Introduction
Chronic glomerulonephritis (CGN), the most common
form of glomerular disease, accounts for ~20% of chronic kidney
disease cases in many countries (1,2). CGN
is associated with immune-mediated inflammatory diseases and is
characterized by proteinuria, edema, hematuria and hypertension,
which are accompanied by renal dysfunction which is a primary cause
of end-stage of renal disease (ESRD) (3,4).
Numerous pathogenic factors may promote the development of this
disease; however, the molecular mechanisms remain unknown (5,6).
In the authors previous experiments, differentially
regulated genes were screened and analyzed. The results revealed
that Fos and spleen-associated tyrosine kinase (Syk) were potent
hub genes and that CGN pathogenesis may be associated with the
disordered inflammatory response in addition to abnormal metabolism
(7). Therefore, it is important to
explain the specific mechanism of Fos and Syk in CGN, which may
contribute to understanding the pathogenesis of CGN and developing
novel diagnostic markers.
The functions of B lymphocytes are adjusted by a
number of signaling pathways, some of which involve the B-cell
receptor (BCR) (8). Syk exhibits a
central role in the activation of the BCR (9). The Fos gene family encode leucine
zipper proteins that form the transcription factor complex
activating protein (AP)-1, and can regulate the expression of tumor
necrosis factor-α, interleukin (IL)-6 and IL-8 by phosphorylation
of mitogen-activated protein kinase (MAPK) and the BCR signaling
pathway, which participates in inflammation in CGN (10). The Syk/Ras/c-Fos signaling pathway
has a critical role in B cells, including ontogeny, autoimmunity,
immune response and immunoglobulin production.
By searching relevant literature, we found that LPS
can be used as an inducer for cell viability of glomerular
mesangial cells. And this is consistent with our CGN pathology
(11,12).
In the present study, Adriamycin (ADR)-induced CGN
rats and lipopolysaccharide (LPS)-stimulated HBZY-1 cells were used
as experimental models to identify the differentially expressed
mRNAs and proteins of the Syk/Ras/c-Fos signaling pathway, and
elucidate the potential pathogenesis of CGN.
Materials and methods
Materials
ADR was obtained from Hisun Pfizer Pharmaceuticals
Ltd. (cat. no. 15029611; Zhejiang, China). Sodium pentobarbital was
obtained from Shanghai Chemical Reagent Company (cat. no. 127K1005;
Shanghai, China). Total RNA from renal cortex tissues was extracted
by TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) following the manufacturer's
protocol. Antibodies against phosphorylated (p)-Syk, Ras, p-MAPK
extracellular signal regulated kinase (ERK; MEK)1/2, p-ERK1/2,
c-Fos and β-actin were purchased from Abcam (Shanghai, China; cat.
nos. ab79193, ab16907, ab194754, ab76299, ab209794, ab8226). The
Syk/Ras/c-Fos pathway inhibitor R406 (inhibitor of Syk) was
purchased from AbMole BioScience, (Shanghai, China). All the
materials under current study were non-toxic to animals and cell
cultures, including all biological and synthetical agents used for
immunopharmacological studies.
Animals and cell cultures
The HBZY-1 cell line was obtained from the Cell Bank
of Chinese Academy of Sciences (Shanghai, China) and incubated with
Dulbecco's modified Eagle's/F-12 medium [10% (v/v) fetal calf serum
and 1% (v/v) antibiotics mixture] in 95% air and 5% CO2
at 37°C (13). Specific
pathogen-free (SPF), male Sprague-Dawley (SD) rats (weighing
280–320 g, 9 weeks old) were provided by the Laboratory Animal
Center of Anhui Medical University (Hefei, China). All rats were
kept in standard cages under 40–60% humidity at 18–22°C with free
access to food and water. All animal experiments were approved by
the Committee on the Ethics of Animal Experiments of The First
Affiliated Hospital of Anhui University of Chinese Medicine (Hefei,
China). All surgeries were performed under sodium pentobarbital
anesthesia, and all efforts were made to minimize suffering.
CGN rat model establishment and
experimental protocols
Following acclimatization for 2 weeks, all animals
were divided randomly into the control group and experimental model
group (n=10 per group). CGN was induced in the rats by tail
intravenous injection with ADR: 3.5 mg/kg ADR was given on the 1st
day and 3.0 mg/kg on the 14th day (7,14),
whereas the control group was administered a saline solution for
comparison at the same time. On the 21st day, all rats were placed
into metabolism cages and urine was collected over 24 h to
determine the urinary protein levels. A successful model was
considered to be indicated by a 24 h urinary protein quantitation
of >50 mg/kg. The rats were anesthetized with intraperitoneal
sodium pentobarbital (2 ml/kg), and serum samples were obtained
from the abdominal aorta for measuring biochemical parameters. All
urine and serum samples were stored at −70°C prior to analysis.
Animals were then sacrificed and each kidney was retrieved to
determine kidney viscera index, and then one half of each kidney
was frozen in liquid nitrogen for RNA preparation and protein
extraction, while the other half was fixed in 10% neutral formalin
for histological evaluation.
Biochemical determination
The 24-h urinary protein, blood urea nitrogen (BUN)
and creatinine (Crn) were measured using an automatic biochemistry
analyzer.
Hematoxylin and eosin (HE)
staining
Glomerular specimens were fixed in 10% neutral
formalin, and 4-µm-thick paraffin-embedded sections were stained
with HE and observed microscopically.
