Introduction
Prostate cancer (PCa) is the third most common cause
of cancer-related deaths among men in the United States, accounting
for an estimated 26,730 deaths in 2016 (1). Recently, the incidence of PCa in
Asian countries has also markedly increased. The majority of PCa
patients who respond well to androgen deprivation therapy (ADT)
will eventually progress to castration-resistant prostate cancer
(CRPC) within 1–3 years, at which point the median survival rate is
reduced to only 12–15 months (2).
Bone metastasis occurs in approximately 90% of patients with
advanced PCa (3), and this
severely affects the life quality of PCa patients (4). Therefore, it is necessary to identify
novel targets for therapeutic intervention in PCa.
The epithelial-mesenchymal transition (EMT) is
recognized as an important process during several phases of
embryonic development. It is often marked by a loss of expression
of E-cadherin and a gain expression of N-cadherin and vimentin.
Accumulated evidence has indicated that the EMT is a critical
process to facilitate metastasis in various types of cancer
including CRPC (5,6). The EMT can be induced by a variety of
stimuli including growth factors, cytokines, and hypoxia (7). Transforming growth factor (TGF)-β is
one of the most prominent extracellular inducers of the EMT.
Although studies have demonstrated that TGF-β can inhibit the
proliferation of tumor cells during the early stages of tumor
development, it can locally result in greater tumor invasiveness
through the induction of the EMT during the advanced stages of
cancer (8–10). At present, it is known that TGF-β
participates in the EMT through the canonical and non-canonical
TGF-β signaling pathways. For the canonical TGF-β/Smad signaling
pathway, the direct binding of TGF-β to its receptors leads to the
phosphorylation of the Smad proteins, which form transcription
factor complexes that regulate the expression of TGF-β-responsive
genes (11,12). TGF-β also activates a variety of
non-canonical pathways, including the MAPK, Rho-like GTPase, and
PI3K-AKT-mTOR pathways (13).
Elevated TGF-β expression was observed in advanced PCa with higher
Gleason scores, where it showed a negative correlation with
clinical outcome (14,15).
Differentiated embryonic chondrocyte gene (DEC) 1
and DEC2 are members of the basic helix-loop-helix (bHLH)
transcription factor family and have been implicated as signaling
mediators of diverse biological events including cell
proliferation, circadian rhythms, apoptosis, tumorigenesis, and the
response to hypoxia (16–19). In our preliminary study, we showed
that DEC1 promoted TGF-β-induced EMT in human pancreatic cancer
PANC-1 cells (20). However, the
roles of DEC1 and DEC2 in the EMT progress as well as their
relations with TGF-β in PCa cells remain undetermined.
The aim of the present study was to elucidate the
effects of DEC on the TGF-β-induced EMT in PCa cells and explore
the underlying downstream signaling mechanism. Our results
demonstrated that DEC1 and DEC2 positively and negatively
correlated with EMT in PCa PC-3 cells, respectively.
Materials and methods
Cell culture and treatment
Human prostate cancer PC-3 cells from RIKEN
BioResource Center (Tsukuba, Japan) were cultured as described
previously (21). Cells were
incubated with various concentrations of recombinant human TGF-β
(R&D Systems, Inc., Minneapolis, MN, USA) for the indicated
period of time.
Short interfering RNA (siRNA) and
transfection
The siRNAs targeting DEC1 and DEC2, as well as a
scrambled non-specific sequence that served as a negative control,
were synthesized by Invitrogen; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The sequences of DEC1, DEC2, and the negative
control siRNA have been described previously (22). PC-3 cells were seeded in six-well
plates at a density of 5×104 per well and then
transfected with the negative control siRNA or the siRNA against
DEC1 or DEC2 using Lipofectamine RNA iMAX reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's instructions. Twenty-four h after transfection, the
medium was replaced with antibiotics-free medium, and the cells
were treated with TGF-β for another 24 h and subjected to various
analyses.
DEC1 and DEC2 overexpression
Human DEC1 and DEC2 plasmids were kindly gifted from
Dr Katsumi Fujimoto (Hiroshima University) (17). PC-3 cells were seeded at
5×104 cells per 35-mm well. DEC1 or DEC2 expression
plasmid was introduced into cells using Lipofectamine LTX
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. After 18 h of transfection, the cells
were incubated with 10 ng/ml TGF-β for an additional 24 h and
subjected to reverse transcription-polymerase chain reaction
(RT-PCR) or western blot analysis.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells with an RNeasy
Mini kit (Qiagen GmbH, Hilden, Germany) and was used for reverse
transcription. First-strand cDNA was synthesized from 1 µg of total
RNA using ReverTra Ace (Toyobo Co., Ltd., Osaka, Japan). RT-PCR was
performed using Taq PCR Master mix (Qiagen). The sequences of the
primers and the sizes of the amplification products are shown in
Table I. The products were
analyzed by electrophoresis on 1.5% (w/v) agarose gels that were
stained with ethidium bromide.
 | Table I.Sequences of the primer sets and the
product sizes of RT-PCR. |
Table I.
