Introduction
Mammalian cells express a number of peptide
antibiotics that function as effector components in innate host
defense systems (1–3). Cathelicidin is a family of
antimicrobial peptides, characterized by the highly conserved
cathelin-like prosequences and variable C-terminal sequences that
correspond to the mature antibacterial peptides (4). LL-37 is the sole antibacterial
peptide of human cathelicidin comprising of 37 amino acids, which
is expressed mainly in epithelial cells and neutrophils, and
cleaved from the 18-kDa human cationic antibacterial polypeptide
(5). LL-37 has an α-helical
amphiphilic structure, and can disrupt the outer and inner
membranes of bacteria. In addition its broad killing activity
against bacteria, fungi, and certain viruses (6), LL-37 has diverse immunomodulatory
effects, including the regulation of pro- and anti-inflammatory
mediator production (7,8), wound healing (9), angiogenesis (10,11),
and expression of nerve elongation factors (12). Additionally, it was reported that
LL-37 induces chemotaxis and histamine release by mast cells
(13).
Mast cells are usually present in submucosal tissues
and connective tissues, and play a pivotal role in innate immunity
by releasing several mediators such as histamine, leukotrienes, and
tryptase (14,15). We previously found that LL-37
activates mast cells to induce chemotaxis, degranulation, and the
production of cytokines and inflammatory mediators (13,16,17).
As mast cells and LL-37-expressing epidermal cells are located
close to each other, we hypothesized that LL-37 activates mast
cells locally at the sites of infection/inflammation, and controls
the immune response. Recently, a G protein-coupled receptor,
Mas-related gene X2 (MrgX2), was identified as a putative receptor
for LL-37 for mast cell degranulation (18). This suggests that LL-37 interacts
with MrgX2 and activates the G protein signaling cascade. However,
little is known about how LL-37 activates MrgX2, thereby leading to
mast cell degranulation. In contrast, some pruritogenic basic
peptides, such as substance P, have been reported to induce mast
cell degranulation by translocating (internalizing) into the cells
(19). LL-37 has affinity for the
cell membrane based on its α-helical and amphipathic structure
(20). Thus, we speculate that
LL-37 also internalizes into the cells and activates MrgX2, thereby
inducing the degranulation of mast cells. Therefore, in this study,
we investigated the relationship between the internalization of
LL-37 and MrgX2-mediated mast cell degranulation using the LAD2
human mast cell line.
Materials and methods
Reagents and antibodies
Chlorpromazine hydrochloride and genistein were
purchased from Nacalai Tesque (Kyoto, Japan). Dynasore and
neuraminidase were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Pertussis toxin was purchased from Fujifilm
Wako Pure Chemical (Osaka, Japan). A 37-mer peptide of hCAP18
(LL-37;
L1LGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES37) was
synthesized by the solid-phase method on a peptide synthesizer
(model PSSM-8; Shimadzu Scientific Instruments, Kyoto, Japan) by
fluorenylmethoxycarbonyl chemistry, as described previously
(21). The concentration of the
LL-37 stock solution was measured using the bicinchoninic acid
method with bovine serum albumin (BSA) as a standard (Pierce BCA
Protein Assay kit; Pierce; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Anti-LL-37 serum was raised in rabbits using LL-37
covalently coupled to keyhole limpet hemocyanin, as described
previously (5). Rabbit anti-human
MrgX2 polyclonal antibodies (pAbs) were purchased from Abcam
(ab129548, Cambridge, MA, USA) and MyBioSource (MBS7006480; San
Diego, CA, USA). Mouse anti-LL-37 monoclonal antibody (mAb) was
purchased from Santa Cruz Biotechnology, Inc. (sc-166770; Santa
Cruz, CA, USA). Phycoerythrin-conjugated mouse anti-human MrgX2 mAb
and its isotype control were purchased from BioLegend (359004 and
400314; San Diego, CA, USA).
Cell culture
The human mast cell line LAD2 was a kind gift from
Dr Dean D. Metcalfe (National Institutes of Health, Bethesda, MD,
USA), and was maintained in StemPro-34 serum-free media
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with
penicillin (50 IU/ml), streptomycin (50 µg/ml), L-glutamine (2 mM),
and recombinant human stem cell factor (100 ng/ml). Another human
mast cell line, HMC-1, was obtained from Merck Millipore
(Darmstadt, Germany), and maintained in Iscove's modified
Dulbecco's medium (IMDM) supplemented with 10% fetal bovine serum
(FBS), penicillin (100 IU/ml), streptomycin (100 µg/ml), and
α-thioglycerol (1.2 mM). The human embryonic kidney cell line 293
was supplied by American Type Culture Collection (Manassas, VA,
USA), and maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% FBS, penicillin (100 IU/ml), and streptomycin
(100 µg/ml).
β-Hexosaminidase release
LAD2 mast cells (1×105 cells) were
suspended in 200 µl of Tyrode's buffer (130 mM NaCl, 5 mM KCl, 3.2
mM KH2PO4, 1.4 mM
CaCl2.2H2O, 1 mM
MgCl2.6H2O, 10 mM HEPES, 5.6 mM D-glucose,
and 0.1% BSA), and then stimulated with differing concentrations of
LL-37 (1–10 µM) for 40 min at 37°C. The activity of
β-hexosaminidase in the supernatants and total cell lysates, which
were solubilized with 1% Triton X-100, was quantified by hydrolysis
of 4 mM p-nitrophenyl-N-acetyl-β-D-glucopyranoside in 0.1 M sodium
citrate buffer (pH 4.5) for 30 min at 37°C, and the reaction was
stopped by the addition 0.2 M glycine buffer (pH 11), as previously
reported (22). The absorbance was
measured with a microplate reader at a wavelength of 405 nm. The
percentage of β-hexosaminidase release was calculated using the
formula: % release=(OD of stimulated supernatant-OD of unstimulated
supernatant) ×100/(OD of total cell lysate-OD of unstimulated
supernatant). In some experiments, mast cells were pre-treated with
pertussis toxin (250 ng/ml) for 60 min, endocytosis inhibitors for
30 min, or neuraminidase for 40 min; thereafter, the cells were
washed and stimulated with LL-37.
Determination of LL-37
internalization
LAD2 mast cells (1×105 cells) were
suspended in 200 µl of Tyrode's buffer and then incubated with
LL-37 (5 µM) for 40 min at 37°C, unless otherwise stated. Cells
were washed twice with ice-cold phosphate-buffered saline (PBS)
containing 1% BSA and resuspended in PBS. Cells were
cytocentrifuged with Cytospin 4 (Shandon, Runcorn, UK) at 300 × g
for 2 min, fixed with 4% paraformaldehyde for 10 min, permeabilized
with 0.2% saponin, and blocked by Blocking One (Nacalai Tesque,
Kyoto, Japan). Cells were then incubated with rabbit anti-LL-37
serum or mouse anti-LL-37 mAb overnight at 1:1,000, followed by
incubation with the respective secondary antibodies conjugated with
Alexa Fluor 488 or 594 overnight at 1:1,000 at 4°C. Alternatively,
for double staining of LL-37 and MrgX2, cells were further
incubated with anti-MrgX2 rabbit polyclonal antibody (MyBioSource)
overnight at 1:200, followed by incubation with the secondary
antibodies conjugated with Alexa Fluor 488. After washing, the
cells were mounted in Vectorshield mounting media containing
4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories,
Burlingame, CA, USA). Images were obtained by using the
fluorescence microscope BZ-X700 (Keyence Japan, Osaka, Japan). The
percentage of LL-37-internilized cells was calculated using the
following formula: The LL-37-containing cell number ×100/the total
cell number. Alternatively, the internalization of LL-37 was
determined by measuring the fluorescence intensities using ImageJ
software (NIH Image, National Institutes of Health, Bethesda, MD,
USA). The fluorescence intensity values were calculated as the
average of two distinct regions on the same slide glass. Data
represent the ratio of controls cells incubated with LL-37, but not
treated with pertussis toxin, MrgX2 knockdown, MrgX2 stable
expression, neuraminidase, or endocytosis inhibitors.
