Introduction
Globally, type 2 diabetes (T2D) is a common chronic
disease, which is characterized by high blood glucose levels and
insulin resistance (1). Insulin is
involved in the pathogenesis of T2D and metabolic syndrome. An
inappropriate amount of insulin as a result of b-cell dysfunction
is the hallmark of T2D. Decreased glycogen level is a hallmark of
insulin resistance in hepatocytes (2). Members of the phosphatidylinositol
3-kinase (PI3K) family function in the regulation of islet mass and
function (3). Although three
different PI3K isoforms are expressed in the endocrine pancreas
(4), the class 1 PI3K isoforms are
the most investigated groups, with opposing roles in the regulation
of insulin secretion (5). For
example, the PI3K catalytic subunit α (PI3Kα/PIK3CA), also termed
p110α, acts as a negative regulator of insulin secretion, while
PI3Kβ/PIK3CB, also termed p110β, is a positive regulator of insulin
secretion through distinct mechanisms that are separated from the
catalytic activity (6,7). However, the regulation of PI3Kα in
T2D is poorly understood.
MicroRNAs (miRNA/miRs) are a group of endogenous
non-coding small RNAs that have a length of between 21 and 23
nucleotides and act as key regulators of post-transcriptional gene
expression (8). miRNAs function by
base pairing to the 3′ untranslated region of the target mRNA and
act as specific gene silencers (9). Various studies have indicated that
miRNAs regulate the multiple aspects in various metabolic diseases,
including diabetes mellitus, endothelial function, obesity and
metabolic syndrome (10,11). Among miRNAs, miR-152 was reported
to regulate hepatic insulin resistance and glucose metabolism, and
high glucose levels reduced the expression of miR-152 and
subsequently impaired the synthesis of glycogen in hepatocytes
(12). However, the mechanism
underlying the miR-152 regulation cascade remains unclear.
The investigation of the molecular mechanisms
involved in T2D strongly indicates that excess genes impair cell
functions, leading to metabolically relevant cellular dysfunction,
inflammation and oxidative stress.
Materials and methods
Cell culture
INS-1 and MIN6 cells were purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in low-glucose Dulbecco's modified Eagle's medium (DMEM; 5 mmol/l
glucose; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc.), 5.5 mM 2-mercaptoethanol, 100 U/ml
penicillin and 0.1 mg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified chamber atmosphere with
5% CO2.
Reagents and transfection of INS-1 and
MIN6 cells
The miR-152 mimic, miR-152 inhibitor, and miRNA
mimic and inhibitor controls, were purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China). The miR-152 mimic,
GCAGTCTCAGTGCATGACAGA, miR-152 inhibitor, CCAUCUUUACCAGACAGUGUUA,
inhibitor controls CAGUACUUUUGUGUAGUAC. HiPerFect transfection
reagent (Qiagen GmbH, Hilden, Germany) was used for the
transfection of miR-152 mimic and inhibitors, and controls.
MIN6/INS-1 cells were cultured by plating 6×105 cells into 100-mm
Petri dishes. A total of 250 pmol miR-152 mimic or inhibitor, or
respective controls were transfected into cells. At 48 h following
transfection, the expression of miR-152 and target genes were
detected reverse transfection-quantitative polymerase chain
reaction (RT-qPCR). Insulin (1 nmol/l) was purchased from United
States Biological (Salem, MA, USA).
Blood sample collection
The present study was approved by the Ethics
Committee of the Second Clinical Medical College, Yangtze
University (Jingzhou, China) and written informed consent was
obtained from all patients and healthy volunteers. Blood samples
were collected from patients with T2D (n=50), of whom 24 were
female and 26 were male, and patients were aged between 45 and 60.
Normal subjects (n=20), of which 10 were female and 10 were male,
were aged between 44 and 60. The samples were collected between
January 2014 and November 2015 in Jingzhou Central Hospital.
Patients with serious kidney diseases, malignancy and acute heart
failure were excluded.
Experimental animals
A total of 40 Male C57BL/6J mice (age, 12 weeks;
weight, 20–25 g) were provided by the Chinese Academy of Sciences
affiliated Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China), and were originally purchased from Jackson Laboratory (Bar
Harbor, ME, USA). All animal procedures were performed in
accordance with the National Institutes of Health Animal Care and
Use Guidelines (13) and approved
by the Animal Ethics Committee at the Shanghai Institute of
Geriatrics (Shanghai, China). Mice were housed in a clean animal
laboratory at the Second Clinical Medical College, Yangtze
University Experimental Animal Center. Mice were housed under
specific pathogen free conditions with controlled room temperature
(22.0±1.0°C), 0.05% CO2, a 12 h light/dark cycle (light,
8 am-8 pm), humidity of 40–60% and free access to water and food.
Mice islets were isolated from the pancreata according to a
previously described method by Lau et al (14). Adenovirus with miR-152
overexpression construct or the miR-152 inhibitor, and the relative
controls were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China) and were transfected in freshly 2 ug isolated
mouse pancreatic islets at a dose of 1×109 plaque
forming units in 0.2 ml PBS for 72 h and collected for the
following experiments.
Determination of glucose in blood
samples
The glucose levels were determined by a routine
laboratory method. The blood glucose levels in whole blood were
examined at the Department of Clinical Laboratory of Jingzhou
Central Hospital, The Second Clinical Medical College, Yangtze
University using a Glucose assay kit (BioVision, Inc., Milpitas,
CA, USA), according to the manufacturer's protocol.
Western blot analysis
Total protein was extracted from INS-1 and MIN6
cells using radioimmunoprecipitation assay lysis buffer
(Sigma-Aldrich; Merck KGaA). After centrifugation at 12,000 × g for
20 min at 4°C, the supernatant was collected. The BCA protein assay
was used to determine the protein concentration. Following mixing
with 4X SDS loading buffer, proteins (30 µg) in each group were
heated at 100°C for 5 min and separated on 10% SDS-polyacrylamide
gels and transferred to a polyvinylidene fluoride membrane (EMD
Millipore, Billerica, MA, USA). Following blocking with 8% non-fat
dry milk at room temperature for 30 min, the membranes were probed
with primary antibodies overnight at 4°C, followed by incubation
with horseradish peroxidase (HRP)-conjugated second antibodies at
room temperature for 1 h and detection with Signal
Boost™ Immunoreaction Enhancer Kit (407207, EMD
Millipore). The signals were recorded using X-ray film. The
following antibodies were employed: PI3Kα (cat. no. sc-7174; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA, 1:1,000), β-actin (cat.
no. sc-130300; Santa Cruz Biotechnology, Inc, 1:2,000), goat
anti-mouse IgG-HRP, (cat. no. sc-2005; Santa Cruz Biotechnology,
Inc. 1:3,000) and goat anti-rabbit IgG-HRP (cat. no. sc-2004; Santa
Cruz Biotechnology, Inc. 1:3,000).
