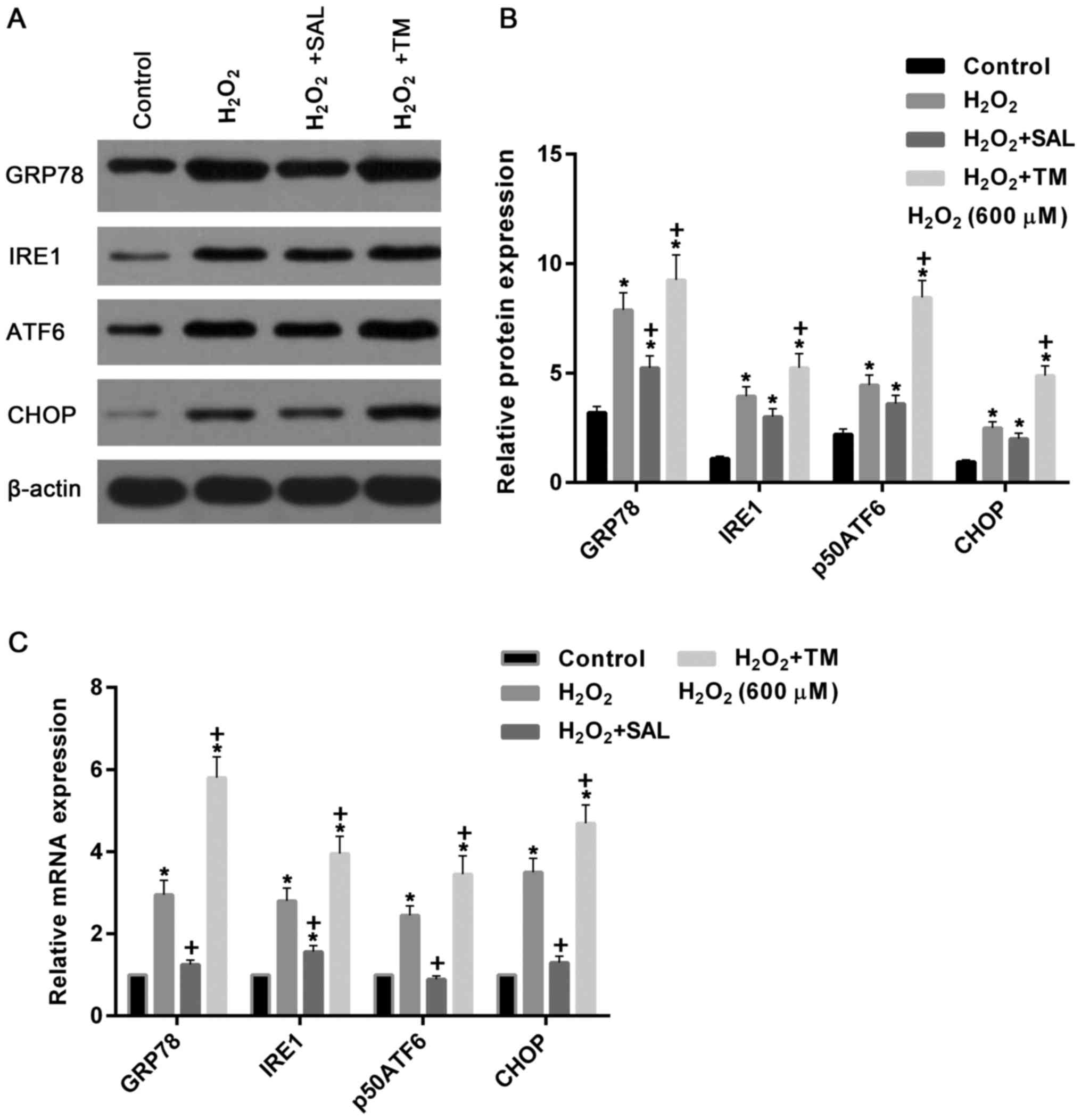
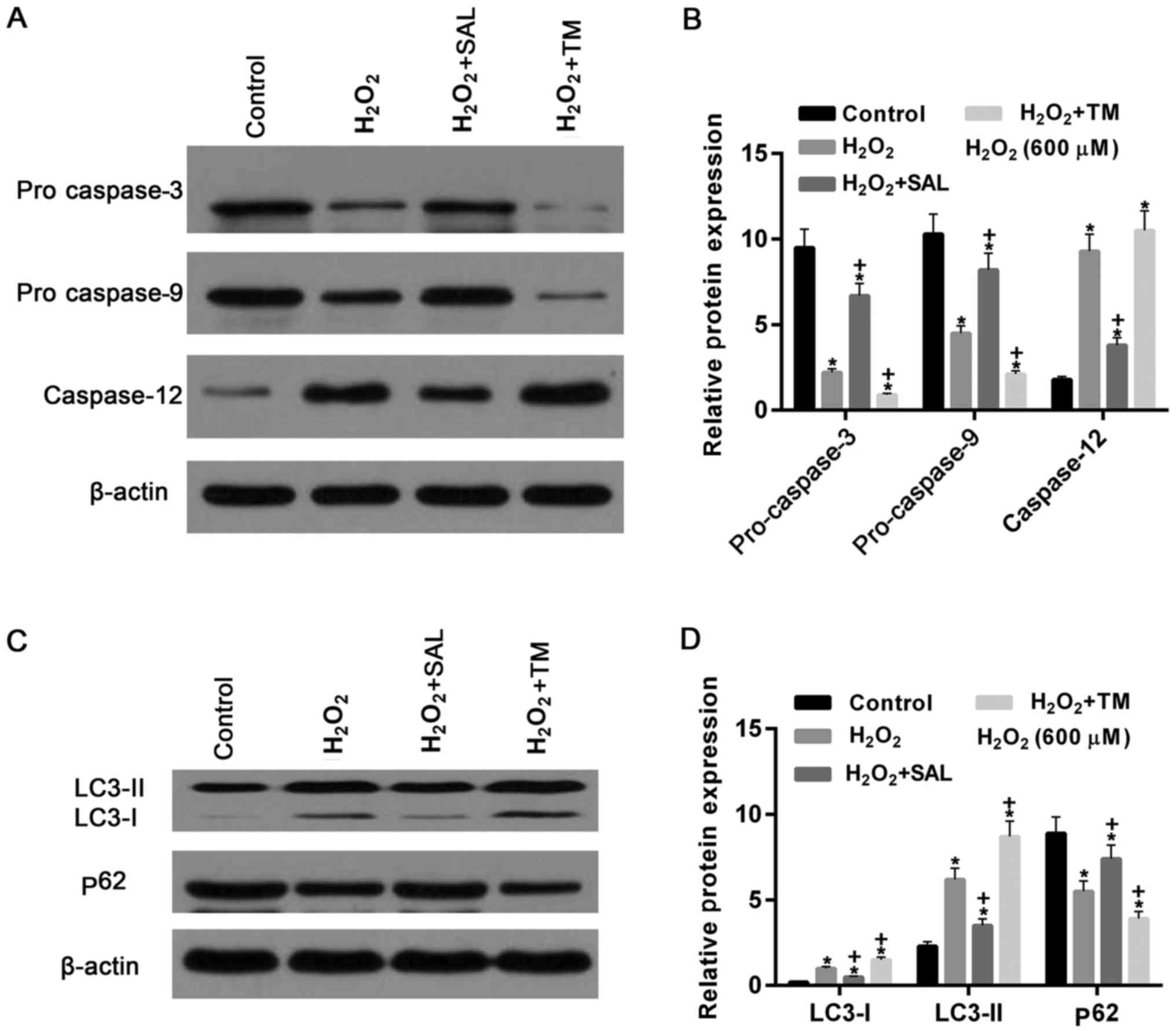
|
1
|
Benham AM: Protein folding and disulfide
bond formation in the eukaryotic cell: Meeting report based on the
presentations at the European Network Meeting on Protein Folding
and Disulfide Bond Formation 2009 (Elsinore, Denmark). FEBS J.
276:6905–6911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kato H and Nishitoh H: Stress responses
from the endoplasmic reticulum in cancer. Front Oncol. 5:932015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henderson KA: Boric acid localization and
effects on storage calcium release and the endoplasmic reticulum in
prostate cancer cells. Dissertations & Theses-Gradworks.