Sequences of the primer sets and the
product sizes of RT-PCR.
| Gene | Product size
(bp) | Cycles | Primer sequences |
|---|
| DEC1 | 534 | 27 | F:
5′-GTCTGTGAGTCACTCTTCAG-3′ |
|
|
|
| R:
5′-GAGTCTAGTTCTGTTTGAAGG-3′ |
| DEC2 | 501 | 27 | F:
5′-CACCTTTGACGTCTTTGGAG-3′ |
|
|
|
| R:
5′-GAGAGTGGGAATAGATGCAC-3′ |
|
E-cadherin | 200 | 27 | F:
5′-TGCCCAGAAAATGAAAAAGG-3′ |
|
|
|
| R:
5′-GTGTATGTGGCAATGCGTTC-3′ |
|
N-cadherin | 201 | 29 | F:
5′-ACAGTGGCCACCTACAAAGG-3′ |
|
|
|
| R:
5′-CCGAGATGGGGTTGATAATG-3′ |
| Vimentin | 301 | 29 | F:
5′-CTTCGCCAACTACATCGACAA-3′ |
|
|
|
| R:
5′-CGCATTGTCAACATCCTGTC-3′ |
| 18s rRNA | 151 | 20 | F:
5′-GTAACCCGTTGAACCCCATT-3′ |
|
|
|
| R:
5′-CCATCCAATCGGTAGTAGCG-3′ |
Western blot analysis
Cells were lysed using M-PER lysis buffer (Thermo
Fisher Scientific, Inc.), and protein concentrations were
quantified by the bicinchoninic acid (BCA) assay. The obtained
lysates (10 µg protein) were run on a 10% polyacrylamide gel to
separate proteins and were then transferred to polyvinylidene
difluoride (PVDF) membranes (Immobilon P; EMD Millipore, Billerica,
MA, USA). After blocking with 5% non-fat milk solution, the
membranes were probed overnight at 4°C with primary antibodies,
followed by incubating with a horseradish peroxidase-conjugated
secondary antibody. The bands were visualized using a Bio-Rad
western blotting system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) with the ECL-prime western blot analysis detection system (GE
Healthcare Life Sciences, Little Chalfont, UK).
Immunofluorescence staining
PC-3 cells were seeded in a four-well chamber slide
and fixed with 4% paraformaldehyde (Wako Pure Chemical Industries,
Ltd., Osaka, Japan). The permeabilized cells were incubated with
anti-E-cadherin (1:100), anti-N-cadherin (1:100), or anti-vimentin
(1:200) antibodies at 4°C overnight, followed by Alexa Fluor
488-conjugated secondary antibodies (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 1 h. Nuclear staining was performed
using 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent confocal
imaging was performed using a fluorescent microscope (BZ-X700;
Keyence Corporation, Osaka, Japan).
Wound healing assay
PC-3 cells were seeded in a four-well chamber slide
to reach confluence and then a ‘wound’ was created at 0 h in the
cell monolayers with a P-200 pipette tip. Subsequently, the medium
was immediately changed and the cells were transfected with control
siRNA, or siRNA against DEC1 or DEC2 accompanied with TGF-β. Images
were taken at 0, 24, and 48 h with a phase-contrast inverted
microscope.
Results
Effects of TGF-β on DEC expression and
EMT-related factors in PC-3 cells
Our previous study reported that TGF-β induced the
expression of DEC1, while it reduced that of DEC2 in human
esophageal squamous cell carcinoma TE-11 cells (23). In the present study, we firstly
examined the expression of DEC1 and DEC2 in PC-3 cells in response
to various concentrations of TGF-β by western blotting and RT-PCR.
In PC-3 cells, TGF-β increased the expression of DEC1, but
decreased that of DEC2 in a dose-dependent manner. The expression
of the EMT-related factor E-cadherin was inhibited, whereas
N-cadherin and vimentin were upregulated by TGF-β (Fig. 1A and B). Moreover, TGF-β led to the
phosphorylation of Smad2 but not of Smad3 (Fig. 1B, data for pSmad3 not shown).
