Introduction
Diabetes mellitus (DM) is a chronic non-infectious
disease. Of them, disease resulted from islet B cell damage is
called type 1 DM (T1DM), while disease in which the body can
produce insulin but the cells can not utilize the insulin is called
type 2 DM (T2DM) (1). DM morbidity
shows an increasing trend globally with the improvement in people's
living standard and changes in lifestyle (2). It is conservatively estimated that,
the global DM cases would increase from 0.382 billion in 2013 to
0.592 billion in 2035 (3). Large
epidemiological survey in China discovers that, DM morbidity in
people aged over 20 years is 9.7%. Of them, T2DM accounts for 90%,
and patients combined with diabetic nephropathy (DN) have taken up
20–40% (3). DN is a common chronic
complication of DM, which manifests as proteinuria and
hypertension. Typically, it is characteristic of the sign of
urinary albumin excretion rate (4). Meanwhile, it is also the most
critical cause of end-stage renal disease (ESRD) (4). The growing ESRD cases have caused
tremendous economic burdens on the country (5). Research also reports that, DN-induced
deaths account for 60% of the total DM-related deaths (5).
The pathogenesis of DN is mainly explained from
genetic susceptibility factor, abnormal glucose metabolism pathway,
kidney hemodynamic changes, inflammatory response theory and
cytokine theory (6). An increasing
number of scholars accept the inflammatory response theory
(7). In other words, DM is a
natural focal disease, with inflammatory response in its course
(8). It is an inflammatory disease
induced by metabolic disorder (7).
In the genesis and development of microvascular complications such
as DN, inflammatory response also plays a vital role. Similarly,
this view is verified in related clinical and laboratory research
(6). However, the genesis of
inflammatory response is complicated. It is a disease resistance
response occurring in systemic tissues and multiple organs
(7). Meanwhile, it is accompanying
with body fever and leukocytosis (6). In essence, it is a process in which
inflammatory factors fight against the body (6).
Existing studies suggest that, Toll-like receptor 4
(TLR4) is a major receptor of the natural immune system to
recognize pathogenic microorganism (9). The TLR4 signaling pathway is
activated upon the stimulation of lipopolysaccharide (LPS). As a
result, the lipid of LPS A binds with LPS-binding protein (LBP)
outside the relevant cells to form the LPS-LBP complex. Such
complex binds with CD14 on cell membrane surface, thus activating
TLR4. The activated TLR4 binds with the homodomain of myeloid
differentiation factor 88 (MyD88) through the internal segment of
its cytoplasma (10). In addition,
the death domain (DD) of MyD88 can recruit the downstream molecules
containing DD (11). Therefore, it
can induce the release of pro-inflammatory cytokines tumor necrosis
factor (TNF)-α, interleukin (IL)-1 and IL-6, thus exerting the
immune response effect (11).
Ursolic acid (UA) is the anti-hepatitis active
ingredient extracted from the Caprifoliaceae plant Sambucus
chinensis. It is a pentacyclic triterpenoid extensively distributed
in the nature (12). Typically, it
exists in the free form or binds with sugar to form glycoside in
plants like Sambucus chinensis, Fructus crataegus, Arbutus
menziesii, Prunella vulgaris, glossy privet fruit, plantain herb,
Incarvillea arguta, and oldenlandia diffusa (13,14).
It has multiple clinical pharmacological effects, including
antitumor, anti-hepatitis, anti-inflammation and anti-bacterium. It
is the ideal drug to treat viral hepatitis, with low toxicity and
little side effects (13). The aim
of this in vitro study is to examine the effects of UA
alleviates diabetes-induced nephropathy and its possible
mechanism.
Materials and methods
Animals and experimental rat
model
Adult male Wistar rats (200–230 g) were obtained
from Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China) and were housed in standard environmental
conditions maintained at 22±2°C, 55–60% humidness, freely access to
food and water with 12 h light-dark cycle. All rats were randomly
divided into three groups: i) Sham + vehicle group (n=6); ii)
Nephropathy + vehicle group (n=6); and iii) Nephropathy + 25 mg/kg
UA group (n=6). All rats of Nephropathy + vehicle group or
Nephropathy + 25 mg/kg UA group were received 50 mg/kg of
intra-peritoneal injection of streptozocin for 60 days. All rats of
Nephropathy + 25 mg/kg UA group were gavaged with 25 mg/kg UA for
60 days. This study was approved by the Ethics Committee of Chinese
PLA General Hospital. At the end of these experiments, all animals
were anesthetized by 35 mg/kg pentobarbital sodium and sacrificed
using decollation.
Hematoxylin and eosin (H&E)
staining
After treatment with UA, kidney tissue samples were
collected and fixed with 4% paraformaldehyde for 24 h. Kidney
tissue samples were dehydrated, embedded, and sliced, and cut into
5 µm thickness. Sections of 5 µm thickness were stained with
H&E sassy for 5 min and then examined using transmission
electron microscopy (H7650).
ELISA kits for inflammation
After treatment with UA, peripheral blood or cells
were collected and centrifuged at 1,000 × g for 5 min at 4°C.
TNF-α, IL-1β, IL-6 and IL-18 levels were measured using ELISA
kits.
Western blot analysis
After treatment with UA, kidney tissue samples or
cells were collected and homogenated using RIPA assay for 30 min at
4°C. Proteins were collected, electrophoresed via 10% SDS-PAGE, and
transferred to polyvinylidene fluoride membranes. The membranes
were blocked with 5% non-milk in TBST for 1 h at 37°C and incubated
at 4°C overnight with the following primary antibodies: TLR4,
MyD88, NF-κB and GAPDH (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). The membranes were washed with TBST for 15 min and incubated
with species-specific horseradish peroxidase-conjugated secondary
antibodies (Santa Cruz Biotechnology, Inc.) for 1 h at 37°C.
Protein bands were detected using a Western Bright Enhanced
Chemiluminescence detection system (Advansta, Inc., Menlo Park, CA,
USA).
Statistical analysis
Values were expressed as mean ± SEM. One-way ANOVA
was followed by Tukey's post hoc multiple comparison test for
statistical analysis.
Results
UA prevented diabetes-induced
nephropathy in rat
First, we explored whether the effect of UA
prevented diabetes-induced nephropathy. The structural formula of
UA was showed at Fig. 1A. As
observed in Fig. 1B, glomerulus
appeared damage in rat with diabetes-induced nephropathy, compared
with sham group. As showed in Fig. 1C
and D, body weight was inhibited, blood glucose levels were
increased in rat with diabetes-induced nephropathy, compared with
sham group. Then, treatment with UA prevented glomerular damage,
increased body weight and reduced blood glucose levels in rat with
diabetes-induced nephropathy, compared with sham group (Fig. 1B-D).