RNA isolation and RT-qPCR
Enriched miRNA was isolated using an miRNA isolation
kit using whole blood samples from T2D patients and healthy
controls (Takara Bio, Inc., Otsu, Japan). Total RNA was extracted
from the pancreatic tissues or blood samples from T2D patients or
cell lines using TRIzol method (Invitrogen; Thermo Fisher
Scientific, Inc.). The concentration and purity of RNA was
confirmed. First-strand cDNA synthesis was performed using a
reverse transcription kit (iScript cDNA Synthesis kit; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The following temperature
protocol was used for reverse transcription: 25°C for 10 min, 42°C
for 30 min and 85°C for 3 min. qPCR was performed using the TB
Green™ Premix Dimer Eraser™, according to the
manufacturer's protocol (RR091A, Takara Bio, Inc.) based on an ABI
7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Cycling parameters were as follows: 95°C for 5 min and then 40
cycles of 95°C for 15 sec and annealing/extension at 60°C for 1
min. The U6 small nucleolar RNA or GAPDH were used as the reference
genes. The relative gene expression was normalized to U6 or GAPDH
using the 2−ΔΔCq method (15). PI3Ka 5′-ATATGATGCAGCCATTGACC-3′
(forward) 5′-CTCCTGAAACCTCTCAAATTCTC-3′ (reverse) GAPDH,
GAGAAGTATGACAACAGCCTC-3′ (forward) and 5′-ATGGACTGTGGTCATGAGTC-3′
(reverse). miR-152, 5′-ACTCTCGAGGCTTCTAAGCTGGGAACTTTGTC-3′
(forward) and 5′-ACTGAATTCCGCTTGTCTTGGACATATGGCACT-3′ (reverse);
U6, 5′-CTCGCTTCGGCAGCACA-3′ (forward) and
5′-AACGCTTCACGAATTTGCGT-3′ (reverse). Each reaction was performed
in triplicate.
MTT assay
An MTT kit (Roche Diagnostics, Indianapolis, IN,
USA) was used to determine the proportion of viable MIN6 and INS-1
cells, 5×103 cells were seeded into 12-well plates,
according to manufacturer's protocol. MTT (500 µg/ml) was added to
DMEM medium for 3 h at 37°C. At the end of the incubation, MTT
reagent was removed and the cells were dissolved in dimethyl
sulfoxide. The absorbance value was measured at 570 nm on a
microplate reader (Perkin Elmer EnSpire; Perkin Elmer, Inc.,
Waltham, MA, USA). Experiments were performed at least three
times.
Insulin secretion measurements
Insulin secretion measurements were performed at
37°C in Krebs-Ringer bicarbonate buffer (115 mmol/l NaCl, 5 mmol/l
KCl, 24 mmol/l NaHCO3, 2.5 mmol/l CaCl2, 1
mmol/l MgCl2, 10 mmol/l HEPES and 0.1% bovine serum
albumin (Pierce; Thermo Fisher Scientific, Inc. pH 7.4). A total of
10 mouse islets were preincubated for 2 h in KRB per group,
followed by incubation for 1 h in these basal buffers and further
30 min or 24 h incubation with 3 or 20 mM glucose. Acid/ethanol
extraction was used to extract insulin from islets. Insulin
secretion was also measured in 106 INS-1 and MIN6 cells
following incubation with 3 or 20 mM glucose for 2 h incubation at
37°C. Samples were stored at −20°C until insulin levels were
detected using an ELISA kit (cat. no. 80-INSMSU-E01, Mouse
Ultrasensitive Insulin ELISA, ALPCO, Salem, NH, USA). Each
experiment was repeated at least three times.
Luciferase reporter assay
TargetScan Human version 7.0 (www.targetscan.org) predicted that PI3Kα was a
potential target of miR-152. The wild-type (WT) or mutant (MUT;
without miR-152 binding site) human PI3Kα 3′UTR sequences were
synthesized using QuikChange Multi Site-Directed Mutagenesis kit
(Agilent Technologies, Inc., Santa Clara, CA, USA) and separately
cloned into the pGL-3 luciferase reporter plasmid (Promega
Corporation, Madison, WI, USA). The recombinant plasmids were
termed pGL3-PI3Kα-WT and pGL3-PI3Kα-MUT. These plasmids were
co-transfected with 50 nm miR-152 mimic or inhibitor or their
negative control using Lipofectamine® 2000. Cell lysates
were prepared and luciferase assays were performed 48 h following
transfection. Luciferase activity was normalized to Renilla
luciferase activity.
Statistical analysis
All statistical analyses were performed using
GraphPad prism 6.0 (GraphPad Software, Inc. La Jolla, CA, USA).
Data are presented as the mean ± standard deviation and P<0.05
was considered to indicate a statistically significant difference.
One-way analysis of variance, followed by Fisher's least
significant difference test, was performed to determine the
statistical significance of differences between two groups.
Pearson's correlation analysis was performed to determine the
correlation between miR-152 and glucose levels in the blood of
patients with T2D and healthy controls.
