Introduction
Autism spectrum disorder (ASD) is a disorder of the
central nervous system (CNS) that is characterized by impairments
in social communication skills, including difficulties with forming
and maintaining relationships (1).
According to the data of the 2007 and 2011–2012 US National Survey
of Children's Health (NSCH), the prevalence of ASD increased
between 1.16 and 2.00% (2). At
present, the diagnosis of ASD is primarily based on Childhood
Autism Rating Scale (CARS) (3) and
Autism Behavior Checklist (ABC) (4) scores. Furthermore, there is no cure
for ASD, and medical therapy is limited to managing behavioral
symptoms (5).
In addition to exposure to environmental toxins at
critical periods during brain development, nutritional influences,
genetic predisposition and epigenetic modifications contribute to
the development of ASD (6). DNA
methylation was suggested as a potential mechanism by which
environmental factors confer the risk of ASD (7). Evidence additionally suggested that
alterations to DNA methylation are common in multiple brain regions
of autistic people (8).
Excessive apoptosis impaired brain maturation and
mediated autism-like behaviors (9), and results in CNS dysfunction
(10). Transforming growth
factor β 1 (TGFB1) serves an important role in cell
proliferation, differentiation, invasion, altering the cellular
microenvironment and apoptosis (11). TGFB1 expression levels were
previously observed to be significantly decreased in the plasma of
autistic children (12). Apoptosis
regulator BAX protein mediates the translocation of cytochrome c
between the outer mitochondrial membrane and the cytosol in the
apoptotic process (13). C-C
motif chemokine ligand 2 (CCL2) is important in the protection
of human neurons and astrocytes from N-methyl-D-aspartate or human
immunodeficiency virus-tat-induced apoptosis (14). Insulin like growth factor
binding protein 3 (IGFBP3) activates pro-apoptotic factors in
various cell lines (15).
Protein kinase C β 1 (PRKCB1) was demonstrated to
serve a pivotal role in ischemia/reperfusion-induced apoptosis
(16). Presenilin 2
(PSEN2) overexpression was previously reported to be a cause
of aberrant apoptosis (17).
At present, to the best of the authors' knowledge,
there are no existing studies regarding the DNA methylation status
of the above six apoptosis-associated genes in ASD, the purpose of
the present study was to test the association between ASD and the
methylation of these genes.
Materials and methods
Subjects
A total of 68 subjects were recruited for the
present study. The ASD group included 42 children diagnosed with
ASD (35 males, 7 females; mean age, 4.07±2.78 years). All of the
children with autism and the control group were recruited from the
Children's Psychiatric Clinic of Ningbo Kangning Hospital (Ningbo,
China), and their peripheral blood samples were collected (2 ml)
between September 2015 and September 2017. They were diagnosed with
ASD by at least two psychiatric chief physicians according to the
Diagnostic and Statistical Manual-5 diagnostic criteria (DSM-5,
American Psychiatric Association, 2013) (18). The CARS and the ABC was
additionally used to confirm the accuracy of the diagnosis
(19). The tested phenotypes
comprised 21 clinical characteristics, including interaction
ability, athletic ability and emotional response. The ABC scale
score of the 24 participating children with autism that were tested
was 30.10±10.30, and the CARS scale score was 78.00±36.15. Patients
diagnosed with mental retardation, congenital/genetic disease or
severe physical illness were excluded from the present study. The
cases included in this study had a CARS score of >30 points, or
an ABC scale score of >31 points. A total of 26 children whose
physical examination results were completely normal were included
in the control group (22 males, 4 females; mean age, 5.85±0.78
years); none of the control subjects had a family or personal
medical history of neurological or psychiatric disorders. The
general information of the recruited subjects is presented in
Table I. All the participants were
Han Chinese. The present study was approved by the Bioethics
Committees of Ningbo Kangning Hospital and Ningbo University
(Ningbo, China). All the parents or guardians of the participating
children provided written informed consent.
 | Table I.General information of the
individuals in the present study. |
Table I.
General information of the
individuals in the present study.
| Variables | ASD | Control | χ2
value | P-value |
|---|
| No. | 42 | 26 | – | – |
| Sex, M/F | 35/7 | 22/4 | 0.019 | 0.889 |
| Age, years | 4.07±2.78 | 5.85±0.78 | – |
9.5×10−6 |
Quantitative methylation-specific
(qMSP) polymerase chain reaction (PCR) assay
The tested fragments of the six genes were obtained
from the UCSC genome browser (http://genome.ucsc.edu/). The details of genomic DNA
extraction and bisulfite conversion were as described in our
previous study (20). The details
of the qMSP and the validation of the qMSP products were the same
as previously described (21–24).