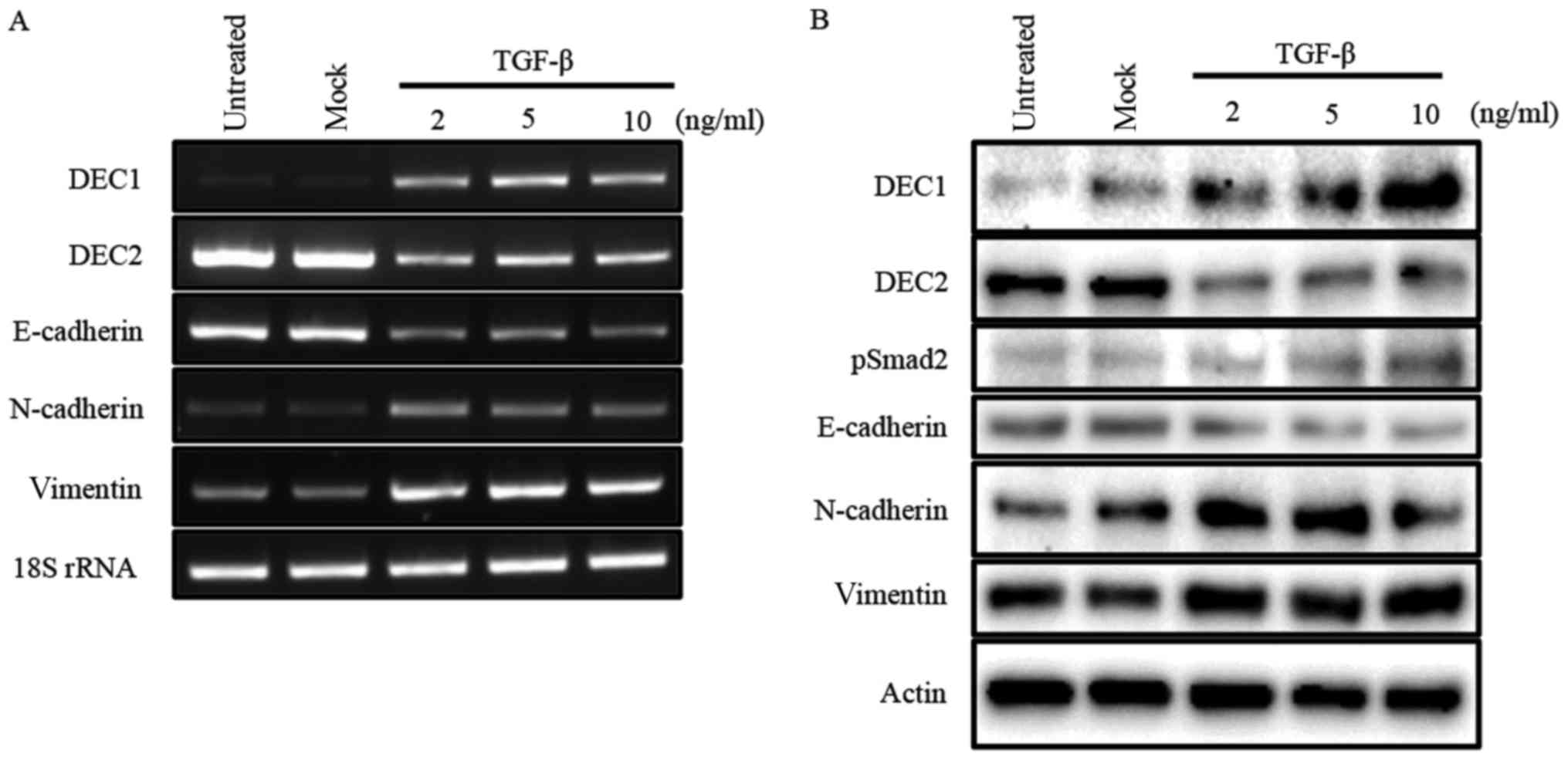 | Figure 1.TGF-β effects the expression of DEC1,
DEC2, and EMT-related factors. (A) PC-3 cells were untreated,
mock-treated (buffer alone), or treated with 2, 5, 10 ng/ml of
TGF-β. After 24 h, total RNA was prepared and subjected to RT-PCR
analyses of DEC1, DEC2, E-cadherin, N-cadherin, vimentin, and 18S
rRNA. PC-3 cells were treated with TGF-β for 24 h. (B) The cells
were lysed and the lysates were subjected to western blot analyses
of DEC1, DEC2, pSmad2, E-cadherin, N-cadherin, vimentin, and
actin. |
DEC1 expression positively correlated
with the expression of EMT-related factors in PC-3 cells, whereas
DEC2 expression showed the opposite trend
To investigate the roles of DEC1/DEC2 in the
TGF-β-induced EMT of PC-3 cells, the knockdown or overexpression of
DEC1/DEC2 combined with TGF-β treatment was carried out in PC-3
cells. The effect of DEC1 on Smad2 phosphorylation was not clearly
detected without TGF-β (Fig. 2B and
D). However, DEC1 knockdown significantly inhibited
TGF-β-induced Smad2 phosphorylation when compared with the control
siRNA group (Fig. 2B).
Consequently, the upregulation of the epithelial marker E-cadherin
and the downregulation of the mesenchymal markers N-cadherin and
vimentin were observed in DEC1 siRNA-transfected PC-3 cells
(Fig. 2A and B). As a further
experiment, we examined the same genes and proteins in PC-3 cells
engineered to overexpress DEC1 pcDNA. The results of this
experiment also showed that DEC1 expression positively correlated
with that of the mesenchymal markers but negatively correlated with
that of the epithelial markers (Fig.