HBZY-1 cell model establishment and
experimental protocols
HBZY-1 cells were seeded into 6-well plates at a
density of 3×105 cells per well and allowed to grow
until 70–80% confluent. The cells were divided into three groups:
Normal control (normal HBZY-1 cells), an LPS model group (cells
were incubated with 0.5 µg/ml LPS for 48 h) and an LPS + R406 group
(cells were incubated with 1.5 µg/ml R406 for 48 h following model
establishment). Each treatment and control were performed at least
in triplicate.
mRNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA samples from glomerular specimens and
HBZY-1 cells were extracted by TRIzol reagent according to the
manufacturer's protocol. The total RNA was used as a template to
synthesize first-strand cDNA using a ThermoScript RT-qPCR system
(Thermo Fisher Scientific, Inc.) The primers for Syk, Ras, MEK1/2,
ERK1/2, c-Fos and β-actin were synthesized by Thermo Fisher
Scientific, Inc. RT-qPCR was completed in a final volume of 25 µl
and the following thermal cycling profile for SYBR Green PCR was
used: 95°C for 5 min, followed by 40 cycles of 95°C for 10 sec and
60°C for 30 sec. To confirm that only one PCR product was amplified
and detected, a dissociation curve analysis of amplification
products was performed at the end of each PCR. The comparative Cq
method (2−ΔΔCq method) was used to quantify the
expression levels of the different genes (15). Primer sequences are listed in
Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Forward and reverse
sequences (5′-3′) | Product length
(bp) |
|---|
| β-actin | F:
CAGCGGAACCGCTCATTGATGG | 155 |
|
| R:
TCACCCACACTGTGCCCAACGA |
|
| Syk | F:
AGAGGGGAGCTCAGACATGA | 138 |
|
| R:
TCTTGTACACACCCTTGGCA |
|
| Ras | F:
GAGTACAGTGCAATGAGGGAC | 130 |
|
| R:
CCTGAGCCTGTTTTGTGTCTAC |
|
| MEK1/2 | F:
GACGAGCAGCAGCGG | 126 |
|
| R:
CTTGAACACCACTCCACCATTG |
|
| ERK1/2 | F:
TCATAGGCATCCGAGACATC | 129 |
|
| R:
TGGTAGAGGAAGTAGCAGATG |
|
| c-Fos | F:
TACTACCATTCCCCAGCCGA | 113 |
|
| R:
GCTGTCACCGTGGGGATAAA |
|
Protein extraction and western blot
analysis
Total protein samples were extracted from glomerular
specimens and rat HBZY-1 cells using a Total Protein Extraction
kit, according to the manufacturer's protocol. Protein
concentrations were determined by BCA assay. An aliquot of 30 µg of
denatured protein from each sample was subjected to 10% SDS-PAGE,
transferred onto a polyvinylidene difluoride membrane, and then
