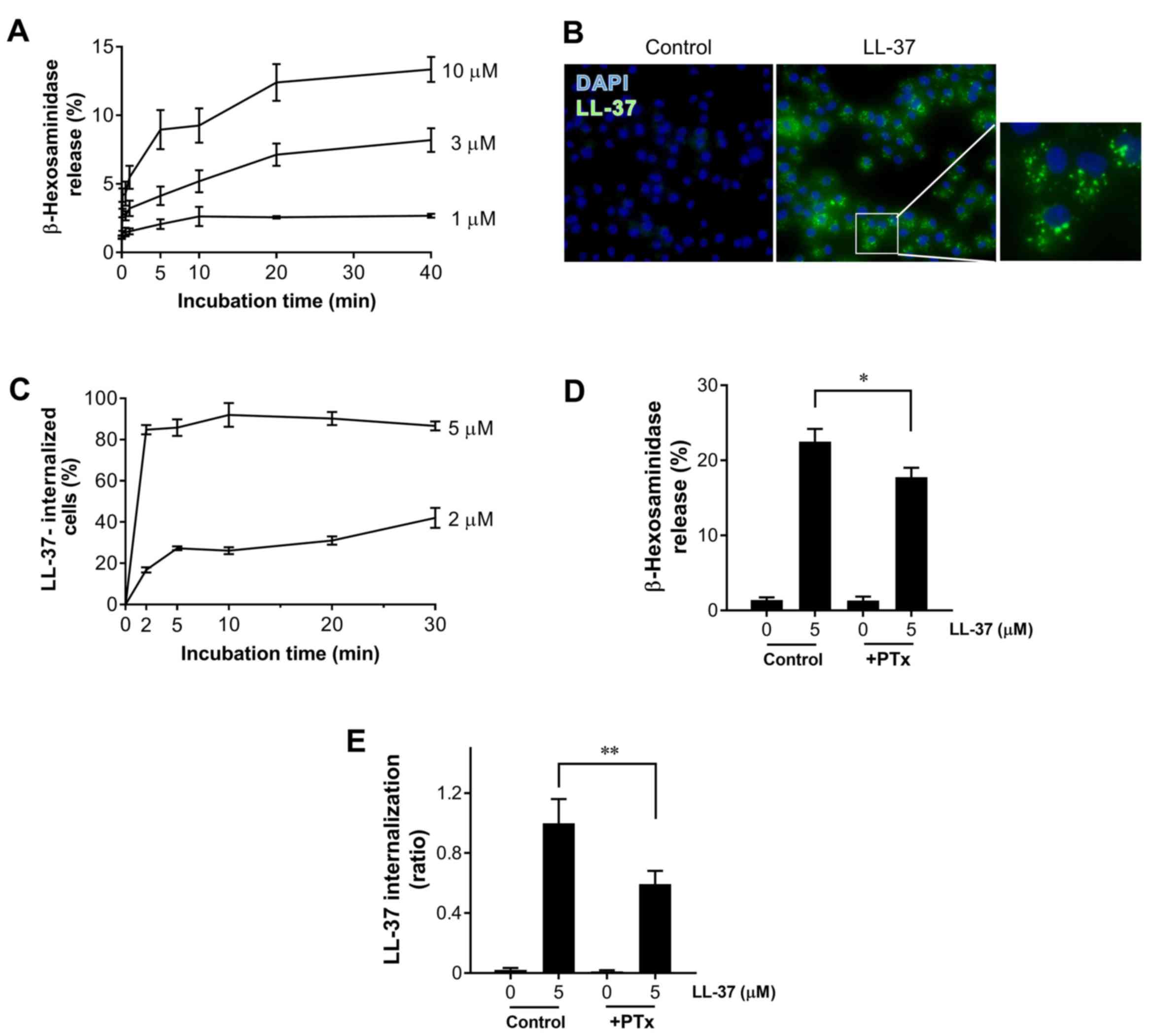
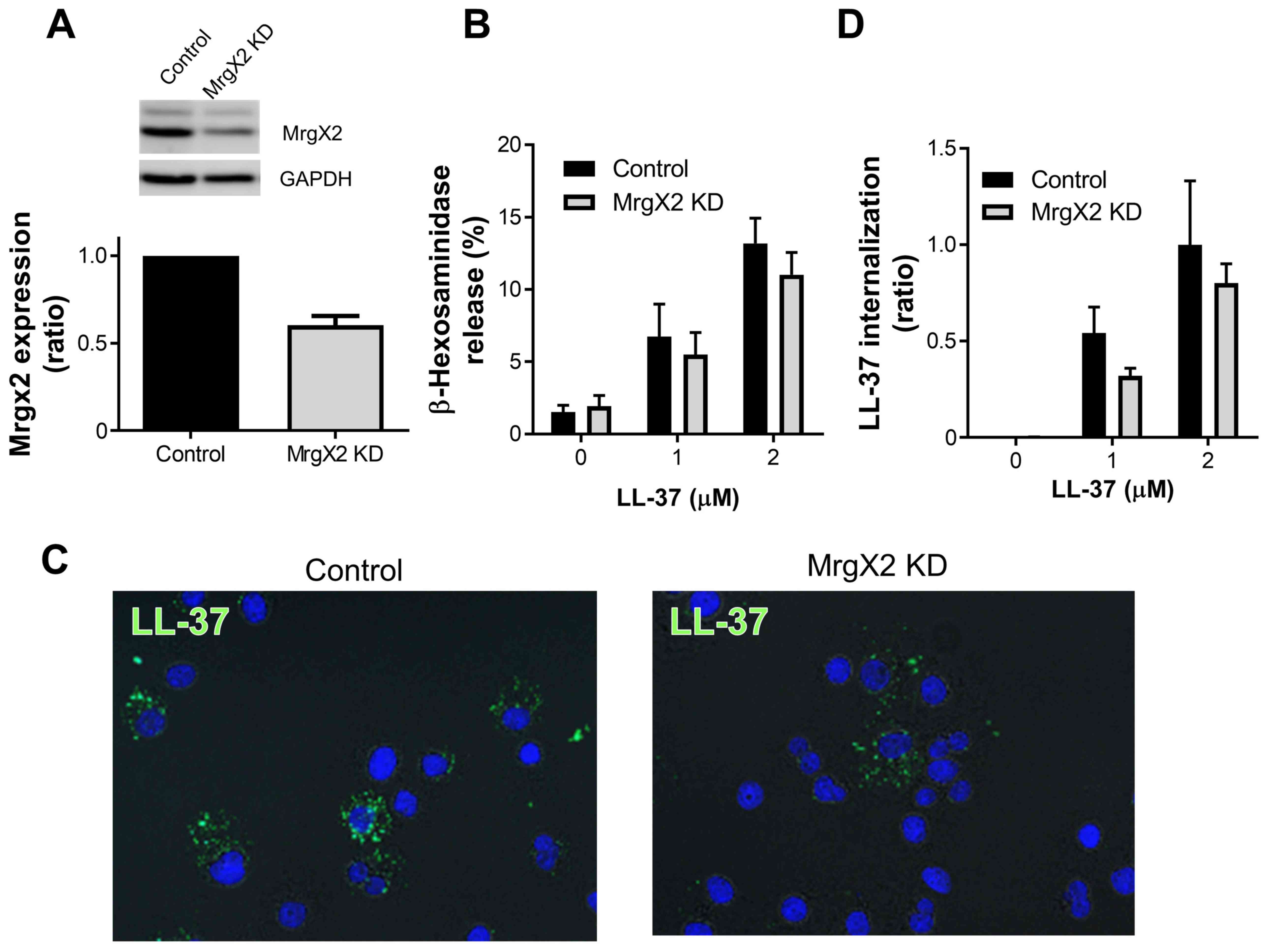
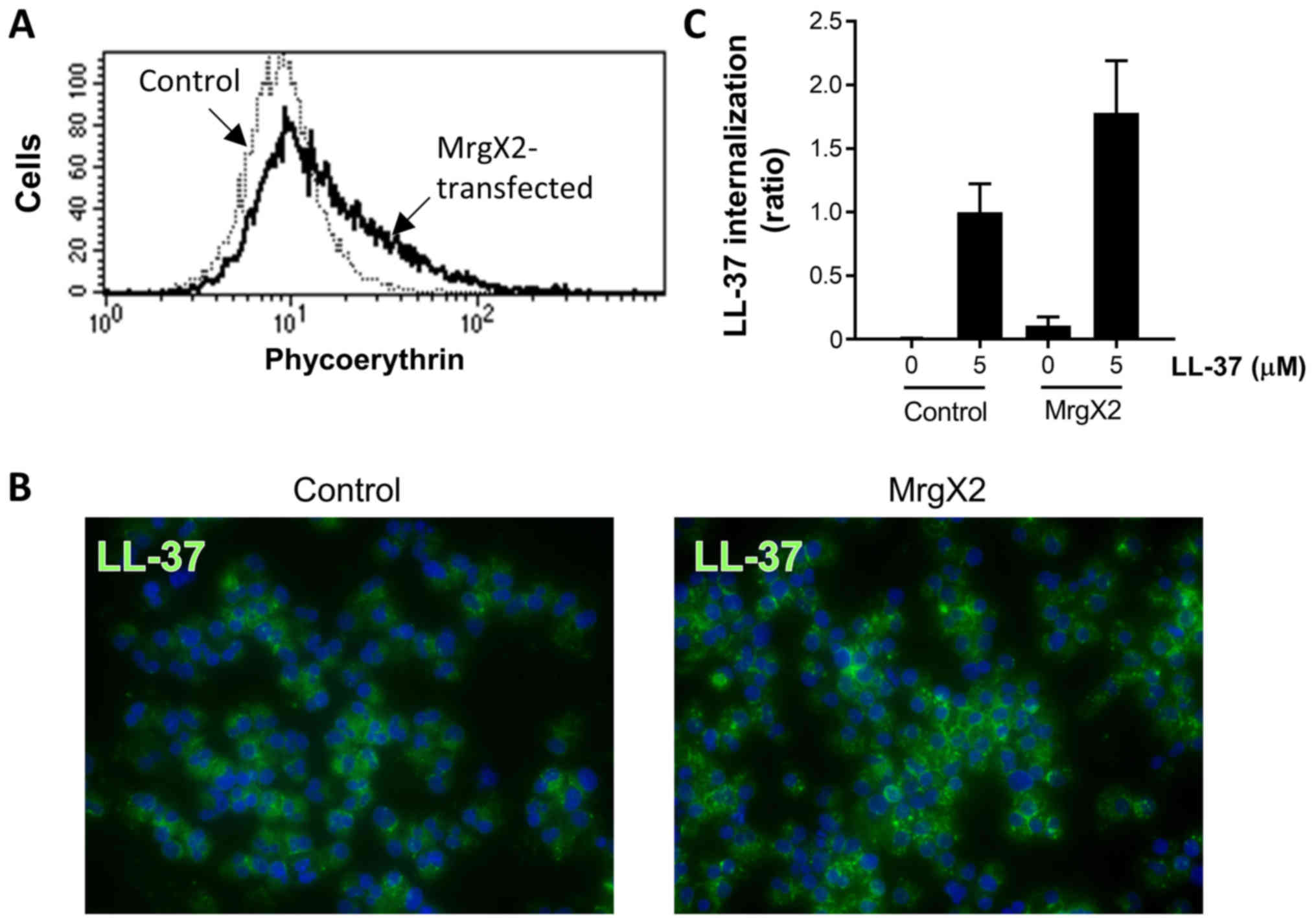
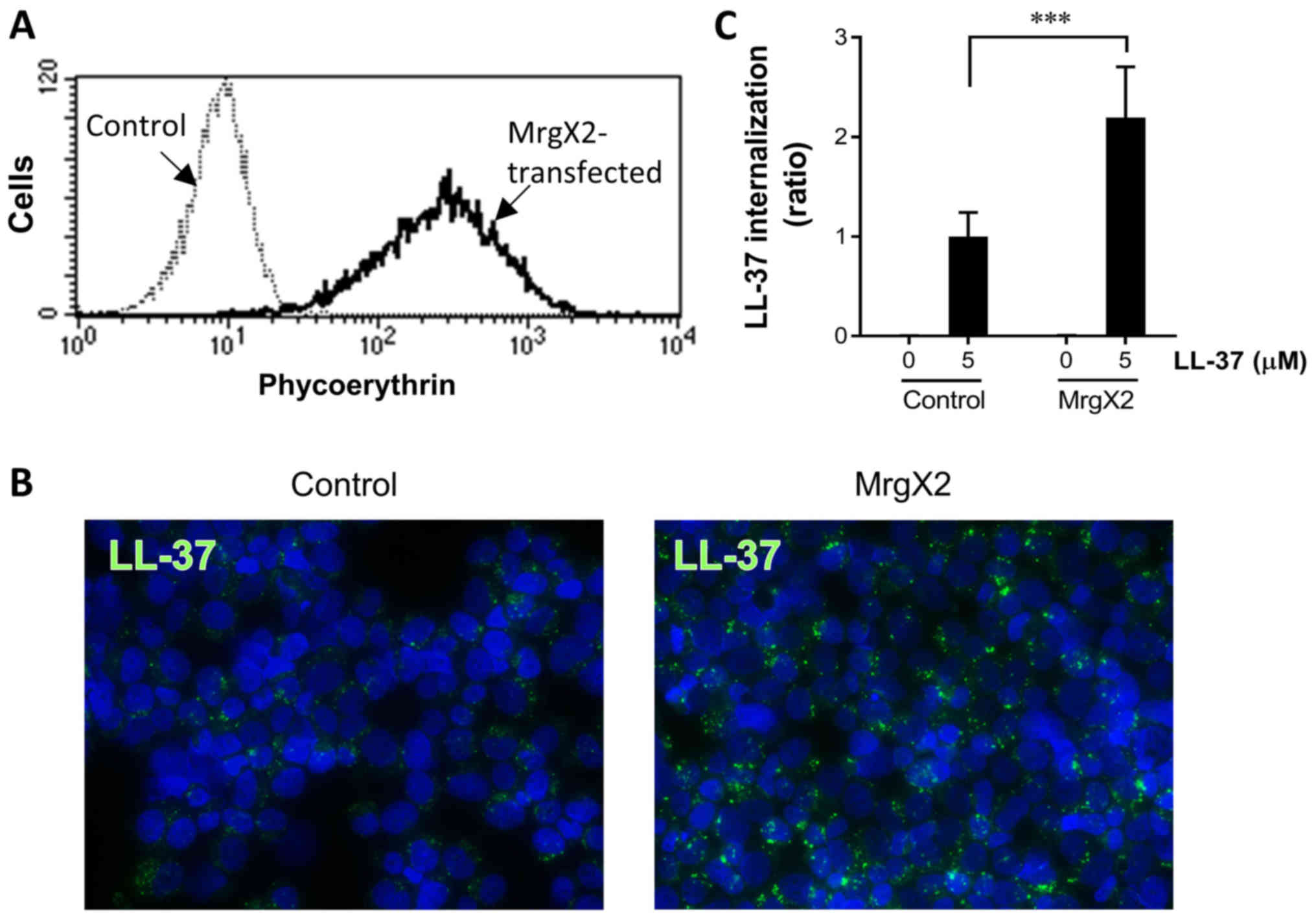
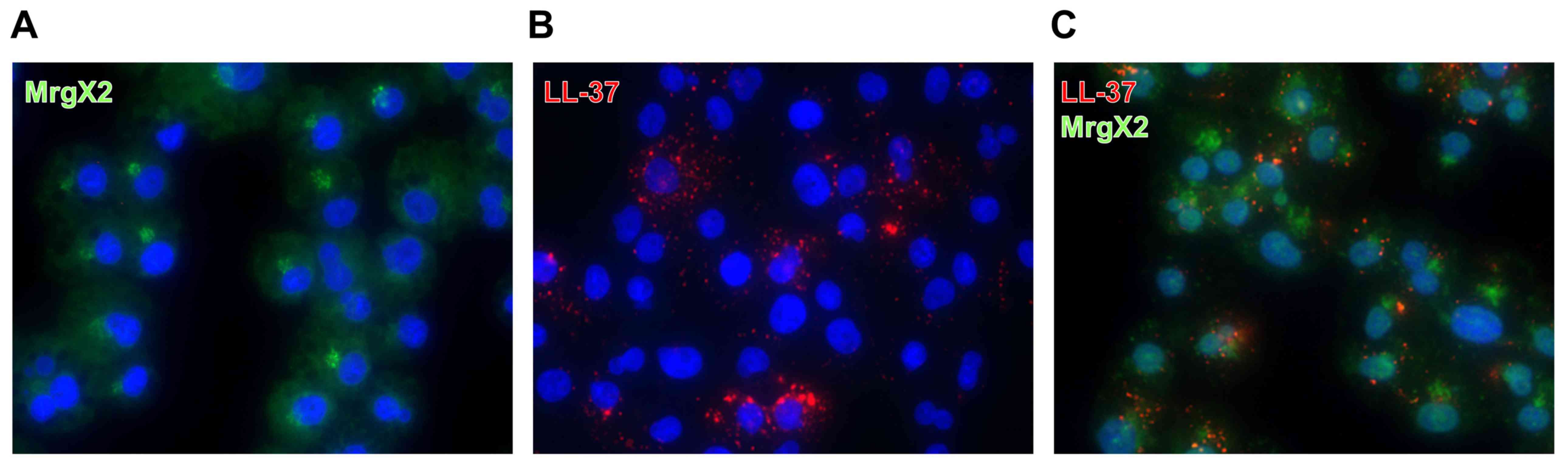
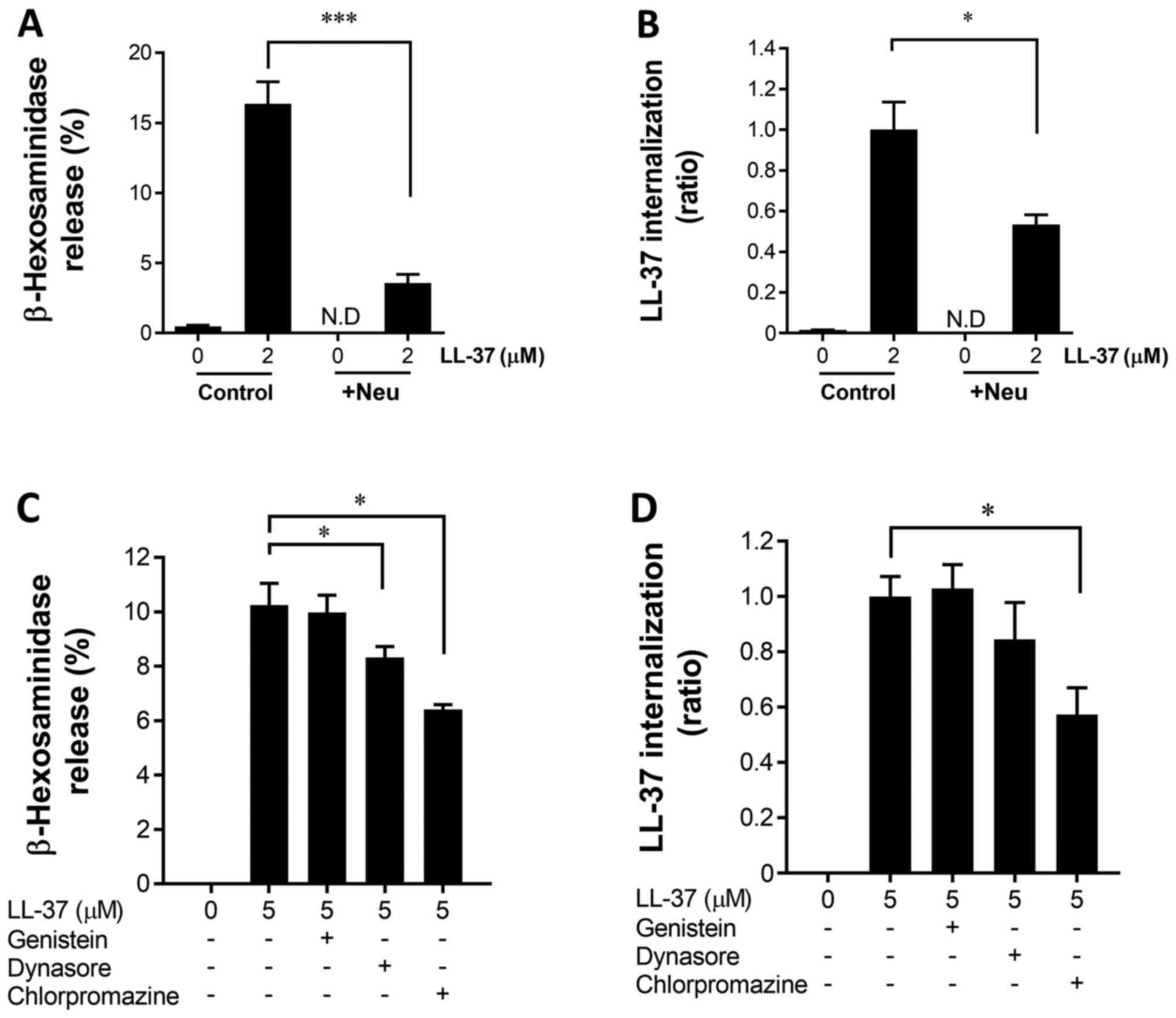
|
1
|
Selsted ME and Ouellette AJ: Mammalian
defensins in the antimicrobial immune response. Nat Immunol.
6:551–557. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagaoka I: Have host defense peptides been
acting in innate immunity since the trilobites of the Cambrian
period 540 million years ago? Juntendo Med J. 62:96–97. 2016.