2C and D).
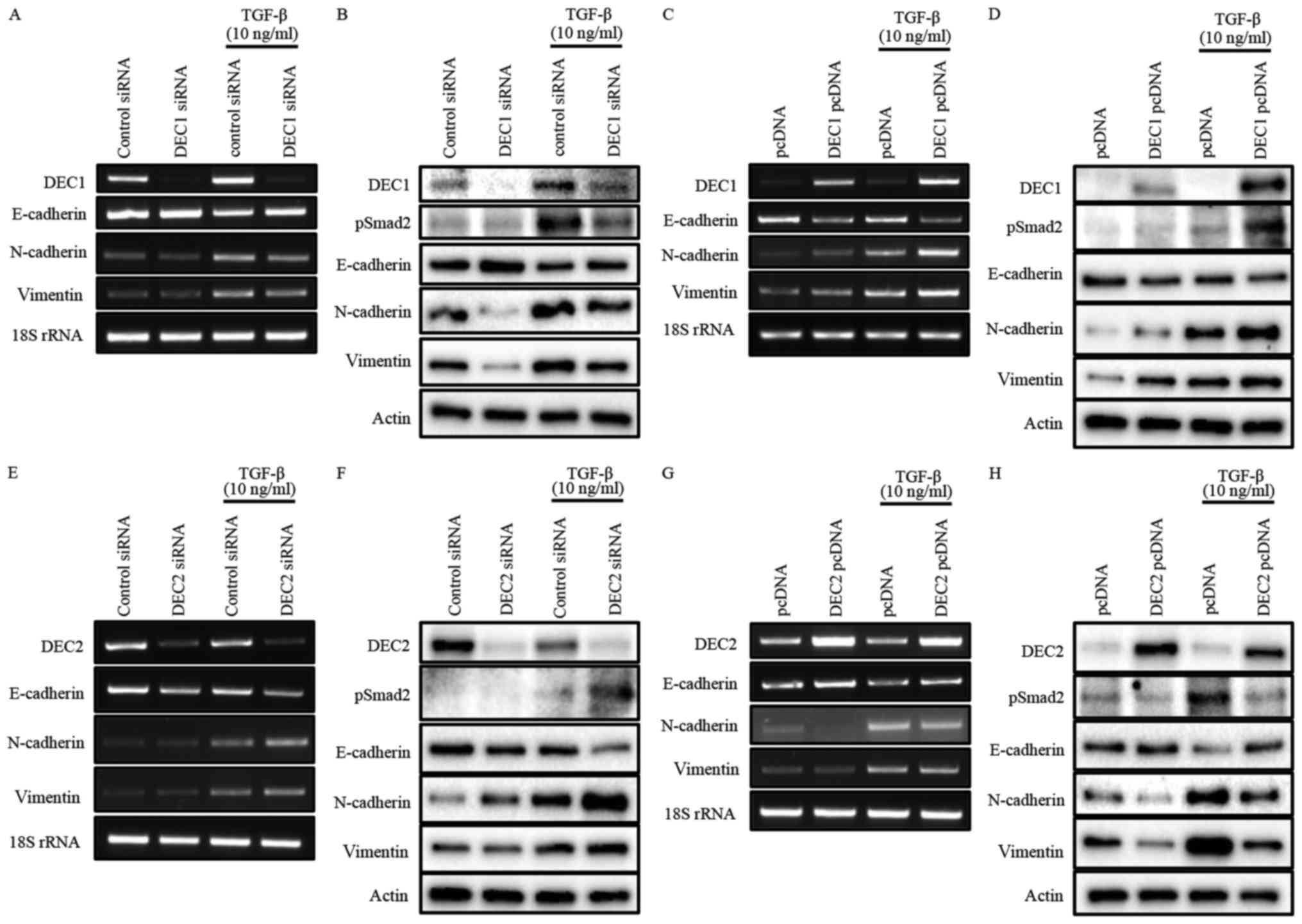 | Figure 2.(A-H) Opposite roles of DEC1 and DEC2
in regulating EMT induced by TGF-β. PC-3 cells were transfected
with the siRNAs (24 h transfection) or the expression plasmids (18
h transfection) of DEC1 and DEC2; subsequently, the cells were
incubated with or without TGF-β (10 ng/ml) for an additional 24 h.