Results
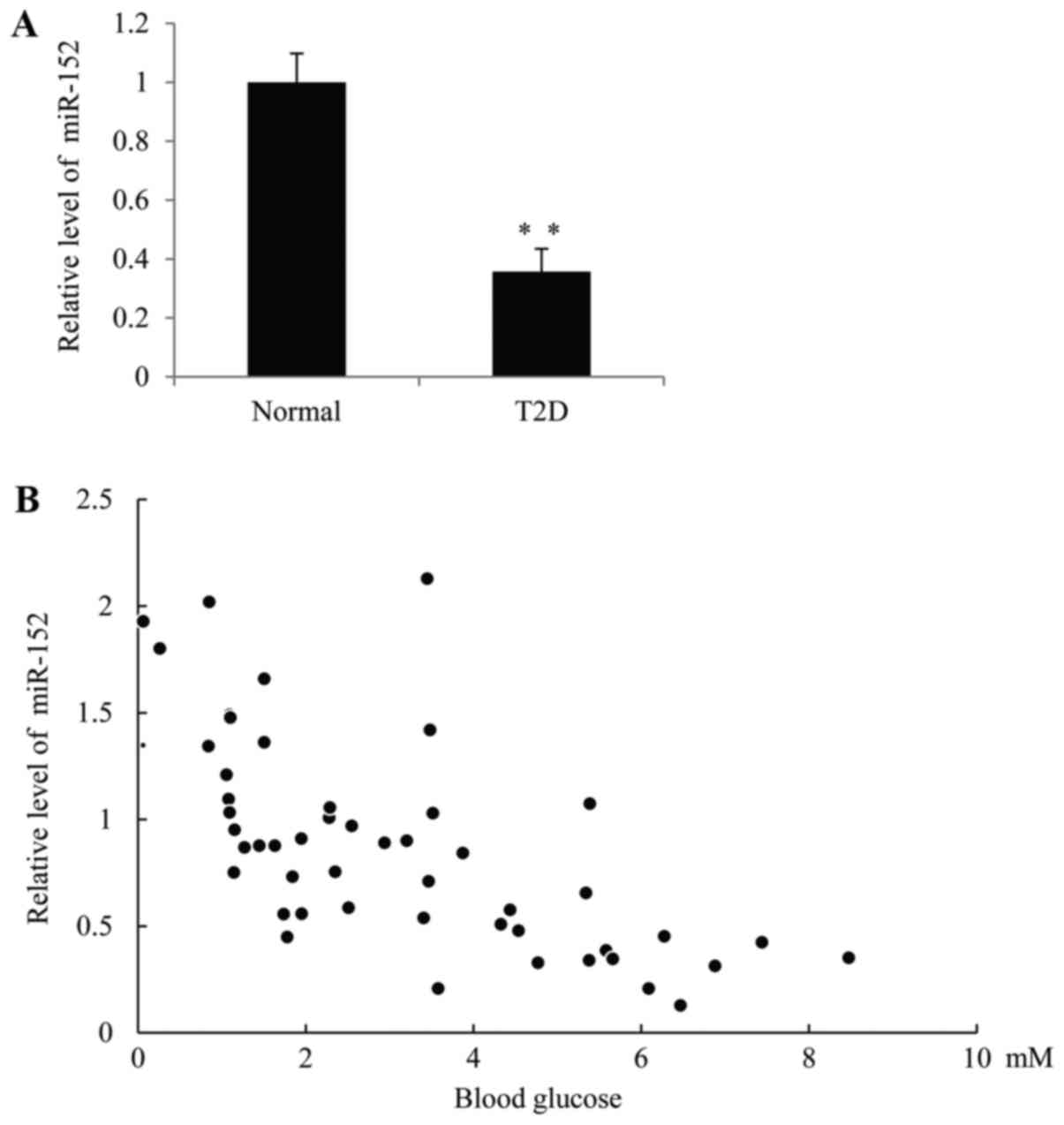
The expression of miR-152 is
significantly downregulated in patients with T2D
In order to investigate the function of miR-152 in
diabetes, RT-qPCR was performed to measure miR-152 levels in the
plasma of patients with T2D and healthy volunteers. As demonstrated
in Fig. 1A, the expression of
miR-152 was significantly decreased in patients with T2D compared
with the control group. Furthermore, a significant inverse
correlation between the miR-152 expression and blood glucose
concentration was observed in patients with T2D, R=0.5424, P=0.0078
(Fig. 1B).
miR-152 enhances the insulin secretion
and proliferation of pancreatic β cell lines
In order to investigate the exact function of
miR-152 in pancreatic β cells, two pancreatic β cell lines, INS-1
and MIN6, were used. miR-152 mimic or miR-152 inhibitor, or
respective controls, were transfected into INS-1 and MIN6 cells. At
48 h after transfection, RT-qPCR was performed to determine the
miR-152 expression levels. As demonstrated in Fig. 2A and B, the expression of miR-152
was significantly higher in the miR-152 mimic-transfected cells,
while the expression of miR-152 was significantly lower in miR-152
inhibitor-transfected cells, compared with respective control
groups. Furthermore, in response to glucose stimulation (20 mM),
transient transfection of miR-152 mimics significantly increased
the insulin secretion, while the transfection of miR-152 inhibitor
reduced insulin secretion stimulated by high glucose levels,
compared with the respective transfection control groups (Fig. 2C and D). In addition, the effects
of miR-152 on cell viability were also investigated using an MTT
assay to confirm whether overexpression or knockdown of miR-152
affected cell viability. The results demonstrated that miR-152
overexpression markedly enhanced cell proliferation, while
knockdown of miR-152 reduced cell proliferation, compared with
their respective control groups (Fig.
2E and F).