incubated with 5% skimmed milk for 1 h. Primary antibodies against
p-Syk (1:500 dilution; ab79193), Ras (1:25 dilution; ab16907),
p-MEK1/2 (1:500 dilution; ab194754), p-ERK1/2 (1:5,000 dilution;
ab76299), c-Fos (1:100 dilution; ab209794) and β-actin (1:500
dilution; ab8226; all from Abcam) were added and incubated at 4°C
overnight. Following washing with TBST, the membranes were
incubated with goat anti-rabbit or anti-mouse IgG secondary
antibodies conjugated with horseradish peroxidase (1:5,000
dilution) for 1 h at 37°C. The blots were visualized using an ECL
western blot detection system and scanned with a Gel Imaging
System.
Statistical analysis
Data are presented as the mean ± standard deviation.
All data were analyzed using SPSS software, v.17.0 (SPSS, Inc.,
Chicago, IL, USA). Two groups were compared with t-test, and
one-way analysis of variance with Tukey's post hoc test was used to
determine the significance of three groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of experimental
rats
Table II presents
the laboratory data of the two groups of rats at the end of the
experimental period. Compared with the normal group, body weights
were significantly lower (P<0.05) and the kidney viscera index
and 24 h urine protein were significantly increased (both
P<0.01; Table II) in the model
group. Furthermore, the levels of BUN and Crn in serum samples were
significantly increased in the model group (both P<0.01;
Table II), which was in accord
with previous studies (16,17).
 | Table II.Body weight, kidney viscera index, 24
h urine protein, blood urea nitrogen and Syk in the different
groups. |
Table II.
Body weight, kidney viscera index, 24
h urine protein, blood urea nitrogen and Syk in the different
groups.
| Parameter | Normal | Model | P-value |
|---|
| Body weight
(g) | 315.15±23.61 |
281.91±44.95a | 0.046 |
| Kidney viscera
index (%) | 0.64±0.04 |
0.90±0.17b | <0.001 |
| 24 h urine protein
(mg/24 h) | 27.32±5.99 |
292.99±44.21b | <0.001 |
| BUN (mmol/l) | 5.53±1.89 |
12.19±3.60b | <0.001 |
| Crn (µmol/l) | 38.58±6.65 |
65.75±13.78b | <0.001 |
Histopathology
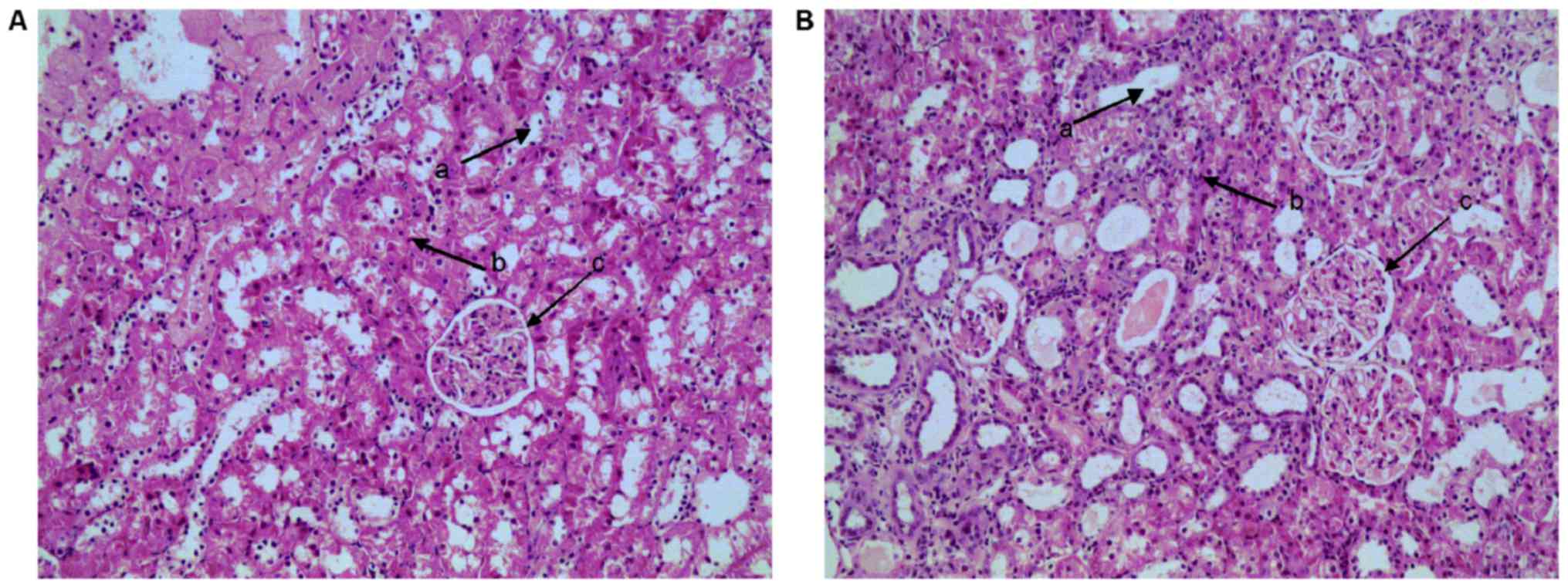
HE staining is presented in Fig. 1. Rats from the control group
invariably exhibited normal glomerular structure and glomerular
basement membrane thickness, clear Bowman's capsule structure and
convoluted tubule structure, and opened capillary loops. However,
in the model group, there were incrassations of the capillary loops