2009.
|
|
4
|
Schapansky J, Morissette M, Odero G,
Albensi B and Glazner G: Neuregulin beta1 enhances peak
glutamate-induced intracellular calcium levels through endoplasmic
reticulum calcium release in cultured hippocampal neurons. Can J
Physiol Pharmacol. 87:883–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mandl J, Mészáros T, Bánhegyi G and Csala
M: Minireview: Endoplasmic reticulum stress: Control in protein,
lipid, and signal homeostasis. Mol Endocrinol. 27:384–393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rangelaldao R: The unfolded protein
response, inflammation, oscillators, and disease: A systems
biologyapproach. Endo Reticulum Stress Dis. 2:30–52. 2015.
|
|
7
|
Richie DL, Feng X, Hartl L, Aimanianda V,
Krishnan K, Powers-Fletcher MV, Watson DS, Galande AK, White SM,
Willett T, et al: The virulence of the opportunistic fungal
pathogen requires cooperation between the endoplasmic
reticulum-associated degradation pathway (ERAD) and the unfolded
protein response (UPR). Virulence. 2:12–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Townsend DM, Manevich Y, He L, Xiong Y,
Bowers RR Jr, Hutchens S and Tew KD: Nitrosative-stress induced
S-glutathionylation of PDI leads to activation of the unfolded
protein response. Cancer Res. 69:7626–7634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reddy RK, Mao C, Baumeister P, Austin RC,
Kaufman RJ and Lee AS: Endoplasmic reticulum chaperone protein
GRP78 protects cells from apoptosis induced by topoisomerase
inhibitors: Role of ATP binding site in suppression of caspase-7
activation. J Biol Chem. 278:20915–20924. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bennett HL, Fleming JT, O'Prey J, Ryan KM
and Leung HY: Androgens modulate autophagy and cell death via
regulation of the endoplasmic reticulum chaperone glucose-regulated
protein 78/BiP in prostate cancer cells. Cell Death Dis. 1:e722010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu T, Yang W, Wang Z, Hu Z, Zeng X, Yang
C, Wang Y, Zhang Y, Li F, Liu Z, Wang D and Ye Z: Knockdown of
glucose-regulated protein 78/binding immunoglobulin heavy chain
protein expression by asymmetric small interfering RNA induces
apoptosis in prostate cancer cells and attenuates migratory
capability. Mol Med Rep. 11:2492015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maiuolo J, Bulotta S, Verderio C, Benfante
R and Borgese N: Selective activation of the transcription factor
ATF6 mediates endoplasmic reticulum proliferation triggered by a
membrane protein. Proc Natl Acad Sci USA. 108:7832–1837. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Umebayashi K, Hirata A, Horiuchi H, Ohta A
and Takagi M: Unfolded protein response-induced BiP/Kar2p
production protects cell growth against accumulation of misfolded
protein aggregates in the yeast endoplasmic reticulum. Eur J Cell
Biol. 78:726–738. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gardner BM and Walter P: Unfolded proteins
are Ire1-activating ligands that directly induce the unfolded
protein response. Science. 333:1891–1894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang J, Guo YS, Zhang Y, Yu XL, Li L,
Huang W, Li Y, Chen B, Jiang JL and Chen ZN: CD147 induces UPR to
inhibit apoptosis and chemosensitivity by increasing the
transcription of Bip in hepatocellular carcinoma. Cell Death
Differ. 19:1779–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hiss DC and Gabriels GA: Implications of
endoplasmic reticulum stress, the unfolded protein response and
apoptosis for molecular cancer therapy. Part II: Targeting cell
cycle events, caspases, NF-κB and the proteasome. Exp Opin Drug
Discov. 4:907–921. 2009. View Article : Google Scholar
|
|
17
|
Svetlana S, Patricia C, Mnich K, Ayo A,
Pakos-Zebrucka K, Patterson JB, Logue SE and Samali A: Endoplasmic
reticulum stress-mediated induction of SESTRIN 2 potentiates cell
survival. Oncotarget. 7:12254–12266. 2016.PubMed/NCBI
|
|
18
|
Netherton CL, Parsley JC and Wileman T:
African swine fever virus inhibits induction of the stress-induced
proapoptotic transcription factor CHOP/GADD153. J Virol.
78:10825–10828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moriya S, Miyazawa K, Kawaguchi T, Che XF
and Tomoda A: Involvement of endoplasmic reticulum stress-mediated
CHOP (GADD153) induction in the cytotoxicity of
2-aminophenoxazine-3-one in cancer cells. Int J Oncol. 39:981–988.
2011.PubMed/NCBI
|
|
20
|
Tan Y, Dourdin N, Wu C, De VT, Elce JS and
Greer PA: Ubiquitous calpains promote caspase-12 and JNK activation
during endoplasmic reticulum stress-induced apoptosis. J Biol Chem.