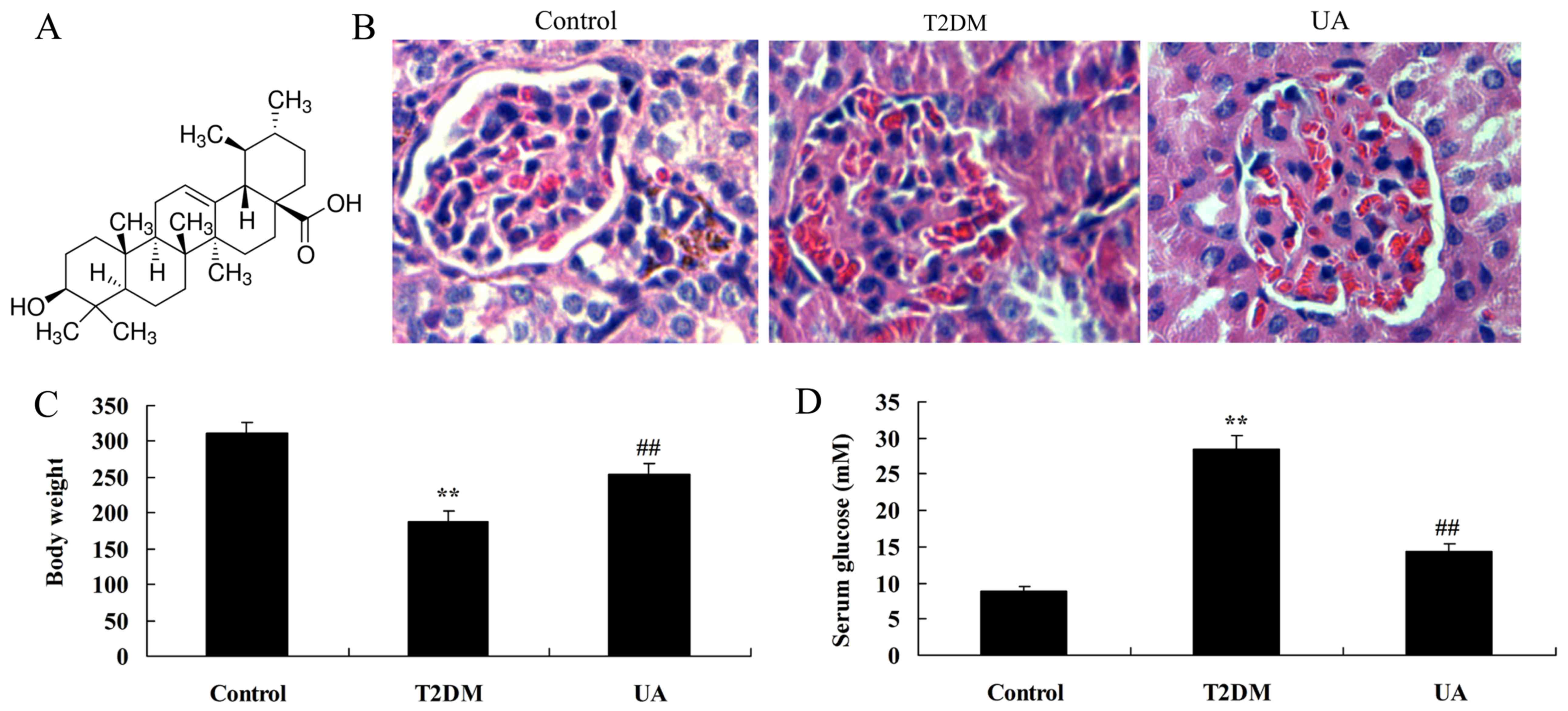 | Figure 1.UA prevented diabetes-induced
nephropathy in rats. (A) The structural formula of UA, (B) H&E
staining in the glomerulus (magnification, ×400), (C) body weight
and (D) blood glucose levels were indicated. **P<0.01 compared
with control rat group, ##P<0.01 compared with
diabetes-induced nephropathy group. Control, control rat group;
T2DM, diabetes-induced nephropathy in rat model group; UA,
treatment with UA in rat of diabetes-induced nephropathy group. UA,
ursolic acid; H&E, hematoxylin and eosin; T2DM, type 2 diabetes
mellitus. |
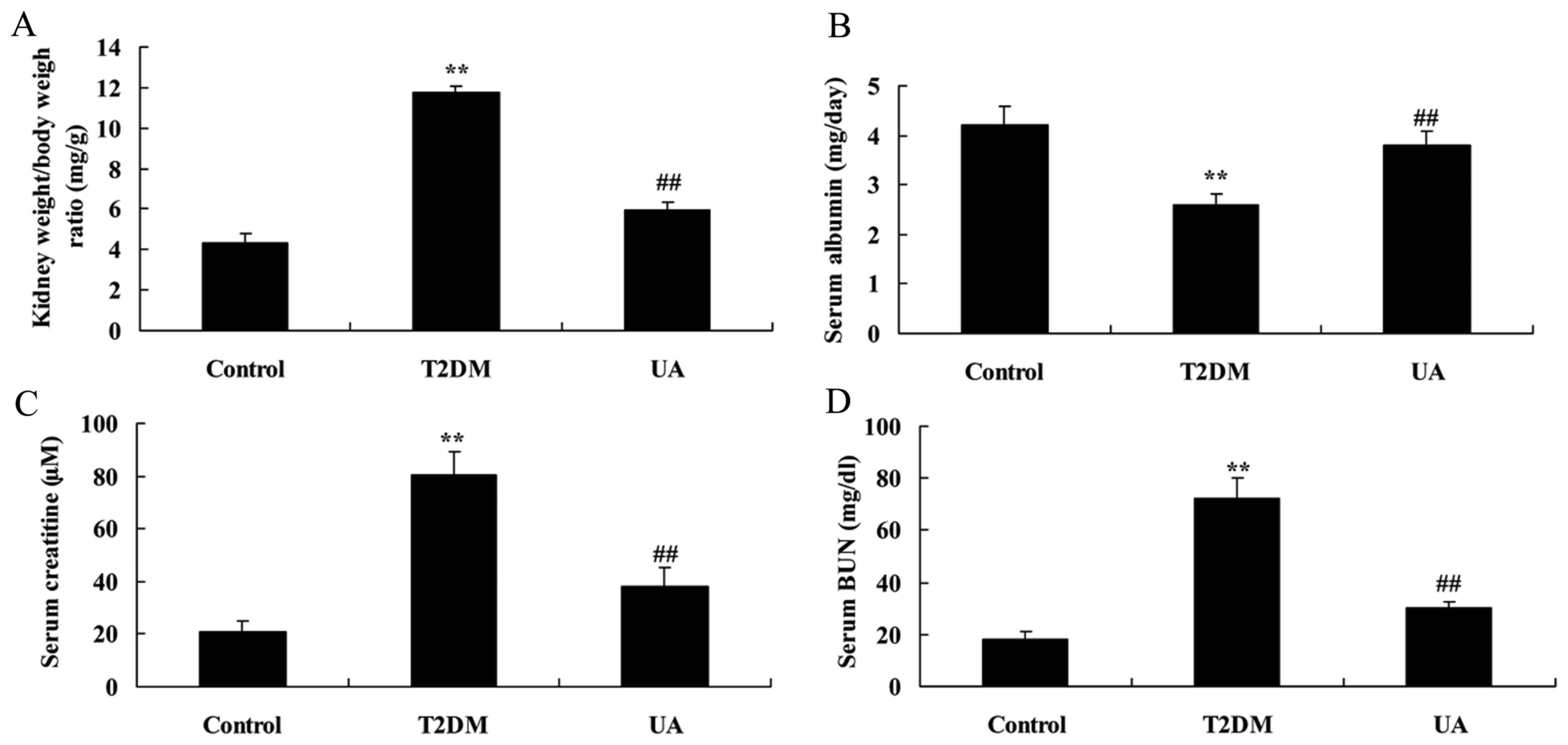
UA protected kidney cell in rat with
diabetes-induced nephropathy
Kidney weight/body weight ratio was decreased, blood
urea nitrogen (BUN) and Creatinine in serum were promoted, and
Albumin in serum was increased in serum of rat with
diabetes-induced nephropathy, compared with sham group (Fig. 2). These indexes were reversed by UA
in rat with diabetes-induced nephropathy, compared with
diabetes-induced nephropathy model group (Fig. 2).
UA prevented inflammation in rat with
diabetes-induced nephropathy through TLR4 pathway
Diabetic rats showed a significant elevation of
TNF-α, IL-1β, IL-6 and IL-18 levels in rat with diabetes-induced
nephropathy, compared with sham group (Fig. 3). Treatment with UA reduced TNF-α,