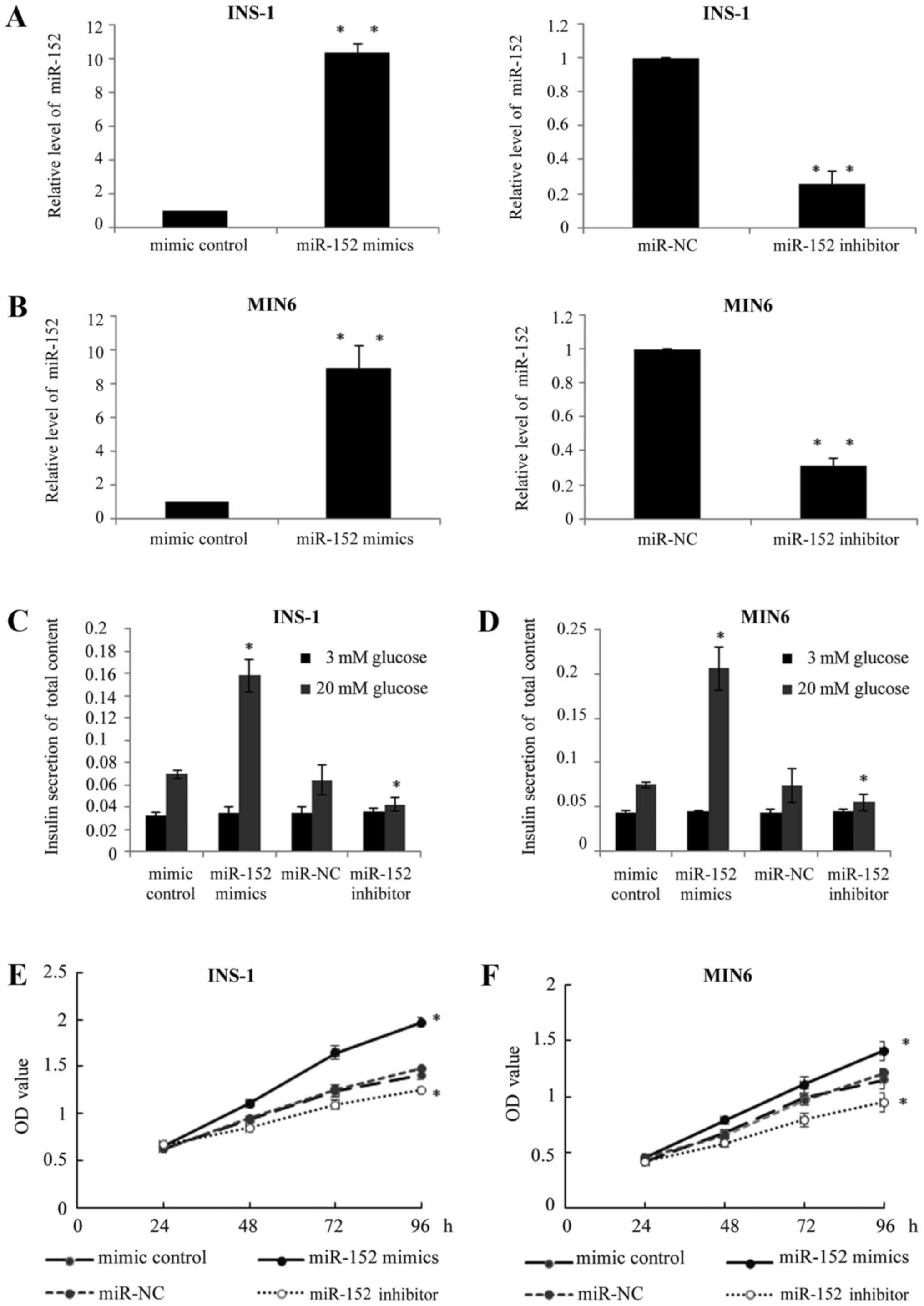 | Figure 2.miR-152 enhanced the insulin secretion
and proliferation of pancreatic β cell lines. Reverse
transcription-quantitative polymerase chain reaction was performed
to measure the miR-152 levels in (A) INS-1 and (B) MIN6 cells
transfected with miR-152 mimic, miRNA mimic control, miR-152
inhibitor or miR-NC inhibitor control, n=5. (C) INS-1 and (D) MIN6
cells were transfected with miR-152 mimic, miRNA mimic control,
miR-152 inhibitor or miR-NC inhibitor control for 48 h, followed by
incubation with 3 or 20 mM glucose for 1 h. Glucose-stimulated
insulin secretion was subsequently determined in each group, n=3.
MTT assay was performed to investigate cell proliferation in (E)
INS-1 and (F) MIN6 cells transfected with miR-152 mimic, miRNA
mimic control, miR-152 inhibitor or miR-NC inhibitor control, n=5.
*P<0.05 and **P<0.01 vs. mimic control or miR-NC. miR,
microRNA; NC, negative control; miRNA, microRNA; OD, optical
density. |
Identification of PI3K-alpha as a
target gene of miR-152
The above observations indicated that miR-152 may be
required for glucose-stimulated insulin secretion and pancreatic β
cell proliferation. Therefore, the present study subsequently
investigated the specific genes suppressed by miR-152. TargetScan
software was used to predict the putative target genes of miR-152.
As demonstrated in Fig. 3A, PI3Kα
was identified as a potential target gene of miR-152. To verify the
targeting of PI3Kα by miR-152, wild-type PI3Kα 3′-untranslated
region (UTR) containing the predicted miR-152 binding sites, or
mutated versions of the PI3Kα 3′-UTR without the predicted miR-152
binding sites, were cloned into firefly luciferase reporter
plasmids. Results of the luciferase reporter activity assay
demonstrated that co-transfection of INS-1 and MIN6 cells with the
wild-type PI3Kα 3′-UTR and miR-152 mimic reduced the luciferase
activity, compared with control mimic-transfected cells (Fig. 3B). By contrast, co-transfection
with the wild-type 3′-UTR and miR-152 inhibitors enhanced PI3Kα
3′-UTR luciferase activity, compared with the transfection control
group (Fig. 3C). However,
luciferase activity was not altered in cells that were
co-transfected with the mutant PI3Kα 3′-UTR vector and miR-152
mimics, compared with the mimic control transfection group
(Fig. 3D).