and Bowman's capsule. In addition, degeneration of renal tubule
epithelial cells, infiltration of inflammatory cells and casts
(protein) in the lumen were also observed, which was in agreement
with the authors previous research and indicated that the CGN model
was successfully established (6,14).
mRNA and protein expression of
Syk/Ras/c-Fos signaling pathway components in the kidney of CGN
rats
In order to evaluate Syk/Ras/c-Fos signaling pathway
whether involved in CGN lesion, the key genes mRNA and protein
expression level were detected in kidney of CGN rats (Figs. 2 and 3). According to western blot results,
levels of p-Syk, Ras, p-MEK1/2, p-ERK1/2 and c-Fos were higher in
CGN model group than in the control group (Fig. 3). Similar results were found in the
relative mRNA levels of Syk, Ras, MEK1/2, ERK1/2 and c-Fos mRNA
(Fig. 2).
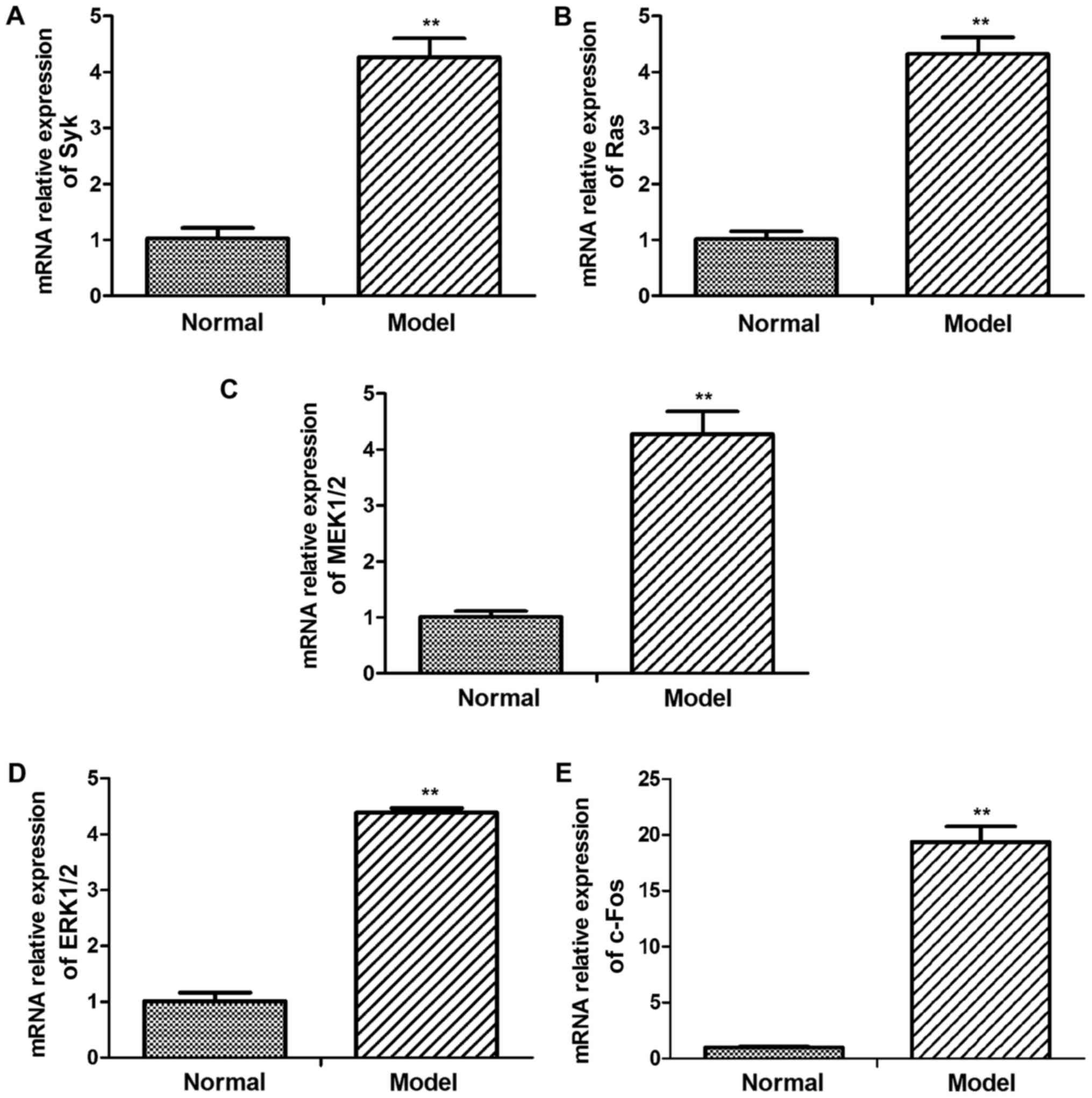 | Figure 2.mRNA levels of Syk, Ras, MEK1/2,
ERK1/2 and c-Fos in glomerular tissues of adriamycin-treated and
normal rats. The mRNA expression levels of (A) Syk, (B) Ras, (C)
MEK1/2, (D) ERK1/2 and (E) c-Fos were assessed using reverse
transcription-quantitative polymerase chain reaction. Syk, Ras,
MEK1/2, ERK1/2 and c-Fos mRNA levels were significantly increased
in the model group. Data are presented as the mean ± standard
deviation of at least three independent experiments. **P<0.01
vs. normal (control) group. Syk, spleen associated tyrosine kinase;
ERK, extracellular signal regulated kinase; MEK, mitogen activated
protein kinase kinase. |
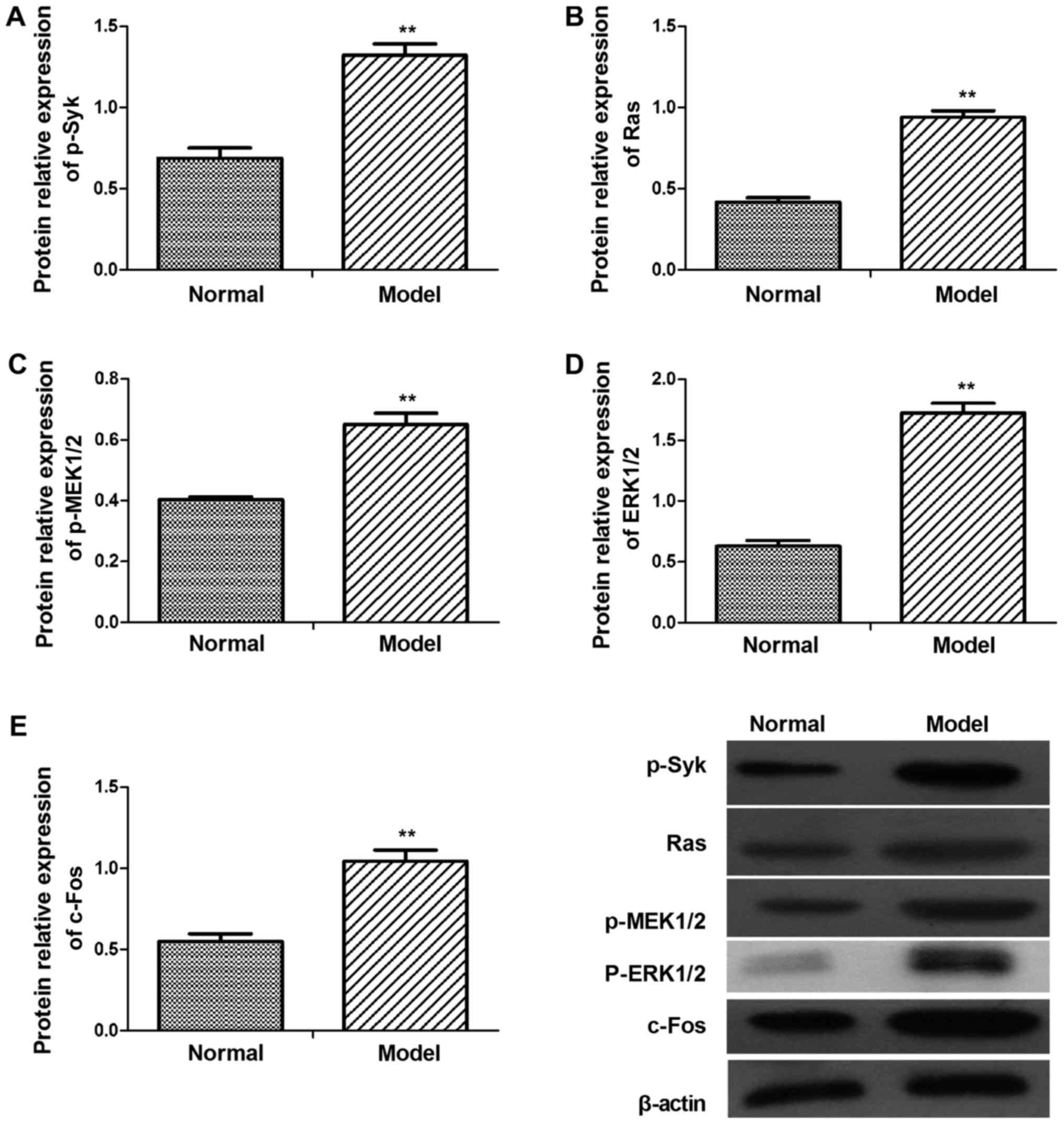 | Figure 3.Protein levels of p-Syk, Ras,
p-MEK1/2, p-ERK1/2 and c-Fos in the glomerular tissues of
adriamycin-treated and normal rats. Protein expression levels of
(A) p-Syk, (B) Ras, (C) p-MEK1/2, (D) p-ERK1/2 and (E) c-Fos were
assessed by western blot analysis. p-Syk, Ras, p-MEK1/2, p-ERK1/2
and c-Fos protein levels were upregulated. Data are presented as
the mean ± standard deviation of at least three independent
experiments. **P<0.01 vs. normal (control) group. Syk, spleen
associated tyrosine kinase; MEK, mitogen activated protein kinase
kinase; ERK, extracellular signal regulated kinase; p-,
phosphorylated. |
mRNA and protein levels of
Syk/Ras/c-Fos signaling pathway components in LPS-stimulated HBZY-1
cells
The literature shows that LPS can be used as an
inducer to induce cell viability of mesangial cells. And this is
consistent with our CGN pathology (11,12).
