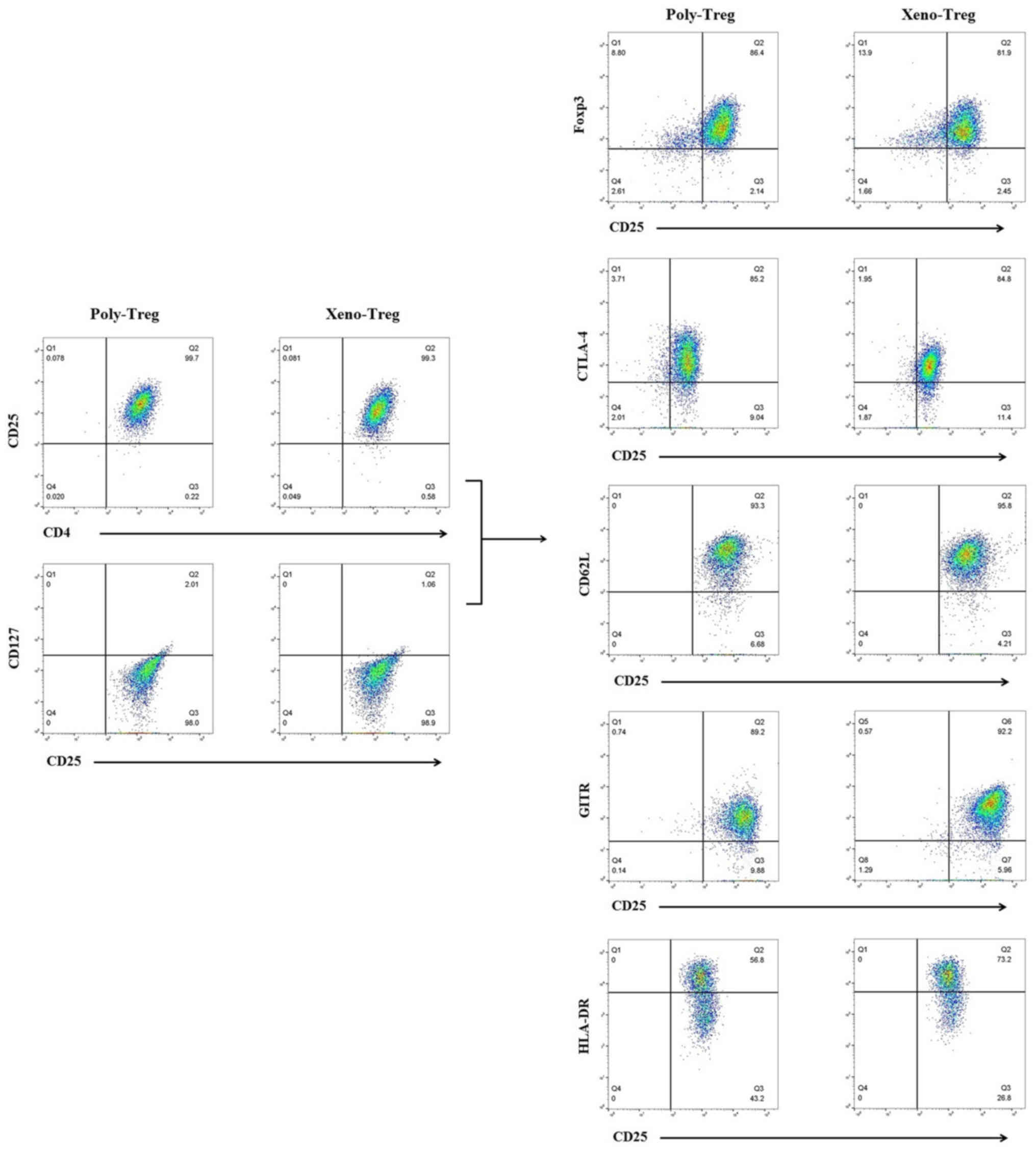
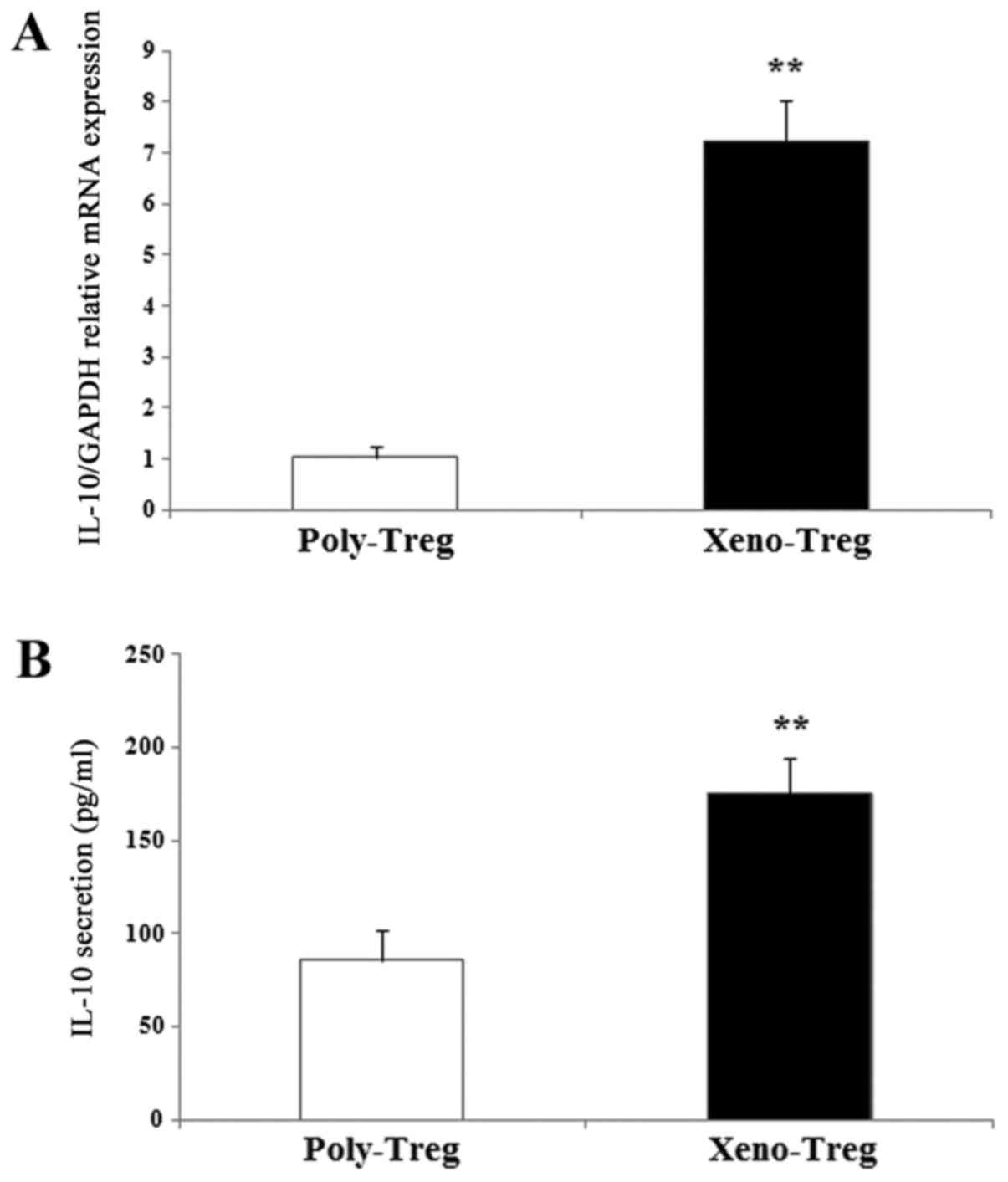
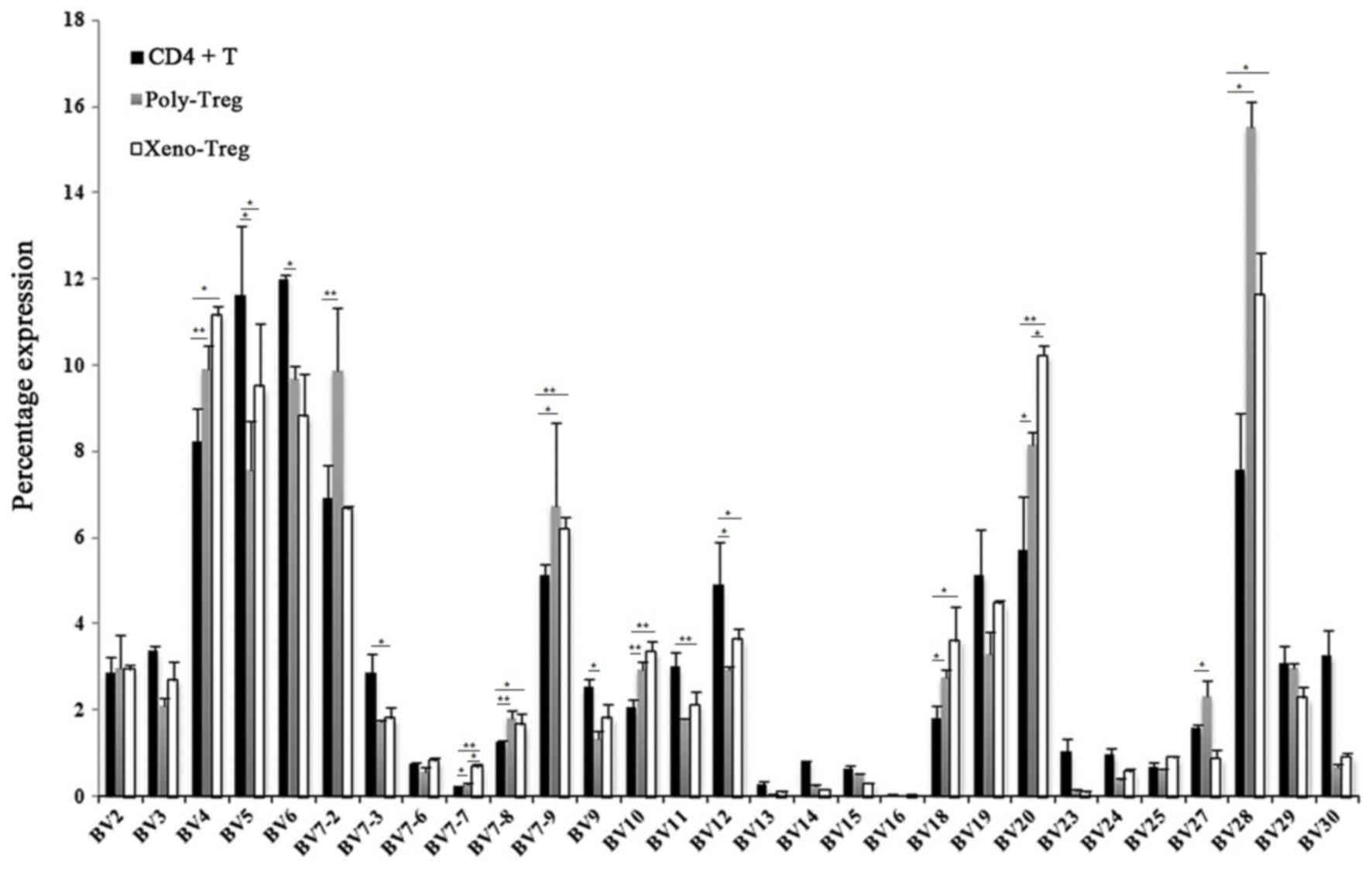
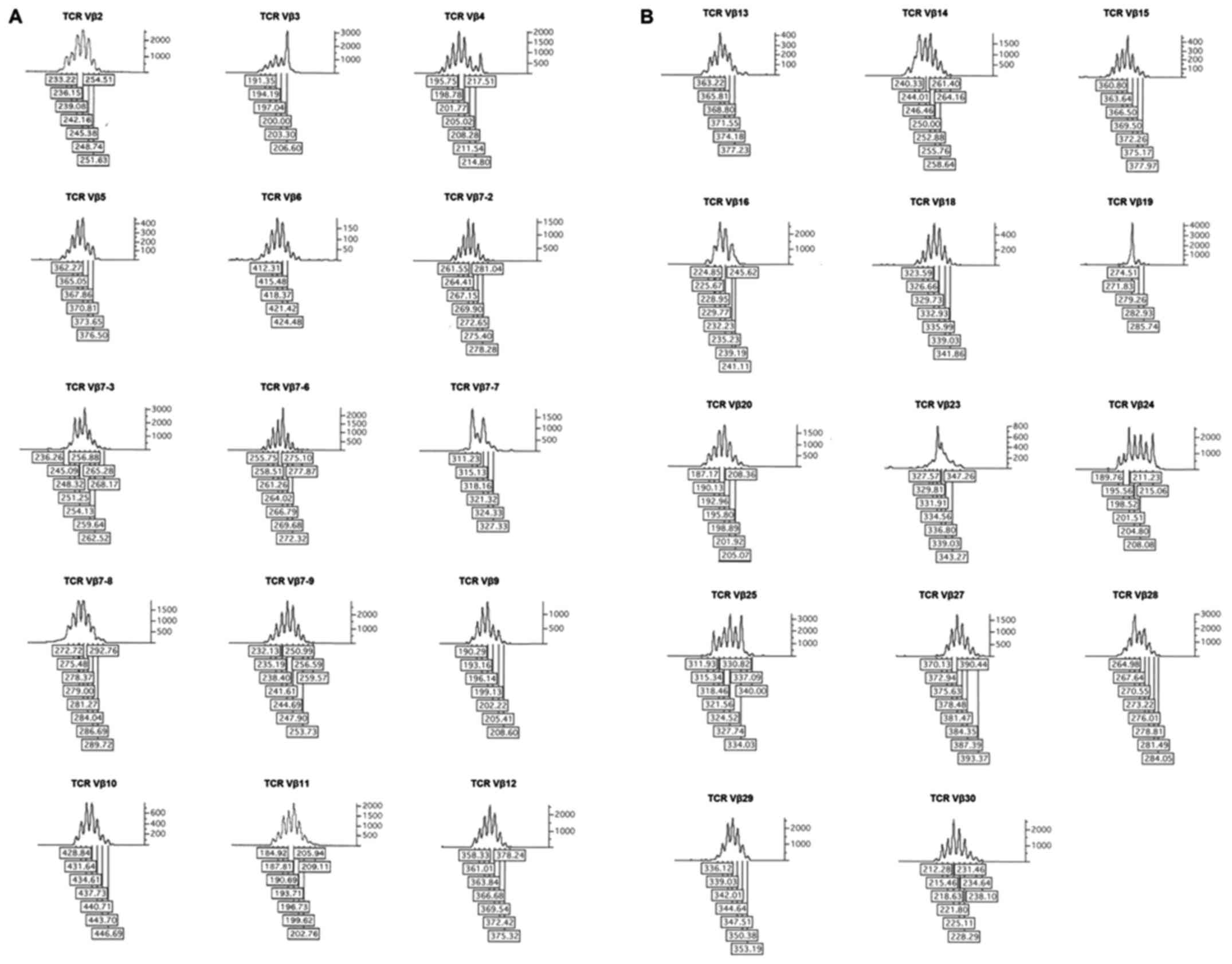
|
1
|
Vadori M and Cozzi E: The immunological
barriers to xenotransplantation. Tissue Antigens. 86:239–253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butler JR, Wang ZY, Martens GR, Ladowski
JM, Li P, Tector M and Tector AJ: Modified glycan models of
pig-to-human xenotransplantation do not enhance the human-anti-pig
T cell response. Transpl Immunol. 35:47–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin JS, Kim JM, Min BH, Yoon IH, Kim HJ,
Kim JS, Kim YH, Kang SJ, Kim J, Kang HJ, et al: Pre-clinical
results in pig-to-non-human primate islet xenotransplantation using
anti-CD40 antibody (2C10R4)-based immunosuppression.