(A, C, E and G) Total RNA was prepared and subjected to RT-PCR
analyses of DEC1 or DEC2, E-cadherin, N-cadherin, vimentin, and 18S
rRNA. (B, D, F, and H) Protein was prepared from the cells and
subjected to western blot analyses of DEC1 or DEC2, pSmad2,
E-cadherin, N-cadherin, vimentin and actin. One representative
result of at least three independent experiments with similar
results is shown. DEC1, differentiated embryonic chondrocyte gene
1; DEC2, differentiated embryonic chondrocyte gene 2; EMT,
epithelial-mesenchymal transition; RT-PCR, reverse
transcription-polymerase chain reaction. |
Our results also showed that DEC2 negatively
regulated the phosphorylation of Smad2 in the presence of TGF-β
(Fig. 2F and H). However, even in
the absence of TGF-β, DEC2 siRNA-transfected PC-3 cells partly lost
their expression of E-cadherin, while they gained the expression of
N-cadherin and vimentin. Correspondingly with this, DEC2
overexpression dramatically attenuated the expression of pSmad2,
N-cadherin, and vimentin, whereas it increased that of E-cadherin
(Fig. 2G and H). The presence of
TGF-β further augmented these changes at both the mRNA and protein
levels (Fig. 2E-H).
Effects of DEC1/DEC2 knockdown on cell
morphological changes and the expression of EMT-related
factors
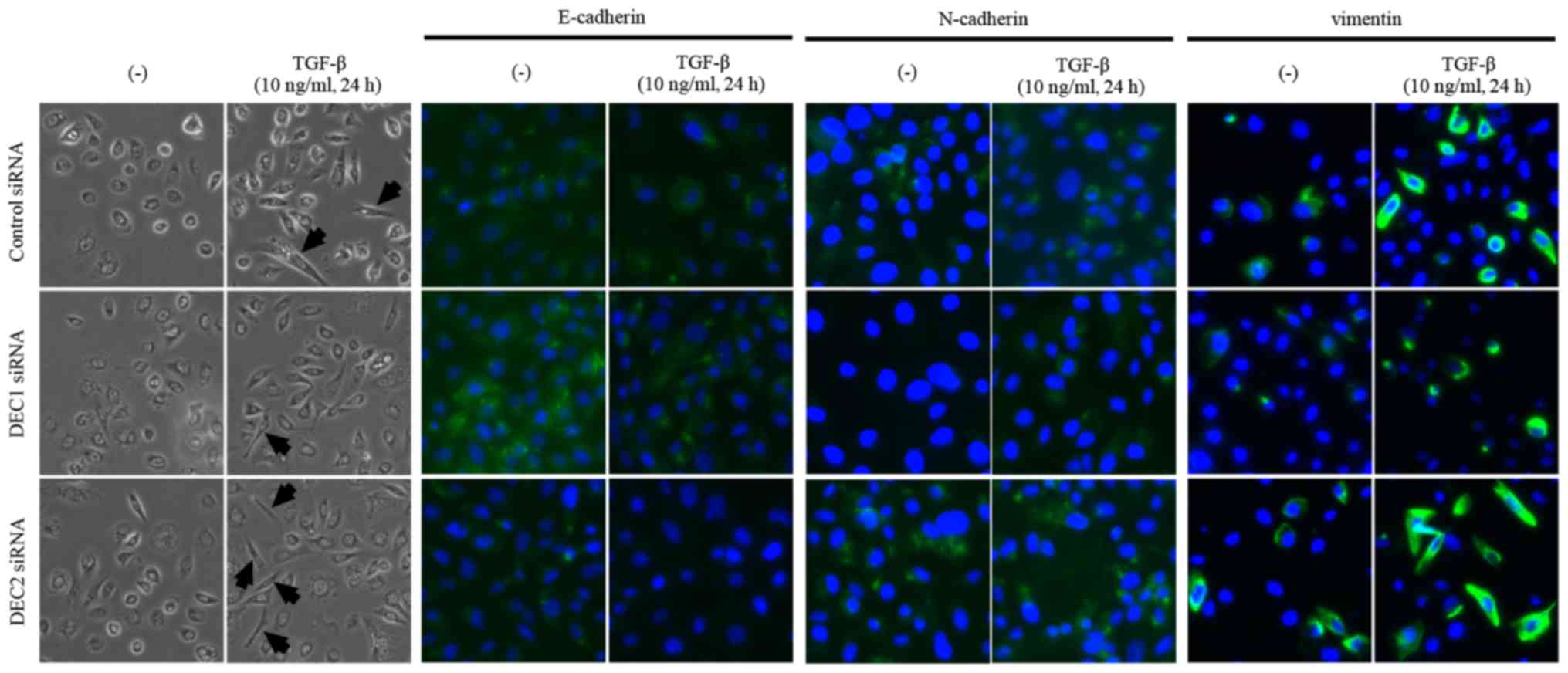
Since a fibroblast-like change in cell morphology is
an important feature of the EMT, we observed the morphology of PC-3
cells that were treated with the combination of DEC1 or DEC2
knockdown and TGF-β. The treatment with TGF-β increased the number
of cells with elongated and fibroblast-like shapes as compared with
the untreated cells. We also found that a pre-incubation with DEC1
siRNA resulted in less fibroblast-like shaped cells than were
observed in those transfected with control siRNA in the presence of
TGF-β, while more spindle-like cells were observed in the DEC2
siRNA-transfected group (Fig. 3,
the left two panels, middle and bottom images). Based on the
immunofluorescence staining results, E-cadherin expression was
slightly increased (Fig. 3, third
and fourth panels from the left, middle images), while the
expression of N-cadherin and vimentin was decreased (Fig. 3, fifth to eighth panels from the
left, middle images) in DEC1 siRNA-transfected cells when compared
with the control siRNA group. Conversely, PC-3 cells transfected
with DEC2 siRNA displayed a reverse pattern to that exhibited in
the DEC1 siRNA-transfected cells (Fig.