So in this experiment, LPS-stimulated HBZY-1 cells were used as
experimental models to elucidate the potential pathogenesis of CGN.
The results revealed that Syk, Ras, MEK1/2, ERK1/2 and c-Fos mRNA
and p-Syk, Ras, p-MEK1/2, p-ERK1/2 and c-Fos protein levels
markedly increased in the LPS model group (Figs. 4 and 5). This may suggest that the key genes
mRNA and protein expression level increased evidently in
Syk/Ras/c-Fos signaling pathway in LPS-stimulated HBZY-1 cells.
Furthermore, R406 was revealed to inhibit the LPS-induced
activation of the Syk/Ras/c-Fos signaling pathway.
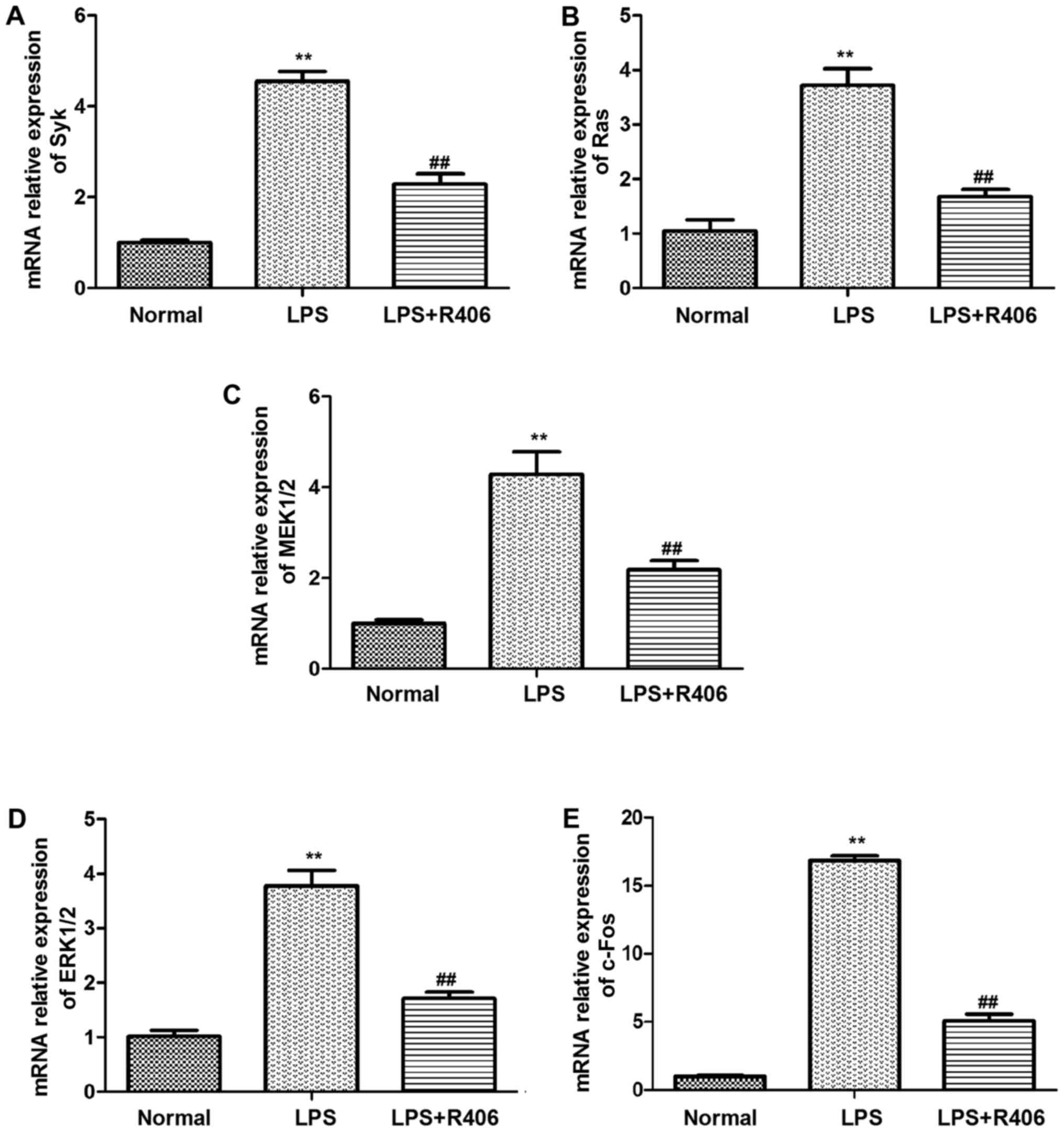 | Figure 4.mRNA levels of (A) Syk, (B) Ras, (C)
MEK1/2, (D) ERK1/2 and (E) c-Fos in HBZY-1 cells were determined by
reverse transcription-quantitative polymerase chain reaction. Syk,
Ras, MEK1/2, ERK1/2 and c-Fos mRNA levels significantly increased
in the LPS model group. The inhibitor R406 inhibited the
LPS-induced activation of the Syk/Ras/c-Fos signaling pathway. Data
are presented as the mean ± standard deviation of at least three
independent experiments. **P<0.01 vs. normal group;
##P<0.01 vs. LPS group. Syk, spleen associated
tyrosine kinase; MEK, mitogen activated protein kinase kinase; ERK,
extracellular signal regulated kinase; p-, phosphorylated; LPS,
lipopolysaccharide. |
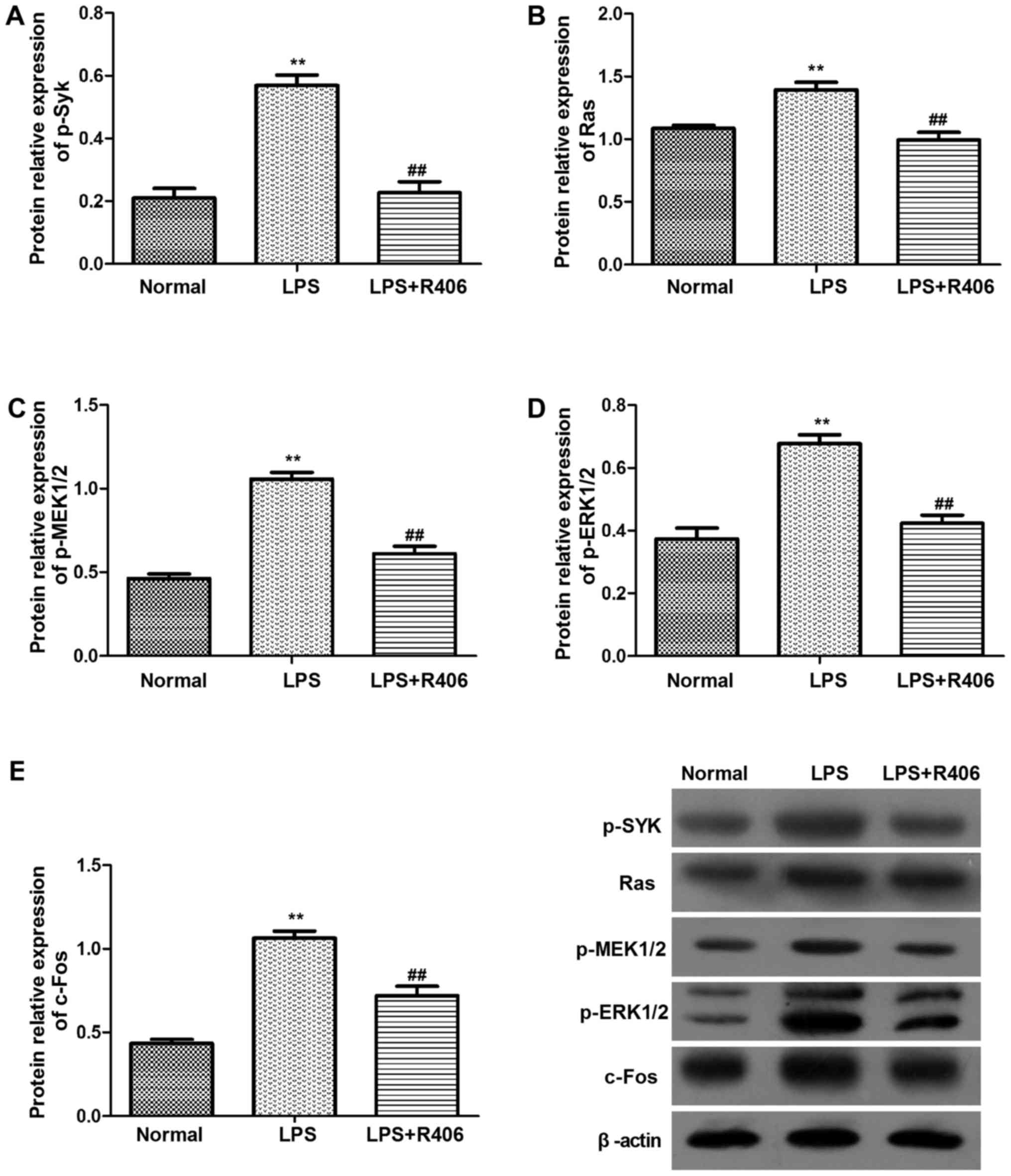 | Figure 5.Protein levels of (A) p-Syk, (B) Ras,
(C) p-MEK1/2, (D) p-ERK1/2 and (E) c-Fos in HBZY-1 cells were
determined by western blotting. p-Syk, Ras, p-MEK1/2, p-ERK1/2 and
c-Fos protein levels significantly increased in the LPS model
group. The inhibitor R406 inhibited the LPS-induced activation of
the Syk/Ras/c-Fos signaling pathway. Data are presented as the mean
± standard deviation of at least three independent experiments.
**P<0.01 vs. normal group; ##P<0.01 vs. LPS group.
Syk, spleen associated tyrosine kinase; MEK, mitogen activated
protein kinase kinase; ERK, extracellular signal regulated kinase;
p-, phosphorylated; LPS, lipopolysaccharide. |
Discussion
CGN, which is associated with immune-mediated