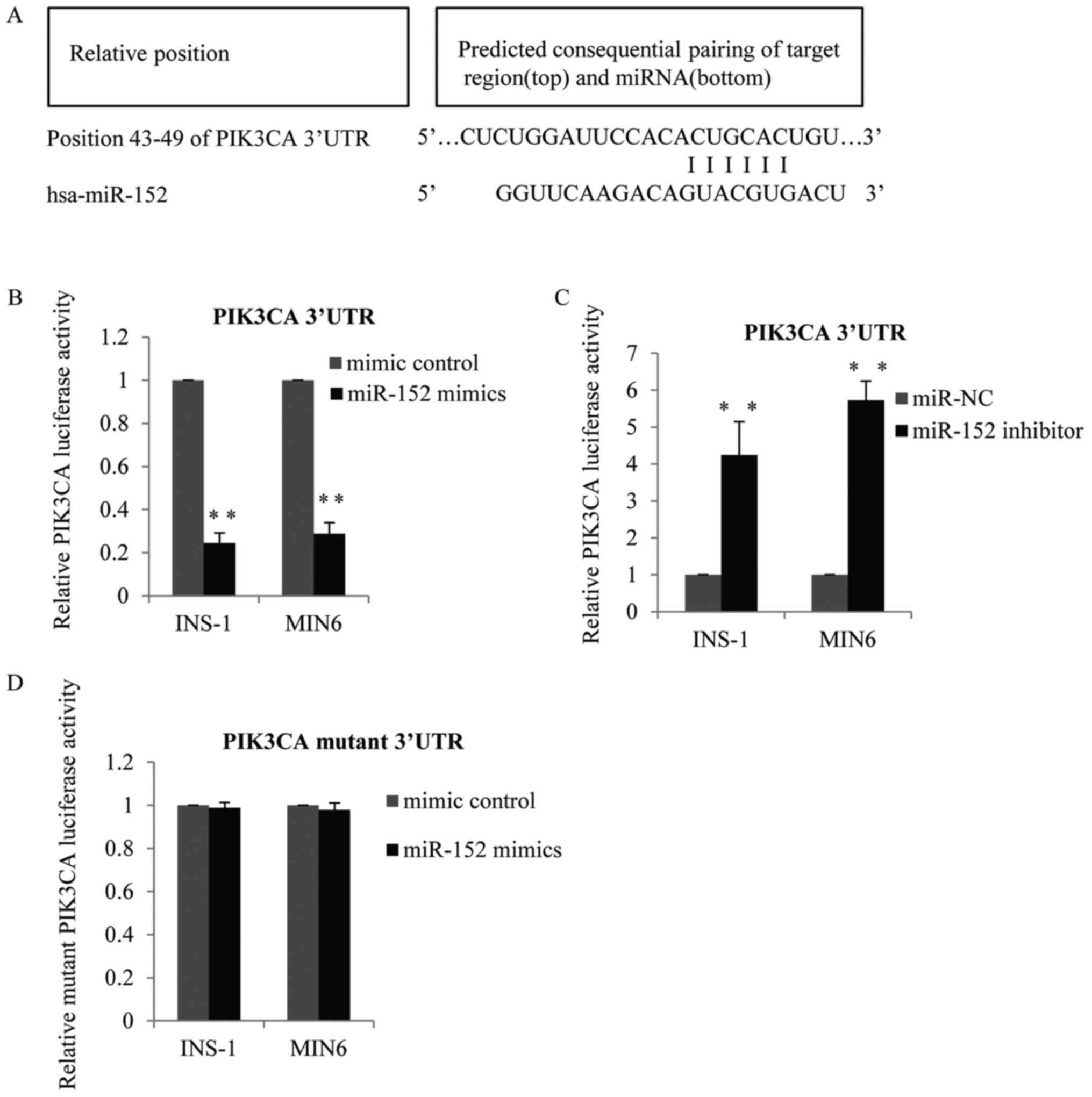 | Figure 3.Identification of PI3Kα as a target
gene of miR-152. (A) PI3Kα was predicted to be a potential target
gene of miR-152. Matches in seed regions are marked. The wild-type
PI3Kα 3′-UTR with the putative miR-152 binding sites was subcloned
into the pMiR-report vector, and a luciferase reporter assay was
performed in MIN6 and INS-1 cells transfected with (B) miR-152
mimic or miRNA mimic control, or (C) miR-152 inhibitor or miR-NC
inhibitor control, n=3. (D) Mutant PI3Kα 3′-UTR was subcloned into
the pMiR-report vector, and a luciferase reporter assay was
performed in MIN6 cells and INS-1 cells transfected with miR-152
mimic or miRNA mimic control, n=3. **P<0.01 vs. mimic control or
miR-NC. PI3Kα, phosphatidylinositol 3-kinase catalytic subunit α;
miR, microRNA; UTR, untranslated region; miRNA, microRNA; NC,
negative control. |
PI3Kα is involved in the
miR-152-mediated pancreatic β cell proliferation and insulin
secretion
It is established that the major function of miRNA
is the negative regulation of target genes at the
post-transcriptional level; therefore, the present study
investigated the effects of miR-152 on the endogenous protein
expression of PI3Kα. Results of western blot analysis demonstrated
that, in miR-152 overexpressing INS-1 and MIN6 cells, the protein
expression of PI3Kα was reduced, compared with transfection control
cells (Fig. 4A). Conversely, the
protein levels of PI3Kα were markedly increased following the
transfection of miR-152 inhibitors, compared with the transfection
control group (Fig. 4B). Based on
these results, we hypothesized that PI3Kα may function as a
downstream effector of miR-152. To verify this hypothesis, the
pcDNA3.1-PI3Kα plasmid was constructed to restore the expression of
PI3Kα in INS-1 and MIN6 cells. At 48 h following transfection,
RT-qPCR was performed to analyze the mRNA levels of PI3Kα. As
demonstrated in Fig. 4C, PI3Kα
levels were significantly higher in the INS-1 and MIN6 cells
transfected with PI3Kα overexpression plasmid, compared with the
vector group. Subsequently, the effect of PI3Kα overexpression on
miR-152-mediated insulin secretion and cell proliferation was
investigated. As demonstrated in Fig.
4D, compared with the cells transfected with miR-152 mimic
alone, transfection of INS-1 and MIN6 cells with the miR-152 mimic
and PI3Kα overexpression plasmid abolished the effect of miR-152
mimics on glucose-stimulated insulin secretion. Similarly, the MTT
assay revealed that PI3Kα overexpression reduced the effect of the
miR-152 on the proliferation of INS-1 and MIN6 cells (Fig. 4E).