3, third to eighth panels from the left, bottom images).
Effects of DEC1/DEC2 knockdown on
migration capacity of PC-3 cells
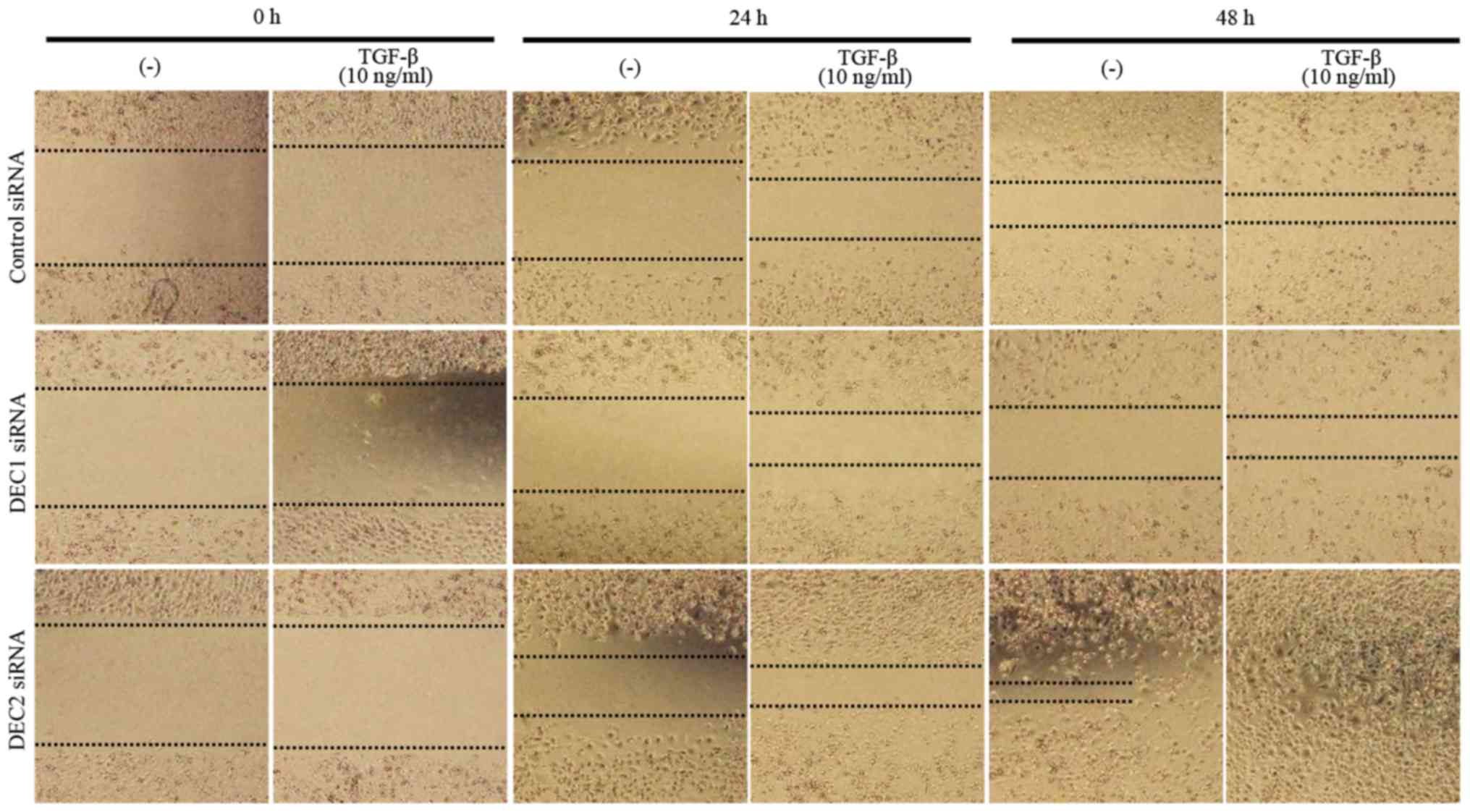
Metastasis is the principal cause of cancer death,
and cell migration is a hallmark of cancer metastasis. Thus, a
wound healing assay was employed to evaluate the effects of DEC on
the migration ability of PC-3 cells. DEC1 siRNA-transfected cells
treated with TGF-β for both 24 and 48 h exhibited a slower
migration speed when compared with the control siRNA-transfected
cells (Fig. 4, images of middle
line), whereas DEC2 siRNA-transfected PC-3 cells migrated quickly,
especially in the presence of TGF-β (Fig. 4, images of bottom line).