Xenotransplantation. 25:2018. View Article : Google Scholar
|
|
4
|
Lee JI, Kim J, Choi YJ, Park HJ, Park HJ,
Wi HJ, Yoon S, Shin JS, Park JK, Jung KC, et al: The effect of
epitope-based ligation of ICAM-1 on survival and retransplantation
of pig islets in nonhuman primates. Xenotransplantation. 25:2018.
View Article : Google Scholar
|
|
5
|
Qiu F, Liu H, Liang CL, Nie GD and Dai Z:
A new immunosuppressive molecule emodin induces both
CD4+FoxP3+ and
CD8+CD122+ regulatory T cells and suppresses
murine allograft rejection. Front Immunol. 8:15192017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garakani R and Saidi RF: Recent progress
in cell therapy in solid organ transplantation. Int J Organ
Transplant Med. 8:125–131. 2017.PubMed/NCBI
|
|
7
|
Marek-Trzonkowska N, Myśliwiec M,
Iwaszkiewicz-Grześ D, Gliwiński M, Derkowska I, Żalińska M,
Zieliński M, Grabowska M, Zielińska H, Piekarska K, et al: Factors
affecting long-term efficacy of T regulatory cell-based therapy in
type 1 diabetes. J Transl Med. 14:3322016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kasper IR, Apostolidis SA, Sharabi A and
Tsokos GC: Empowering regulatory T cells in autoimmunity. Trends
Mol Med. 22:784–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu L, Barbi J and Pan F: The regulation of
immune tolerance by FOXP3. Nat Rev Immunol. 17:703–717. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi S, Ji M, Wu J, Ma X, Phillips P,
Hawthorne WJ and O'Connell PJ: Adoptive transfer with in vitro
expanded human regulatory T cells protects against porcine islet
xenograft rejection via interleukin-10 in humanized mice. Diabetes.
61:1180–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin X, Wang Y, Hawthorne WJ, Hu M, Yi S
and O'Connell P: Enhanced suppression of the xenogeneic T-cell
response in vitro by xenoantigen stimulated and expanded regulatory
T cells. Transplantation. 97:30–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dawson NAJ, Vent-Schmidt J and Levings MK:
Engineered tolerance: Tailoring development, function, and
antigen-specificity of regulatory T cells. Front Immunol.
8:14602017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore C, Tejon G, Fuentes C, Hidalgo Y,
Bono MR, Maldonado P, Fernandez R, Wood KJ, Fierro JA, Rosemblatt
M, et al: Alloreactive regulatory T cells generated with retinoic
acid prevent skin allograft rejection. Eur J Immunol. 45:452–463.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sagoo P, Lombardi G and Lechler RI:
Relevance of regulatory T cell promotion of donor-specific
tolerance in solid organ transplantation. Front Immunol. 3:1842012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
The OLAW office of NIH, . Institutional
animal care and use committee guidebook. 2nd. 2002
|
|
16
|
Korbutt GS, Elliott JF, Ao Z, Smith DK,
Warnock GL and Rajotte RV: Large scale isolation, growth, and
function of porcine neonatal islet cells. J Clin Invest.
97:2119–2129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walters G and Alexander SI: T cell
receptor BV repertoires using real time PCR: A comparison of SYBR
green and a dual-labelled HuTrec fluorescent probe. J Immunol
Methods. 294:43–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi S, Hawthorne WJ, Lehnert AM, Ha H, Wong
JK, van Rooijen N, Davey K, Patel AT, Walters SN, Chandra A and
O'Connell PJ: T cell-activated macrophages are capable of both
recognition and rejection of pancreatic islet xenografts. J
Immunol. 170:2750–2758. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baecher-Allan C, Wolf E and Hafler DA: MHC
class II expression identifies functionally distinct human
regulatory T cells. J Immunol. 176:4622–4631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fountoulakis S, Vartholomatos G, Kolaitis
N, Frillingos S, Philippou G and Tsatsoulis A: HLA-DR expressing
peripheral T regulatory cells in newly diagnosed patients with
different forms of autoimmune thyroid disease. Thyroid.