IL-1β, IL-6 and IL-18 levels in rat with diabetes-induced
nephropathy, compared with diabetes-induced nephropathy model group
(Fig. 3). Diabetic rats showed
increase of protein expression levels of TLR4, MyD88 and NF-κB in
rat with diabetes-induced nephropathy, compared with sham group
(Fig. 4). Treatment with UA
suppressed TLR4, MyD88 and NF-κB protein expression in rat with
diabetes-induced nephropathy, compared with diabetes-induced
nephropathy model group (Fig.
4).
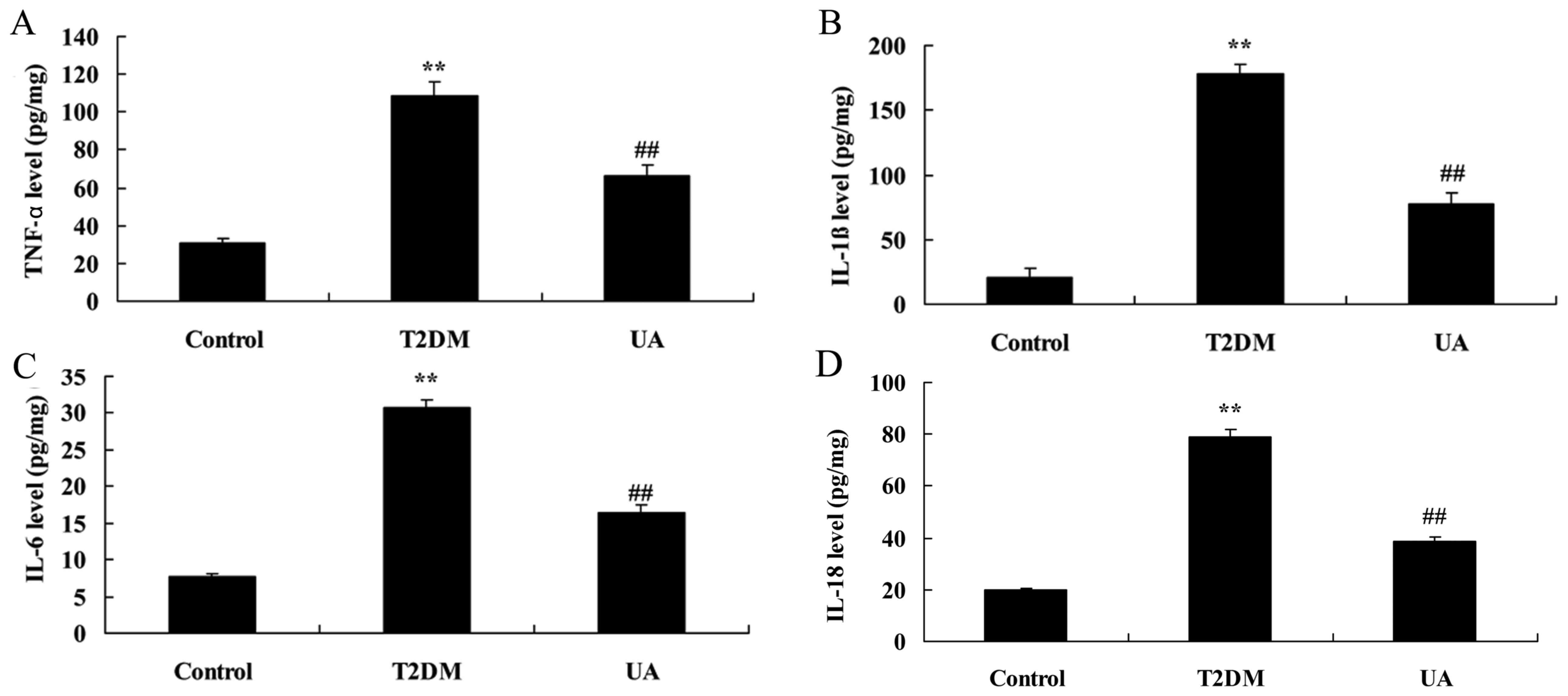 | Figure 3.UA prevented inflammation in rat with
diabetes-induced nephropathy through TLR4 pathway. (A) TNF-α, (B)
IL-1β, (C) IL-6 and (D) IL-18 levels. **P<0.01 compared with
control rat group, ##P<0.01 compared with
diabetes-induced nephropathy group. Control, control rat group;
T2DM, diabetes-induced nephropathy in rat model group; UA,
treatment with UA in rat of diabetes-induced nephropathy group. UA,
ursolic acid; TLR4, Toll-like receptor 4; T2DM, type 2 diabetes
mellitus; TNF, tumor necrosis factor; IL, interleukin. |
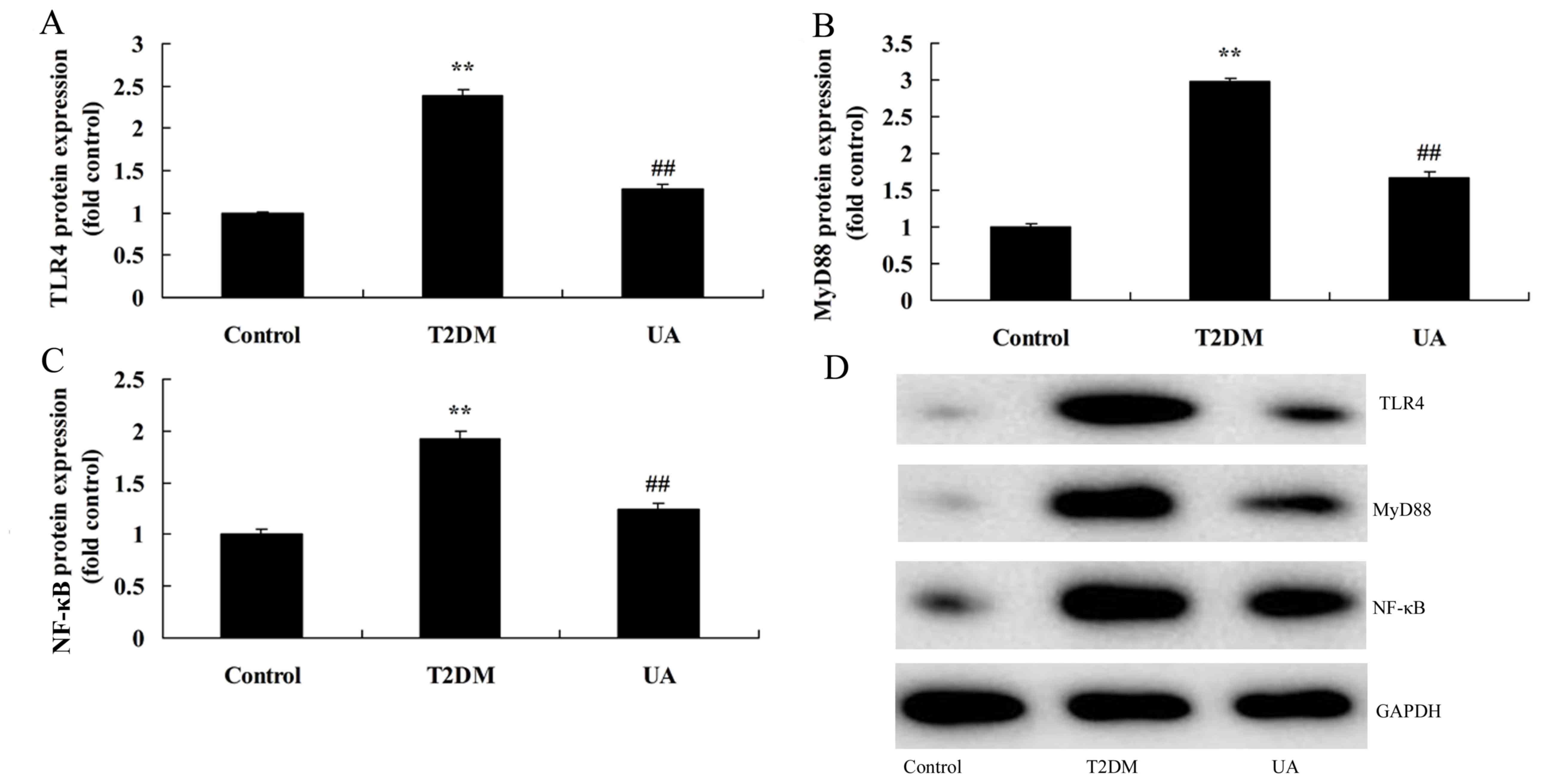 | Figure 4.UA suppressed TLR4 pathway in rat with
diabetes-induced nephropathy through TLR4 pathway. (A-C) TLR4,
MyD88 and NF-κB protein expression by statistical analysis and (D)
western blotting assays. **P<0.01 compared with control rat
