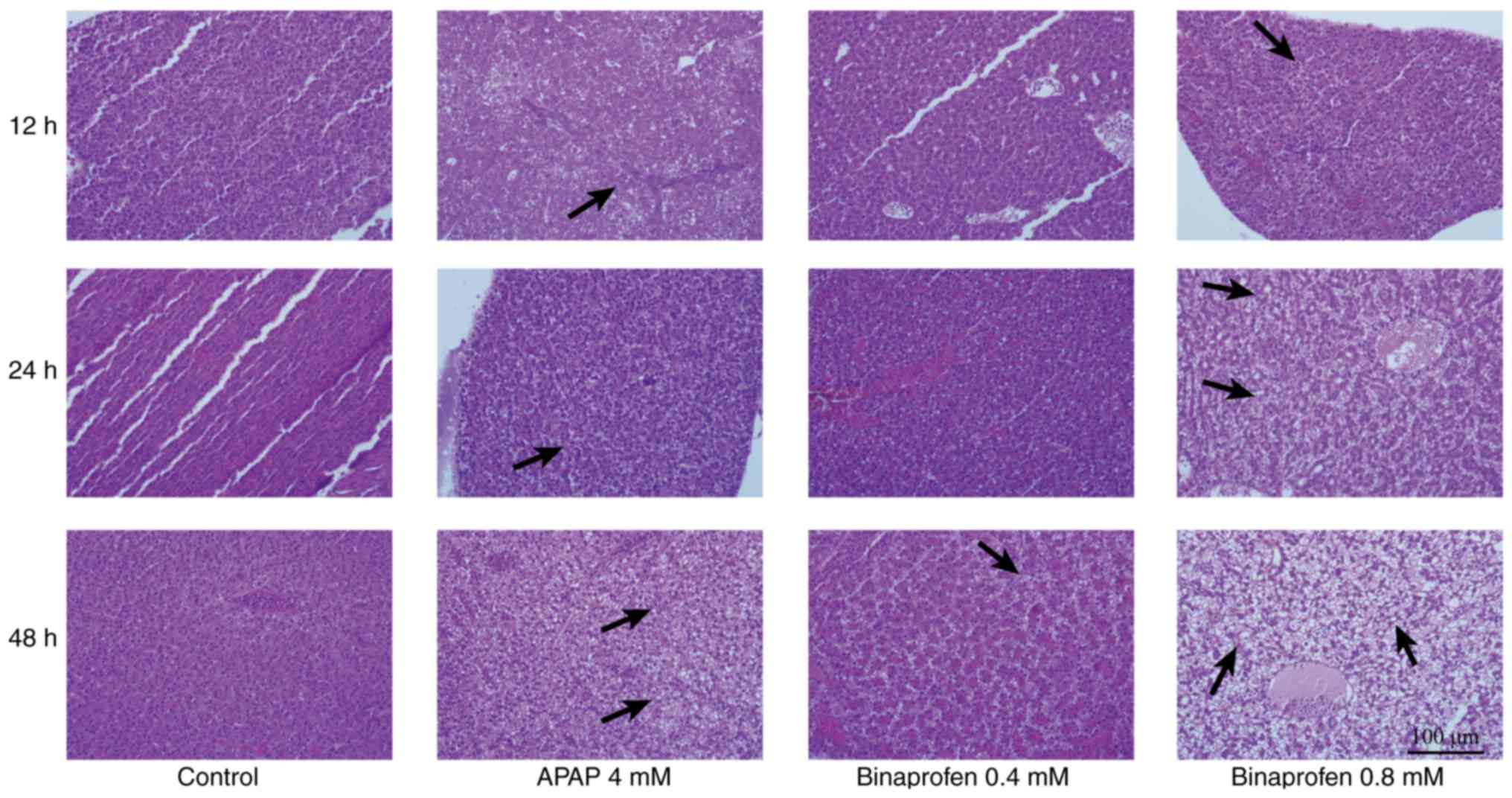
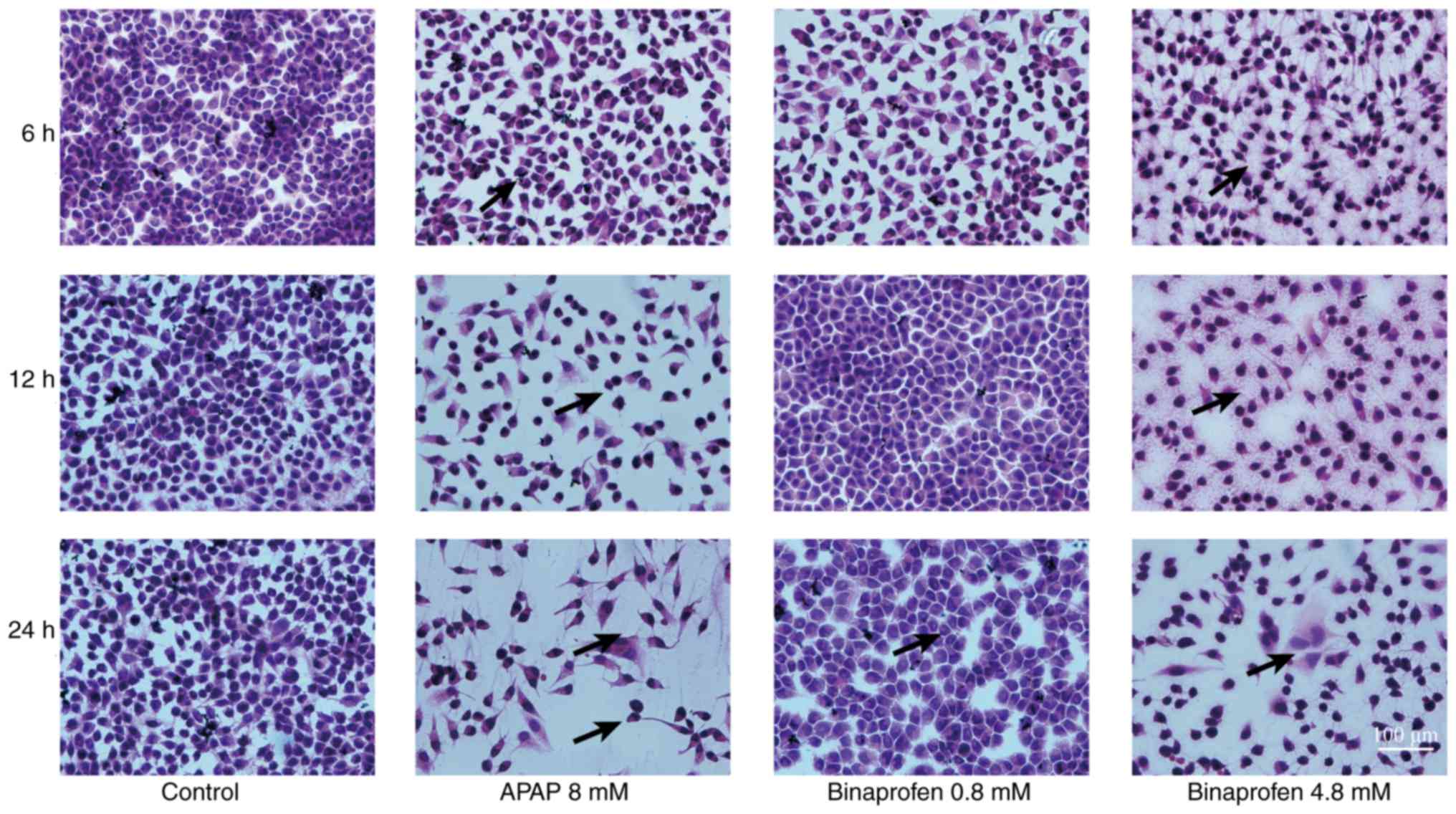
|
1
|
Koller T, Galambosova M, Filakovska S,
Kubincova M, Hlavaty T, Toth J, Krajcovicova A and Payer J:
Drug-induced liver injury in inflammatory bowel disease: 1-year
prospective observational study. World J Gastroenterol.
23:4102–4111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kotsampasakou E, Montanari F and Ecker GF:
Predicting drug-induced liver injury: The importance of data
curation. Toxicology. 389:139–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McEuen K, Borlak J, Tong W and Chen M:
Associations of drug lipophilicity and extent of metabolism with
drug-induced liver injury. Int J Mol Sci. 18:E13352017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Juthani VV, Clearfield E and Chuck RS:
Non-steroidal anti-inflammatory drugs versus corticosteroids for
controlling inflammation after uncomplicated cataract surgery.
Cochrane Database Syst Rev. 7:CD0105162017.PubMed/NCBI
|
|
5
|
Mishra A, Amalakara J, Avula H and Reddy
K: Effect of diclofenac mouthwash on postoperative pain after
periodontal surgery. J Clin Diagn Res. 11:ZC24–ZC26.
2017.PubMed/NCBI
|
|
6
|
Zamir Q and Nadeem A: Non-steroidal
anti-inflammatory drugs vs. Paracetamol: Drug availability,
patients' preference and knowledge of toxicity. J Ayub Med Coll
Abbottabad. 28:746–749. 2016.PubMed/NCBI
|
|
7
|
Savjani JK, Mulamkattil S, Variya B and
Patel S: Molecular docking, synthesis and biological screening of