18:1195–1200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gagne K, Brouard S, Giral M, Sebille F,
Moreau A, Guillet M, Bignon JD, Imbert BM, Cuturi MC and Soulillou
JP: Highly altered V beta repertoire of T cells infiltrating
long-term rejected kidney allografts. J Immunol. 164:1553–1563.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baron C, McMorrow I, Sachs DH and LeGuern
C: Persistence of dominant T cell clones in accepted solid organ
transplants. J Immunol. 167:4154–4160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walters G, Habib AM, Reynolds J, Wu H,
Knight JF and Pusey CD: Glomerular T cells are of restricted
clonality and express multiple CDR3 motifs across different Vbeta
T-cell receptor families in experimental autoimmune
glomerulonephritis. Nephron Exp Nephrol. 98:e71–e81. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adair PR, Kim YC, Zhang AH, Yoon J and
Scott DW: Human tregs made antigen specific by gene modification:
The power to treat autoimmunity and antidrug antibodies with
precision. Front Immunol. 8:11172017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma B, Yang JY, Song WJ, Ding R, Zhang ZC,
Ji HC, Zhang X, Wang JL, Yang XS, Tao KS, et al: Combining exosomes
derived from immature DCs with donor antigen-specific treg cells
induces tolerance in a rat liver allograft model. Sci Rep.
6:329712016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng J, Liu Y, Qin G, Chan PL, Mao H, Lam
KT, Lewis DB, Lau YL and Tu W: Efficient induction and expansion of
human alloantigen-specific CD8 regulatory T cells from naive
precursors by CD40-activated B cells. J Immunol. 183:3742–3750.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Veerapathran A, Pidala J, Beato F, Yu XZ
and Anasetti C: Ex vivo expansion of human Tregs specific for
alloantigens presented directly or indirectly. Blood.
118:5671–5680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheraï M, Hamel Y, Baillou C, Touil S,
Guillot-Delost M, Charlotte F, Kossir L, Simonin G, Maury S, Cohen
JL and Lemoine FM: Generation of human alloantigen-specific
regulatory T cells under good manufacturing practice-compliant
conditions for cell therapy. Cell Transplant. 24:2527–2540. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peters JH, Hilbrands LB, Koenen HJ and
Joosten I: Ex vivo generation of human alloantigen-specific
regulatory T cells from CD4(pos)CD25(high) T cells for
immunotherapy. PLoS One. 3:e22332008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boardman DA, Philippeos C, Fruhwirth GO,
Ibrahim MA, Hannen RF, Cooper D, Marelli-Berg FM, Watt FM, Lechler
RI, Maher J, et al: Expression of a chimeric antigen receptor
specific for donor HLA class I enhances the potency of human
regulatory T cells in preventing human skin transplant rejection.