inflammatory diseases, frequently occurs during ESRD and seriously
affects patient survival. Biological and clinical observations
indicate that focal infection, caused by hematuria, proteinuria,
arterial hypertension and edema, primarily manifests as glomerular
injury (18). Autoimmunity,
infection and the inflammatory response are known to be involved in
the pathogenesis of CGN (19).
However, despite ongoing investigation, the exact molecular
mechanisms remain unclear.
In the current study, ADR-induced CGN rats and
LPS-stimulated HBZY-1 cells were used to explore the molecular
pathogenesis of CGN (7). The
results indicated that the kidney viscera index and the 24 h
urinary protein, BUN and Crn levels were significantly increased
while body weight decreased. The Syk/Ras/c-Fos signaling pathway
was activated both in vitro and in vivo. Therefore,
it was hypothesized that activation of Syk/Ras/c-Fos signaling may
be involved in the inflammatory reaction and proteinuria during the
process of CGN. For all that, The LPS as ADR-induced CGN may not be
accurate, but it can be used as an inducer for glomerular cell
viability and it as a limitation of the present study.
The establishment of appropriate models is critical
for disease research. In the current study, the ADR-induced CGN rat
model was selected as it has previously been demonstrated to be
similar to human CGN progression (20). LPS was used in the in vitro
studies, however not in the animal models. In the present study,
ADR-induced rats developed expansion of the convoluted tubules,
degeneration of renal tubule epithelial cells, infiltration of
inflammatory cells, and casts (protein) in the lumen, which were
consistent with results from a previous study (7). The present study focused on cell
viability of the glomerular mesangial cells, and used the classical
proliferation and inflammatory inducer LPS to simulate CGN in the
cells. However, LPS is considered to be one of the strong
stimulating factors for glomerular mesangial cells, it may be used
as an inducer for glomerular cell viability.