The primer sequences for the six apoptosis-associated genes [TGFB1,
BCL2 associated X, apoptosis regulator (BAX), IGFBP3, PRKC1,
PSEN2 and CCL2] are presented in Table II.
 | Table II.Primer sequences of the six apoptotic
genes. |
Table II.
Primer sequences of the six apoptotic
genes.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Product, bp | Tm, °C |
|---|
| TGFB1 |
TTGTAGGTGGATAGTTTC |
CTACTACCGCTACTACTA | 80 | 58 |
| BAX |
GAAGGTATTAGAGTTGCGATT |
CCAATAAACATCTCCCGATAA | 78 | 58 |
| IGFBP3 |
GGTTGTTTAGGGCGAAGTAC |
GAAACTATAAAATCCAAACAAAAAACG | 209 | 58 |
| PRKCB |
CGGCGTGTTTGATGTTATGAT |
GCAACCATCCAACCAACTC | 113 | 58 |
| PSEN2 |
GGTAGGGTCGTAGGTTTA |
ACTTCTAACTATCTCCTCACTA | 98 | 58 |
| CCL2 |
TGTATTGTTAGGGAGTCGGTTA |
TCGCTACCAACTTACCTTCA | 89 | 56 |
Capillary electrophoresis of qMSP
product
To verify that the fragment size matched the
theoretical fragment length, the qMSP product was analyzed by the
fully automatic capillary electrophoresis apparatus
(Qsep100™ Capillary Gel Electrophoresis System; BiOptic,
Inc. Taiwan, China). Equipment and reagents used in the present
study were self-contained, including a replaceable gel-cartridge
kit, 96-well auto-sampler, buffer tank, burette, centrifugal tubes
used for Alignment marker, Alignment marker (20 and 1,000 bp),
Alignment marker mineral oil, dilution buffer and separation
buffer. The gel-cartridges were stored at 4°C and the Alignment
marker was stored at −20°C. The percentage of gel matrix in the
gel-cartridge was suitable for the analysis of DNA samples of
100–500 bp. One gel-cartridge provides 200 sample injections. All
operations were conducted according to the manufacturer's
protocol.
Gene expression omnibus (GEO)
data-mining study
The mRNA expression data was extracted from the
GSE30192 (25) and GSE5230
(26) GEO datasets (www.ncbi.nlm.nih.gov/gds/).
5-Aza-2′-deoxycytidine (5-AZA) was a demethylation agent. The
expression values of genes in 5-AZA-treated C2C12 and HepG2 cells
were compared with negative controls. Notably, the C2C12 cell line
is a mouse myoblast cell line, and the HepG2 cell line was
originally identified as a hepatocellular carcinoma cell line;
however, has been demonstrated to be derived from hepatoblastoma
(27). An independent samples
t-test was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA)
for the comparison of expression values.
Statistical analysis
Data with normal distribution was presented as the
mean ± standard deviation; otherwise, it was presented as the
median with interquartile range. Each sample was subjected to three
experimental replicates and the mean value was calculated to
represent final methylation data. An independent samples t-test was
applied to compare the gene methylation data between the case and
control groups. A Mann-Whitney U test was used to compare the age
distribution between the groups. Pearson's χ2 test was
used to compare the sex distribution between the groups. As the age
distribution difference was statistically significant between cases
and controls, the binary logistic method (28) was used to correct the age
difference. The Pearson's correlation test (normal distribution)
and Spearman's rank test (abnormal distribution) were used to
determine the associations between gene methylation and ABC or CARS
scores. An independent samples t-test was applied to compare gene
expression between 5-AZA-treated cells and negative controls.
Two-tailed P<0.05 was considered to indicate statistically
significant difference. The statistical analysis was performed by
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). All figures were
produced with GraphPad Prism 6 software (GraphPad Software, Inc.,
San Diego, CA, USA).
