Introduction
Myocardial infarction is a leading cause of
mortality worldwide, and timely blood/oxygen reperfusion using
either thrombolytic therapy or primary percutaneous coronary
intervention may efficiently limit the myocardial infarct size,
preserve left ventricular systolic function and reduce the onset of
heart failure (1). However,
immediate reperfusion may produce a large amount of reactive oxygen
species (ROS), subsequently inducing myocardial cell apoptosis and
finally causing severe ischemia/reperfusion (I/R) cardiac injury
(2). At present, efforts are being
made to relieve ROS-induced myocardial cell damage. Among those
efforts, emerging evidence suggests that the modulation of
autophagy may be a novel therapeutic strategy for myocardial I/R
injury (3).
Autophagy is a degradative process used to eliminate
long-lived proteins and damaged organelles. Autophagy involves
multiple steps, including the generation of autophagosomes, fusion
of autophagosomes and lysosomes, and protein degradation in
lysosomes (4). Previous research
has demonstrated that autophagy dysfunction may lead to numerous
health issues, including neurodegeneration, cardiomyopathy, cancer
and I/R injury (4–7). It is widely accepted that increased
autophagic flux may protect the cell from I/R injury, as autophagy
eliminates impaired organelles and thus limits the spread of damage
inside the cell. I/R preconditioning is one of the best-researched
and commonly used methods of relieving I/R injury. It has been
demonstrated that I/R preconditioning may protect heart cells
through pre-activation of autophagy (8,9).
However, in a recent in vivo animal study, researchers
demonstrated that in heart tissues from aged rats, preconditioning
was unable to induce autophagic activity efficiently, which finally
led to more severe heart I/R injury in aged rats (10).
Traditional Chinese medicines, including Radix
Astragali, have been widely used to treat cardiovascular
disease (11). As a principal
isoflavone compound extracted from Radix Astragali,
formononetin has been reported to have numerous pharmacological
properties, including anticancer, anti-inflammatory, antioxidant,
antiviral and neuroprotective activity, and wound healing (12–14).
However, the molecular mechanisms underlying the cardioprotective
potential of formononetin within the context of myocardial
infarction and subsequent I/R injury remain unclear. Considering
that autophagy has been proven to protect myocardial cell from I/R
injury, the present study raised the hypothesis that formononetin
may protect aged myocardial cells from I/R damage by regulating
autophagy.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), and penicillin/streptomycin (10,000 U/ml each)
were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Dimethyl sulfoxide and berberine were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The rabbit
monoclonal antibody against β-actin (cat. no. ab8227) was obtained
from Abcam (Cambridge, UK). Anti-Caspase-3 (cat. no. 9662),
cytochrome c (Cyt-c; cat. no. 12963), apoptosis regulator
Bcl-2 (Bcl-2; cat. no. 15071), Beclin-1 (cat. no. 3738), autophagy
protein 5 (Atg-5; cat. no. 2630) and p62 (cat. no. 23214)
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibodies (cat. no. A-21109) used in western
blotting were obtained from Invitrogen (Thermo Fisher Scientific,
Inc.).
Plasmids
EGFP-LC3b (cat. no. 11546) and pLV-mCherry (cat. no.
36084) were obtained from Addgene, Inc. (Cambridge, MA, USA). To
construct the mCherry-GFP-LC3 plasmid, PCR was performed for
mCherry from the pLV-mCherry vector with a pair of primers
(Forward, 5′-GCTAGCGCCTGGAGCTGCTTGGCCACCATGCCCCAGACTGTGAGTTGC-3′
and reverse,
5′-GCTAGCATAAAAGACACCAAGGAAGCTGACAAGATAGAGGAAGAGCAA-3′) containing
the Nhe1 target sequence, 21 random nucleotides in between
as linker and a 21 nucleotide matching mCherry sequence. PCR was
performed with PfuUltra High-Fidelity DNA polymerase (Agilent
Technologies, USA). dNTPs were purchased from New England Biolabs,
Inc. (Ipswich, MA, USA). The following thermocycling conditions
were used: 94°C 5 min, followed by 30 cycles of 94°C for 30 sec,
55°C for 30 sec and 72°C for 1 min, with a final extension at 72°C
for 10 min. The PCR product was subjected to Nhe1 digestion
at 37°C overnight (New England Biolabs, Inc.) and gel extraction.
The Nhe1 site between the CMV promoter and EGFP in EGFP-LC3b
vector was also digested by Nhe1 for 1 h at 37°C, followed
by CIP (calf alkaline phosphatase; New England Biolabs, Inc.)
treatment for 1 h at 37°C (New England Biolabs, Inc.). Following
gel extraction, both vector and PCR inserts were ligated with
T4-ligase (New England Biolabs, Inc.).
Isolated heart preparation
Aged male mice (n=60; 10 months old; 35–45 g) were
used for the present study. All animal experiments were conducted
in compliance with the Guide for the Care and Use of Laboratory
Animals published by the National Institutes of Health, and were
approved by the Animal Care Committees of Southern Medical
University (Guangzhou, China). Mice were obtained from the
Experimental Animal Center of Southern Medical University (ID:
2015030856). All mice were housed in plastic cages at 25°C and
50±5% relative humidity under a 12 h light/dark cycle with free
access to rodent chow and water. They were housed for one week to
adapt to their environment prior to experimentation. Mice were
anesthetized with 2% pentobarbital sodium (50 mg/kg
intraperitoneally), and the hearts were mounted in a Langendorff
perfusion apparatus and subjected to simulated I/R as described
previously (15). The
Krebs-Henseleit buffer (KH buffer; 118 mM NaCl, 4.7 mM KCl, 1.2 mM
MgSO4, 1.25 mM CaCl2, 1.2 mM
KH2PO4, 25 mM NaHCO3 and 11 mM
glucose; pH 7.4) was equilibrated with 95% O2 and 5%
CO2 at 37°C for 30 min. The coronary flow rate was
maintained at 6 ml/min during the stabilization, with a constant
pressure of 80 mm H2O throughout the experiment.
Experimental protocols
Each heart was stabilized for 20 min. Following
stabilization, isolated hearts were divided into four groups, and
each group included 10 hearts (n=10). In the control group, after
20 min stabilization, the heart was directly perfused with KH
buffer for 100 min. In the I/R group (I/R), following
stabilization, the hearts were exposed to 40 min ischemia followed
by 60 min reperfusion. In the I/R+preconditioning group (I/R+PC),
following stabilization, the hearts were exposed to ischemia for 40
min and then subjected to an I/R cycle including 10 sec reperfusion
and 10 sec simulated ischemia, repeated six times. Hearts were
subsequently reperfused for 60 min. In the I/R+formononetin group
(I/R+Form), following stabilization, the hearts were exposed to
ischemia for 40 min, and 5 mM formononetin was administered at the
onset of reperfusion for 10 sec. This protocol was repeated a
further five times and followed by 60 min reperfusion.