siRNA-mediated knockdown of MrgX2
Short-interfering RNA products (MISSION esiRNA) for
MrgX2 (EHU145411) and universal negative control siRNA (SIC-001)
were purchased from Sigma-Aldrich (Merck KGaA). The complex of
siRNAs and Lipofectamine 3000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) were formed in Opti-MEM
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. After incubating for 5 min at room
temperature, the complexes were added to LAD2 cells suspended in
StemPro-34 media containing antibiotics. The knockdown efficiency
of MrgX2 was confirmed after 72 h by Western blotting. For western
blotting, LAD2 cells (2×105) were washed twice with
Tyrode's buffer, and lysed in 50 µl RIPA buffer (1% Triton X-100,
1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 150 mM NaCl,
and 10 mM Tris-HCl, pH 7.2) containing protease inhibitor cocktail
(Nacalai Tesque, Kyoto, Japan). Lysed samples were subjected to 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to polyvinylidene fluoride membranes
(Immobilon-P; Merck Millipore). The membranes were blocked with
Blocking One (Nacalai Tesque), and sequentially probed with a 1:250
dilution of rabbit anti-MrgX2 polyclonal antibody (ab129548; Abcam)
and a 1:5,000 dilution of horseradish peroxidase-conjugated goat
anti-rabbit IgG (Merck Millipore). The expression of GAPDH was
evaluated as an internal control. The membranes were reprobed with
a 1:5,000 dilution of anti-GAPDH mAb (MAB374, Merck Millipore) and
a 1:5,000 dilution of horseradish peroxidase-conjugated goat
anti-mouse IgG (115-055-044; Jackson ImmunoResearch, West Grove,
PA, USA). Signals were detected with Super Signal West Dura
Chemiluminescent Substrate (Pierce; Thermo Fisher Scientific,
Inc.), and the detected bands were analyzed with Image Studio
software Ver 4.0 and C-DiGit blot scanner (LI-COR, Lincoln, NB,
USA). Using Mrgx2-knockdown cells, LL-37-induced β-hexosaminidase
release and LL-37 internalization were evaluated as described
above.
Preparation of MrgX2-expressing stable
transfectants
Total RNA was extracted from LAD2 mast cells with
RNeasy (Qiagen, Hilden, Germany) and reverse transcribed into cDNA
using Oligo(dT) primer (KOD-Plus-; Toyobo, Osaka, Japan). MrgX2
cDNA was amplified with the following primers: Forward primer
5′-TATAAGCTTACCATGGATCCAACCACCCCGGC-3′ and reverse primer
5′-GCCGAATTCCTACACCAGACTGCTTCTCGACATC-3′. The forward and reverse
primers contained the sequences for HindIII and EcoRI
digestion sites, respectively. The amplified product was digested
and ligated into the pcDNA3 mammalian expression vector
(Sigma-Aldrich; Merck KGaA) with Ligation high ver.2 (Toyobo) for
subcloning of pcDNA3-MrgX2. 293 and HMC-1 cells were transfected
with pcDNA3-MrgX2 using Lipofectamine 3000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) based on the
manufacturer's protocol, and Mrgx2-expressing 293 and HMC-1 cells
were selected in the presence of 0.4 and 1 mg/ml G418,
respectively. MrgX2 expression was confirmed by flow cytometry as
follows: The selected cells (5×105) were stained with
1:200 diluted phycoerythrin-labelled anti-MrgX2 mAb or isotype
control IgG, and were measured by using BD FACS Calibur (BD
Biosciences, Franklin Lakes, NJ, USA). Internalization of LL-37 was
evaluated as described above.
Statistical analysis
Data are shown as the mean ± standard error of the
mean. Significance was determined by one-way analysis of variance
with Tukey's post hoc test using GraphPad Prism 7.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
LL-37-induced degranulation and LL-37
internalization by the human mast cell line LAD2
We first examined whether LL-37-stimulation induces
the degranulation of LAD2. As shown in Fig. 1A, LL-37 induced degranulation, as
determined by β-hexosaminidase release, in time- and
concentration-dependent manners. β-Hexosaminidase release was
observed within 5–10 min after LL-37 stimulation and reached a
plateau at 20 min; 13.4% after 40-min stimulation with 5 µM of
LL-37. Next, to investigate the relationship between the LL-37
internalization and mast cell degranulation, LAD2 cells were
incubated with or without LL-37 (5 µM) for 40 min, and internalized
LL-37 was detected by anti-LL-37 rabbit pAbs and Alexa Fluor
488-conjugated secondary antibody. As shown in Fig. 1B, the intracellular fluorescence
signals of LL-37 were visualized as dot patterns (middle panel and
right panel, an enlarged photo of the boxed area); however, the
control cells without LL-37 incubation had no signal (left panel).
Of note, the percentage of LL-37-internilized cells reached a
plateau within 2–5 min, and prolonged incubation up to 30 min did
not increase the internalization of LL-37 (Fig. 1C), indicating that LL-37 is quickly
internalized into mast cells prior to degranulation.