Results
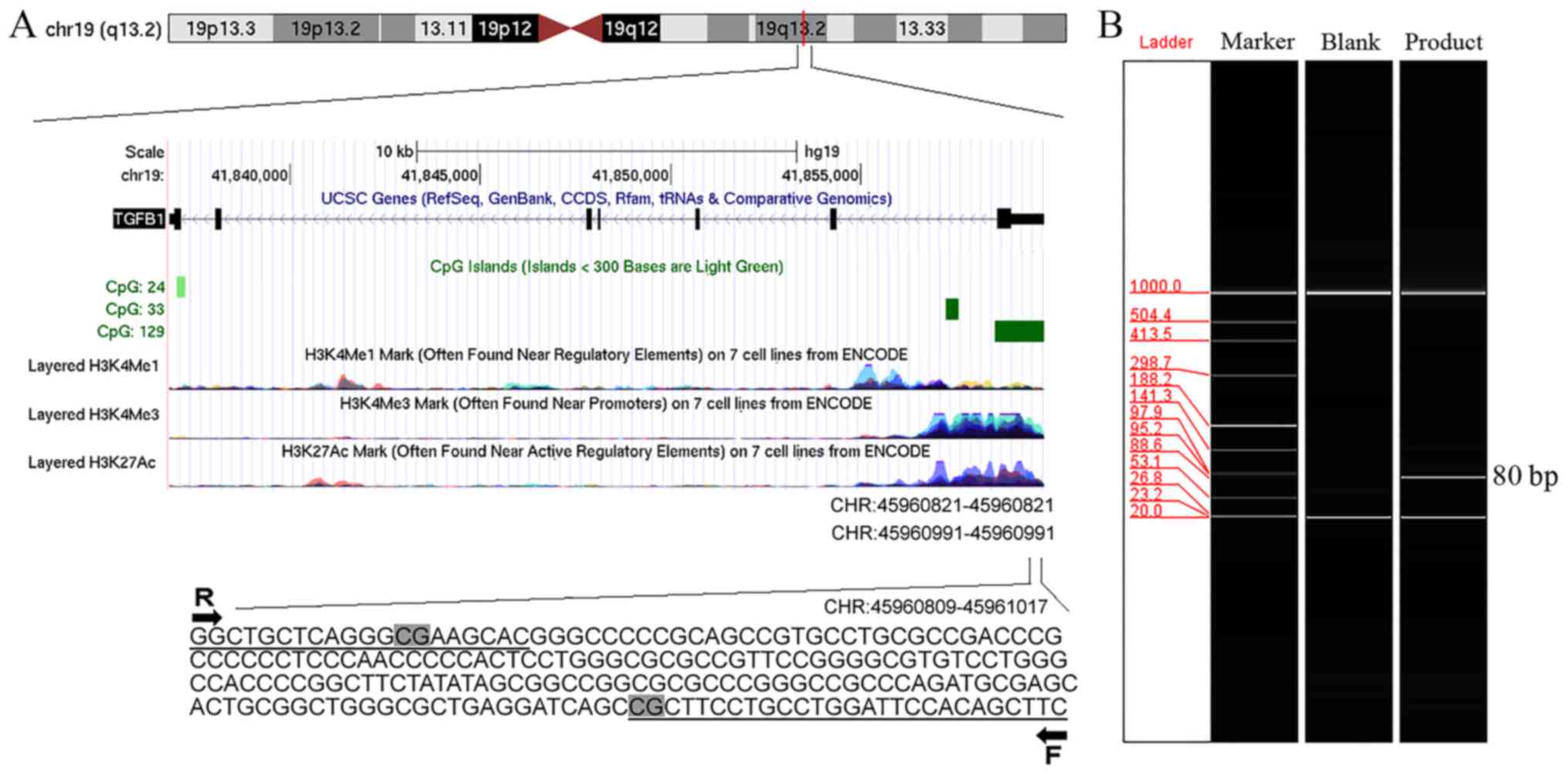
Location of the tested fragments is
identified by the qMSP assay
The tested fragments in the qMSP assay were located
in the promoter CpG islands of TGFB1 [chromosome
(chr)19:45960809-45961017; Fig.
1A], BAX (chr19:49457911-49457988; data not shown),
IGFBP3 (chr7:45960809-45961017; data not shown),
PRKC1 (chr3:169940046-169940158; data not shown),
PSEN2 (chr1:227058897-227058994; data not shown) and
CCL2 (chr17:32581869-32581947; data not shown). The PCR
products were verified by capillary electrophoresis (Fig. 1B).