Evaluation of myocardial infarct
size
At the end of reperfusion, all hearts were rapidly
frozen for 1.5 h at −20°C and subsequently cut into six transverse
slices (~3 mm thick) parallel to the atrioventricular groove. Then
each slice was incubated for 15 min in a 1% solution of
triphenyltetrazolium chloride (TTC) in phosphate buffer at 37°C.
This method is used to reliably distinguish the necrotic myocardium
(which appears pale) from the viable myocardium that stains
brick-red. The extent of the area of necrosis was quantified by
computerized planimetry with ImageJ software (version 1.50i;
National Institutes of Health, Bethesda, MD, USA), correcting for
the weight of the tissue slices. The total weight of the area of
necrosis was calculated and expressed as a percentage of the total
left ventricular weight.
Cardiac function
A catheter was inserted into the left ventricle of
the mice through the left atrium as described previously (16). The left ventricular end-diastolic
pressure was adjusted to 5–7 mm Hg during the initial equilibrium
phase. The distal end of the catheter was connected to a Power Lab
8/SP™ data acquisition system (ADInstruments, Dunedin,
New Zealand) via a pressure transducer for the continuous recording
of cardiac function. Cardiac function was evaluated based on left
ventricular developed pressure. Fractional shortening (FS) was
calculated as follows: FS = [left ventricular end diastolic
diameter (LVEDD)-left ventricular end systolic diameter
(LVESD)]/LVEDD ×100. The ejection fraction (EF) was calculated from
left ventricular end-diastolic volume (LVEDV) and end-systolic
volume (LVESD) using the equation (LVEDV-LVESV)/LVEDV ×100.
Measurement of apoptosis by terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay in myocardial tissues
At the end of reperfusion, the left ventricle free
wall was collected and sliced into 1-mm2 sections from
each group. Myocardial sections were fixed with 10% formalin for 48
h at room temperature, and subsequently sliced into 4–5 µm
sections, as described previously (17). The apoptotic myocytes were stained
via a TUNEL assay using a TUNEL kit (Beyotime Institute of
Biotechnology, Beijing, China) according to the manufacturer's
instructions. Nuclei were stained with 0.5 µg/ml DAPI in glycerol
mounting buffer (Beyotime Biotechnology) at room temperature for 1
h. A total of three sections from each myocardial sample were
randomly selected, and 10 microscopic fields per section were
evaluated by single photon confocal microscopy (×10 objective lens;
Olympus Corporation, Tokyo, Japan). The apoptotic index was
determined by dividing the cell number of TUNEL-positive nuclei by
the total number of cells and multiplying by 100.
Hypoxia/reoxygenation (H/R) model of
D-galactose-induced aging in H9C2 cells
The D-galactose induction was conducted as
previously described (18).
D-galactose (10 g/l) was added to H9C2 cells for 48 h at 37°C.
Hypoxic conditions were produced using D-Hank's solution (Beyotime
Institute of Biotechnology) saturated with 95% N2 and 5%
CO2 at 37°C. The pH was regulated to 6.8 using lactate
to mimic ischemic conditions. Aged H9C2 cells were put into a
hypoxic incubator which was equilibrated with 1% O2, 5%
CO2 and 94% N2 at 37°C. Following the hypoxia
period, the culture medium was rapidly replaced with fresh DMEM
with 10% FBS (normoxic culture solution) to mimic
reoxygenation.
Cell viability assay
Cell viability was measured using a Cell Counting
Kit-8 (CCK-8, BiYunTian, Beijing, China). Cells were seeded in
96-well plates at a concentration of 3×103 cells/well.
After 24 h of each treatment as described above (Control, I/R,
I/R+PC and I/R+Form), 10 µl of CCK-8 was immediately added to each
well. Subsequently, cells were incubated for 2 h at 37°C. Using a
microplate spectrophotometer, the plates were read at 570 nm to
determine their optical density.
Please add a subsection containing the full protocol
for western blotting, including the following details:
Western blotting
Total proteins were extracted from heart tissues or
aged cells. Samples were lysed with radioimmunoprecipitation assay
buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl,
1 mM EDTA, and 50 mM Tris-HCl; pH 7.4) with a protease inhibitor
cocktail (Sigma-Aldrich; Merck KGaA) and centrifuged at 12,000 × g
for 10 min at 4°C. Protein concentrations in supernatants were
measured with a bicinchoninic acid protein assay(BiYunTian,
Beijing, China). Protein samples (50 µg/lane) were separated by 12%
SDS-PAGE and subsequently transferred onto polyvinylidene fluoride
membranes. Membranes were blocked in 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) with Tris-buffered saline with 1%
Tween-20 for 1 h at room temperature and then incubated overnight
at 4°C with primary antibodies against Caspase-3, Cyt-c, Bcl-2,
Beclin-1, Atg-5, p62 and β-actin (all 1:2,000; Cell Signaling
Technology, Inc.). Following this, membranes were incubated with
HRP-conjugated goat anti-rabbit secondary antibodies (1:5,000,
Abcam, China) for 1 h at room temperature. Target bands were
visualized by enhanced chemiluminescence (BiYunTian, Beijing,
China). The density of specific bands was analyzed using with
ImageJ software (version 1.50i; National Institutes of Health) and
normalized to β-actin.
Plasmids transfection and live cell
imaging
H9C2 cells were transfected for 24 h with different
constructs using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Live cell images for H9C2 cells were captured by a
single photon confocal microscopy (×60 objective lens; Olympus
Corporation). LysoTracker-Red staining was observed 5 min
subsequent to reagent administration at 37°C. The colocalization of
different vesicles or the number of single vesicles was quantified
using ImageJ software (version 1.50i, National Institutes of
Health, Bethesda, MD, USA) with Coloc 2 plugin (version 2.1.0;
imagej.net/Coloc_2). A two-tailed, two-sample,
unequal variance Student's t-test was used to calculate the
P-values. Error bars represent the standard deviation of the
samples.
Statistical analysis
All data are expressed as the mean ± standard
deviation and represent at least three independent experiments.
Statistical comparisons were performed using SPSS 11.0 (SPSS, Inc.,
Chicago, IL, USA). using a Student's t-test or one-way analysis of
variance followed by a post hoc analysis (Tukey's test) where
applicable. P<0.05 was considered to indicate a statistically
significant difference.