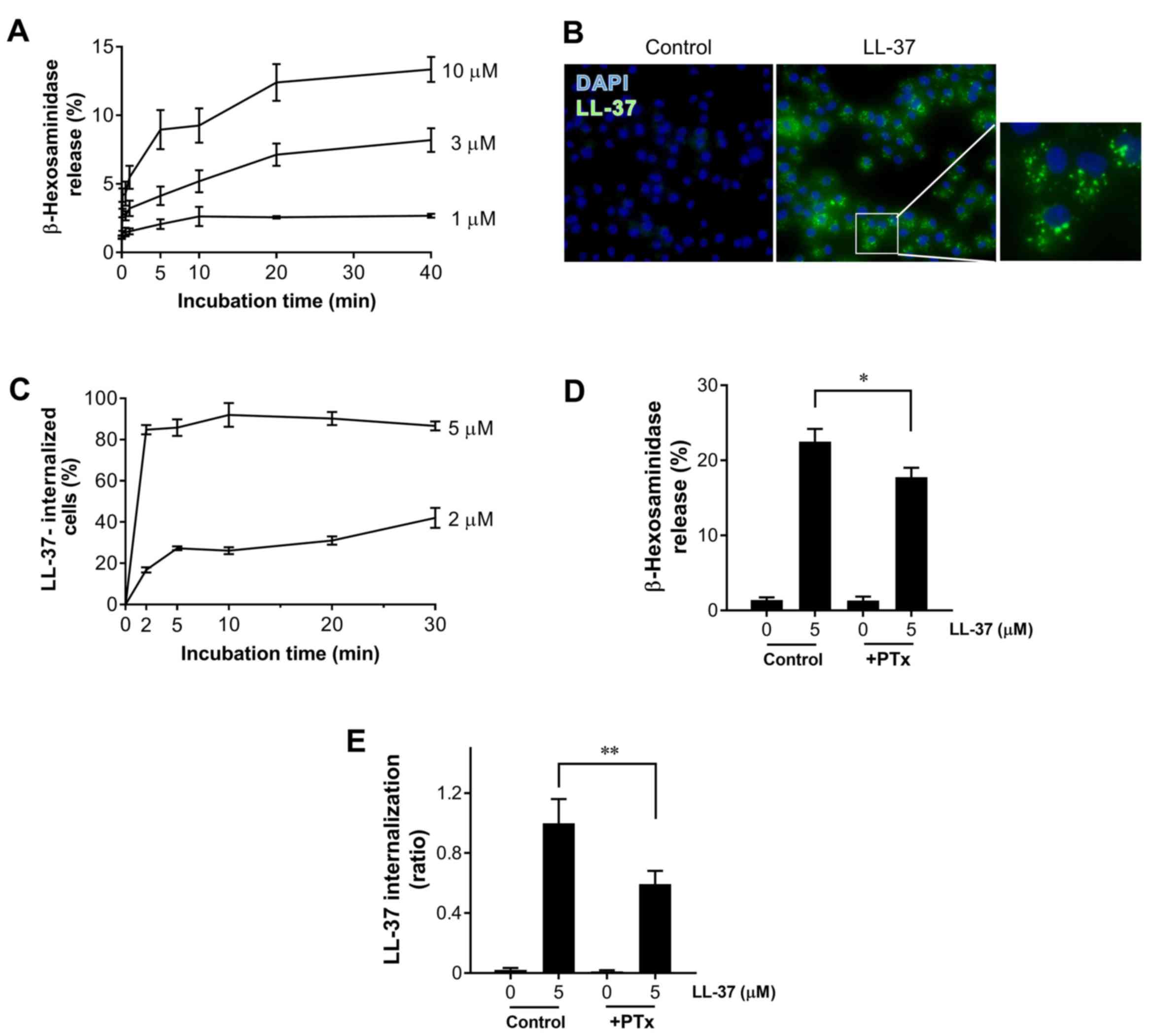 | Figure 1.LL-37 induces degranulation and
internalization into LAD2 cells. LAD2 cells were incubated with
LL-37 (1–10 µM) at 37°C for the indicated time periods, and (A) the
release of β-hexosaminidase was measured. (B) LAD2 cells were
cytocentrifuged following incubation without (Control, left panel)
or with 5 µM LL-37 (LL-37, middle panel) at 37°C for 40 min, and
LL-37 was stained with anti-LL-37 rabbit polyclonal antibodies
followed by anti-rabbit Immunoglobulin-G conjugated with Alexa
Fluor 488 (green staining). Magnification, ×400. The right-hand
panel presents the enlarged photo of the boxed area in the middle
panel. LAD2 cells were incubated with LL-37 (2–5 µM) at 37°C for
the indicated time periods, and (C) the percentage of
LL-37-internilized cells was calculated using the following
formula: (LL-37-containing cell number ×100)/the total cell number.
LAD2 cells were untreated (Control) or treated with PTx (250 ng/ml,
+PTx) at 37°C for 1 h, and further incubated with or without LL-37
(5 µM) for 40 min. The release of (D) β-hexosaminidase and (E)
LL-37 internalization was evaluated. The internalization of LL-37
was determined by measuring the fluorescence intensities using
ImageJ software, and represented as a ratio of controls cells
incubated with LL-37 (5 µM) but not treated with pertussis toxin.
Data are presented as the mean ± standard error of the mean of 4 to
8 independent experiments. *P<0.05 and **P<0.01, as
indicated. PTx, pertussis toxin. |
It has been reported that the mast cell
degranulation response by LL-37 is susceptible to pertussis toxin
(PTx) and involves G protein-coupled receptor activation (13). However, it is unclear whether the
internalization of LL-37 is also susceptible to pertussis toxin.
Thus, LAD2 cells were stimulated with LL-37 (5 µM) in the presence
or absence of pertussis toxin, and degranulation and
LL-37-internalization were evaluated. As previously reported
(13), degranulation was
significantly suppressed by pertussis toxin (Fig. 1D). Moreover, LL-37-internalization
was also significantly suppressed by pertussis toxin (Fig. 1E).
These observations suggest that not only the
LL-37-induced degranulation but also the internalization of LL-37
are elicited via the G protein-coupled pathway.
Effects of MrgX2 knockdown on the
degranulation and LL-37-internalization of LAD2
It was previously reported that a G protein-coupled
receptor, MrgX2, functions in the LL-37-induced mast cell
degranulation (18). Thus, we next
investigated whether MrgX2 is also involved in the internalization
of LL-37 into LAD2 cells using siRNA-mediated MrgX2-knockdown
cells. The expression of MrgX2 in MrgX2-knockdown cells was reduced
to approximately 30% of that in control siRNA-transfected cells
(Fig. 2A). Based on the reduced
MrgX2 expression, the β-hexosaminidase release was slightly reduced
in MrgX2-knockdown cells by 10 and 20% at 1 and 2 µM LL-37,
respectively, compared with that in control siRNA-transfected cells
(Fig. 2B). Similarly, the LL-37
internalization was markedly suppressed by MrgX2-knockdown
(Fig. 2C), and reduced in
MrgX2-knockdown cells by 30 and 20% at 1 and 2 µM LL-37,
respectively, compared with that in control siRNA-transfected cells
(Fig. 2D). These observations
suggest that MrgX2 is likely involved in both the degranulation and
internalization of LL-37 by LAD2 cells.