group, ##P<0.01 compared with diabetes-induced
nephropathy group. Control, control rat group; T2DM,
diabetes-induced nephropathy in rat model group; UA, treatment with
UA in rat of diabetes-induced nephropathy group. UA, ursolic acid;
TLR4, Toll-like receptor 4; T2DM, type 2 diabetes mellitus; MyD88,
myeloid differentiation factor 88; NF-κB, nuclear factor-κB. |
The inhibition of TLR4 increased the
anti-inflammation of UA on inflammation in rat with
diabetes-induced nephropathy through TLR4 pathway
To assess the role of TLR4 in the anti-inflammation
of UA on inflammation in vitro model, TLR4 inhibitor
(TAK-242) suppressed TLR4 protein expression in vitro model
by UA. As showed in Fig. 5, TLR4
inhibitor suppressed TLR4, MyD88 and NF-κB protein expression in
rat with diabetes-induced nephropathy by UA, compared with only
treatment with UA group. However, TLR4 inhibitor promoted TNF-α,
IL-1β, IL-6 and IL-18 levels in rat with diabetes-induced
nephropathy by UA, compared with only treatment with UA group
(Fig. 6). TLR4 inhibitor reduced
glomerulus appeared damage, kidney weight/body weight ratio, BUN
and Creatinine in serum, albumin and protein of urine, and
increased albumin in serum of rat with diabetes-induced nephropathy
by UA, compared with only treatment with UA group (Fig. 7).