Rate of TGFB1 methylation is
significantly decreased in children with ASD
The present analysis demonstrated that the sex
distribution was not different between the ASD and control groups
(χ2=0.019; P=0.889; Table
I); whereas, age distribution differed significantly between
the patients with ASD and the controls (4.07±2.78 vs. 5.85±0.78;
P<0.0001; Table I). A logistic
regression analysis was subsequently conducted to adjust for the
difference in age in the analysis of the association of the six
apoptosis-associated genes with ASD. The results suggested that the
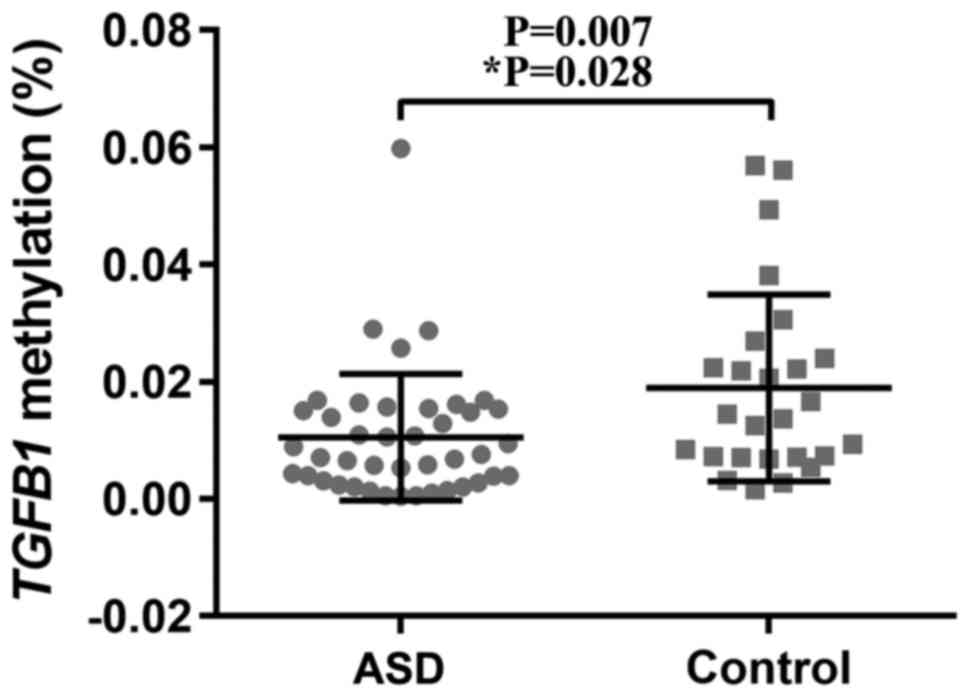
rate of TGFB1 methylation in the blood samples from the
children with ASD was significantly decreased compared with the
control blood samples (mean percentage of methylated reference,
0.011% vs. 0.019%; adjusted P=0.028; Table III and Fig. 2). There were no significant
associations of the remaining five genes with ASD subsequent to age
adjustment (Table III).
 | Table III.Comparison of apoptotic gene
methylation between patients with ASD and the controls. |
Table III.
Comparison of apoptotic gene
methylation between patients with ASD and the controls.
| Gene | PMR of ASD, % | PMR of controls,
% | P-value |
P-valuea |
|---|
| TGFB1 | 0.011±0.011 | 0.019±0.016 | 0.007 | 0.028 |
| BAX | 1.104±0.868 | 1.238 (0.033,
3.859) | 0.595 | 0.644 |
| IGFBP3 | 0.032 (0.018,
0.048) | 0.021 (0.011,
0.030) | 0.003 | 0.483 |
| CCL2 | 3.123 (2.747,
3.848) | 2.552 (2.064,
3.102) |
2×10−4 | 0.995 |
| PSEN2 | 0.716±0.396 | 0.894 (0.376,
1.592) | 0.107 | 0.095 |
| PRKCB | 1.366±0.725 | 0.519 (0.365,
0.814) |
3×10−14 | 0.281 |
TGFB1 methylation is positively
correlated with the interaction ability of the ASD group
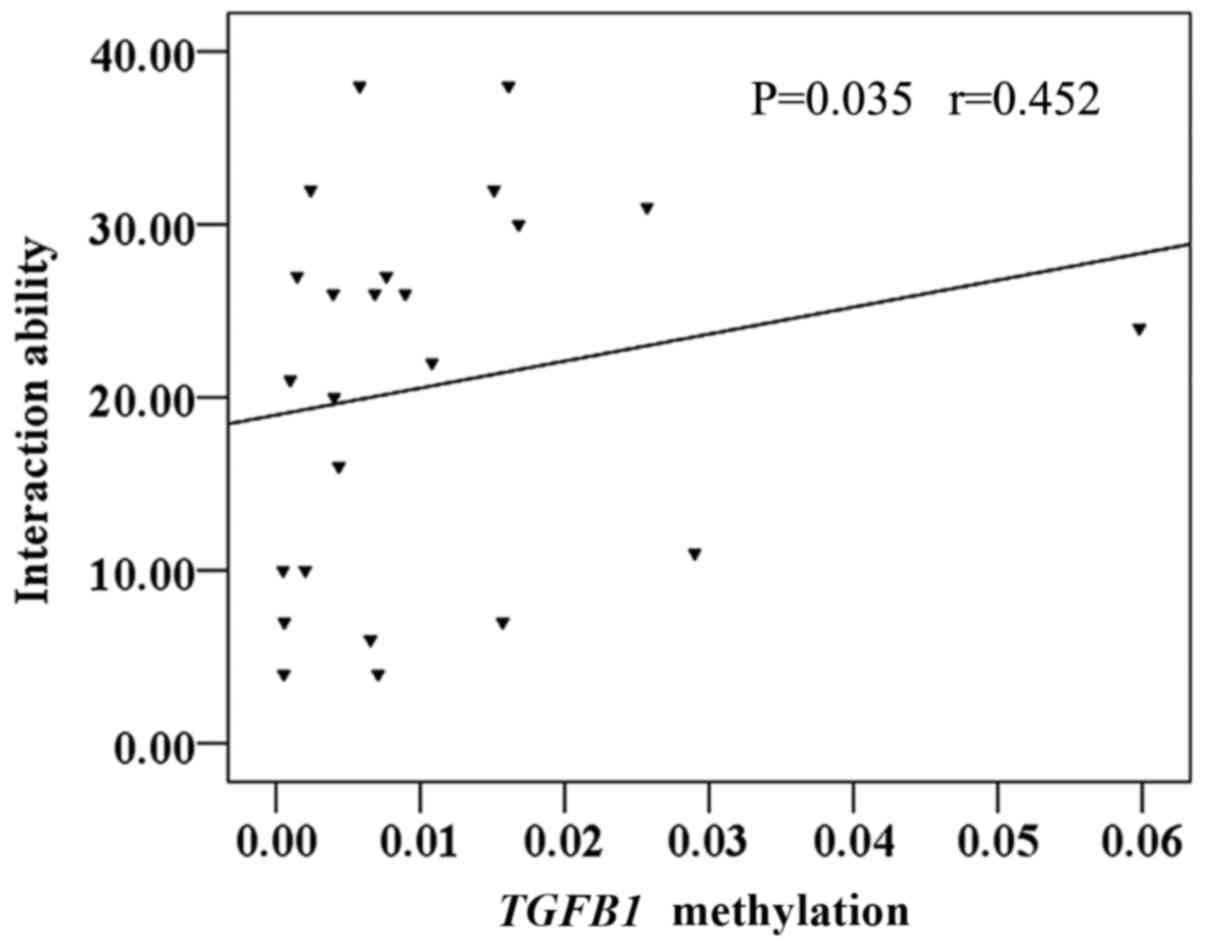
In the present study, 24 participants with autism
were included, and the CARS and ABC were used to assess ASD
clinical phenotypes. Using the Spearman's rank correlation
coefficient test, it was identified that TGFB1 methylation
was positively correlated with the interaction ability of the ASD
group (r=0.452; P=0.035; Fig. 3).
There was no correlation between TGFB1 methylation and other
clinical characteristics (P>0.05; data not shown).