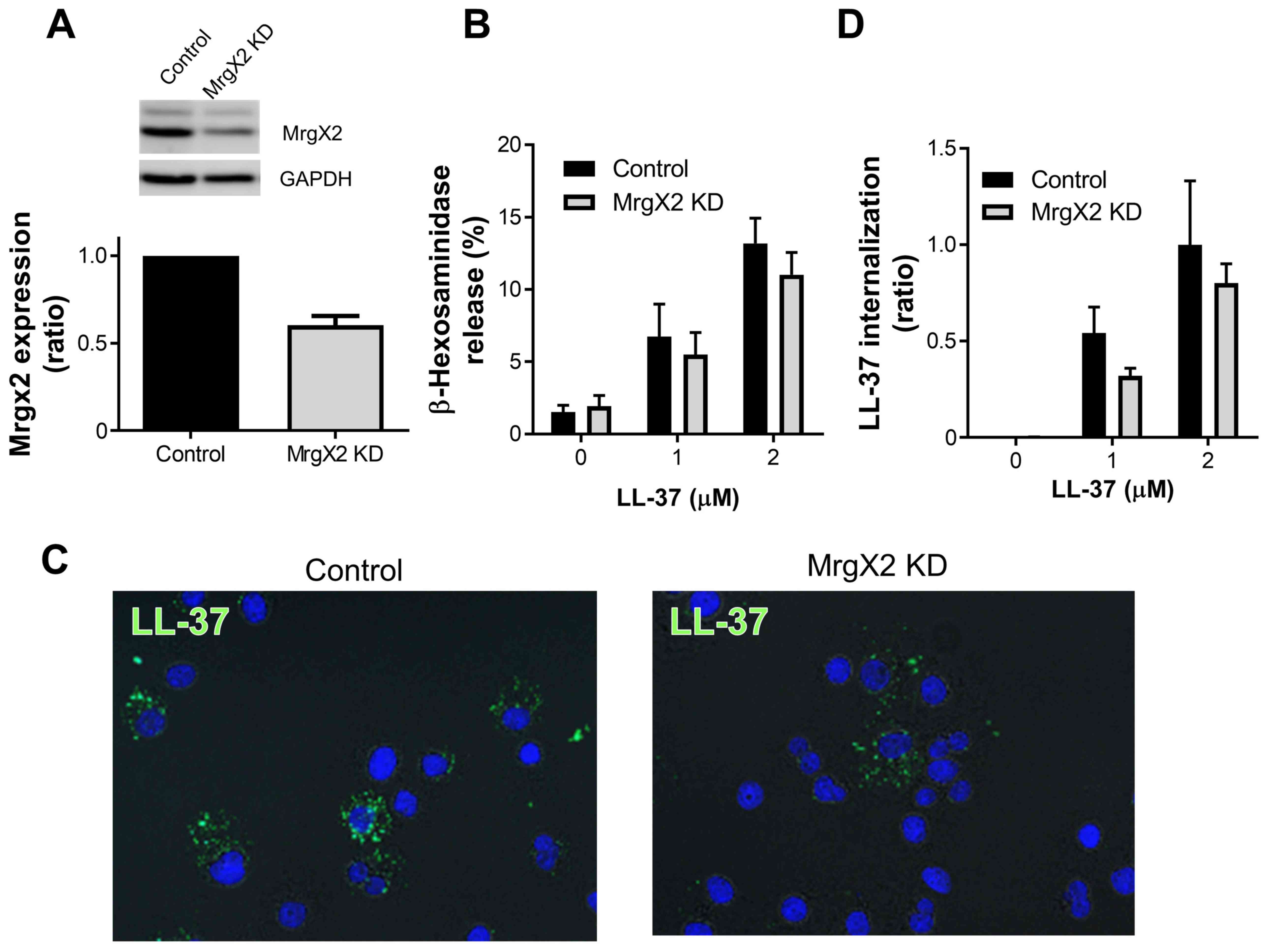 | Figure 2.MrgX2 knockdown reduces degranulation
and LL-37 internalization. Mrgx2 was knocked-down by MrgX2-targeted
siRNA transfection, and confirmed by western blotting. The
expression of GAPDH was evaluated as the internal control. Images
are representative of 3 independent experiments. (A) MrgX2
expression was quantified by densitometry, and the expression in
MrgX2-knockdown LAD2 cells (MrgX2 KD group) was expressed as a
ratio of that in the control siRNA-transfected cells (Control
group). (B) The control siRNA-transfected LAD2 cells (Control
group) and MrgX2-knockdown cells (MrgX2 KD group) were incubated
with or without LL-37 (1–2 µM) at 37°C for 40 min, and the release
of β-hexosaminidase was measured. (C) The control siRNA-transfected
LAD2 cells (Control group, left panel) and MrgX2-knockdown cells
(MrgX2 KD group, right panel) were cytocentrifuged following
incubation with LL-37 (5 µM) at 37°C for 40 min, and LL-37 was
stained with anti-LL-37 rabbit polyclonal antibodies followed by
anti-rabbit Immunoglobulin-G-Alexa Fluor 488 (green staining).
Nuclei were stained with DAPI (blue staining). Images are
representative of 3 independent experiments. Magnification, ×400.
(D) The control siRNA-transfected LAD2 cells (Control group) and
MrgX2-knockdown cells (MrgX2 KD group) were incubated with or
without LL-37 (1–2 µM) at 37°C for 40 min. The internalization of
LL-37 was determined by measuring the fluorescence intensities
using ImageJ software, and represented as a ratio of the control
siRNA-transfected LAD2 cells (Control group) incubated with LL-37
(2 µM). Data are presented as the mean ± standard error of the mean
of 3 independent experiments. MrgX2, Mas-related gene X2; siRNA,
small interfering RNA; KD, knockdown. |