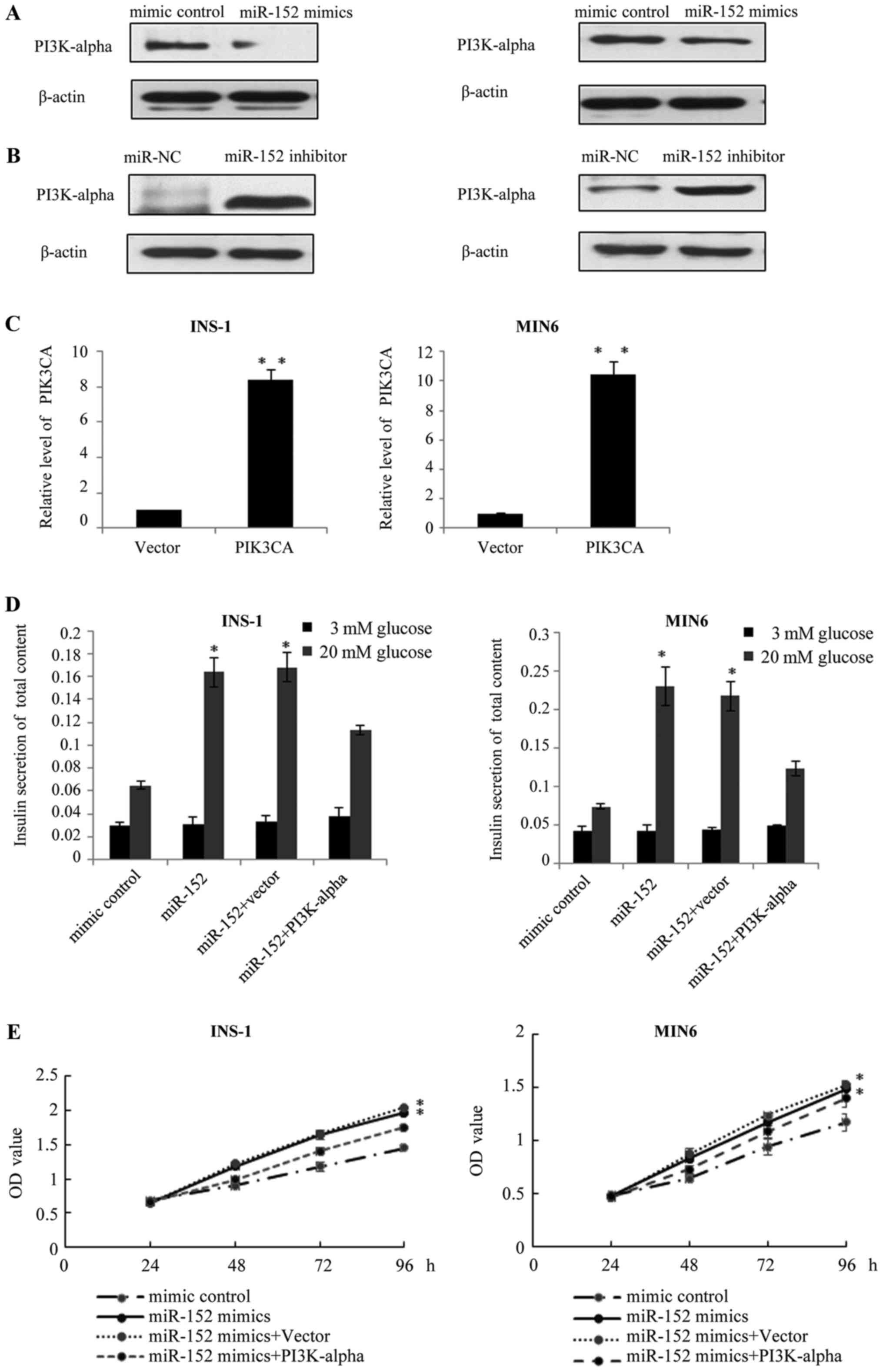 | Figure 4.PI3Kα is involved in miR-152-mediated
pancreatic β cell proliferation and insulin secretion. Western blot
analysis was performed to measure the protein expression of PI3Kα
in INS-1 and MIN6 cells transfected with (A) miR-152 mimic or miRNA
mimic control, or transfected with (B) miR-152 inhibitor or miR-NC
inhibitor control, n=3. (C) Reverse transcription-quantitative
polymerase chain reaction was performed to measure the mRNA
expression of PI3Kα in INS-1 and MIN6 cells transfected with PI3Kα
overexpression plasmid or vector, n=3. **P<0.01 vs. vector
group. (D) Glucose-stimulated insulin secretion was measured in
INS-1 and MIN6 cells transfected with miR-152 mimic, miRNA mimic
control, miR-152 mimic + vector or miR-152 mimic + PI3Kα
overexpression plasmid. Transfected cells were treated with 3 or 20
mM glucose. *P<0.05 vs. mimic control, n=5. (E) MTT assay was
performed in INS-1 and MIN6 cells transfected with miR-152 mimic,
miRNA mimic control, miR-152 mimic + vector or miR-152 mimic +
PI3Kα overexpression plasmid. *P<0.05. n=3. PI3Kα,
phosphatidylinositol 3-kinase catalytic subunit α; miR, microRNA;
miRNA, microRNA; NC, negative control; OD, optical density. |
The miR-152/PI3Kα axis regulates
glucose-stimulated insulin secretion in murine pancreatic islets,
and in patients with T2DM
The present study further determined the role of the
miR-152/PI3Kα axis in glucose-stimulated insulin secretion in
islets isolated from mice. As demonstrated in Fig. 5A and B, high-glucose treatment
upregulated the expression of miR-152 and downregulated PI3Kα
expression in murine pancreatic islets. Furthermore, in miR-152
overexpression islets, insulin secretion was markedly increased
compared with mimic control-transfected islets following treatment
with 20 mM glucose (Fig. 5C). By
contrast, insulin secretion was suppressed when miR-152 was
inhibited in islets, compared with control-transfected cells,
following treatment with 20 mM glucose (Fig. 5D). In addition, the plasma PI3Kα
levels were determined in patients with T2D and healthy controls
using ELISA. As demonstrated in Fig.
5E, in the blood samples of patients with T2D, PI3Kα levels
were significantly increased compared with healthy individuals
(Fig. 5E). These observations
strongly indicated that dysregulation of the miR-152 and PI3Kα axis
may be involved in the development of T2D.