281:16016–16024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niso-Santano M, Bravo-San Pedro JM,
Gómez-Sánchez R, Climent V, Soler G, Fuentes JM and González-Polo
RA: ASK1 overexpression accelerates paraquat-induced autophagy via
endoplasmic reticulum stress. Toxicol Sci. 119:1562011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakka VP, Prakash-Babu P and Vemuganti R:
Crosstalk between endoplasmic reticulum stress, oxidative stress,
and autophagy: Potential therapeutic targets for acute CNS
Injuries. Mol Neurobiol. 53:532–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Devenish RJ and Klionsky DJ: Autophagy:
Mechanism and physiological relevance ‘brewed’ from yeast studies.
Front Biosci (Schol Ed). 4:1354–1363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deegan S, Koryga I, Glynn SA, Gupta S,
Gorman AM and Samali A: A close connection between the PERK and IRE
arms of the UPR and the transcriptional regulation of autophagy.
Biochem Biophys Res Commun. 456:305–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drake KR, Kang M and Kenworthy AK:
Nucleocytoplasmic distribution and dynamics of the autophagosome
marker EGFP-LC3. PLoS One. 5:e98062010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamura H, Shibata M, Koike M, Sasaki M and
Uchiyama Y: Atg9A, an autophagy-related membrane protein, is
localized in neurons of mouse brain. Neuroscience Research.
68:443–453. 2010. View Article : Google Scholar
|
|
27
|
Jo YK, Kim SC, Park IJ, Park SJ, Jin DH,
Hong SW, Cho DH and Kim JC: Increased expression of ATG10 in
colorectal cancer is associated with lymphovascular invasion and
lymph node metastasis. PLoS One. 7:e527052012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen ZH, Cao JF, Zhou JS, Liu H, Che LQ,
Mizumura K, Li W, Choi AM and Shen HH: Interaction of caveolin-1
with ATG12-ATG5 system suppresses autophagy in lung epithelial
cells. Am J Physiol Lung Cell Mol Physiol. 306:1016–1025. 2014.
View Article : Google Scholar
|
|
29
|
Hwang S, Maloney NS, Bruinsma MW, Goel G,
Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, et
al: Nondegradative role of Atg5-Atg12/Atg16L1 autophagy protein
complex in antiviral activity of interferon gamma. Cell Host
Microbe. 11:397–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kung CP, Budina A, Balaburski G,
Bergenstock MK and Murphy M: Autophagy in tumor suppression and
cancer therapy. Critical Reviews in Eukaryotic Gene Exp. 21:71–100.
2011. View Article : Google Scholar
|
|
31
|
Lebovitz CB, Bortnik SB and Gorski SM:
Here, there be dragons: Charting autophagy-related alterations in
human tumors. Clin Cancer Res. 18:1214–1226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
BenYounès A, Tajeddine N, Tailler M, Malik
SA, Shen S, Métivier D, Kepp O, Vitale I, Maiuri MC and Kroemer G:
A fluorescence-microscopic and cytofluorometric system for
monitoring the turnover of the autophagic substrate p62/SQSTM1.
Autophagy. 7:883–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Komatsu M, Kurokawa H, Waguri S, Taguchi
K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et
al: The selective autophagy substrate p62 activates the stress
responsive transcription factor Nrf2 through inactivation of Keap1.
Nat Cell Biol. 12:213–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kundu M: ULK1, Mammalian Target of
Rapamycin, and Mitochondria: Linking Nutrient Availability and
Autophagy. Antioxid Redox Signal. 14:1953–1958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheong H and Klionsky DJ: Dual role of
Atg1 in regulation of autophagy-specific PAS assembly in
Saccharomyces cerevisiae. Autophagy. 4:724–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luciani MF, Giusti C, Harms B, Oshima Y,
Kikuchi H, Kubohara Y and Golstein P: Atg1 allows second-signaled
autophagic cell death in Dictyostelium. Autophagy. 7:501–508. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yorimitsu T, Nair U, Yang Z and Klionsky
DJ: Endoplasmic reticulum stress triggers autophagy. J Biol Chem.
281:30299–30304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tekirdag KA, Korkmaz G, Ozturk DG, Agami R
and Gozuacik D: MIR181A regulates starvation- and rapamycin-induced
autophagy through targeting of ATG5. Autophagy. 9:374–385. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lopez-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kenneth J and Livak TD: Analysis of
relative gene expression data using rea l-time quantitative PCR a
nd the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
41
|
Simasi J, Schubert A, Oelkrug C, Gillissen
A and Nieber K: Primary and secondary resistance to tyrosine kinase
inhibitors in lung cancer. Anticancer Res. 34:2841–2850.