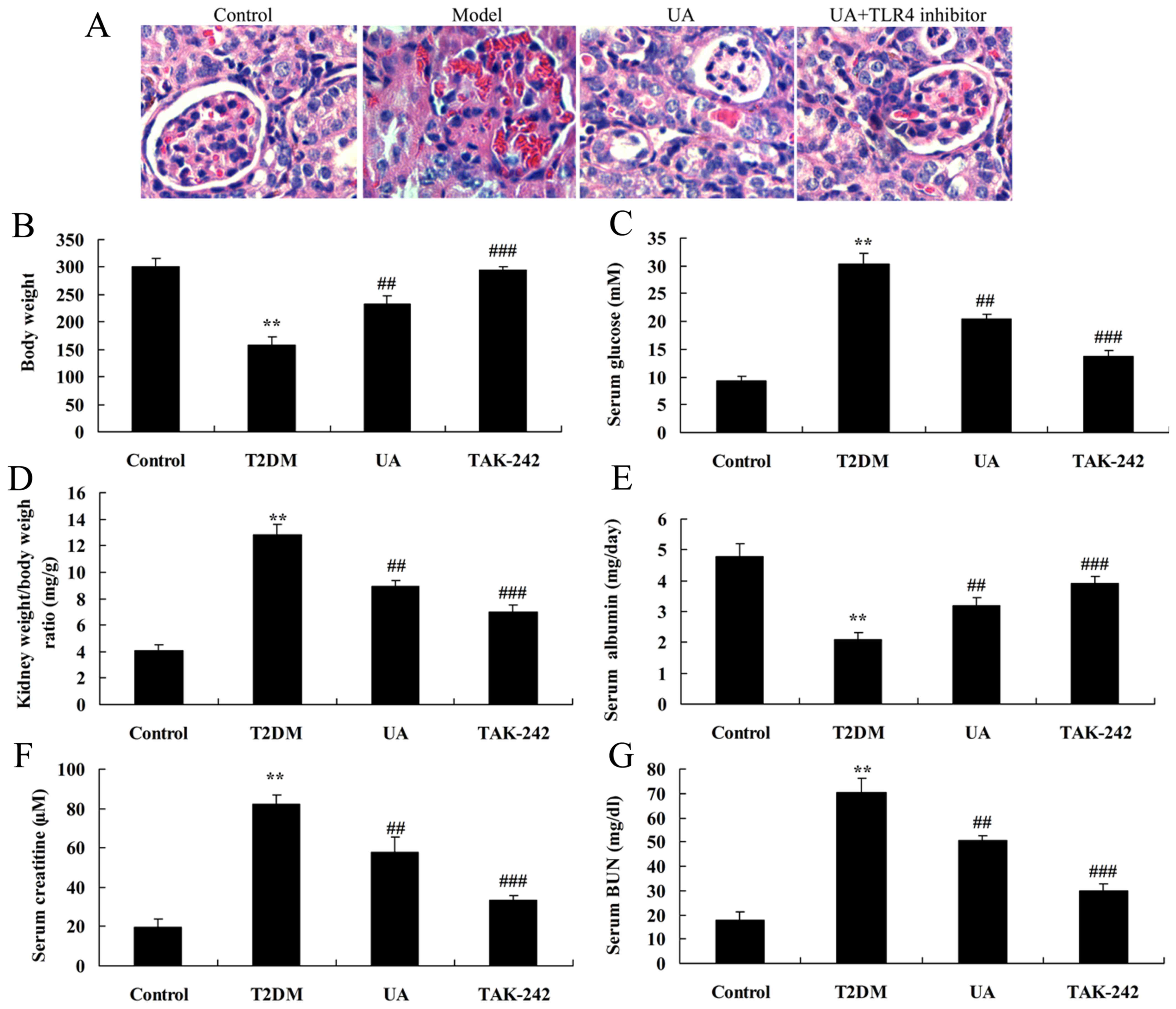 | Figure 5.Inhibition of TLR4 increased the
anti-inflammation of UA on nephropathy in rat with diabetes-induced
nephropathy through TLR4 pathway. (A) H&E staining in the
glomerulus (magnification, ×400), (B) body weight, (C) blood
glucose levels, (D) kidney weight/body weight ratio, (E) albumin,
(F) BUN and (G) creatinine levels were indicated. **P<0.01
compared with control rat group, ##P<0.01 compared
with diabetes-induced nephropathy group, ###P<0.01
compared with treatment with UA in rat of diabetes-induced
nephropathy group. Control, control rat group; T2DM,
diabetes-induced nephropathy in rat model group; UA, treatment with
UA in rat of diabetes-induced nephropathy group; TAK-242, treatment
with UA and TAK-242 in rat of diabetes-induced nephropathy group.