Discussion
It is well accepted that the EMT plays very
important roles in tumor progression, especially in cancer cell
invasion and metastasis. EMT is mainly controlled by a cadherin
switch that is localized in the cell membrane and is characterized
by a shift from apical-basal polarity to front-back end polarity, a
modification of cell morphology, and a gain of cellular migration
activity. A reduction or absence of E-cadherin expression and
increased N-cadherin and/or vimentin expression were observed in
PCa with high Gleason Scores, and the expression of these markers
has been reported to correlate with the metastasis and
aggressiveness of PCa (24,25).
Among the numerous genes and signaling pathways involved in the
EMT, TGF-β is recognized as the primary inducer of EMT by
activating canonical or non-canonical pathways (26). In canonical TGF-β signaling,
binding to the type II/I receptor complex leads to the
phosphorylation of Smad 2/3, followed by an interaction of Smad2/3
with Smad4 and nuclear translocation (11). The expression of the representative
epithelial and mesenchymal markers E-cadherin and N-cadherin then
changes, resulting in decreased cell adhesion and enhancement of
migration. The inhibition of TGF-β signaling has been emerging as a
therapeutic strategy owing to its functions in the development of
cancer, including its roles in the EMT and metastasis (27,28).
Our previous report showed that DEC1 promoted the
EMT through the activation of the TGF-β/Smad signaling pathway in
human pancreatic cancer PANC-1 cells (20). In the present study, we
demonstrated that TGF-β increased DEC1 but decreased DEC2
expression in PC-3 cells, accompanied by the phosphorylation of
Smad2, as well as an altered expression of E-cadherin, N-cadherin,
and vimentin. Additionally, the treatment of PC-3 cells with 10
ng/ml TGF-β induced a change to a spindle-shaped morphology, which
indicated the alteration of cell polarity. Since upregulated cell
motility is another major feature of the EMT, a wound healing assay
was carried out to estimate the effects of DEC on the migration
capacity of TGF-β-treated PC-3 cells. The results showed that the
migratory capacity was slightly decreased by DEC1 siRNA, while it
was significantly increased by DEC2 siRNA. These findings
implicated that TGF-β is sufficient for EMT induction in PC-3 cells
and TGF-β-induced DEC expression might somewhat relate to the EMT
process.
As a crucial downstream mediator of the TGF-β
signaling pathway, Smad2 protein is phosphorylated by the TGF-β
receptor and mediates the intracellular signal transduction of
TGF-β. The present study demonstrated that in the absence of TGF-β,
the knockdown or overexpression of DEC altered the expression of
EMT-related factors without Smad2 phosphorylation. In the presence
of TGF-β, EMT-related factors were remarkably changed by DEC siRNA
or expression plasmids. We concluded that DEC could regulate
EMT-related factors independently of pSmad2, but as an enhancer,
DEC collaborated with Smad2 and augmented its function of
regulating the translation of the target proteins. Notably, DEC1
and DEC2 exhibited different effects on EMT-related markers. The
differences between DEC1 and DEC2 with regard to their functions
may be partially caused by the differences in the protein structure
of their C-terminals.
Based on these results, we propose the hypothesis
that DEC1 and DEC2 have distinct roles in the process of EMT in
cancer cells. This hypothesis merits addressing in future studies,
especially, the details of the molecular mechanisms for the
transcriptional regulation of EMT markers by DECs should be further
investigated. Furthermore, future studies should investigate
whether DECs may represent potential molecular targets for the
management of aggressive PCa.
Acknowledgements
The authors would like to thank Professor Yukio Kato
and Dr Katsumi Fujimoto at the Department of Dental and Medical
Biochemistry, Hiroshima University Graduate School of Biomedical
for kindly providing the plasmids.
Funding
The present study was supported by Grants-in-Aid for
Science from the Ministry of Education, Culture, Sports, Science
and Technology of Japan (grant nos. 17K17575 and 17H04057).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and HK conceived and designed the study. YW and
QL designed the methods, analyzed the data, interpreted the results
and drafted and reviewed the manuscript. QL executed all of the
experiments. HS, TH, TY and SM contributed to data interpretation
and critically reviewed the manuscript. All authors contributed to,
read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DEC1
|
differentiated embryonic chondrocyte
gene 1
|
|
DEC2
|
differentiated embryonic chondrocyte
gene 2
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ADT
|
androgen deprivation therapy
|
|
TGF-β
|
transforming growth factor-beta
|
|
DAPI
|
4′, 6-diamidino-2-phenylindole
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Denmeade SR and Isaacs JT: Development of
prostate cancer treatment: The good news. Prostate. 58:211–224.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carlin BI and Andriole GL: The natural
history, skeletal complications, and management of bone metastases
in patients with prostate carcinoma. Cancer. 88 12
Suppl:S2989–S2994. 2000. View Article : Google Scholar
|
|
4
|
Bubendorf L, Schöpfer A, Wagner U, Sauter
G, Moch H, Willi N, Gasser TC and Mihatsch MJ: Metastatic patterns
of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol.