TGFB1 mRNA expression levels are
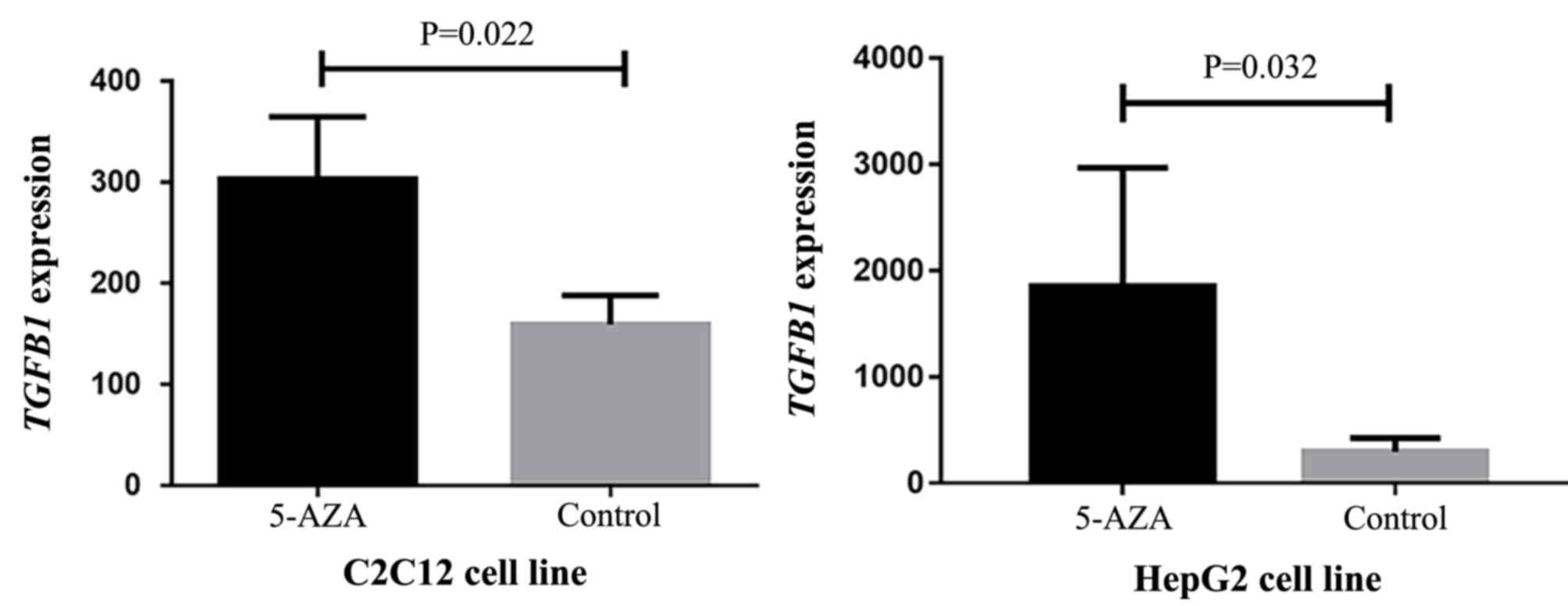
increased in 5-AZA-treated cell lines
Due to the lack of TGFB1 expression data for
the study participants, it was not possible to assess the effect of
TGFB1 methylation on TGFB1 expression. Therefore, a
GEO dataset was analyzed to identify that the TGFB1 mRNA
expression levels in 5-AZA-treated cell lines were significantly
increased compared with the negative controls (C2C12, P=0.022, fold
change >1.24; HepG2, P=0.032, fold change >1.96; Fig. 4). These results suggested that
TGFB1 expression may be upregulated by TGFB1
hypomethylation, although the cell lines were not CNS-specific.
Discussion
In the present study, it was identified that
TGFB1 hypomethylation was significantly associated with ASD.
Additionally, aberrant TGFB1 methylation was positively
correlated with the interaction ability score of the subjects.
These data suggested epigenetic dysregulation as a potential
mechanism for the development of ASD.
Previous studies have identified that alterations in
TGFB1 methylation are associated with the occurrence of CNS
disease. Impairment of the TGFB1 signaling pathway is associated
with Alzheimer's disease (AD), supporting a role for an alteration
in the DNA methylation of TGFB1 in AD pathogenesis (29). As a complex CNS condition, ASD was
previously associated with TGFB1-associated apoptotic
activity in a number of previous studies (12,30,31).
In the present study, it was reported for the first time, to the
best of the authors' knowledge, that the hypomethylation of
TGFB1 may be associated with ASD. Additionally, it was
observed that the hypomethylation of TGFB1 is associated
with a decreased interaction ability score, which is an important
element of social communication ability.
DNA methylation levels of protein-coding genes are
generally negatively correlated with expression levels (32,33).
To confirm that the TGFB1 gene methylation identified in the
present study affected TGFB1 expression, the association
between TGFB1 methylation and TGFB1 expression was
examined by GEO data-mining. The present analysis demonstrated that