UA, ursolic acid; TLR4, Toll-like receptor 4; T2DM, type 2 diabetes
mellitus; BUN, blood urea nitrogen. |
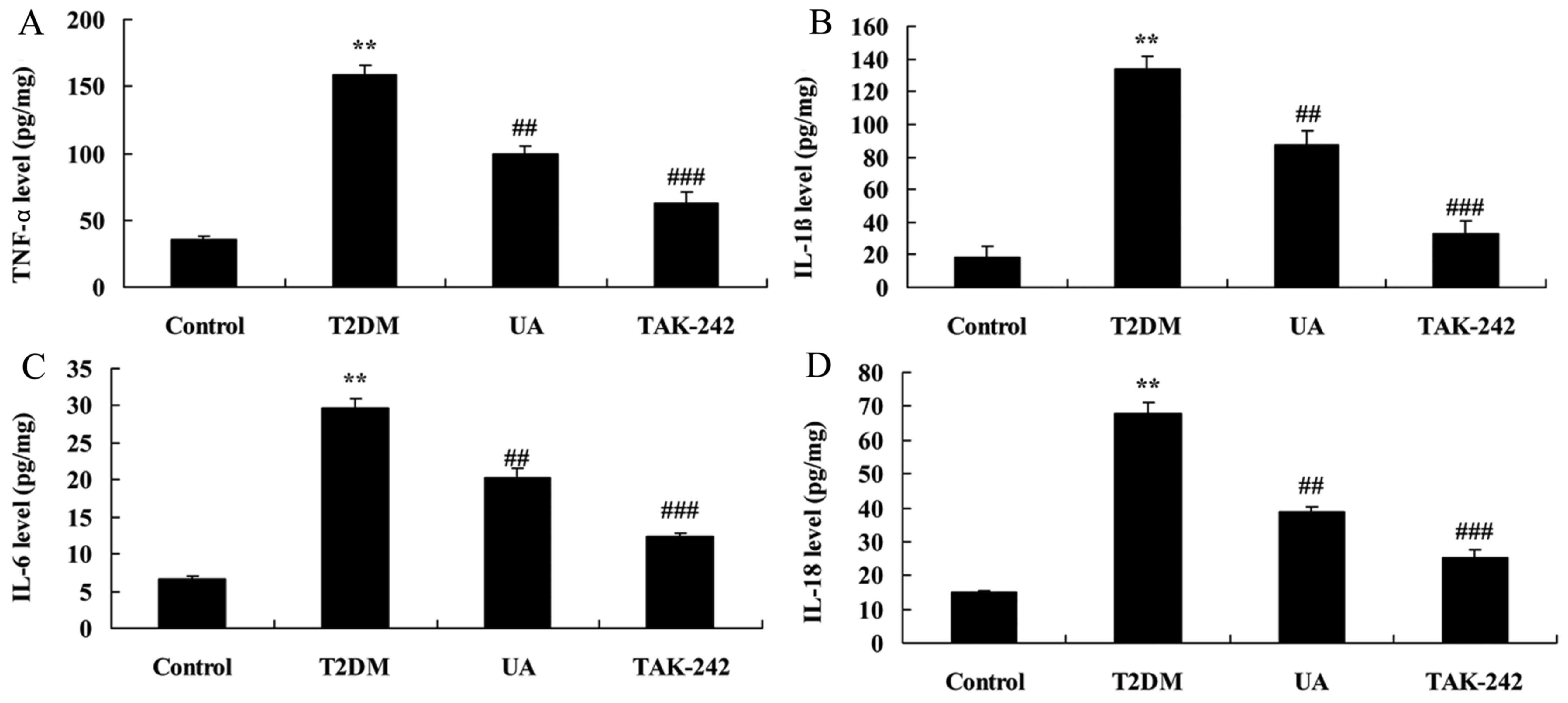 | Figure 6.Inhibition of TLR4 increased the
anti-inflammation of UA on inflammation in rat with
diabetes-induced nephropathy. (A) TNF-α, (B) IL-1β, (C) IL-6 and
(D) IL-18 levels. **P<0.01 compared with control rat group,
##P<0.01 compared with diabetes-induced nephropathy
group, ###P<0.01 compared with treatment with UA in
rat of diabetes-induced nephropathy group. Control, control rat
group; T2DM, diabetes-induced nephropathy in rat model group; UA,
treatment with UA in rat of diabetes-induced nephropathy group;
TAK-242, treatment with UA and TAK-242 in rat of diabetes-induced
nephropathy group. UA, ursolic acid; TLR4, Toll-like receptor 4;
T2DM, type 2 diabetes mellitus; TNF, tumor necrosis factor; IL,
interleukin. |
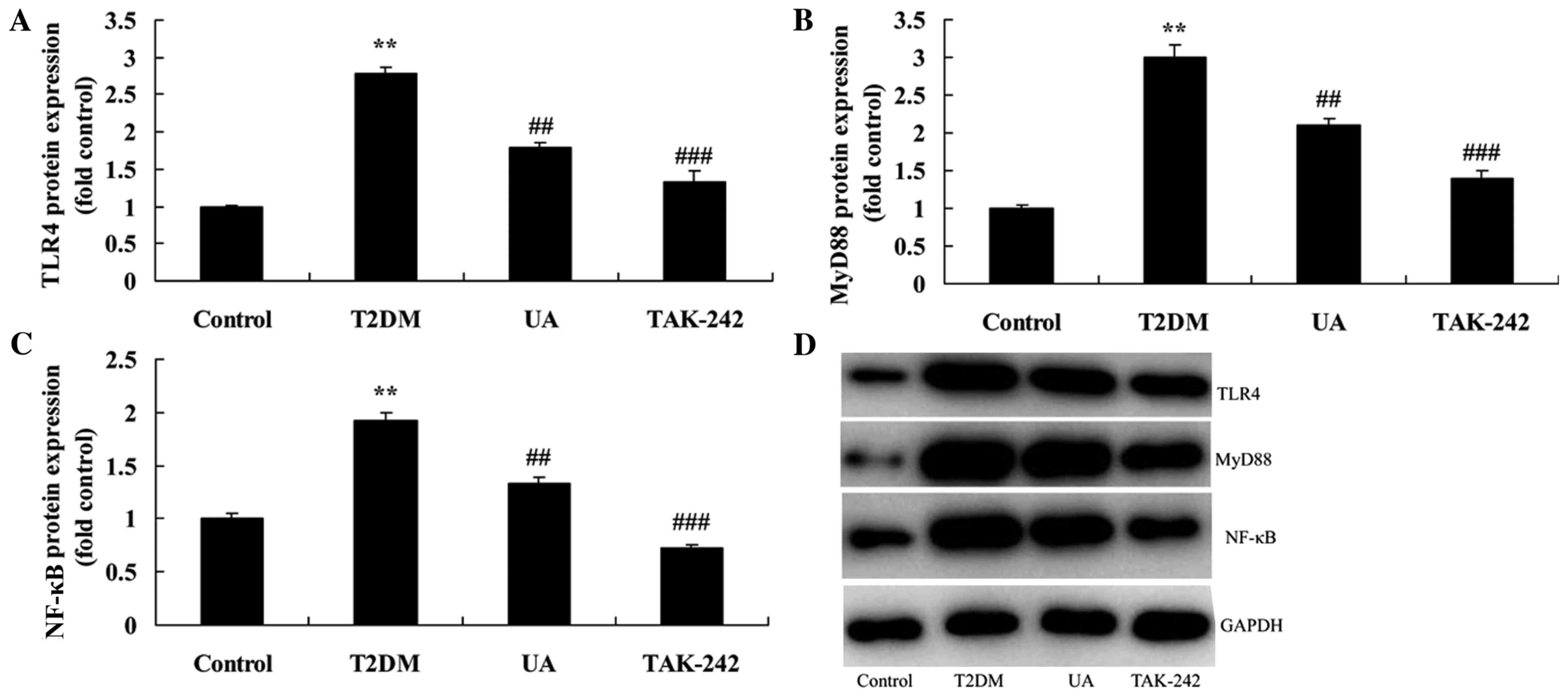 | Figure 7.Inhibition of TLR4 increased the
anti-inflammation of UA on TLR4 pathway in rat with
diabetes-induced nephropathy. (A-C) TLR4, MyD88 and NF-κB protein
expression by statistical analysis and (D) western blotting assays.