31:578–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Investig. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miettinen PJ, Ebner R, Lopez AR and
Derynck R: TGF-beta induced transdifferentiation of mammary
epithelial cells to mesenchymal cells: Involvement of type I
receptors. J Cell Biol. 127:2021–2036. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyazono K: Transforming growth
factor-beta signaling in epithelial-mesenchymal transition and
progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 85:pp.
314–323. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown KA, Pietenpol JA and Moses HL: A
tale of two proteins: Differential roles and regulation of Smad2
and Smad3 in TGF-beta signaling. J Cell Biochem. 101:9–33. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuzaki K: Smad phosphoisoform signaling
specificity: The right place at the right time. Carcinogenesis.
32:1578–1588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reis ST, Pontes-Júnior J, Antunes AA,
Sousa-Canavez JM, Abe DK, Cruz JA, Dall'oglio MF, Crippa A,
Passerotti CC, Ribeiro-Filho LA, et al: Tgf-β1 expression as a
biomarker of poor prognosis in prostate cancer. Clinics (Sao
Paulo). 66:1143–1147. 2011.PubMed/NCBI
|
|
15
|
Wikström P, Stattin P, Franck-Lissbrant I,
Damber JE and Bergh A: Transforming growth factor beta1 is
associated with angiogenesis, metastasis, and poor clinical outcome
in prostate cancer. Prostate. 37:19–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun H and Taneja R: Stra13 expression is
associated with growth arrest and represses transcription through
histone deacetylase (HDAC)-dependent and HDAC-independent
mechanisms. Proc Natl Acad Sci USA. 97:pp. 4058–4063. 2000;
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honma S, Kawamoto T, Takagi Y, Fujimoto K,
Sato F, Noshiro M, Kato Y and Honma K: Dec1 and Dec2 are regulators
of the mammalian molecular clock. Nature. 419:841–844. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Xie M, Yang J, Yang D, Deng R, Wan Y
and Yan B: The expression of antiapoptotic protein survivin is
transcriptionally upregulated by DEC1 primarily through multiple
sp1 binding sites in the proximal promoter. Oncogene. 25:3296–3306.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chakrabarti J, Turley H, Campo L, Han C,
Harris AL, Gatter KC and Fox SB: The transcription factor DEC1
(stra13, SHARP2) is associated with the hypoxic response and high
tumour grade in human breast cancers. Brit J Cancer. 91:954–958.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Sato F, Yamada T, Bhawal UK,
Kawamoto T, Fujimoto K, Noshiro M, Seino H, Morohashi S, Hakamada
K, et al: The BHLH transcription factor DEC1 plays an important
role in the epithelial-mesenchymal transition of pancreatic cancer.
Int J Oncol. 41:1337–1346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Q, Wu Y, Yoshizawa T, Yan X, Morohashi
S, Seino H, Kato Y and Kijima H: Basic helix-loop-helix
transcription factor DEC2 functions as an anti-apoptotic factor
during paclitaxel-induced apoptosis in human prostate cancer cells.
Int J Mol Med. 38:1727–1733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Sato F, Kawamoto T, Fujimoto K,
Morohashi S, Akasaka H, Kondo J, Wu Y, Noshiro M, Kato Y and Kijima
H: Anti-apoptotic effect of the basic helix-loop-helix (bHLH)
transcription factor DEC2 in human breast cancer cells. Genes
Cells. 15:315–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Liu Q, Yan X, Kato Y, Tanaka M,
Inokuchi S, Yoshizawa T, Morohashi S and Kijima H:
Podoplanin-mediated TGF-β-induced epithelial-mesenchymal transition
and its correlation with bHLH transcription factor DEC in TE-11
cells. Int J Oncol. 48:2310–2320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomita K, van Bokhoven A, van Leenders GJ,
Ruijter ET, Jansen CF, Bussemakers MJ and Schalken JA: Cadherin
switching in human prostate cancer progression. Cancer Res.
60:3650–3654. 2000.PubMed/NCBI
|
|
25
|
Jennbacken K, Tesan T, Wang W, Gustavsson
H, Damber JE and Welén K: N-cadherin increases after androgen
deprivation and is associated with metastasis in prostate cancer.
Endocr Relat Cancer. 17:469–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones E, Pu H and Kyprianou N: Targeting
TGF-beta in prostate cancer: Therapeutic possibilities during tumor
progression. Expert Opin Ther Targets. 13:227–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|