Results
Pretreatment with formononetin reduces
myocardial tissue injury, improves cardiac function and decreases
apoptosis in heart tissue
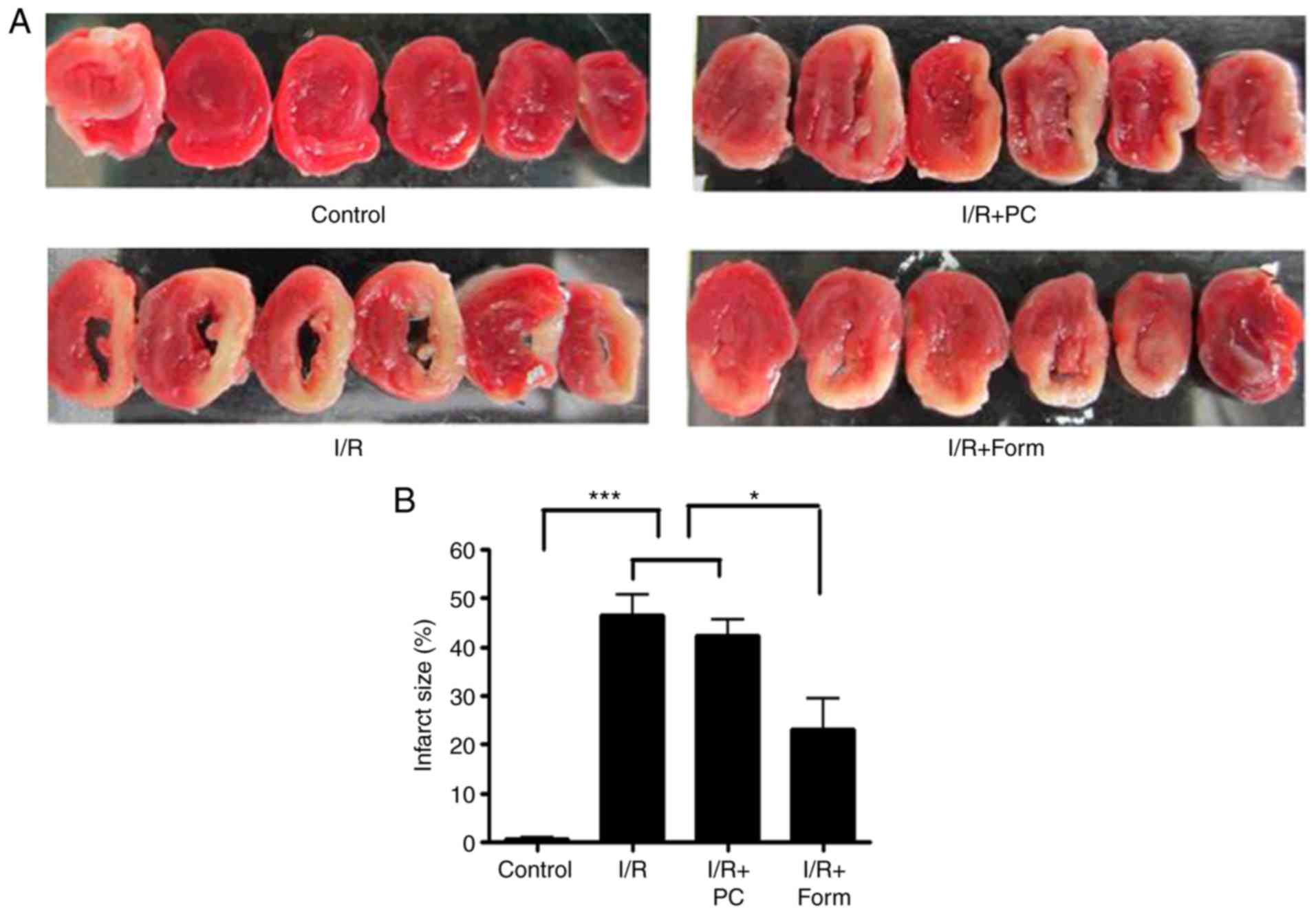
As hypothesized, the results of the present study
demonstrated that I/R treatment induced extensive infarct injury
(pale tissue sections following TTC staining) compared with the
control group. Notably, although preconditioning was unable to
effectively rescue heart infarct injury, pretreatment with
formononetin at 5 mM was able to significantly reduce the areas of
the pale sections (Fig. 1A and B).
The average infarct size was 46% in the I/R group and was reduced
to 23% following pretreatment with formononetin. These data
indicated that pretreatment with formononetin may relieve cardiac
infarct injury in heart tissue from aged mice. To examine whether
pretreatment with formononetin improved cardiac function following
I/R injury, echocardiography was used to measure cardiac functional
indices, including LVESD, LVEDD, FS and EF.
As presented in Fig.
1, LVESD in the I/R group increased to 4.14±0.75 mm compared
with the control group (2.50±0.44 mm), and treatment with
formononetin reduced this to 3.1±0.27 mm (Fig. 1C). Similarly, LVEDD was increased
to 4.61±0.69 mm in the I/R group compared with 3.01±0.43 mm in the
control group, and treatment with formononetin reduced this to
3.52±0.16 mm (Fig. 1D). These two
measurements suggested that treatment with formononetin alleviated
cardiac dilation following I/R injury. Furthermore, values of FS
(16.16±5.31%) and EF (29.29±6.97%) in the I/R group were reduced
when compared with the control group (33.22±3.56% and 66.07±9.15%,
respectively) and treatment with formononetin significantly
increased these two indices (Fig. 1E
and F). Therefore, treatment with formononetin reduced the size
of infarct lesions and improved cardiac function in I/R-induced
heart failure.