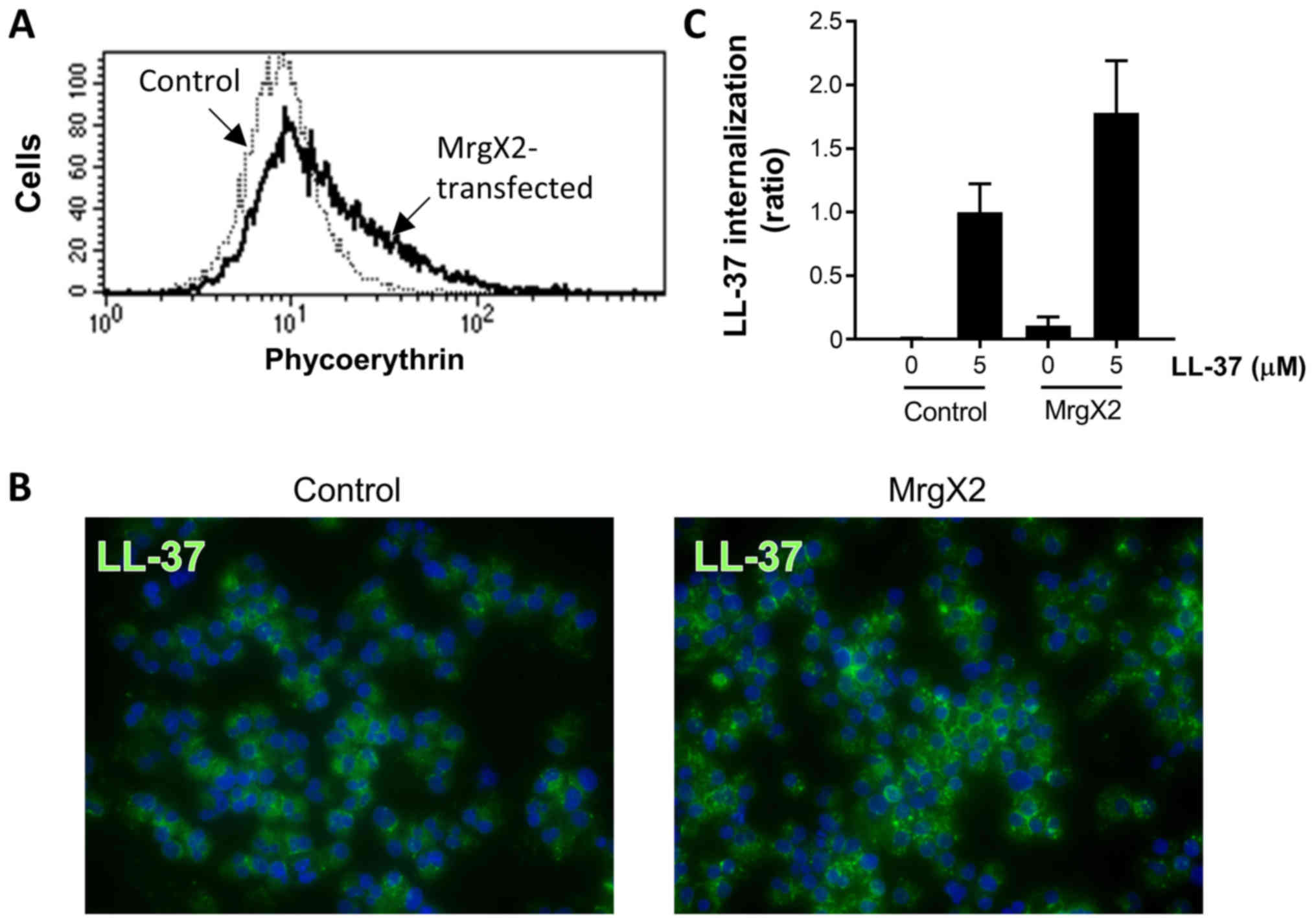
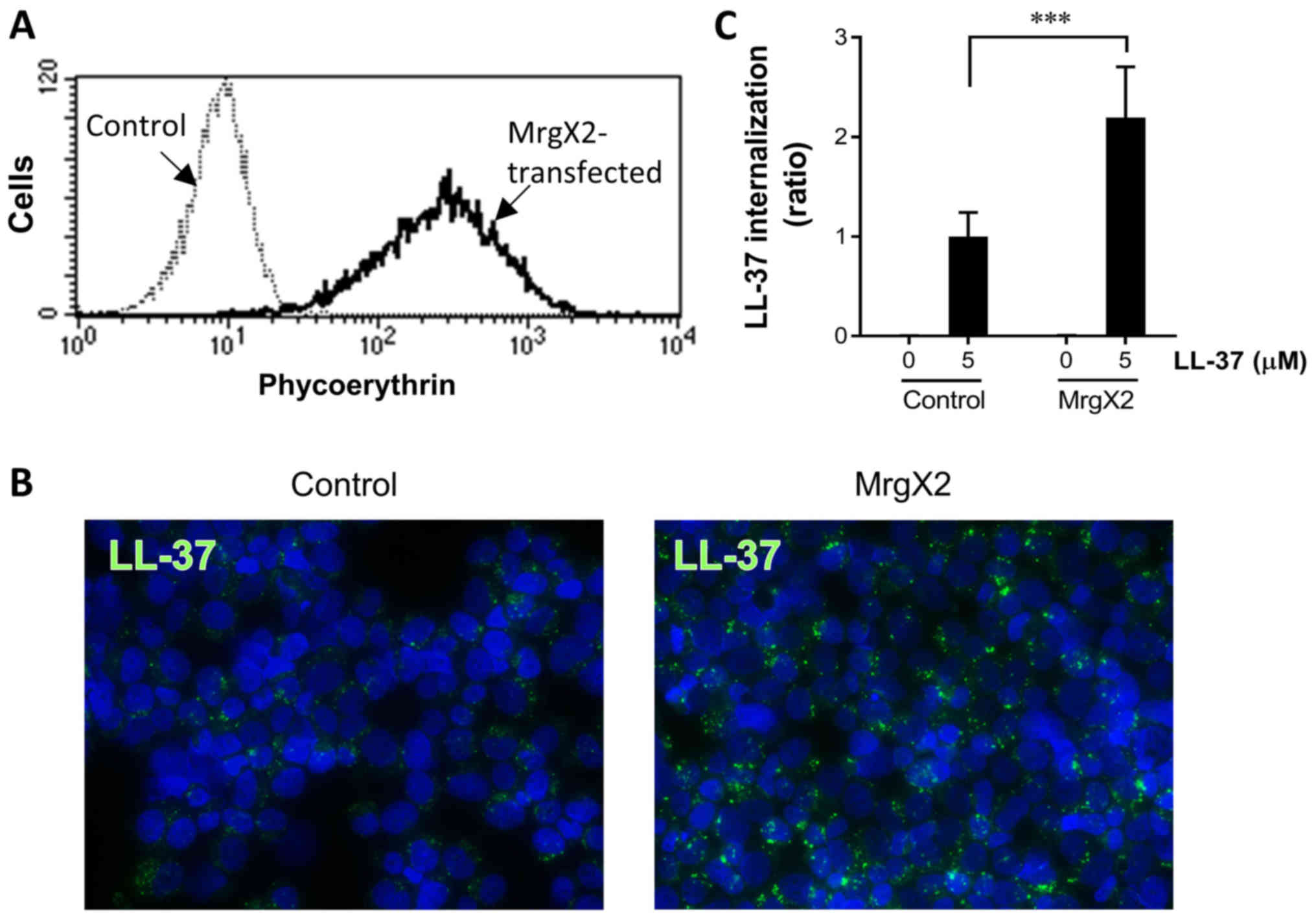
Effects of stable MrgX2 expression on
the LL-37-internalization by HMC-1 and 293 cells
To further clarify the involvement of MrgX2
expression in LL-37 internalization, we established MrgX2-stable
transfectants of the human mast cell line HMC-1 and embryonic
kidney 293 cells, both of which do not endogenously express MrgX2.
As shown in Figs. 3A and 4A, the cells transfected with the
MrgX2-expression plasmid exhibited the augmented fluorescence
intensity of MrgX2 as compared with the cells transfected with the
control plasmid on flow cytometry using phycoerythrin-labeled
anti-MrgX2 antibody. Furthermore, when stimulated with 5 µM LL-37,
both MrgX2-expressing HMC-1 and 293 cells demonstrated enhanced
LL-37 internalization compared with control plasmid-transfected
HMC-1 and 293 cells (Figs. 3B and
C, and 4B and C). It should be
noted that LL-37 internalization was moderately observed in control
plasmid-transfected HMC-1 and 293 cells, suggesting that LL-37 is
internalized into the cells independent of MrgX2 (Figs. 3B and C, and 4B and C). These observations clarified
that MrgX2 plays a role in the LL-37 internalization, although
LL-37 is also internalized into the cells in an MrgX2-independent
manner. In addition, in separate experiments, we confirmed that
LL-37 cannot induce β-hexosaminidase release by MrgX2-expressing
HMC-1 and 293 cells nor by control plasmid-transfected HMC-1 and
293 cells, although these cells possess comparable β-hexosaminidase
activity with LAD2 cells (data not shown). These observations
suggest that signaling molecules or machineries essential for
LL-37-induced degranulation are missing in these cells regardless
of the expression of MrgX2.