Syk and c-Fos were demonstrated to be involved in
the BCR signaling pathway. BCR signaling is a complex process that
involves a number of kinases, phosphatases and adaptor proteins
that transmit, modulate or terminate the signal (21). Once activated, Syk propagates the
BCR signal through an important signaling intermediate associated
with the phosphorylation of adapter proteins, including B-cell
linker protein and phospholipase Cγ2 (22). The signaling cascade then proceeds
to activate downstream signaling molecules that regulate the
cellular response, including Ras GTPase-activating protein (Ras
GAP). Ras GAP regulates Ras by converting the active GTP-bound form
of Ras into the inactive GDP-bound form and may also function as an
effector of Ras (23,24).
MAPKs are important mediators of the intracellular
signal transduction pathways that are responsible for cell growth
and differentiation (25). Ras may
induce cell proliferation by activating the MAPK survival pathway
and regulating the expression of IL-8, IL-2 and IL-6. A previous
study suggested that the expression of p-ERK is significantly
increased in the anti-Thy1 nephritis group as compared with the
sham group (P<0.01), and it was suggested that kidney injury may
be directly associated with the inactivation of the ERK signaling
pathway, thereby inhibiting the abnormal cell viability of
intravascular cells (26). In
another study, the development of diabetic nephropathy is
accelerated with a decrease in Raf kinase inhibitor protein and an
increase in p-ERK1/2 (27).
Activated ERK1/2 is transferred from the cytoplasm
to the nucleus, where it further mediates the transcriptional
activation of c-Fos and c-Jun. c-Fos is an important member of the
AP-1 transcription complex, which is involved in major cellular
functions including proliferation, transformation, differentiation
and apoptosis (28). Zu et
al (29) concluded that
saikosaponin-D inhibits the proliferation of glomerular mesangial
cells and the synthesis of extracellular matrix proteins through
the downregulation of the cyclin dependent kinase 4, c-Jun and
c-Fos genes. Therefore, members of the Fos gene family are known to
be regulators of cell proliferation, differentiation,
transformation and inflammation, which are involved in inflammation
in CGN (10). In the present
study, the expression levels of Ras, p-MEK, p-ERK1/2 and c-Fos were
increased in the model group rats and HBZY-1 cells after LPS
treatment compared with control group, which was in accordance with
the literature (30).
Although the inhibitor R406 is used in cell
experiments, there is no interference experiment with syk on animal
models, is a great regret of our project and also the limitation of
this experiment. In addition, we should increase the expression of
Syk/Ras/c-fos by immunohistochemical staining, which will give us a
direct and vivid expression.
In conclusion, the Syk/Ras/c-Fos signaling pathway
was activated significantly in ADR-induced CGN rats and LPS-induced
HBZY-1 cells. The results of the present study provide novel
insights suggesting that Syk/Ras/c-Fos signaling may be directly
associated with CGN.
Acknowledgements
The authors are grateful to Mr. Qiang Fan (Ao Ji
Bio-tech Co., Ltd., Shanghai, China) for assisting with data
analysis.
Funding
The present study was financially supported by the
Science Foundation Projects of Anhui University of Chinese Medicine
(grant no. 2015fy004), the Second Science and Technology Planning
Project in Anhui Province (grant no. 15011d04007), the Natural
Science Foundation of the Anhui Higher Education Institutions of
China (grant no. KJ2017A284) and the Traditional Chinese Medicine
Research Projects of Health and Family Planning Commission of Anhui
Province (grant no. 2016zy17).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JG, LW and HJ conceived and designed the study. YG,
XW and SX performed the experiments. JS participated in the
analysis and processing of animal experiments and data, and wrote
the paper. HJ critically revised the manuscript for important
intellectual content. All authors read and approved the
manuscript.
Ethical approval and consent to
participate
All animal experiments were approved by the
Committee on the Ethics of Animal Experiments of The First
Affiliated Hospital of Anhui University of Chinese Medicine (Hefei,
China). All surgeries were performed under sodium pentobarbital
anesthesia, and all efforts were made to minimize suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Floege J and Amann K: Primary
glomerulonephritides. Lancet. 387:2036–2048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H, Ying Y, Chen Y, Lu X and Huang Y:
Effect of chronic glomerulonephritis on the semen quality and
cytokines in the semen of infertile males. Am J Reprod Immunol.
77:2017.https://doi.org/10.1111/aji.12598simple10.1111/aji.12598
View Article : Google Scholar
|
|
3
|
Chebotareva NV, Bobkova IN, Neprintseva
NV, Kozlovskaia LV and Malkandueva ZT: Urinary biomarkers for
podocyte injury: Significance for evaluating the course and
prognosis of chronic glomerulonephritis. Ter Arkh. 87:34–39. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Satirapoj B, Jirawatsiwaporn K,
Tangwonglert T and Choovichian P: Performance of the estimated
glomerular filtration rate creatinine and cystatin C based
equations in Thai patients with chronic glomerulonephritis. Int J
Nephrol Renovasc Dis. 8:145–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dudnyk V, Zvenigorodska A and Guminska G:
Genetic aspects of chronic glomerulonephritis. Lik Sprava. 159–160.
