 1 as a negative feedback
mechanism (22). The present study
demonstrated the effect of miR-449b on cell growth is via the
modulation of cell proliferation rather than via apoptosis.
The further functional analysis in the present study
indicates that miR-449b-3p serves an inhibitory role in promoting
tubulogenesis of HUVECs, whereas it has no effect on cell
invasiveness. Similar to tumors, the survival and growth of
endometrium requires a blood supply. It has been demonstrated that
eutopic endometrium from patients with endometriosis exhibits
increased angiogenic potential in comparison with disease-free
women, potentially contributing to the initiation of endometriosis
(23).
The study of miR-449b-3p downstream mechanisms will
be investigated further. In the present study, the abnormal
expression of miR-449b-3p in endometriosis was clarified and the
biological functional alterations brought about by the
downregulation of miR-449b-3p were further investigated. Presently,
the present study group is also trying to identify the downstream
molecular targets of miR-449b-3p and hope to further explain the
specific molecular mechanisms of miR-449b-3p.
In conclusion, it was demonstrated that miR-449b-3p
was downregulated in ectopic and eutopic tissues, and the same
expression pattern was also observed in ESCs. Its expression is not
affected by estrogen or progesterone. The upregulated expression of
miR-449b-3p inhibited the proliferation of ESCs and the
supernatants of miR-449b-overexpressing ESCs inhibited the
formation of tubular structures in HUVECs. The present study group
is still investigating targets of this miR that are associated with
cellular functions. The impact of miR-449b on endometriosis in
vivo can be expected to improve the implantation and
establishment of ectopic lesions. These results suggest that
abnormalities in miR-449b expression lead to the development and
progression of endometriosis.
Acknowledgements
Not applicable.
Funding
This study was supported by funds from the National
Natural Science Foundation of China (grant no. 81471438).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL conducted all the experiments and arranged the
figures and the manuscript. XZ, LT and XZ assisted with sample
collection. JC assisted with analyzing the data and revising the
manuscript critically. YS initiated and supervised the project,
analyzed the data and formed the conclusion, and edited the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Obstetrics and Gynecology Hospital and patients
consented to tissue donation prior to surgery. Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pillai RS: MicroRNA function: Multiple
mechanisms for a tiny RNA? RNA. 11:1753–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engels BM and Hutvagner G: Principles and
effects of microRNA-mediated post-transcriptional gene regulation.
Oncogene. 25:6163–6169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nematian SE, Mamillapalli R, Kadakia TS,
Zolbin Majidi M, Moustafa S and Taylor HS: Systemic inflammation
induced by microRNAs: Endometriosis derived alterations in
circulating microRNA 125b-5p and Let7b-5p regulate macrophage
cytokine production. J Clin Endocrinol Metab. 103:64–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JH, Lee SK, Kim MK, Lee JH, Yun BH,
Park JH, Seo SK, Cho S and Choi YS: Saponin extracts induced
apoptosis of endometrial cells from women with endometriosis
through modulation of miR-21-5p. Reprod Sci. 25:292–301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joshi NR, Miyadahira EH, Afshar Y, Jeong
JW, Young SL, Lessey BA, Serafini PC and Fazleabas AT: Progesterone
resistance in endometriosis is modulated by the altered expression
of MicroRNA-29c and FKBP4. J Clin Endocrinol Metab. 102:141–149.
2017.PubMed/NCBI
|
|
6
|
Shi YL, Luo XZ, Zhu XY, Hua KQ, Zhu Y and
Li DJ: Effects of combined 17beta-estradiol with TCDD on secretion
of chemokine IL-8 and expression of its receptor CXCR1 in
endometriotic focus-associated cells in co-culture. Hum Reprod.
21:870–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi XY, Gu L, Chen J, Guo XR and Shi YL:
Downregulation of miR-183 inhibits apoptosis and enhances the
invasive potential of endometrial stromal cells in endometriosis.
Int J Mol Med. 33:59–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sandbothe M, Buurman R, Reich N, Greiwe L,
Vajen B, Gürlevik E, Schäffer V, Eilers M, Kühnel F, Vaquero A, et
al: The microRNA-449 family inhibits TGF-β-mediated liver cancer
cell migration by targeting SOX4. J Hepatol. 66:1012–1021. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan H, Chen B, Wang J, Wang X, Hu P, Wu S,
Liu Y, Xu Z, Zhang W, Wang B and Cao Y: The miR-449b polymorphism,
rs10061133 A>G, is associated with premature ovarian
insufficiency. Menopause. 23:1009–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buggele WA, Krause KE and Horvath CM:
Small RNA profiling of influenza A virus-infected cells identifies
miR-449b as a regulator of histone deacetylase 1 and interferon
beta. PLoS One. 8:e765602013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Braza-Boïls A, Marí-Alexandre J, Gilabert
J, Sánchez-Izquierdo D, España F, Estellés A and Gilabert-Estellés
J: MicroRNA expression profile in endometriosis: Its relation to
angiogenesis and fibrinolytic factors. Hum Reprod. 29:978–988.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saare M, Rekker K, Laisk-Podar T,
Rahmioglu N, Zondervan K, Salumets A, Götte M and Peters M:
Challenges in endometriosis miRNA studies-From tissue heterogeneity
to disease specific miRNAs. Biochim Biophys Acta. 1863:2282–2292.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meijer HA, Smith EM and Bushell M:
Regulation of miRNA strand selection: Follow the leader? Biochem
Soc Trans. 42:1135–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sørensen AE, Udesen PB, Wissing ML,
Englund AL and Dalgaard LT: MicroRNAs related to androgen
metabolism and polycystic ovary syndrome. Chem Biol Interact.
259:8–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lam EW, Shah K and Brosens JJ: The
diversity of sex steroid action: The role of micro-RNAs and FOXO
transcription factors in cycling endometrium and cancer. J
Endocrinol. 212:13–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mortensen MM, Høyer S, Orntoft TF,
Sørensen KD, Dyrskjøt L and Borre M: High miR-449b expression in
prostate cancer is associated with biochemical recurrence after
radical prostatectomy. BMC Cancer. 14:8592014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu MC, Yu CL, Chen HC, Yu HC, Huang HB and
Lai NS: Aberrant T cell expression of Ca2+
influx-regulated miRNAs in patients with systemic lupus
erythematosus promotes lupus pathogenesis. Rheumatology (Oxford).
54:343–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Xu L and Wang G: Regulation of
MET-mediated proliferation of thyroid carcinoma cells by miR-449b.
Tumour Biol. 36:8653–8660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Lp, Li N, He Xj and Zhang Q: miR-449b
and miR-34c on inducing down-regulation of cell cycle-related
proteins and cycle arrests in SKOV3-ipl cell, an ovarian cancer
cell line. Beijing Da Xue Xue Bao. 43:129–133. 2011.(In Chinese).
PubMed/NCBI
|
|
21
|
Fang Y, Gu X, Li Z, Xiang J and Chen Z:
miR-449b inhibits the proliferation of SW1116 colon cancer stem
cells through downregulation of CCND1 and E2F3 expression. Oncol
Rep. 30:399–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M
and Yu Q: miR-449a and miR-449b are direct transcriptional targets
of E2F1 and negatively regulate pRb-E2F1 activity through a
feedback loop by targeting CDK6 and CDC25A. Genes Dev. 23:2388–93.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laschke MW and Menger MD: Anti-angiogenic
treatment strategies for the therapy of endometriosis. Hum Reprod
Update. 18:682–702. 2012. View Article : Google Scholar : PubMed/NCBI
|