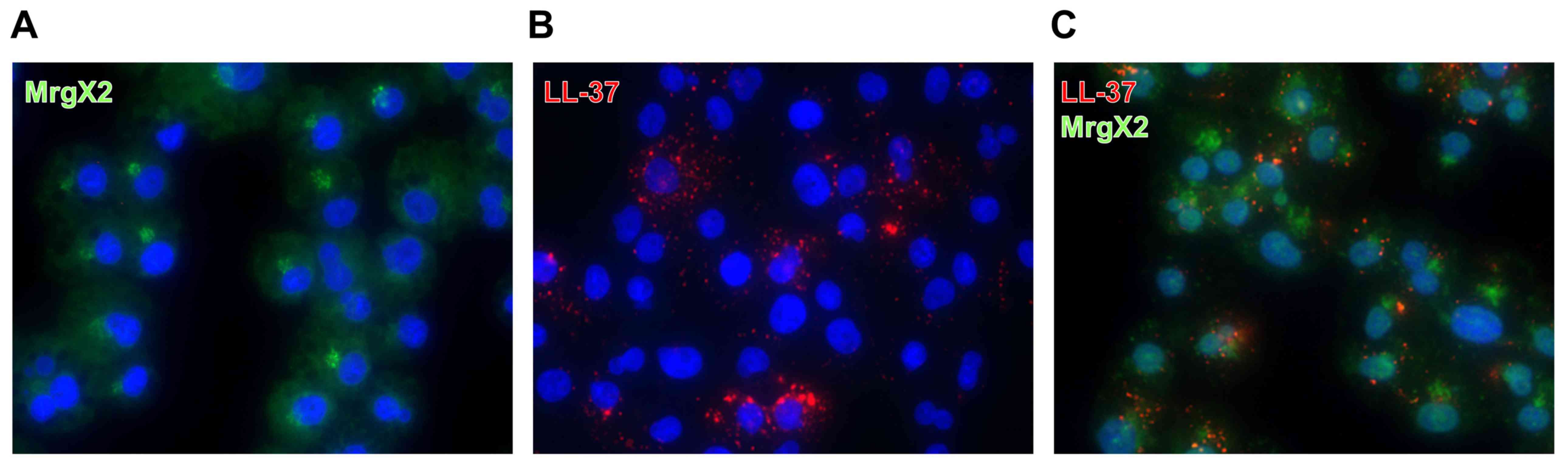
Colocalization of internalized LL-37
and MrgX2
A pruritogenic basic peptide, substance P, was
previously found to be rapidly internalized after interaction and
form a ligand-receptor complex with its receptor, NK1R
(23). Thus, to clarify the
interaction of LL-37 with MrgX2, we next examined the localization
of LL-37 and MrgX2 in the cells by immunofluorescence double
staining of LL-37 and MrgX2. As shown in Fig. 5A, MrgX2 was diffusely detected in
the cytoplasmic region or localized in the perinuclear region of
the control cells without LL-37 stimulation. When cells were
stimulated with LL-37, internalized LL-37 was detected as dot
patterns in the perinuclear region (Fig. 5B). Notably, internalized LL-37
mainly colocalized with MrgX2, although not all MrgX2 colocalized
with the internalized LL-37 (Fig.
5C).
Effects of neuraminidase-treatment and
endocytosis inhibitors on the degranulation and LL-37
internalization of LAD2
Lastly, we evaluated the effects of
neuraminidase-treatment and endocytosis inhibitors on the
degranulation and LL-37 internalization of LAD2. Notably,
neuraminidase treatment (0.2 U/ml) markedly reduced the
degranulation and LL-37 internalization (Fig. 6A and B). Moreover, the
clathrin-mediated endocytosis inhibitors dynasore (10 µM) and
chlorpromazine (2.5 µM) inhibited degranulation, and chlorpromazine
similarly suppressed LL-37 internalization. In contrast, a
caveolar-mediated endocytosis inhibitor, genistein (10 µM), did not
affect degranulation or LL-37 internalization (Fig. 6C and D).
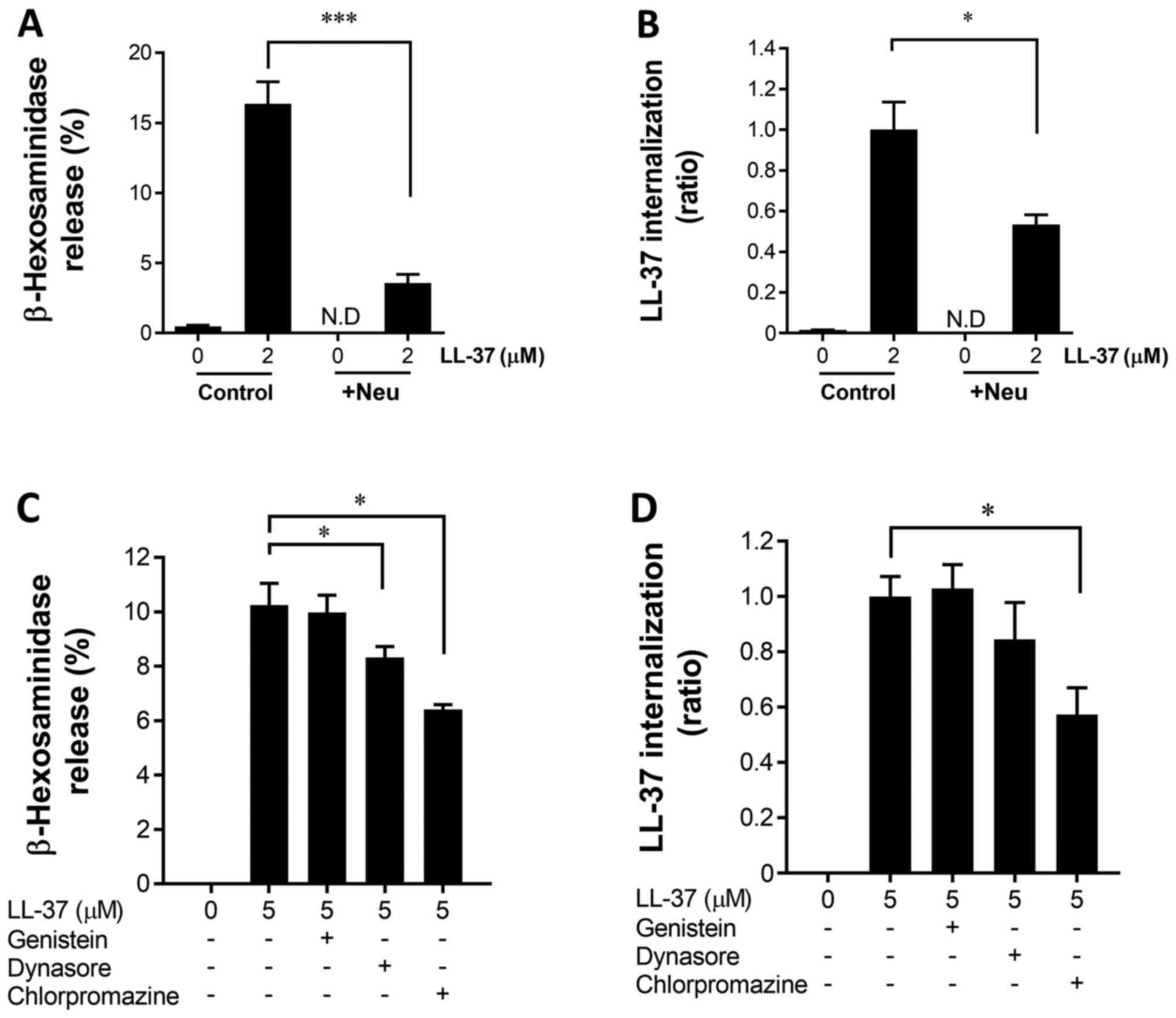 | Figure 6.Effects of neuraminidase treatment and
endocytosis inhibitors on the degranulation and LL-37
internalization of LAD2 cells. LAD2 cells were treated with
neuraminidase (0.2 U/ml, +Neu) or without (Control) at 37°C for 40
min, and further incubated with or without LL-37 (2 µM) for 40 min.
(A) Thereafter, the release of β-hexosaminidase was measured.