**P<0.01 compared with control rat group, ##P<0.01
compared with diabetes-induced nephropathy group,
###P<0.01 compared with treatment with UA in rat of
diabetes-induced nephropathy group. Control, control rat group;
T2DM, diabetes-induced nephropathy in rat model group; UA,
treatment with UA in rat of diabetes-induced nephropathy group;
TAK-242, treatment with UA and TAK-242 in rat of diabetes-induced
nephropathy group. UA, ursolic acid; TLR4, Toll-like receptor 4;
T2DM, type 2 diabetes mellitus; MyD88, myeloid differentiation
factor 88; NF-κB, nuclear factor-κB. |
Discussion
DN morbidity is growing in numerous countries in the
world with the improvement in people's living standard (1). DN is one of the severe complications
of DM-related microvascular lesion. It is also one of the major
causes leading to ESRD. The pathogenesis of DN is complicated,
which is once considered to be a metabolic disorder (15). Existing studies suggest that, DN
genesis is closely related to genetic, immune and environmental
factors (15). These findings
suggest that treatment with UA prevented glomerular damage,
increased body weight and Albumin, reduced blood glucose levels,
BUN, Creatinine in serum levels and kidney weight/body weight ratio
in rat with diabetes-induced nephropathy.
The pathogenesis of DN is complex, which is
previously considered to be related to mechanisms such as gene
polymorphism, abnormal kidney hemodynamics, abnormal growth factor
metabolism and oxidative stress (16). In recent years, an increasing
number of studies find that the genesis and development of DN is
closely related to inflammation (16). Research also discovers that,
metabolic disorders like hyperglycemia will damage the renal cells,
and promote them to secrete and release the inflammatory factors
(8). In this way, they will induce
the cascade reaction and induce renal damage (17). The immunity and inflammation
theories have received wide attention in recent years. It is
suggested that endothelial injury and platelet activation are
closely correlated with the genesis and development of DM (17). Wang et al showed that UA
ameliorates oxidative stress, inflammation and fibrosis in diabetic
cardiomyopathy rats (18). We
found that UA prevented TNF-α, IL-1β, IL-6 and IL-18 levels in rat
with diabetes-induced nephropathy.
From the view of inflammatory factor expression, the
renal tubular epithelial cells HK-2 and macrophages THP-1 are
stimulated by TLR4 endogenous and exogenous ligands under
hyperglycemia status (10). As a
result, expression of inflammatory factors TNF-α, MCP-1, IL-6 and
IL-8 is remarkably up-regulated within a short time (19). These factors play vitals roles in
regulating the innate and adaptive immune response, killing target
cells, inducing apoptosis and inducing the production of acute
phase reactive protein. Particularly, increased expression of these
inflammatory factors have been proved in the serum of DM and DN
patients (20). This finding
suggest that TLR4 ligands can induce the production of rapid and
strong inflammatory response by renal tubular epithelial cells and
macrophages under hyperglycemia environment (19). In addition, it demonstrates that
renal cell itself can be involved in the inflammatory response of
DN through activating TLR4 (20).
Zhang et al indicated that UA alleviates early brain injury
by suppressing TLR4-mediated inflammatory pathway (12). In the present study, Treatment with
UA suppressed TLR4, MyD88 and NF-κB protein expression in rat with
diabetes-induced nephropathy.
Nuclear transcription factor NF-κB is a
transcription factor in eukaryocyte with extensive distribution and
function (21). It can be
activated by multiple extracellular stimulations. In addition, it
can participate in gene regulation under apoptosis and inflammation
(22). Meanwhile, it has important
physiological and pathological actions (23). Recent study indicated that, NF-κB
is closely related to the genesis and development of DN (23). NF-κB can be activated by numerous
physiological and non-physiological stimulations. TLR4 is the major
receptor activating NF-κB. Data suggest that NF-κB can be activated
by some DM factors, thus it can initiate the transcription of many
DN-related genes (24). Moreover,
evidence also suggests that NF-κB activation can promote renal
interstitial fibrosis through its downstream action (24). Luo et al showed that UA
inhibits breast cancer growth via the PI3K/AKT and NF-κB signaling
pathways (25). Furthermore, we
found that the inhibition of TLR4 increased the anti-inflammation
of UA on inflammation in rat with diabetes-induced nephropathy
through TLR4 pathway.
In conclusion, UA alleviates inflammation and
against diabetes-induced nephropathy through TLR4-mediated
inflammatory pathway, and UA as a possible therapeutic agent
against diabetic nephropathy. UA could be applied in the
development of an effective therapeutic strategy for treating
diabetes-induced nephropathy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YS designed the present study. JL, NL, SY, ML, BS
and YL performed the experiments. YS and JL analyzed the data. YS
wrote the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Chinese PLA General Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Boer IH, Sun W, Gao X, Cleary PA,
Lachin JM, Molitch ME, Steffes MW and Zinman B; DCCT/EDIC research
group, : Effect of intensive diabetes treatment on albuminuria in
type 1 diabetes: Long-term follow-up of the diabetes control and
complications trial and epidemiology of diabetes interventions and
complications study. Lancet Diabetes Endocrinol. 2:793–800. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madsen SM, Thorup AC, Bjerre M and
Jeppesen PB: Does 8 weeks of strenuous bicycle exercise improve
diabetes-related inflammatory cytokines and free fatty acids in
type 2 diabetes patients and individuals at high-risk of metabolic
syndrome? Arch Physiol Biochem. 121:129–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aghasi M, Ghazi-Zahedi S, Koohdani F,
Siassi F, Nasli-Esfahani E, Keshavarz A, Qorbani M, Khoshamal H,
Salari-Moghaddam A and Sotoudeh G: The effects of green cardamom
supplementation on blood glucose, lipids profile, oxidative stress,
sirtuin-1 and irisin in type 2 diabetic patients: A study protocol
for a randomized placebo-controlled clinical trial. BMC Complement
Altern Med. 18:182018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strain WD, Lukashevich V, Kothny W,
Hoellinger MJ and Paldánius PM: Individualised treatment targets
for elderly patients with type 2 diabetes using vildagliptin add-on
or lone therapy (INTERVAL): A 24 week, randomised, double-blind,
placebo-controlled study. Lancet. 382:409–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salminen P, Helmiö M, Ovaska J, Juuti A,
Leivonen M, Peromaa-Haavisto P, Hurme S, Soinio M, Nuutila P and
Victorzon M: Effect of laparoscopic sleeve gastrectomy vs
laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years
among patients with morbid obesity: The SLEEVEPASS randomized
clinical trial. JAMA. 319:241–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boss JD, Singh PK, Pandya HK, Tosi J, Kim
C, Tewari A, Juzych MS, Abrams GW and Kumar A: Assessment of
neurotrophins and inflammatory mediators in vitreous of patients
with diabetic retinopathy. Invest Ophthalmol Vis Sci. 58:5594–5603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heier M, Margeirsdottir HD, Brunborg C,
Hanssen KF, Dahl-Jørgensen K and Seljeflot I: Inflammation in
childhood type 1 diabetes; influence of glycemic control.