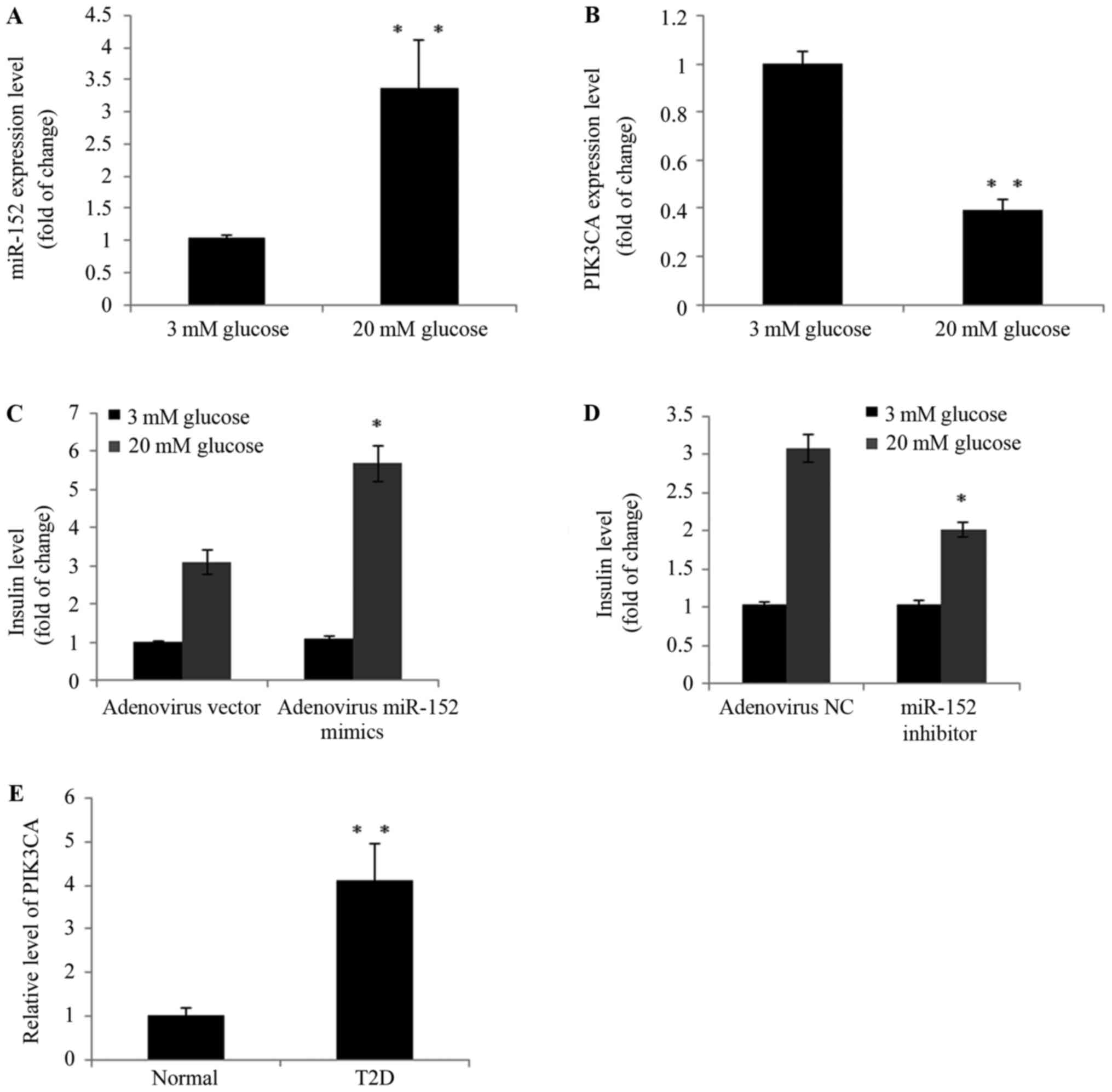 | Figure 5.The miR-152/PI3Kα axis regulated
glucose-stimulated insulin secretion in murine pancreatic islets
and patients with T2D. Freshly isolated mouse pancreatic islets
were isolated and incubated with 3 or 20 mM glucose for 24 h. The
expression of (A) miR-152 and (B) PI3Kα was analyzed using RT-qPCR,
n=3. Pancreatic islets were transduced with (C) adenovirus vector
or adenovirus miR-152, or transduced with (D) adenovirus NC and
adenovirus miR-152 inhibitor. Cells were subsequently incubated
with 3 or 20 mM glucose for 2 h and insulin secretion was measured,
n=3. (E) RT-qPCR was performed to measure the PI3Kα levels in blood
samples from patients with T2D (n=50) and healthy volunteers
(n=20). For parts A and B, **P<0.01 vs. 3 mM glucose. For parts
C and D, *P<0.05 vs. adenovirus vector or adenovirus NC. For
part E, **P<0.01 vs. normal group. miR, microRNA; PI3Kα,
phosphatidylinositol 3-kinase catalytic subunit α; T2D, type 2
diabetes; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; NC, negative control; normal group, healthy
volunteers. |
Discussion
Diabetes is characterized by high levels of blood
glucose, and a lack of insulin-producing pancreatic β cells is the
major cause of type 1 diabetes, while increased insulin resistance
leads to the development of T2D and metabolic syndrome (2,16).
Increasing evidence has indicated that various types of miRNA may
be involved in the pathogenesis of diabetes (17,18).
It was previously reported that miR-9 controlled the expression of
granuphilin-a and the secretory response of insulin-producing cells
(19). In addition, by negatively
regulating hepatic gluconeogenesis, miR-29a-c reduced fasting blood
glucose levels (20), and miR-301a
mediated the effect of interleukin (IL)-6 on the AKT/glycogen
synthase kinase pathway and hepatic glycogenesis through the
regulation of phosphatase and tensin homolog (PTEN) expression
(21). Furthermore, miR-200s
contributed to IL-6-induced insulin resistance in hepatocytes
(22). However, reports concerning
the function of miR-152 were different depending on the cancer and
tissue type. For example, Huang et al (23) reported that miR-152 targeted PTEN
to inhibit cell apoptosis and promote cancer cell proliferation in
nasopharyngeal carcinoma cells. However, in hepatocellular
carcinoma, miR-152 inhibited tumor cell growth by directly
targeting rhotekin (24). These
different functions of miR-152 may be observed as miR-152 has
multiple target genes. The results of the present study
demonstrated that miR-152 promoted insulin secretion and pancreatic
β proliferation, and insulin is a critical factor in the
pathogenesis of diabetes. According to the bioinformatics, although
several potential targets for miR-152 were identified, the binding
of PI3Kα was of significant importance. As PI3Kα was previously
reported to be implicated in the development and progression of T2D
(6), the present study focused on
PI3Kα as a target of miR-152. Overexpression of miR-152 resulted in
a marked decrease in the protein expression of PI3Kα in MIN6 and
INS-1 cells, while inhibition of miR-152 led to increased PI3Kα
expression. Therefore, miR-152 may be involved in the regulation of
insulin secretion and pancreatic β cell proliferation via PI3Kα.