Formononetin rescues I/R-induced
cellular apoptosis in isolated heart tissue
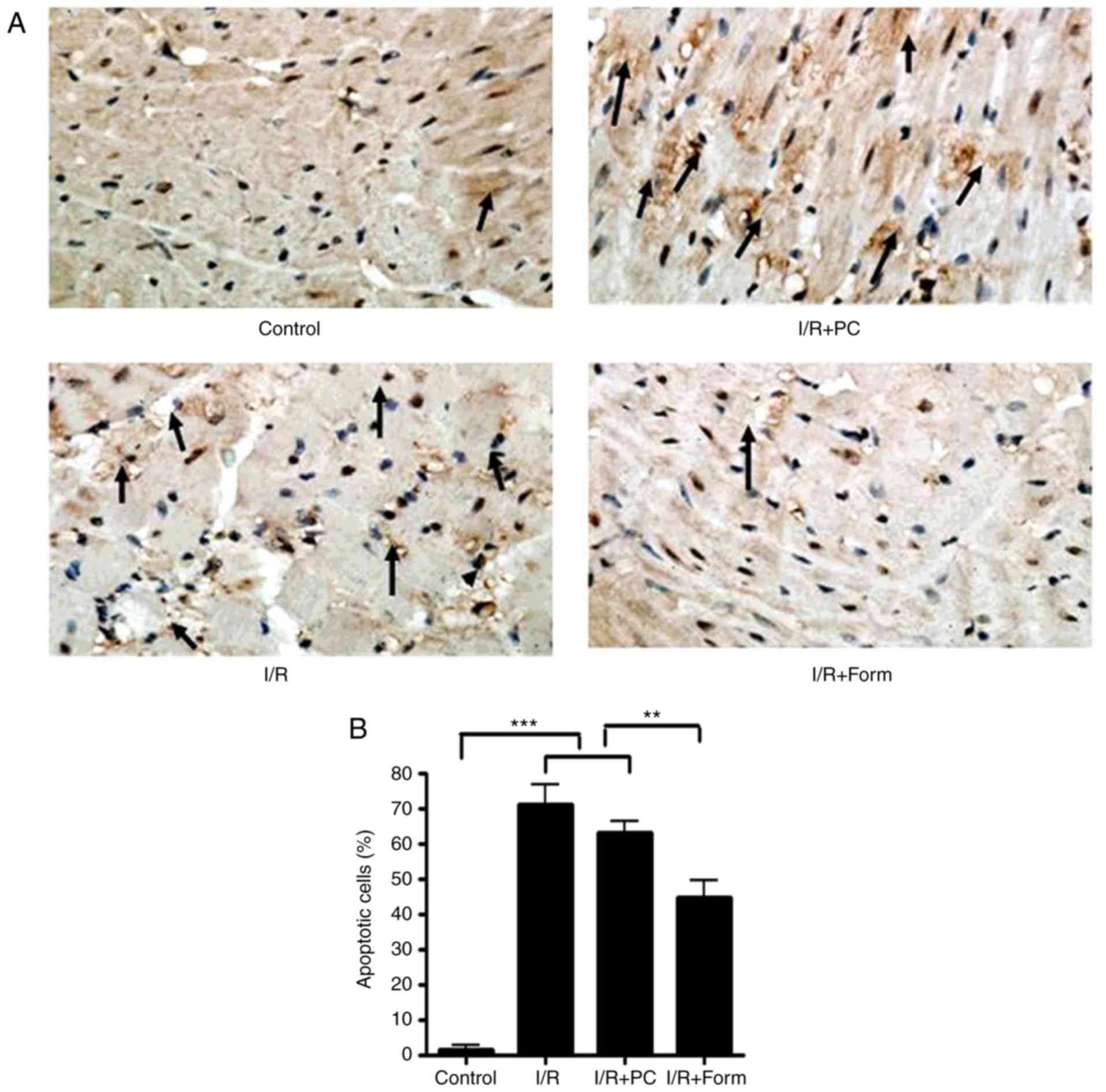
The present study investigated apoptosis markers in
isolated hearts from aged mice. The data in Fig. 2 demonstrate that following I/R
injury, the number of apoptotic cells was significantly increased
in the I/R group, and treatment with formononetin was able to
reduce this number (Fig. 2A and
B). Furthermore, western blot analysis demonstrated that
following I/R or I/R with PC, the density of cleaved caspase-3 and
Cyt-c was significantly increased, while that of Bcl-2 was
decreased. This result additionally indicated that preconditioning
was unable to effectively rescue I/R-induced cellular apoptosis in
the aged heart. However, it was identified that pretreatment with
formononetin reversed the alterations in all of the above markers,
which indicated that formononetin may protect the aged heart from
I/R injury through a mechanism different from that of
preconditioning.
Formononetin alleviates I/R-induced
cellular apoptosis in aged H9C2 cells
To further investigate the detailed mechanism
underlying the cardioprotective effects of formononetin, a
chemically-induced aging H9C2 cell line was used in the present
study. Similar to the isolated hearts, a marked apoptosis phenotype
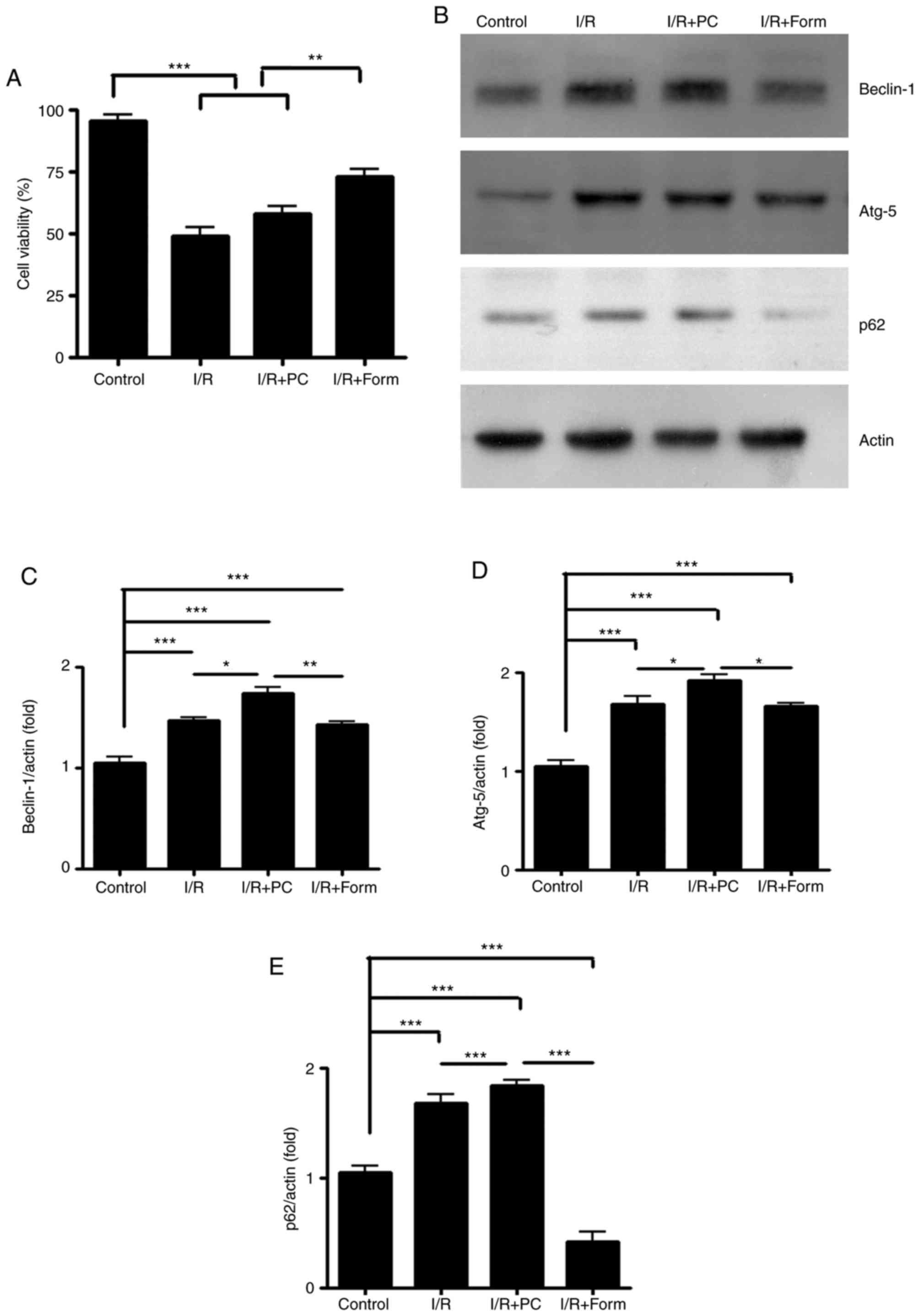
was observed in aged H9C2 cells by CCK-8 assay (Fig. 3A) in both I/R and I/R+PC groups.