TGFB1 expression was increased in cells following treatment
with the demethylation agent 5-AZA. Although the cell lines were
not CNS specific, it is sufficient to demonstrate the negative
correlation between methylation and expression of the TGFB1
gene. Therefore, it was hypothesized that TGFB1
hypomethylation causes TGFB1 overexpression, leading to subsequent
disturbances in apoptosis, inducing cerebral dysplasia and
eventually contributing to the development of ASD.
Additionally, other apoptosis-associated genes were
considered in the present study that encode proteins previously
described to be associated with apoptosis in the brain or ASD.
Psychiatric symptoms were accompanied with altered BAX expression
and increased neuronal apoptosis in the medial prefrontal cortex in
a rat model (34). Children with
autism demonstrated increased deficits in cognitive functions, in
addition to altered expression levels of immunological markers,
including CCL2 (35) and
significantly increased blood IGFBP-3 expression levels (36), compared with children without
autism. Functional variants of PRKCB1 were identified to be
associated with an increased likelihood of ASD (37). PSEN2 overexpression was observed to
induce excessive apoptosis (17).
Although the present study was unable to identify any association
between the methylation of these genes and ASD, further studies
with larger cohorts are required to clarify their roles in ASD.
There were specific limitations to the present
study. The verification of the regulatory mechanism of TGFB1
hypomethylation was based on the GEO data analysis, and the cell
lines analyzed were not CNS-specific. Validation with clinical
samples is required to confirm the association between TGFB1
gene methylation and expression. In addition, as the present
results were based on a relatively small sample size, larger
samples are required to confirm the present results. Furthermore,
as obtaining brain tissue from study subjects is not possible, the
DNA methylation levels of the six genes were only tested in
peripheral blood samples. Further studies are required to examine
whether gene methylation may be detected in brain tissue, similar
to peripheral blood.