Atherosclerosis. 238:33–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
On'kin JBKL, Longo-Mbenza B,
Tchokonte-Nana V, Okwe AN and Kabangu NK: Hyperbolic relation
between beta-cell function and insulin sensitivity for type 2
diabetes mellitus, malaria, influenza, Helicobacter pylori,
Chlamydia pneumoniae, and hepatitis C virus infection-induced
inflammation/oxidative stress and temporary insulin resistance in
Central Africans. Turk J Med Sci. 47:1834–1841. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doody NE, Dowejko MM, Akam EC, Cox NJ,
Bhatti JS, Singh P and Mastana SS: The role of TLR4, TNF-α and
IL-1β in type 2 diabetes mellitus development within a North Indian
population. Ann Hum Genet. 81:141–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perry BD, Rahnert JA, Xie Y, Zheng B,
Woodworth-Hobbs ME and Price SR: Palmitate-induced ER stress and
inhibition of protein synthesis in cultured myotubes does not
require Toll-like receptor 4. PLoS One. 13:e01913132018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang S, Zhang J, Wang S, Shi J and Zhao X:
Knockdown of angiopoietin-like protein 2 ameliorates diabetic
nephropathy by inhibiting TLR4. Cell Physiol Biochem. 43:685–696.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang T, Su J, Guo B, Zhu T, Wang K and Li
X: Ursolic acid alleviates early brain injury after experimental
subarachnoid hemorrhage by suppressing TLR4-mediated inflammatory
pathway. Int Immunopharmacol. 23:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhai M, Guo J, Ma H, Shi W, Jou D, Yan D,
Liu T, Tao J, Duan J, Wang Y, et al: Ursolic acid prevents
angiotensin II-induced abdominal aortic aneurysm in apolipoprotein
E-knockout mice. Atherosclerosis. 271:128–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou W, Lin L, Cheng Y and Liu Y: Ursolic
acid improves liver transplantation and inhibits apoptosis in
miniature pigs using donation after cardiac death. Cell Physiol
Biochem. 43:331–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu EY, Wong CK, Ho SY, Wong SY and Lam CL:
Can HbA1c replace OGTT for the diagnosis of diabetes mellitus among
Chinese patients with impaired fasting glucose? Fam Pract.
32:631–638. 2015.PubMed/NCBI
|
|
16
|
Yang YJ, Wu CT, Ou HY, Lin CH, Cheng HC,
Chang WL, Chen WY, Yang HB, Lu CC and Sheu BS: Male non-insulin
users with type 2 diabetes mellitus are predisposed to gastric
corpus-predominant inflammation after H. pylori infection. J Biomed
Sci. 24:822017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng Y, Jiang CD, Chang AM, Shi Y, Gao J,
Zhu L and Zhang Z: Interactions among insulin resistance,
inflammation factors, obesity-related gene polymorphisms,
environmental risk factors, and diet in the development of
gestational diabetes mellitus. J Matern Fetal Neonatal Med. 1–9.
2018. View Article : Google Scholar
|
|
18
|
Wang XT, Gong Y, Zhou B, Yang JJ, Cheng Y,
Zhao JG and Qi MY: Ursolic acid ameliorates oxidative stress,
inflammation and fibrosis in diabetic cardiomyopathy rats. Biomed
Pharmacother. 97:1461–1467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen G, Sun W, Liang Y, Chen T, Guo W and
Tian W: Maternal diabetes modulates offspring cell proliferation
and apoptosis during odontogenesis via the TLR4/NF-κB signalling
pathway. Cell Prolif. 50:2017. View Article : Google Scholar :
|
|
20
|
Li C, Che LH, Ji TF, Shi L and Yu JL:
Effects of the TLR4 signaling pathway on apoptosis of neuronal
cells in diabetes mellitus complicated with cerebral infarction in
a rat model. Sci Rep. 7:438342017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang ST, Su H, Zhang Q, Tang HQ, Wang CJ,
Zhou Q, Wei W, Zhu HQ and Wang Y: Sitagliptin inhibits endothelin-1
expression in the aortic endothelium of rats with
streptozotocin-induced diabetes by suppressing the nuclear
factor-κB/IκBα system through the activation of AMP-activated
protein kinase. Int J Mol Med. 37:1558–1566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ge Y, Paisie TK, Newman JRB, McIntyre LM
and Concannon P: UBASH3A mediates risk for type 1 diabetes through
inhibition of T-cell receptor-induced NF-κB signaling. Diabetes.
66:2033–2043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo C, Zhang L, Nie L, Zhang N, Xiao D, Ye
X, Ou M, Liu Y, Zhang B, Wang M, et al: Association of
polymorphisms in the MyD88, IRAK4 and TRAF6 genes and
susceptibility to type 2 diabetes mellitus and diabetic nephropathy
in a southern Han Chinese population. Mol Cell Endocrinol.
429:114–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Li D, Fan L, Xiao Q, Zuo H and Li
Z: CAPE-pNO2 ameliorated diabetic nephropathy through regulating
the Akt/NF-κB/iNOS pathway in STZ-induced diabetic mice.
Oncotarget. 8:114506–114525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo J, Hu YL and Wang H: Ursolic acid
inhibits breast cancer growth by inhibiting proliferation, inducing
autophagy and apoptosis, and suppressing inflammatory responses via
the PI3K/AKT and NF-κB signaling pathways in vitro. Exp Ther Med.
14:3623–3631. 2017. View Article : Google Scholar : PubMed/NCBI
|