View Article : Google Scholar
|
|
3
|
Niyonsaba F: Novel insight into the role
of antimicrobial (host defense) peptides/proteins in human skin
diseases. Juntendo Med J. 62:120–131. 2016. View Article : Google Scholar
|
|
4
|
Zanetti M: Cathelicidins, multifunctional
peptides of the innate immunity. J Leukoc Biol. 75:39–48. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagaoka I, Hirata M, Sugimoto K,
Tsutsumi-Ishii Y, Someya A, Saionji K and Igari J: Evaluation of
the expression of human CAP18 gene during neutrophil maturation in
the bone marrow. J Leukoc Biol. 64:845–852. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis SM, Anderson NN, Forsyth WR,
Espiritu C, Conway BD, Greenberg EP, McCray PB Jr, Lehrer RI, Welsh
MJ and Tack BF: Bactericidal activity of mammalian
cathelicidin-derived peptides. Infect Immun. 68:2748–2755. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Z, Murakami T, Suzuki K, Tamura H,
Kuwahara-Arai K, Iba T and Nagaoka I: Antimicrobial cathelicidin
peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of
macrophages by dual mechanism. PLoS One. 9:e857652014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagaoka I, Hirota S, Niyonsaba F, Hirata
M, Adachi Y, Tamura H and Heumann D: Cathelicidin family of
antibacterial peptides CAP18 and CAP11 inhibit the expression of
TNF-alpha by blocking the binding of LPS to CD14(+) cells. J
Immunol. 167:3329–3338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niyonsaba F, Ushio H, Nakano N, Ng W,
Sayama K, Hashimoto K, Nagaoka I, Okumura K and Ogawa H:
Antimicrobial peptides human beta-defensins stimulate epidermal
keratinocyte migration, proliferation and production of
proinflammatory cytokines and chemokines. J Invest Dermatol.
127:594–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodríguez-Martínez S, Cancino-Diaz JC,
Vargas-Zuñiga LM and Cancino-Diaz ME: LL-37 regulates the
overexpression of vascular endothelial growth factor (VEGF) and
c-IAP-2 in human keratinocytes. Int J Dermatol. 47:457–462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koczulla R, von Degenfeld G, Kupatt C,
Krötz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M,
Lebherz C, et al: An angiogenic role for the human peptide
antibiotic LL-37/hCAP-18. J Clin Invest. 111:1665–1672. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Umehara Y, Kamata Y, Tominaga M, Niyonsaba
F, Ogawa H and Takamori K: Antimicrobial peptides human LL-37 and
β-defensin-3 modulate the expression of nerve elongation factors in
human epidermal keratinocytes. J Dermatol Sci. 88:365–367. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niyonsaba F, Someya A, Hirata M, Ogawa H
and Nagaoka I: Evaluation of the effects of peptide antibiotics
human beta-defensins-1/−2 and LL-37 on histamine release and
prostaglandin D(2) production from mast cells. Eur J Immunol.