Consistently, the expression of miR-152 was lower, while the
expression of PI3Kα mRNA was higher, in patients with T2D compared
with healthy controls. However, further research is required to
validate these findings and compare PI3Kα protein expression in
blood samples of patients T2D and healthy volunteers. The
expression of miR-152 in the blood samples may be due to the
secretion of circulating miRNAs. Investigation of the plasma
miR-152 levels in the present study was performed with the aim of
identifying novel blood-based biomarkers for T2D diagnosis and
prognosis. In addition, miR-152 expression was also analyzed in
murine pancreatic islets. The results demonstrated that
high-glucose treatment upregulated the expression of miR-152 and
downregulated PI3Kα expression. Furthermore, in miRNA-152
overexpression islets, compared with mimic control-transfected
islets, insulin secretion was markedly increased following
treatment with high-glucose.
To the best of our knowledge, the present study
provides novel experimental evidence demonstrating that miR-152 may
function as a potential candidate for treating diabetes. However,
further investigation is required in future studies.
References
|
1
|
Danaei G, Finucane MM, Lu Y, Singh GM,
Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA,
et al: National, regional, and global trends in fasting plasma
glucose and diabetes prevalence since 1980: Systematic analysis of
health examination surveys and epidemiological studies with 370
country-years and 2.7 million participants. Lancet. 378:31–40.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meshkani R and Adeli K: Hepatic insulin
resistance, metabolic syndrome and cardiovascular disease. Clin
Biochem. 42:1331–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muller D, Huang GC, Amiel S, Jones PM and
Persaud SJ: Gene expression heterogeneity in human islet endocrine
cells in vitro: The insulin signalling cascade. Diabetologia.
50:1239–1242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dominguez V, Raimondi C, Somanath S,
Bugliani M, Loder MK, Edling CE, Divecha N, da Silva-Xavier G,
Marselli L, Persaud SJ, et al: Class II phosphoinositide 3-kinase
regulates exocytosis of insulin granules in pancreatic beta cells.
J Biol Chem. 286:4216–4225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kolic J, Manning Fox JE, Chepurny OG,
Spigelman AF, Ferdaoussi M, Schwede F, Holz GG and MacDonald PE:
PI3 kinases p110α and PI3K-C2β negatively regulate cAMP via PDE3/8
to control insulin secretion in mouse and human islets. Mol Metab.
5:459–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kolic J, Spigelman AF, Plummer G, Leung E,
Hajmrle C, Kin T, Shapiro AM, Manning Fox JE and MacDonald PE:
Distinct and opposing roles for the phosphatidylinositol 3-OH
kinase catalytic subunits p110α and p110β in the regulation of
insulin secretion from rodent and human beta cells. Diabetologia.
56:1339–1349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoyagi K, Ohara-Imaizumi M, Nishiwaki C,
Nakamichi Y, Ueki K, Kadowaki T and Nagamatsu S: Acute inhibition
of PI3K-PDK1-Akt pathway potentiates insulin secretion through
upregulation of newcomer granule fusions in pancreatic β-cells.
PLoS One. 7:e473812012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herranz H and Cohen SM: MicroRNAs and gene
regulatory networks: Managing the impact of noise in biological
systems. Genes Dev. 24:1339–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA. 104:pp. 9667–9672.
2007; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arunachalam G, Lakshmanan AP, Samuel SM,
Triggle CR and Ding H: Molecular interplay between microRNA-34a and
sirtuin1 in hyperglycemia-mediated impaired angiogenesis in
endothelial cells: Effects of metformin. J Pharmacol Exp Ther.
356:314–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima
S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P
and Stoffel M: A pancreatic islet-specific microRNA regulates
insulin secretion. Nature. 432:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Wang L, Dou L, Guo J, Fang W, Li
M, Meng X, Man Y, Shen T, Huang X and Li J: MicroRNA 152 regulates
hepatic glycogenesis by targeting PTEN. FEBS J. 283:1935–1946.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council (US) Institute
for Laboratory Animal Research, . The Development of Science-based
Guidelines for Laboratory Animal Care: Proceedings of the November
2003 International Workshop. Washington (DC). National Academies
Press (US); 2004;
|
|
14
|
Lau T, Carlsson PO and Leung PS: Evidence
for a local angiotensin-generating system and dose-dependent
inhibition of glucose-stimulated insulin release by angiotensin II
in isolated pancreatic islets. Diabetologia. 47:240–248. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leclercq IA, Da Silva Morais A, Schroyen
B, Van Hul N and Geerts A: Insulin resistance in hepatocytes and
sinusoidal liver cells: Mechanisms and consequences. J Hepatol.
47:142–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joglekar MV, Parekh VS and Hardikar AA:
Islet-specific microRNAs in pancreas development, regeneration and
diabetes. Indian J Exp Biol. 49:401–408. 2011.PubMed/NCBI
|
|
18
|
Mao Y, Mohan R, Zhang S and Tang X:
MicroRNAs as pharmacological targets in diabetes. Pharmacol Res.
75:37–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Plaisance V, Abderrahmani A, Perret-Menoud
V, Jacquemin P, Lemaigre F and Regazzi R: MicroRNA-9 controls the
expression of Granuphilin/Slp4 and the secretory response of
insulin-producing cells. J Biol Chem. 281:26932–26942. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang J, Liu C, Qiao A, Cui Y, Zhang H,
Cui A, Zhang S, Yang Y, Xiao X, Chen Y, et al: MicroRNA-29a-c
decrease fasting blood glucose levels by negatively regulating
hepatic gluconeogenesis. J Hepatol. 58:535–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dou L, Wang S, Sui X, Meng X, Shen T,
Huang X, Guo J, Fang W, Man Y, Xi J and Li J: MiR-301a mediates the
effect of IL-6 on the AKT/GSK pathway and hepatic glycogenesis by
regulating PTEN expression. Cell Physiol Biochem. 35:1413–1424.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dou L, Zhao T, Wang L, Huang X, Jiao J,
Gao D, Zhang H, Shen T, Man Y, Wang S and Li J: miR-200s contribute
to interleukin-6 (IL-6)-induced insulin resistance in hepatocytes.
J Biol Chem. 288:22596–22606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang S, Li X and Zhu H: MicroRNA-152
targets phosphatase and tensin homolog to inhibit apoptosis and
promote cell migration of nasopharyngeal carcinoma cells. Med Sci
Monit. 22:4330–4337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Zhang Y, Qi Y, Yu D, Shao Q and
Liang J: MicroRNA-152 inhibits tumor cell growth by directly
targeting RTKN in hepatocellular carcinoma. Oncol Rep.
37:1227–1234. 2017. View Article : Google Scholar : PubMed/NCBI
|