This result indicated that the in vitro chemically-induced
aging H9C2 cells were able to be used to mimic I/R injury. The
present study subsequently assessed whether formononetin
administration to aged H9C2 cells alleviated I/R-induced apoptosis.
The results in Fig. 3 demonstrated
that formononetin decreased the cellular apoptosis rate following
I/R injury.
Formononetin enhances cellular
autophagic activity in aged H9C2 cells
As autophagy is an important mechanism for cell
survival following I/R injury, the present study sought to
investigate whether autophagy was involved in the I/R model.
Through western blotting, it was observed that in aged H9C2 cells,
I/R increased the expression of Beclin-1 and Atg5, two well-known
autophagy indicators. Furthermore, preconditioning elevated the
Beclin-1 and Atg5 expression levels to a greater extent. However,
compared with preconditioning, formononetin led to a more modest
elevation of Beclin-1 and Atg5 protein expression (Fig. 3B-D). p62, an autophagy degradation
marker, was detected and it was observed that although I/R and
preconditioning increased Beclin-1 and Atg5 efficiently, they
failed to decrease p62 expression compared with the control or
formononetin groups (Fig. 3E).
This result indicated that although I/R and PC initiated the
autophagy process in aged H9C2 cells, they did not lead to its
completion due to unknown reasons. By contrast, although
formononetin increased Beclin-1 expression modestly, it was able to
effectively decrease p62 expression following I/R injury.
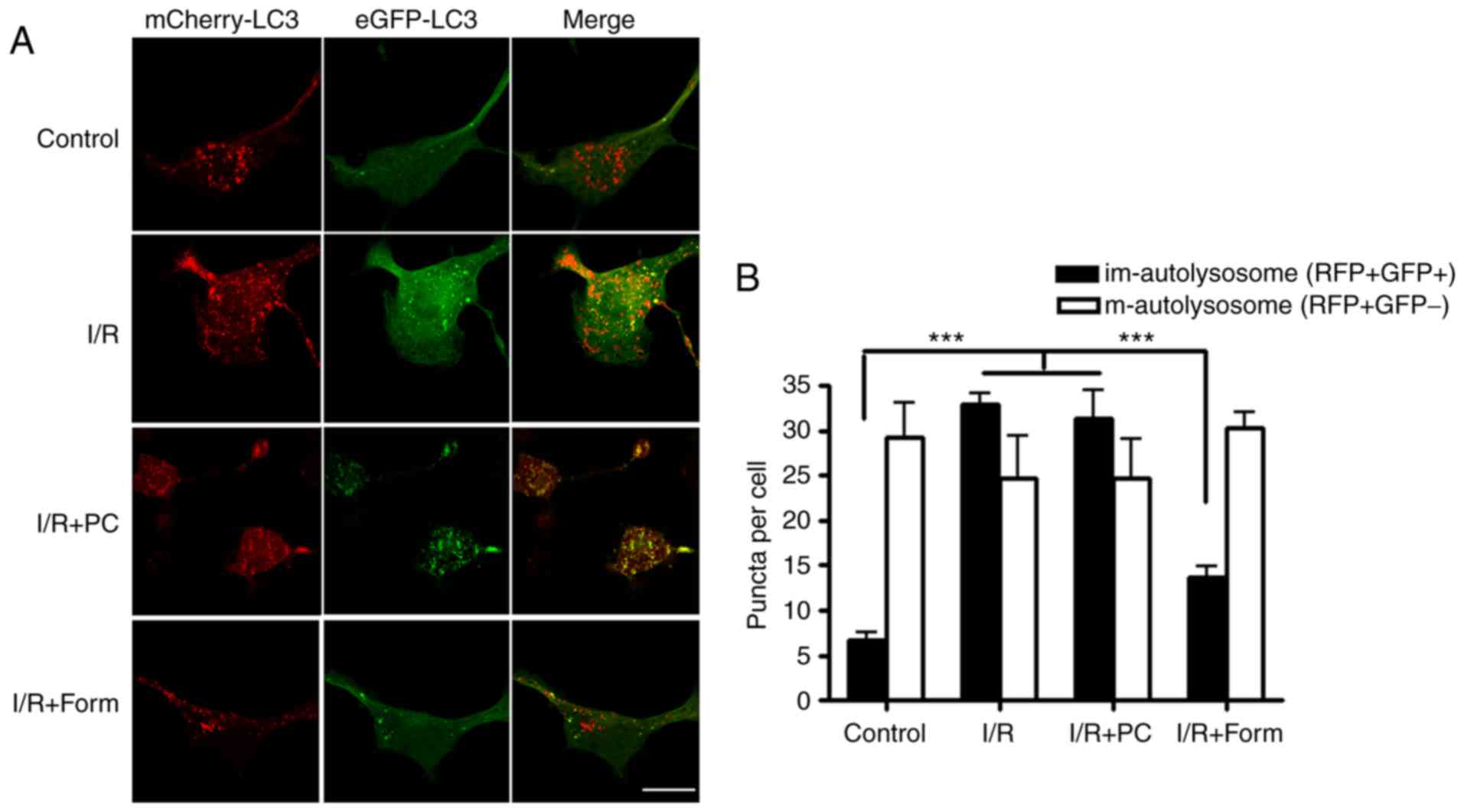
To further investigate how autophagy is involved in
I/R-induced cellular apoptosis, mCherry-EGFP-LC3B was used. As GFP
and mCherry have different pKa values, GFP does not exhibit
fluorescence in an environment with a pH <4.5; however, mCherry
may fluoresce in an environment with a pH as low as 1.0. Determined
by the different physical characteristics of mCherry and GFP, a
green vesicle was taken to indicate a newly-formed autophagosome, a
red vesicle indicated a mature autolysosome and a yellow vesicle
indicated an immature autolysosome. By transfecting this construct
into aged cells, it was identified that there were more yellow
vesicles in the I/R and I/R+PC groups. However, pretreatment with
formononetin was able to decrease the number of yellow
autolysosomes and increase the number of mature autolysosomes
(Fig. 4). These results indicated
that I/R led to a greater number of immature autolysosomes in aged
H9C2 cells, while pretreatment with formononetin rescued this
phenotype.