31:1066–1075. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Metcalfe DD, Baram D and Mekori YA: Mast
cells. Physiol Rev. 77:1033–1079. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoth M and Penner R: Depletion of
intracellular calcium stores activates a calcium current in mast
cells. Nature. 355:353–356. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niyonsaba F, Ushio H, Hara M, Yokoi H,
Tominaga M, Takamori K, Kajiwara N, Saito H, Nagaoka I, Ogawa H and
Okumura K: Antimicrobial peptides human beta-defensins and
cathelicidin LL-37 induce the secretion of a pruritogenic cytokine
IL-31 by human mast cells. J Immunol. 184:3526–3534. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niyonsaba F, Iwabuchi K, Someya A, Hirata
M, Matsuda H, Ogawa H and Nagaoka I: A cathelicidin family of human
antibacterial peptide LL-37 induces mast cell chemotaxis.
Immunology. 106:20–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Subramanian H, Gupta K, Guo Q, Price R and
Ali H: Mas-related gene X2 (MrgX2) is a novel G protein-coupled
receptor for the antimicrobial peptide LL-37 in human mast cells:
Resistance to receptor phosphorylation, desensitization, and
internalization. J Biol Chem. 286:44739–44749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lorenz D, Wiesner B, Zipper J, Winkler A,
Krause E, Beyermann M, Lindau M and Bienert M: Mechanism of
peptide-induced mast cell degranulation. Translocation and
patch-clamp studies. J Gen Physiol. 112:577–591. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wildman Henzler KA, Lee DK and Ramamoorthy
A: Mechanism of lipid bilayer disruption by the human antimicrobial
peptide, LL-37. Biochemistry. 42:6545–6558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagaoka I, Hirota S, Yomogida S, Ohwada A
and Hirata M: Synergistic actions of antibacterial neutrophil
defensins and cathelicidins. Inflamm Res. 49:73–79. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Supajatura V, Ushio H, Nakao A, Akira S,
Okumura K, Ra C and Ogawa H: Differential responses of mast cell
Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin
Invest. 109:1351–1359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garland AM, Grady EF, Payan DG, Vigna SR
and Bunnett NW: Agonist-induced internalization of the substance P
(NK1) receptor expressed in epithelial cells. Biochem J.
303:177–186. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferry X, Brehin S, Kamel R and Landry Y: G
protein-dependent activation of mast cell by peptides and basic
secretagogues. Peptides. 23:1507–1515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tatemoto K, Nozaki Y, Tsuda R, Konno S,
Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, et
al: Immunoglobulin E-independent activation of mast cell is
mediated by Mrg receptors. Biochem Biophys Res Commun.
349:1322–1328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Yang, Chen Q, Schmidt AP, Anderson GM,
Wang JM, Wooters J, Oppenheim JJ and Chertov O: LL-37, the
neutrophil granule- and epithelial cell-derived cathelicidin,
utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to
chemoattract human peripheral blood neutrophils, monocytes and T
cells. J Exp Med. 192:1069–1074. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagaoka I, Tamura H and Hirata M: An
antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses
neutrophil apoptosis via the activation of formyl-peptide
receptor-like 1 and P2X7. J Immunol. 176:3044–3052. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki K, Murakami T, Hu Z, Tamura H,
Kuwahara-Arai K, Iba T and Nagaoka I: Human host defense
cathelicidin peptide LL-37 enhances the lipopolysaccharide uptake
by liver sinusoidal endothelial cells without cell activation. J
Immunol. 196:1338–1347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coleman JW, Huang Q and Stanworth DR: The
mast cell response to substance P: Effects of neuraminidase,
limulin, and some novel synthetic peptide antagonists. Peptides.
7:171–175. 1986. View Article : Google Scholar : PubMed/NCBI
|