In conclusion, the present data suggested that
TGFB1 hypomethylation may be associated with ASD. The
correlation between TGFB1 hypomethylation and interaction
ability may provide novel molecular insight for the understanding
of the development of ASD.
Acknowledgements
Not applicable.
Funding
This research was supported by grants from the
Medical Science and Technology Project of Zhejiang Province (grant
no. 2017207569) and the K.C. Wong Magna Fund of Ningbo
University.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SD, ZH and YZ contributed to the conception and
design of the study, and the final approval of the manuscript. CZ,
HanY, WZ, FC, HaiY, DZ, BL, JL, JD, JZ, MC, TH and RP performed the
data analyses and conducted the experiments. YZ and CZ created the
figures and tables, and wrote the paper. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Bioethics
Committees in Ningbo Kangning Hospital (Ningbo, China) and Ningbo
University (Ningbo, China). All the parents or guardians of the
participating children provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jones RM, Pickles A and Lord C: Evaluating
the quality of peer interactions in children and adolescents with
autism with the Penn Interactive Peer Play Scale (PIPPS). Mol
Autism. 8:282017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blumberg SJ, Bramlett MD, Kogan MD,
Schieve LA, Jones JR and Lu MC: Changes in prevalence of
parent-reported autism spectrum disorder in school-aged U.S.
children: 2007 to 2011–2012. Natl Health Stat Rep. 1–11. 2013.
|
|
3
|
Avcil S, Baykara B, Baydur H, Munir KM and
Inal Emiroglu N: The validity and reliability of the Social
Communication Questionnaire-Turkish form in autistics aged 4–18
years. Turk Psikiyatri Derg. 26:56–64. 2015.(In Turkish).
PubMed/NCBI
|
|
4
|
Krug DA, Arick J and Almond P: Behavior
checklist for identifying severely handicapped individuals with
high levels of autistic behavior. J Child Psychol Psychiatry.
21:221–229. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wink LK, Plawecki MH, Erickson CA, Stigler
KA and McDougle CJ: Emerging drugs for the treatment of symptoms
associated with autism spectrum disorders. Expert Opin Emerg Drugs.
15:481–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siniscalco D, Cirillo A, Bradstreet JJ and
Antonucci N: Epigenetic findings in autism: New perspectives for
therapy. Int J Environ Res Public Health. 10:4261–4273. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keil KP and Lein PJ: DNA methylation: A
mechanism linking environmental chemical exposures to risk of
autism spectrum disorders? Environ Epigenet. 2(pii): dvv0122016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ladd-Acosta C, Hansen KD, Briem E, Fallin
MD, Kaufmann WE and Feinberg AP: Common DNA methylation alterations
in multiple brain regions in autism. Mol Psychiatry. 19:862–871.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei H, Alberts I and Li X: The apoptotic
perspective of autism. Int J Dev Neurosci. 36:13–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JE, Shin MS, Seo TB, Ji ES, Baek SS,
Lee SJ, Park JK and Kim CJ: Treadmill exercise ameliorates motor
disturbance through inhibition of apoptosis in the cerebellum of
valproic acid-induced autistic rat pups. Mol Med Rep. 8:327–334.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong J, Liu C, Zhang QH, Chen L, Shen YY,
Chen YJ, Zeng X, Zu XY and Cao RX: TGF-β1 induces HMGA1 expression:
The role of HMGA1 in thyroid cancer proliferation and invasion. Int
J Oncol. 50:1567–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashwood P, Enstrom A, Krakowiak P,
Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN and
Van de Water J: Decreased transforming growth factor beta1 in
autism: A potential link between immune dysregulation and
impairment in clinical behavioral outcomes. J Neuroimmunol.
204:149–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simonyan L, Renault TT, Novais MJ, Sousa
MJ, Côrte-Real M, Camougrand N, Gonzalez C and Manon S: Regulation
of Bax/mitochondria interaction by AKT. FEBS Lett. 590:13–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eugenin EA, D'Aversa TG, Lopez L, Calderon
TM and Berman JW: MCP-1 (CCL2) protects human neurons and
astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem.
85:1299–1311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo LL, Zhao L, Xi M, He LR, Shen JX, Li
QQ, Liu SL, Zhang P, Xie D and Liu MZ: Association of insulin-like
growth factor-binding protein-3 with radiotherapy response and
prognosis of esophageal squamous cell carcinoma. Chin J Cancer.
34:514–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schechter B, Rosing MA, Wilchek M and
Arnon R: Blood levels and serum protein binding of cis-platinum(II)
complexed to carboxymethyl-dextran. Cancer Chemother Pharmacol.