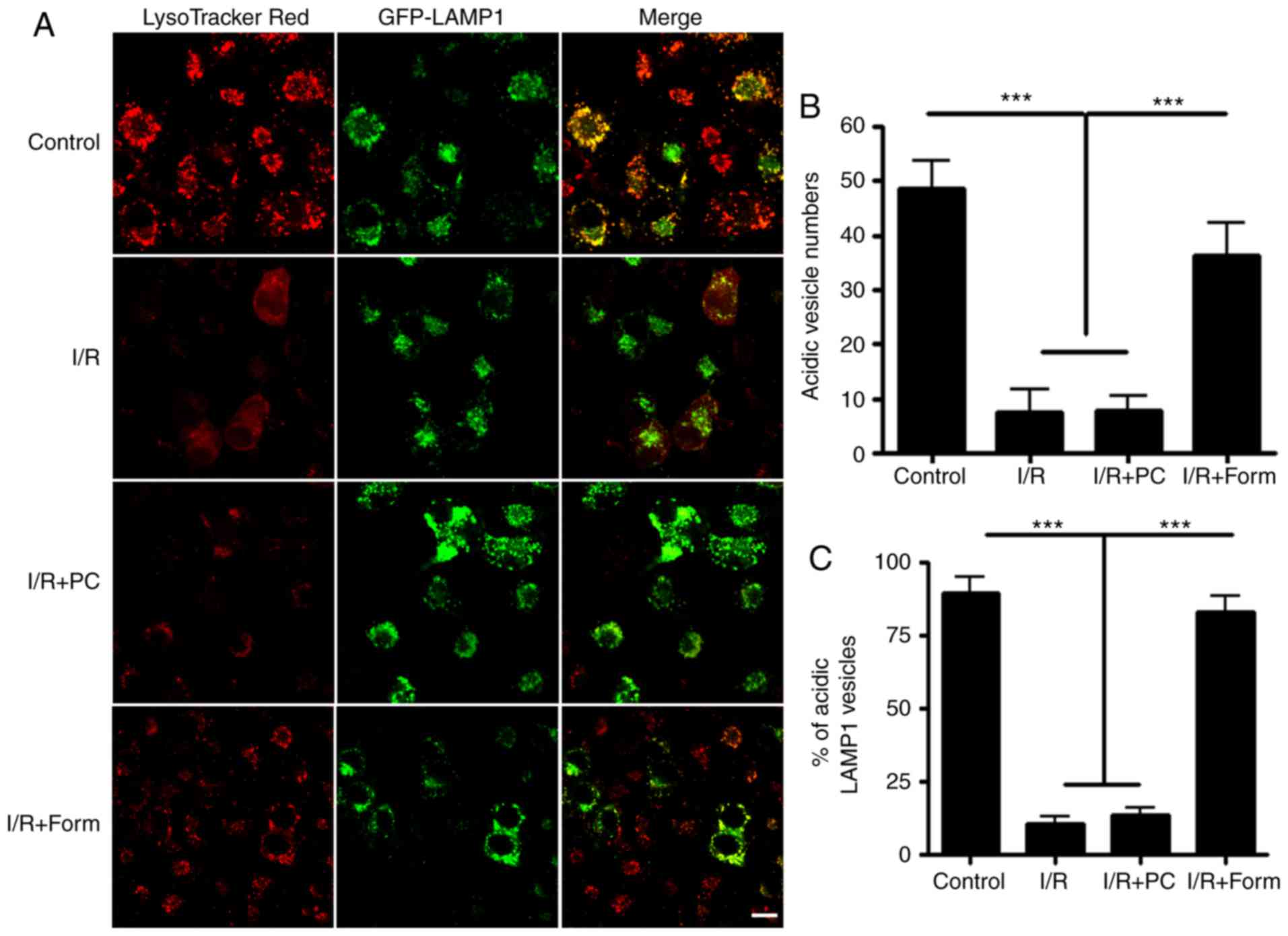
Formononetin regulates autophagy by
modulating lysosomal acidification
To further investigate the mechanisms underlying how
formononetin regulated autophagy, the cells were treated with
LysoTracker Red, an acidic vesicle indicator. By co-transfecting
with GFP-lysosome-associated membrane glycoprotein 1, a lysosome
indicator, it was observed that the majority of lysosomes were
colocalized with LysoTracker in the control group. However,
following I/R injury, there was a significantly decreased number of
acidic vesicles in the aged cells. Finally, it was identified that
formononetin was able to restore the number of acidic vesicles
compared with the I/R or I/R + PC groups (Fig. 5).
Discussion
Myocardial infarction is the irreversible death of
heart cells caused by a prolonged period of oxygen deprivation. A
total of ~1.5 million cases of myocardial infarction occur annually
in the USA (19). Immediate oxygen
recovery is the most efficient way to treat an infarction. However,
the excess of ROS generated following reperfusion may cause severe
side effects, including heart cell apoptosis (20–23).
Therefore, either enhancing endogenous ROS elimination pathways or
applying exogenous medication to inhibit ROS production have become
the most promising methods of avoiding I/R injury.