2015.(In Ukrainian). PubMed/NCBI
|
|
6
|
Hule GP, Karmarkar MG, Cameron A, Hase N,
Khopkar U, Mehta PR, McNeilly CL, McMillan D and Sriprakash KS:
Seropositivity for antibodies to DRS-G, a virulence factor from
streptococcus dysgalactiae subsp. equisimilis, is an independent
risk factor for poststreptococcus glomerulonephritis and chronic
kidney disease in Mumbai, India. Clin Vaccine Immunol. 22:938–942.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao JR, Qin XJ, Jiang H, Wang T, Song JM
and Xu SZ: Screening and functional analysis of differentially
expressed genes in chronic glomerulonephritis by whole genome
microarray. Gene. 589:72–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campa MJ, Moody MA, Zhang R, Liao HX,
Gottlin EB and Patz EF Jr: Interrogation of individual intratumoral
B lymphocytes from lung cancer patients for molecular target
discovery. Cancer Immunol Immunother. 65:171–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng KW, Wang S, Dong X, Jiang Y, Jin HW
and Tu PF: Sesquiterpene dimmer (DSF-27) inhibits the release of
neuroinflammatory mediators from microglia by targeting spleen
tyrosine kinase (Syk) and Janus kinase 2 (Jak2): Two major
non-receptor tyrosine signaling proteins involved in inflammatory
events. Toxicol Appl Pharmacol. 275:244–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu L, Zhang L, Zhao J, Ning X, Mu C and
Wang C: Cloning and expression of a transcription factor activator
protein-1 (AP-1) member identified from manila clam Venerupis
philippinarum. Gene. 557:106–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee DS, Yang SH, Kim HL, Joo KW, Lim CS,
Chae DW, Kim S, Lee JS and Kim YS: Recombinant uteroglobin prevents
the experimental crescentic glomerulonephritis. Kidney Int.
66:1061–1067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gohda T, Makita Y, Shike T, Funabiki K,
Shirato I and Tomino Y: Dilazep hydrochloride, an antiplatelet
drug, inhibits lipopolysaccharide-induced mouse mesangial cell IL-6
secretion and proliferation. Kidney Blood Press Res. 24:33–38.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao H, Liu Z, Shen H, Jin S and Zhang S:
Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney
injury via suppressing inflammation, apoptosis and oxidative
stress. Eur J Pharmacol. 781:92–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao JR, Qin XJ, Jiang H, Wang T, Song JM
and Xu SZ: The effects of Qi Teng Xiao Zhuo granules, traditional
Chinese medicine, on the expression of genes in chronic
glomerulonephritis rats. J Ethnopharmacol. 193:140–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun LY, Wang Y, Chen SF, Sun ML and Li XM:
The effects of strict dietary salt restriction on blood pressure
and proteinuria in chronic glomerulonephritis patients. Zhonghua
Nei Ke Za Zhi. 48:995–998. 2009.(In Chinese). PubMed/NCBI
|
|
17
|
Zhong WQ, Liu GX, Yang YM, Cai X and Huang
ZL: Clinical effect of treatment with lipo-prostaglandin E1 on the
patients with chronic glomerulonephritis. Zhongguo Wei Zhong Bing
Ji Jiu Yi Xue. 16:292–294. 2004.(In Chinese). PubMed/NCBI
|
|
18
|
Wang NH: Clinical research on effects of
the integrative medicine on chronic nephritis. Clin J Chin Med.
10:94–95. 2015.(In Chinese).
|
|
19
|
Ding SY, Zheng PD, He LQ, Hou WG, Zou Y
and Gao JD: The research on xiaochaihu decoction improving the
inflammation of chronic glomerulonephritis patients and relieving
the proteninuria. Zhongguo Zhong Xi Yi Jie He Za Zhi. 33:21–26.
2013.(In Chinese). PubMed/NCBI
|
|
20
|
Chiu HY, Huang HL, Li CH, Yin YJ, Chen HA,
Hsu ST, Lin SJ, Tsai TF and Ho SY: Increased risk of
glomerulonephritis and chronic kidney disease in relation to the
severity of psoriasis, concomitant medication, and comorbidity: A
nationwide population-based cohort study. Br J Dermatol.
173:146–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gobessi S, Laurenti L, Longo PG, Carsetti
L, Berno V, Sica S, Leone G and Efremov DG: Inhibition of
constitutive and BCR-induced Syk activation downregulates Mcl-1 and
induces apoptosis in chronic lymphocytic leukemia B cells.
Leukemia. 23:686–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ying H, Li Z, Yang L and Zhang J: Syk
mediates BCR- and CD40-signaling integration during B cell
activation. Immunobiology. 216:566–570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li HL, Forman MS, Kurosaki T and Puré E:
Syk is required for BCR-mediated activation of p90Rsk, but not
p70S6k, via a mitogen-activated protein kinase-independent pathway
in B cells. J Biol Chem. 272:18200–18208. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vogel US, Dixon RA, Schaber MD, Diehl RE,
Marshall MS, Scolnick EM, Sigal IS and Gibbs JB: Cloning of bovine
GAP and its interaction with oncogenic ras p21. Nature. 335:90–93.
1988. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishiyama A, Yao L, Nagai Y, Miyata K,
Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, et
al: Possible contributions of reactive oxygen species and
mitogen-activated protein kinase to renal injury in
aldosterone/salt-induced hypertensive rats. Hypertension.
43:841–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geng W, Wei R, Liu S, Tang L, Zhu H, Chen
P, Wu J, Zhang X, Zhu F, Yin Z and Chen X: Shenhua Tablet inhibits
mesangial cell proliferation in rats with chronic anti-Thy-1
nephritis. Biol Res. 49:172016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang MX, Li L and Wang XY: Expression of
Raf kinase inhibitor protein and p-ERK in renal tissues of diabetic
rats. Chin J Pathophysiol. 29:358–360. 2013.(In Chinese).
|
|
28
|
Chen D, Fong HW and Davis JS: Induction of
c-fos and c-jun messenger ribonucleic acid expression by
prostaglandin F2alpha is mediated by a protein kinase C-dependent
extracellular signal-regulated kinase mitogen-activated protein
kinase pathway in bovine luteal cells. Endocrinology. 142:887–895.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zu N, Li P, Li N, Choy P and Gong Y:
Mechanism of saikosaponin-d in the regulation of rat mesangial cell
proliferation and synthesis of extracellular matrix proteins.
Biochem Cell Biol. 85:169–174. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen D, Li Y, Mei Y, Geng W, Yang J, Hong
Q, Feng Z, Cai G, Zhu H, Shi S, et al: miR-34a regulates mesangial
cell proliferation via the PDGFR-β/Ras-MAPK signaling pathway. Cell
Mol Life Sci. 71:4027–4042. 2014. View Article : Google Scholar : PubMed/NCBI
|