Am J Transplant. 17:931–943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fozza C, Barraqueddu F, Corda G, Contini
S, Virdis P, Dore F, Bonfigli S and Longinotti M: Study of the
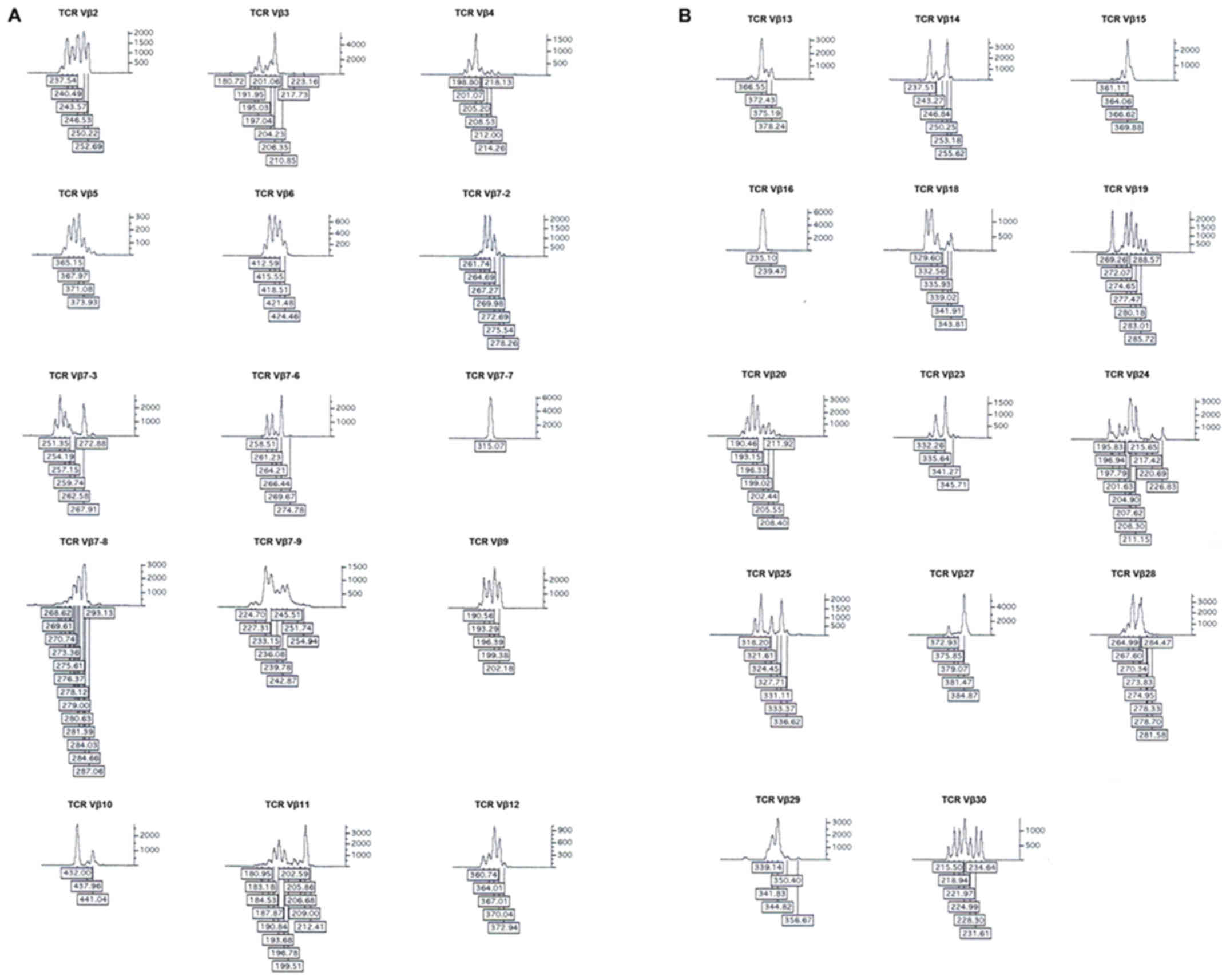
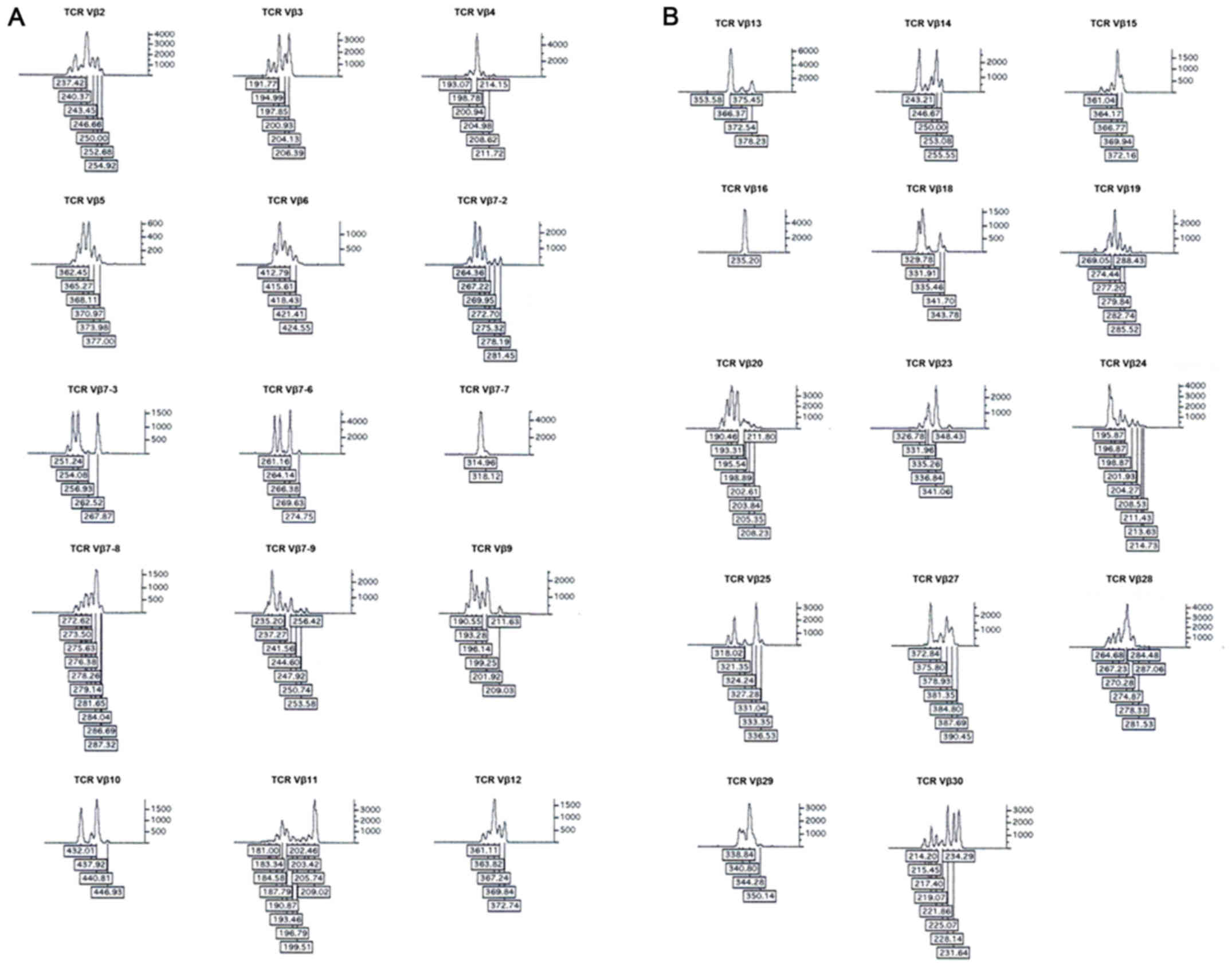
T-cell receptor repertoire by CDR3 spectratyping. J Immunol
Methods. 440:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alvarez CM, Opelz G, Giraldo MC, Pelzl S,
Renner F, Weimer R, Schmidt J, Arbeláez M, García LF and Süsal C:
Evaluation of T-cell receptor repertoires in patients with
long-term renal allograft survival. Am J Transplant. 5:746–756.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li M, Eckl J, Abicht JM, Mayr T, Reichart
B, Schendel DJ and Pohla H: Induction of porcine-specific
regulatory T cells with high specificity and expression of IL-10
and TGF-β1 using baboon-derived tolerogenic dendritic cells.
Xenotransplantation. 25:2018. View Article : Google Scholar
|
|
35
|
Li M, Eckl J, Geiger C, Schendel DJ and
Pohla H: A novel and effective method to generate human
porcine-specific regulatory T cells with high expression of IL-10,
TGF-β1 and IL-35. Sci Rep. 7:39742017. View Article : Google Scholar : PubMed/NCBI
|