Autophagy is an endogenous protein degradation
pathway involving the process of autophagosome generation, fusion
with lysosomes and eventual lysosome degradation. Physiologically,
autophagy may degrade incorrectly folded proteins or eliminate
damaged organelles, and this is an important step in preventing any
potential cytotoxicity or stress inside the cell, and subsequently
preventing cellular apoptosis. Due to the natural functions of
autophagy, methods that are able to evoke autophagic activity
efficiently have attracted increasing research interest. Since the
autophagic process comprises a number of key steps, arresting at
any of these may cause the autophagic process to be incomplete. In
the present study, it was demonstrated that although autophagy was
evoked efficiently in aging cells in the I/R+PC group, the protein
clearance inside the autophagosome was blocked in aged cells
following I/R injury.
Recent studies have reported controversial results
on elevated autophagic activity and I/R injury. Certain studies
have demonstrated that autophagy is critical to the elimination of
I/R-induced ROS (24,25), while other reports have
demonstrated that excess autophagic activity may aggravate cellular
apoptosis (26,27). The differences between previous
reports may come from different cell types, experimental designs or
research methods. For example, although certain studies have
counted GFP-LC3 dots as autophagic activity, this marker may only
indicate the stimulation of autophagy, and not a complete
autophagic clearance process. In the present study, GFP-mCherry
LC-3 constructs were used to indicate autophagosomes and
autolysosomes. Combined with p62 protein level detection and
LysoTracker live staining results, it was observed that following
I/R injury, degradation inside the autolysosome/lysosome was
blocked in aged cells. The results of the present study indicated
there were a number of ‘non-functional’ autolysosomes/lysosomes in
the aged cells, which may be considered to be an excess of
autophagy if inappropriate observation methods are used.
Since the ability of aged cells to maintain
metabolic homeostasis is frequently diminished, it was hypothesized
that I/R may cause more severe injury in aged cells. Notably, by
treating the aged cells with hypoxia preconditioning, a well
established model for cell to evoke autophagy and adapt I/R
condition, the present study demonstrated that cell viability was
not markedly restored, which indicated that autophagy may not be
involved in the response of aged cells to I/R injury. However, upon
investigation of the mechanisms, it was demonstrated that the
acidic level of lysosomes in aged cells was impaired by I/R injury.
Thus, lysosome degradation impairment may be considered as the
reason.
Formononetin is a principal isoflavone compound
extracted from Radix Astragali, which has long been used to
treat cardiovascular diseases in traditional Chinese medicine. For
example, formononetin has been reported to be able to reduce
intracellular ROS by inhibiting mitogen activated protein kinase 8
phosphorylation (28); to inhibit
breast cancer cell proliferation by regulating the
phosphatidylinositol 3-kinase (PI3K)/RAC-α serine/threonine-protein
kinase (AKT)/serine/threonine-protein kinase mTOR signaling pathway
(29); to increase adipocyte
thermogenesis by modulating peroxisome proliferator-activated
receptor-γ activity (30); or to
have neuroprotective effects following traumatic brain injury by
increasing microRNA-155 and heme oxygenase-1 expression (31). Regarding I/R injury, formononetin
has been reported to markedly reduce the infarct volume and brain
water accumulation in a rat model of I/R injury via activation of
the PI3K/Akt signaling pathway (32). In the present study, it was
additionally observed that formononetin was able to alleviate
I/R-induced cellular apoptosis in aged isolated hearts or aged H9C2
cells. Sulfonated formononetin additionally exhibits
cardioprotective effects in acute myocardial infarction in rats,
possibly by regulating energy metabolism in cardiac mitochondria
(33). Apart from the
aforementioned literature, the present study is the first, to the
best of our knowledge, to demonstrate that formononetin may protect
aged heart cells from I/R injury by modulating autophagic
activity.
Although the detailed mechanisms remain largely
unknown, the results of the present study demonstrated that
formononetin was able to restore the lysosome acidic level.
H+ concentrations inside lysosome are largely controlled
by the vascular-type ATPase (v-ATPase) protein channel, and a
recent study reported that formononetin was able to phosphorylate
glycogen synthase kinase-3β (GSK-3B) at the Ser-9 site in a
concentration-dependent manner (34). Considering that GSK-3B may regulate
some members of v-ATPase family (35), it may be hypothesized that
formononetin may modulate lysosome acidic level by regulating
GSK-3B activity. In conclusion, the present study demonstrated that
formononetin was able to regulate lysosome H+
concentrations in aged cells following I/R injury, and thus evoke
autophagy and protect cells from I/R injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH analyzed the data and wrote the manuscript. XH
and YL performed the experiments and collected the data.
Ethics approval and consent to
participate
All animal experiments were conducted in compliance
with the Guide for the Care and Use of Laboratory Animals published
by the National Institutes of Health, and were approved by the
Animal Care Committees of Southern Medical University (Guangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Showkathali R and Natarajan A:
Antiplatelet and antithrombin strategies in acute coronary
syndrome: State-of-the-art review. Curr Cardiol Rev. 8:239–249.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei Y, Ruan L, Zhou G, Zhao L, Qi B,
Ouyang P, Jin Z, Zhang C and Liu S: Local ischemic postconditioning
during primary percutaneous coronary intervention: A meta-analysis.
Cardiology. 123:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu J, Wu P, Wang Y, Du YAN, Liu S, Zhang
Y, Zhou N, Xu Z and Yang Z: Ad-HGF improves the cardiac remodeling
of rat following myocardial infarction by upregulating autophagy
and necroptosis and inhibiting apoptosis. Am J Transl Res.
8:4605–4627. 2016.PubMed/NCBI
|
|
4
|
MS, Li Q and Liu Y: Autophagy and
Alzheimer's Disease. Cellular and molecular neurobiology. 1–12
|
|
5
|
Sun M, Asghar SZ and Zhang H: The polarity
protein Par3 regulates APP trafficking and processing through the
endocytic adaptor protein Numb. Neurobiol Dis. 93:1–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cortese A, Tucci A, Piccolo G, Galimberti
CA, Fratta P, Marchioni E, Grampa G, Cereda C, Grieco G, Ricca I,
et al: Novel CLN3 mutation causing autophagic vacuolar myopathy.
Neurology. 82:2072–2076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song H, Yan C, Tian X, Zhu N, Li Y, Liu D,
Liu Y, Liu M, Peng C, Zhang Q, et al: CREG protects from myocardial
ischemia/reperfusion injury by regulating myocardialautophagy and
apoptosis. Biochim Biophys Acta. 1863:1893–1903. 2017. View Article : Google Scholar
|
|
8
|
Yi J, He G, Yang J, Luo Z, Yang X and Luo
X: Heat acclimation regulates the autophagy-lysosome function to
protect against heat stroke-induced brain injury in mice. Cell
Physiol Biochem. 41:101–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu CL, Chen CH, Hwang CS, Chen SD, Hwang
WC and Yang DI: Roles of p62 in BDNF-dependent autophagy
suppression and neuroprotection against mitochondrial dysfunction
in rat cortical neurons. J Neurochem. 140:845–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Gao J, Sun W, Li L, Wang Y, Bai S,
Li X, Wang R, Wu L, Li H and Xu C: Involvement of exogenous H2S in
recovery of cardioprotection from ischemic post-conditioning via
increase of autophagy in the aged hearts. Int J Cardiol.