2014.PubMed/NCBI
|
|
42
|
Quintás-Cardama A, Kantarjian HM and
Cortes JE: Mechanisms of primary and secondary resistance to
imatinib in chronic myeloid leukemia. Cancer Control. 16:122–131.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schleicher SM, Moretti L, Varki V and Lu
B: Progress in the unraveling of the endoplasmic reticulum
stress/autophagy pathway and cancer: Implications for future
therapeutic approaches. Drug Resist Updat. 13:79–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li T, Su L, Zhong N, Hao X, Zhong D,
Singhal S and Liu X: Salinomycin induces cell death with autophagy
through activation of endoplasmic reticulum stress in human cancer
cells. Autophagy. 9:1057–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Su CC: Tanshinone IIA could inhibit
pancreatic cancer BxPC-3 cells through increasing PERK, ATF6,
Caspase-12 and CHOP expression to induce apoptosis. J Biom Sci Eng.
8:149–159. 2015. View Article : Google Scholar
|
|
46
|
Liu H, Nishitoh H, Ichijo H and Kyriakis
JM: Activation of apoptosis signal-regulating kinase 1 (ASK1) by
tumor necrosis factor receptor-associated factor 2 requires prior
dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol.
20:2198–2208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An Endoplasmic Reticulum Stress-specific
Caspase Cascade in Apoptosis cytochrome c-independent activation of
caspase-9 by caspase-12. J Biol Chem. 277:34287–34294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bae JY and Park HH: Purification and
characterization of a ubiquitin-like system for autophagosome
formation. J Microbiol Biotechnol. 20:1647–1652. 2010.PubMed/NCBI
|
|
50
|
Satoo K, Noda NN, Kumeta H, Fujioka Y,
Mizushima N, Ohsumi Y and Inagaki F: The structure of Atg4B-LC3
complex reveals the mechanism of LC3 processing and delipidation
during autophagy. EMBO J. 28:1341–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tanida I, Minematsuikeguchi N, Ueno T and
Kominami E: Lysosomal turnover, but not a cellular level, of
endogenous LC3 is a marker for autophagy. Autophagy. 1:84–91. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sailaja GS, Praveen B, Bharathi G, Chetty
C, Gogineni VR, Velpula KK, Gondi CS and Rao JS: The secreted
protein acidic and rich in cysteine (SPARC) induces endoplasmic
reticulum stress leading to autophagy-mediated apoptosis in
neuroblastoma. Int J Oncol. 42:188–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang Q, Li F, Shi K, Wu P, An J, Yang Y
and Xu C: Involvement of p38 in signal switching from autophagy to
apoptosis via the PERK/eIF2α/ATF4 axis in selenite-treated NB4
cells. Cell Death Dis. 5:e12702014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kamada Y: Prime-numbered Atg proteins act
at the primary step in autophagy: Unphosphorylatable Atg13 can
induce autophagy without TOR inactivation. Autophagy. 6:415–416.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Saito A, Ochiai K, Kondo S, Tsumagari K,
Murakami T, Cavener DR and Imaizumi K: Endoplasmic reticulum stress
response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved
in osteoblast differentiation induced by BMP2. J Biol Chem.
286:4809–4818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kimura S, Noda T and Yoshimori T:
Dynein-dependent movement of autophagosomes mediates efficient
encounters with lysosomes. Cell Struct Funct. 33:109–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee H, Noh JY, Oh Y, Kim Y, Chang JW,
Chung CW, Lee ST, Kim M, Ryu H and Jung YK: IRE1 plays an essential
role in ER stress-mediated aggregation of mutant huntingtin via the
inhibition of autophagy flux. Hum Mol Genet. 21:101–114. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang C, Kawauchi J, Adachi MT, Hashimoto
Y, Oshiro S, Aso T and Kitajima S: Activation of JNK and
Transcriptional Repressor ATF3/LRF1 through the IRE1/TRAF2 pathway
is implicated in human vascular endothelial cell death by
homocysteine. Biochem Biophys Res Commun. 289:718–724. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shvets E, Fass E, Scherz-Shouval R and
Elazar Z: The N-terminus and Phe52 residue of LC3 recruit
p62/SQSTM1 into autophagosomes. J Cell Sci. 121:2685–2695. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu
HL, Yang C and Liu HF: p62 links the autophagy pathway and the
ubiqutin-proteasome system upon ubiquitinated protein degradation.
Cell Mol Biol Lett. 21:292016. View Article : Google Scholar : PubMed/NCBI
|