24:161–166. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar A, Sivanandam TM and Thakur MK:
Presenilin 2 overexpression is associated with apoptosis in Neuro2a
cells. Transl Neurosci. 7:71–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
American Psychiatric Association, .
Diagnostic and Statistical Manual of Mental Disorders. 5th.
Arlington, VA: American Psychiatric Publishing; 2013
|
|
19
|
Rellini E, Tortolani D, Trillo S, Carbone
S and Montecchi F: Childhood Autism Rating Scale (CARS) and Autism
Behavior Checklist (ABC) correspondence and conflicts with DSM-IV
criteria in diagnosis of autism. J Autism Dev Disord. 34:703–708.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Chen C, Bi X, Zhou C, Huang T, Ni C,
Yang P, Chen S, Ye M and Duan S: DNA methylation of CMTM3, SSTR2
and MDFI genes in colorectal cancer. Gene. 630:1–7. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu H, Chen X, Wang C, Jiang Y, Li J, Ying
X, Yang Y, Li B, Zhou C and Zhong J: The role of TFPI2
hypermethylation in the detection of gastric and colorectal cancer.
Oncotarget. 8:84054–84065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen R, Hong Q, Jiang J, Chen X, Jiang Z,
Wang J, Liu S, Duan S and Shi S: AGTR1 promoter hypermethylation in
lung squamous cell carcinoma but not in lung adenocarcinoma. Oncol
Lett. 14:4989–4994. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Chen X, Jiang Y, Yang Y, Zhong J,
Zhou C, Hu H and Duan S: CCL2 promoter hypomethylation is
associated with gout risk in Chinese Han male population. Immunol
Lett. 190:15–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Chen X, Hu H, Jiang Y, Yu H, Dai
J, Mao Y and Duan S: Elevated UMOD methylation level in peripheral
blood is associated with gout risk. Sci Rep. 7:111962017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hupkes M, Jonsson MK, Scheenen WJ, van
Rotterdam W, Sotoca AM, van Someren EP, van der Heyden MA, van Veen
TA, van Ravestein-van Os RI, Bauerschmidt S, et al: Epigenetics:
DNA demethylation promotes skeletal myotube maturation. FASEB J.
25:3861–3872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dannenberg LO and Edenberg HJ: Epigenetics
of gene expression in human hepatoma cells: Expression profiling
the response to inhibition of DNA methylation and histone
deacetylation. BMC Genomics. 7:1812006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lopez-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong J, Chen X, Ye H, Wu N, Chen X and
Duan S: CDKN2A and CDKN2B methylation in coronary heart disease
cases and controls. Exp Ther Med. 14:6093–6098. 2017.PubMed/NCBI
|
|
29
|
Cong L, Jia J, Qin W, Ren Y and Sun Y:
Genome-wide analysis of DNA methylation in an APP/PS1 mouse model
of Alzheimer's disease. Acta Neurol Belg. 114:195–206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okada K, Hashimoto K, Iwata Y, Nakamura K,
Tsujii M, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, et
al: Decreased serum levels of transforming growth factor-beta1 in
patients with autism. Prog Neuropsychopharmacol Biol Psychiatry.
31:187–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Depino AM, Lucchina L and Pitossi F: Early
and adult hippocampal TGF-β1 overexpression have opposite effects
on behavior. Brain Behav Immun. 25:1582–1591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dunn BK: Hypomethylation: One side of a
larger picture. Ann N Y Acad Sci. 983:28–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moore LD, Le T and Fan G: DNA methylation
and its basic function. Neuropsychopharmacology. 38:23–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Han F and Shi Y: Increased neuronal
apoptosis in medial prefrontal cortex is accompanied with changes
of Bcl-2 and Bax in a rat model of post-traumatic stress disorder.
J Mol Neurosci. 51:127–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han YM, Cheung WK, Wong CK, Sze SL, Cheng
TW, Yeung MK and Chan AS: Distinct Cytokine and Chemokine Profiles
in Autism Spectrum Disorders. Front Immunol. 8:112017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mills JL, Hediger ML, Molloy CA, Chrousos
GP, Manning-Courtney P, Yu KF, Brasington M and England LJ:
Elevated levels of growth-related hormones in autism and autism
spectrum disorder. Clin Endocrinol. 67:230–237. 2007. View Article : Google Scholar
|
|
37
|
Lintas C, Sacco R, Garbett K, Mirnics K,
Militerni R, Bravaccio C, Curatolo P, Manzi B, Schneider C, Melmed
R, et al: Involvement of the PRKCB1 gene in autistic disorder:
Significant genetic association and reduced neocortical gene
expression. Mol Psychiatry. 14:705–718. 2009. View Article : Google Scholar : PubMed/NCBI
|