Alternatively, the cells were cytocentrifuged, and LL-37 was
stained with anti-LL-37 rabbit pAbs followed by anti-rabbit
IgG-Alexa Fluor 488. (B) The internalization of LL-37 was
determined by measuring the fluorescence intensities using ImageJ
software, and represented as a ratio of the control cells (Control)
incubated with LL-37 (2 µM) but without neuraminidase treatment.
(C) In addition, LAD2 cells were treated with or without genistein
(10 µM), and dynasore (10 µM) or chlorpromazine (2.5 µM), and
further incubated with or without LL-37 (5 µM) at 37°C for 40 min,
and the β-hexosaminidase release was measured. Alternatively, the
cells were cytocentrifuged, and LL-37 was stained with anti-LL-37
rabbit pAbs followed by anti-rabbit IgG-Alexa Fluor 488. (D) The
internalization of LL-37 was determined by measuring the
fluorescence intensities using ImageJ software, and represented as
a ratio of the cells incubated with LL-37 (5 µM) but without
endocytosis inhibitors. Data are presented as the mean ± standard
error of the mean of 4 to 8 independent experiments. *P<0.05 and
***P<0.001, as indicated. IgG, immunoglobulin G; pAbs,
polyclonal antibodies; N.D, not detected; MrgX2, Mas-related gene
X2. |
Discussion
Mast cells are usually present in submucosal tissues
and connective tissues, and play a central role in innate immunity
and allergic reactions by releasing several mediators such as
histamine, leukotrienes, tryptase, and several pro- and
anti-inflammatory cytokines (24).
For mast cell activation, two main pathways, immunoglobulin
E-dependent and -independent, have been identified. Immunoglobulin
E/antigen-dependent activation occurs in response to antigens, and
is mediated by the cross-linking of specific immunoglobulin E-bound
FcεRI on the cell surface (25).
Although this classical activation pathway is well investigated,
less is known about the immunoglobulin E-independent pathway. The
activators of the FcεRI-independent pathway mainly comprise basic
secretagogues, such as substance P, venom peptide mastoparan,
neuropeptide Y, and compound 48/80, and some antibacterial
peptides, including LL-37. They commonly activate a pertussis
toxin-sensitive G-protein in mast cells and lead to degranulation;
however, the receptor and signaling mechanism are unclear (24).
LL-37 is the sole anti-microbial peptide of the
cathelicidin family in humans, and is cleaved from an 18-kDa
precursor human cationic antimicrobial polypeptide. In addition to
its broad spectrum of bactericidal activities, LL-37 can alter
numerous immune responses. It has been reported that LL-37 utilizes
formyl peptide receptor-like 1 (FPRL1) to chemoattract human
neutrophils, T cells, and monocytes (26). FPRL1 also functions in the
LL-37-mediated angiogenic activity of endothelial cells (11). Furthermore, LL-37 suppresses
neutrophil apoptosis via action on both FPRL1 and P2X7 (27). Based on this, we investigated
whether WRW4, an FPRL1 antagonist, and KN-62, a P2X7 antagonist,
can inhibit the LL-37-induced degranulation; however, both WRW4 and
KN-62 did not inhibit the degranulation of a LAD2 human mast cell
line (data not shown). The failure of these antagonists to inhibit
the LL-37-mediated mast cell activation suggests that FPRL1 and
P2X7 are not functional, at least regarding LL-37-mediated mast
cell activation.
Recently, MrgX2, a member of the mas-related genes
primarily expressed in mast cells and dorsal root ganglia, was
identified as a putative receptor for LL-37 involved in mast cell
activation; however, the mechanism by which LL-37 activates MrgX2
remains unclear. In contrast, some pruritogenic basic peptides,
such as substance P, induce mast cell degranulation by
internalizing into the cells (23). We recently demonstrated that LL-37
enhances the uptake of bacterial lipopolysaccharide by liver
sinusoidal cells by forming a complex with the lipopolysaccharide
and internalizing into the cells via an endocytosis-mediated
mechanism (28). Thus, we
speculated that LL-37 also internalizes into mast cells and
activates MrgX2, thereby inducing degranulation. In the current
study, we investigated the relationship between the internalization
of LL-37 and MrgX2-mediated mast cell degranulation using a LAD2
mast cell line, and found that LL-37 internalizes into LAD2 mast
cells in dose- and time-dependent manners, and induces
degranulation, possibly via the pertussis toxin-sensitive G
protein-coupled pathway (Fig. 1).
Furthermore, based on the results using siRNA-mediated knockdown
cells, MrgX2 may function in both the degranulation and
internalization of LL-37 (Fig. 2),
and this possibility was further supported by the finding that
LL-37 internalization is enhanced in stably MrgX2-expressing HMC-1
cells and 293 cells (Figs. 3 and
4). To further clarify the
interaction of LL-37 with MrgX2, the localization of LL-37 and
MrgX2 in LAD2 cells was examined by the immunofluorescence double
staining of LL-37 and MrgX2. MrgX2 was diffusely detected in the
cytoplasmic region or localized in the perinuclear region of
control cells without LL-37 stimulation (Fig. 5A). Notably, when cells were
stimulated with LL-37, internalized LL-37 was detected as dot
patterns and colocalized with MrgX2 in the perinuclear region,
although not all MrgX2 colocalized with the internalized LL-37
(Fig. 5B and C). These
observations suggest that LL-37 interacts with MrgX2 after
internalization into the cells for mast cell activation.
It has been reported that a pruritogenic peptide,
substance P, is internalized into cells by endocytosis for mast
cell activation (23). Moreover,
positively charged amino acid residues (Arg and Lys) in the
N-terminal region and hydrophobic amino acid residues (Phe, Leu,
and Met) in the C-terminal region of substance P are essential for
the binding of substance P to the cell surface and subsequent
internalization into the cells. This is because neuraminidase
treatment, which can remove negatively charged sialic acid from the
cell surface, inhibits the binding of substance P to the cell
surface and mast cell activation (29). As LL-37 is an amphipathic molecule
with cationic and hydrophobic features, neuraminidase treatment was
expected to inhibit LL-37 internalization and mast cell activation.
Thus, we evaluated the effects of neuraminidase treatment and
endocytosis inhibitors on LL-37 internalization and LAD2 cell
activation. Indeed, neuraminidase and clathrin-mediated endocytosis
inhibitors suppressed the LL-37-internalization and degranulation
of LAD2 (Fig. 6). These
observations suggest that LL-37, a positively charged amphipathic
molecule, interacts with the negatively charged cell surface
molecules, such as sialic acid, and internalizes into the cells via
clathrin-mediated endocytosis for mast cell activation.
In conclusion, to elucidate the mechanism of mast
cell activation by LL-37, the relationship between the
internalization of LL-37 and MrgX2-mediated mast cell activation
was evaluated in the present study. We found that LL-37 likely
binds with the negatively charged cell surface molecules, rapidly
internalizes into the cells via clathrin-mediated endocytosis, and
interacts with MrgX2 for mast cell activation. Further studies are
needed to clarify the mechanism by which the internalized LL-37
induces the signal leading to mast cell activation after
interacting with MrgX2.
Acknowledgements
The authors would like to thank Ms. Toshiko
Moribayashi (Department of Host Defense and Biochemical Research,
Juntendo University Graduate School of Medicine, Tokyo, Japan), for
her technical assistance in the experiments performed in the
present study.