220:681–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HL, Zhou QH, Xu MB, Zhou XL and Zheng
GQ: Astragaloside IV for experimental focal cerebral ischemia:
Preclinical evidence and possible mechanisms. Oxid Med Cell Longev.
2017:84243262017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian Z, Liu SB, Wang YC, Li XQ, Zheng LH
and Zhao MG: Neuroprotective effects of formononetin against
NMDA-induced apoptosis in cortical neurons. Phytother Res.
27:1770–1775. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q and Sun M: Effects of potassium ion
channels in term pregnant myometrium. J Obstet Gynaecol Res.
38:479author reply 480. –481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Wang Y, Wei C, Bai S, Zhao Y, Li H,
Wu B, Wang R, Wu L and Xu C: Mediation of exogenous hydrogen
sulfide in recovery of ischemic post-conditioning-induced
cardioprotection via down-regulating oxidative stress and
up-regulating PI3K/Akt/GSK-3β pathway in isolated aging rat hearts.
Cell Biosci. 5:112015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Ye Z, Luo H, Sun M, Li M, Fan D and
Chui D: Inhalative formaldehyde exposure enhances aggressive
behavior and disturbs monoamines in frontal cortex synaptosome of
male rats. Neurosci Lett. 464:113–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Canene-Adams K: Preparation of
formalin-fixed paraffin-embedded tissue for immunohistochemistry.
Methods Enzymol. 533:225–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yue Z, Rong J, Ping W, Bing Y, Xin Y, Feng
LD and Yaping W: Gene expression of the p16(INK4a)-Rb and
p19(Arf)-p53-p21(Cip/Waf1) signaling pathways in the regulation of
hematopoietic stem cell aging by ginsenoside Rg1. Genet Mol Res.
13:10086–10096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the American Heart Association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Y, Zhou L, Sun M, Zhou T, Zhong K, Wang
H, Liu Y, Liu X, Xiao R, Ge J, et al: Xylocoside G reduces
amyloid-β induced neurotoxicity by inhibiting NF-κB signaling
pathway in neuronal cells. J Alzheimers Dis. 30:263–275. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu J, Sun M, Chen Z, Lu J, Liu Y, Zhou L,
Xu X, Fan D and Chui D: Magnesium modulates amyloid-beta protein
precursor trafficking and processing. J Alzheimers Dis.
20:1091–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Sun M, Yang H, Tian X, Tong Y,
Zhou T, Zhang T, Fu Y, Guo X, Fan D, et al: Hypoxia inducible
factor 1α mediates up regulation of neprilysin by histone
deacetylase1 under hypoxia condition in neuroblastoma cells. J
Neurochem. 131:4–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding W, Cai Y, Wang W, Ji L, Dong Y and
Zhang X, Su M, Liu J, Lu G and Zhang X: Adiponectin protects the
kidney against chronic intermittent hypoxia-induced injury through
inhibiting endoplasmic reticulum stress. Sleep Breath.
20:1069–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan T, Chen L, Huang Z, Wang W, Zhang B,
Xu Y, Mao Z, Hu H and Geng Q: Autophagy activation by rapamycin
before hypoxia-reoxygenation reduces endoplasmic reticulum stress
in alveolar epithelial cells. Cell Physiol Biochem. 41:79–90. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ning Y, Li Z and Qiu Z: FOXO1 silence
aggravates oxidative stress-promoted apoptosis in cardiomyocytes by
reducing autophagy. J Toxicol Sci. 40:637–645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia Y, Liu Y, Xia T, Li X, Huo C, Jia X,
Wang L, Xu R, Wang N, Zhang M, et al: Activation of
volume-sensitive Cl-channel mediates autophagy-related cell death
in myocardial ischaemia/reperfusion injury. Oncotarget.
7:39345–39362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z,
Dai K, Wang C and Huang W: Berberine alleviates cardiac
ischemia/reperfusion injury by inhibiting excessive autophagy
cardiomyocytes. Eur J Pharmacol. 762:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee H, Lee D, Kang KS, Song JH and Choi
YK: Inhibition of intracellular ROS accumulation by formononetin
attenuates cisplatin-mediated apoptosis in LLC-PK1 cells. Int J Mol
Sci. 19:E8132018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou R, Chen H, Chen J, Chen X, Wen Y and
Xu L: Extract from Astragalus membranaceus inhibit breast cancer
cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC
Complement Altern Med. 18:832018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nie T, Zhao S, Mao L, Yang Y, Sun W, Lin
X, Liu S, Li K, Sun Y, Li P, et al: A natural compound,
formononetin, extracted from astragalus membranaceus increase
adipocyte thermogenesis by modulating PPARγ activity. Br J
Pharmacol. 175:1439–1450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Z, Wang Y, Zeng G, Zheng X, Wang W,
Ling Y, Tang H and Zhang J: Increased miR-155 and heme oxygenase-1
expression is involved in the protective effects of formononetin in
traumatic brain injury in rats. Am J Transl Res. 9:5653–5661.
2017.PubMed/NCBI
|
|
32
|
Liang K, Ye Y, Wang Y, Zhang J and Li C:
Formononetin mediates neuroprotection against cerebral
ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2
ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci.
344:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Tang X, Tian J, Li C, Zhang G,
Jiang W and Zhang Z: Cardioprotective effect of sulphonated
formononetin on acute myocardial infarction in rats. Basic Clin
Pharmacol Toxicol. 108:390–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng Y, Xia Z, Han Y and Rong J: Plant
natural product formononetin protects rat cardiomyocyte H9c2 cells
against oxygen glucose deprivation and reoxygenation via inhibiting
ROS formation and promoting GSK-3β phosphorylation. Oxid Med Cell
Longev. 2016:20608742016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Avrahami L, Farfara D, Shaham-Kol M,
Vassar R, Frenkel D and Eldar-Finkelman H: Inhibition of glycogen
synthase kinase-3 ameliorates β-amyloid pathology and restores
lysosomal acidification and mammalian target of rapamycin activity
in the Alzheimer disease mouse model: In vivo and in vitro studies.
J Biol Chem. 288:1295–1306. 2013. View Article : Google Scholar : PubMed/NCBI
|