Funding
The present study was supported by a Grant-in-Aid
(grant no. S1201013) from the Ministry of Education, Culture,
Sports, Science and Technology Supported Program for the Strategic
Research Foundation at a Private University, 2012–2016.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
TM, KS and IN designed the research. TM and KS
performed the experiments. TM and IN analyzed the data. HyT, FN, JR
and HsT interpreted and discussed the data. TM and IN prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Selsted ME and Ouellette AJ: Mammalian
defensins in the antimicrobial immune response. Nat Immunol.
6:551–557. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagaoka I: Have host defense peptides been
acting in innate immunity since the trilobites of the Cambrian
period 540 million years ago? Juntendo Med J. 62:96–97. 2016.
View Article : Google Scholar
|
|
3
|
Niyonsaba F: Novel insight into the role
of antimicrobial (host defense) peptides/proteins in human skin
diseases. Juntendo Med J. 62:120–131. 2016. View Article : Google Scholar
|
|
4
|
Zanetti M: Cathelicidins, multifunctional
peptides of the innate immunity. J Leukoc Biol. 75:39–48. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagaoka I, Hirata M, Sugimoto K,
Tsutsumi-Ishii Y, Someya A, Saionji K and Igari J: Evaluation of
the expression of human CAP18 gene during neutrophil maturation in
the bone marrow. J Leukoc Biol. 64:845–852. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis SM, Anderson NN, Forsyth WR,
Espiritu C, Conway BD, Greenberg EP, McCray PB Jr, Lehrer RI, Welsh
MJ and Tack BF: Bactericidal activity of mammalian
cathelicidin-derived peptides. Infect Immun. 68:2748–2755. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Z, Murakami T, Suzuki K, Tamura H,
Kuwahara-Arai K, Iba T and Nagaoka I: Antimicrobial cathelicidin
peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of
macrophages by dual mechanism. PLoS One. 9:e857652014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagaoka I, Hirota S, Niyonsaba F, Hirata
M, Adachi Y, Tamura H and Heumann D: Cathelicidin family of
antibacterial peptides CAP18 and CAP11 inhibit the expression of
TNF-alpha by blocking the binding of LPS to CD14(+) cells. J
Immunol. 167:3329–3338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niyonsaba F, Ushio H, Nakano N, Ng W,
Sayama K, Hashimoto K, Nagaoka I, Okumura K and Ogawa H:
Antimicrobial peptides human beta-defensins stimulate epidermal
keratinocyte migration, proliferation and production of
proinflammatory cytokines and chemokines. J Invest Dermatol.
127:594–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodríguez-Martínez S, Cancino-Diaz JC,
Vargas-Zuñiga LM and Cancino-Diaz ME: LL-37 regulates the
overexpression of vascular endothelial growth factor (VEGF) and
c-IAP-2 in human keratinocytes. Int J Dermatol. 47:457–462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koczulla R, von Degenfeld G, Kupatt C,
Krötz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M,
Lebherz C, et al: An angiogenic role for the human peptide
antibiotic LL-37/hCAP-18. J Clin Invest. 111:1665–1672. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Umehara Y, Kamata Y, Tominaga M, Niyonsaba
F, Ogawa H and Takamori K: Antimicrobial peptides human LL-37 and
β-defensin-3 modulate the expression of nerve elongation factors in
human epidermal keratinocytes. J Dermatol Sci. 88:365–367. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niyonsaba F, Someya A, Hirata M, Ogawa H
and Nagaoka I: Evaluation of the effects of peptide antibiotics
human beta-defensins-1/−2 and LL-37 on histamine release and
prostaglandin D(2) production from mast cells. Eur J Immunol.
31:1066–1075. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Metcalfe DD, Baram D and Mekori YA: Mast
cells. Physiol Rev. 77:1033–1079. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoth M and Penner R: Depletion of
intracellular calcium stores activates a calcium current in mast
cells. Nature. 355:353–356. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niyonsaba F, Ushio H, Hara M, Yokoi H,
Tominaga M, Takamori K, Kajiwara N, Saito H, Nagaoka I, Ogawa H and
Okumura K: Antimicrobial peptides human beta-defensins and
cathelicidin LL-37 induce the secretion of a pruritogenic cytokine
IL-31 by human mast cells. J Immunol. 184:3526–3534. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niyonsaba F, Iwabuchi K, Someya A, Hirata
M, Matsuda H, Ogawa H and Nagaoka I: A cathelicidin family of human
antibacterial peptide LL-37 induces mast cell chemotaxis.
Immunology. 106:20–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Subramanian H, Gupta K, Guo Q, Price R and
Ali H: Mas-related gene X2 (MrgX2) is a novel G protein-coupled
receptor for the antimicrobial peptide LL-37 in human mast cells:
Resistance to receptor phosphorylation, desensitization, and
internalization. J Biol Chem. 286:44739–44749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lorenz D, Wiesner B, Zipper J, Winkler A,
Krause E, Beyermann M, Lindau M and Bienert M: Mechanism of
peptide-induced mast cell degranulation. Translocation and
patch-clamp studies. J Gen Physiol. 112:577–591. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wildman Henzler KA, Lee DK and Ramamoorthy
A: Mechanism of lipid bilayer disruption by the human antimicrobial
peptide, LL-37. Biochemistry. 42:6545–6558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagaoka I, Hirota S, Yomogida S, Ohwada A
and Hirata M: Synergistic actions of antibacterial neutrophil
defensins and cathelicidins. Inflamm Res. 49:73–79. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Supajatura V, Ushio H, Nakao A, Akira S,
Okumura K, Ra C and Ogawa H: Differential responses of mast cell
Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin
Invest. 109:1351–1359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garland AM, Grady EF, Payan DG, Vigna SR
and Bunnett NW: Agonist-induced internalization of the substance P
(NK1) receptor expressed in epithelial cells. Biochem J.
303:177–186. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferry X, Brehin S, Kamel R and Landry Y: G
protein-dependent activation of mast cell by peptides and basic
secretagogues. Peptides. 23:1507–1515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tatemoto K, Nozaki Y, Tsuda R, Konno S,
Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, et
al: Immunoglobulin E-independent activation of mast cell is
mediated by Mrg receptors. Biochem Biophys Res Commun.
349:1322–1328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Yang, Chen Q, Schmidt AP, Anderson GM,
Wang JM, Wooters J, Oppenheim JJ and Chertov O: LL-37, the
neutrophil granule- and epithelial cell-derived cathelicidin,
utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to
chemoattract human peripheral blood neutrophils, monocytes and T
cells. J Exp Med. 192:1069–1074. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagaoka I, Tamura H and Hirata M: An
antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses
neutrophil apoptosis via the activation of formyl-peptide
receptor-like 1 and P2X7. J Immunol. 176:3044–3052. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki K, Murakami T, Hu Z, Tamura H,
Kuwahara-Arai K, Iba T and Nagaoka I: Human host defense
cathelicidin peptide LL-37 enhances the lipopolysaccharide uptake
by liver sinusoidal endothelial cells without cell activation. J
Immunol. 196:1338–1347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coleman JW, Huang Q and Stanworth DR: The
mast cell response to substance P: Effects of neuraminidase,
limulin, and some novel synthetic peptide antagonists. Peptides.
7:171–175. 1986. View Article : Google Scholar : PubMed/NCBI
|