mefenamic acid derivatives as anti-inflammatory agents. Eur J
Pharmacol. 801:28–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin JY, Song I, Lee JH, Yoon JL, Kwon JS
and Park BJ: Differential risk of peptic ulcer among users of
antidepressants combined with nonsteroidal anti-inflammatory drugs.
J Clin Psychopharmacol. 37:239–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rostom A, Goldkind L and Laine L:
Nonsteroidal anti-inflammatory drugs and hepatic toxicity: A
systematic review of randomized controlled trials in arthritis
patients. Clin Gastroenterol Hepatol. 3:489–498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rathi C, Pipaliya N, Patel R, Ingle M,
Phadke A and Sawant P: Drug induced liver injury at a tertiary
hospital in India: Etiology, clinical features and predictors of
mortality. Ann Hepatol. 16:442–450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soleimanpour M, Imani F, Safari S, Sanaie
S, Soleimanpour H, Ameli H and Alavian SM: The role of
non-steroidal anti-inflammatory drugs (NSAIDs) in the treatment of
patients with hepatic disease: A review article. Anesth Pain Med.
6:e378222016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bunchorntavakul C and Reddy KR:
Acetaminophen (APAP or N-Acetyl-p-Aminophenol) and acute liver
failure. Clin Liver Dis. 22:325–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Q, Chen G, Zhou Q and Jin R:
Comparison of hepatotoxicity and toxic mechanisms of matrine and
oxymatrine using in vivo and in vitro models. Chinese J Comparative
Med. 28:44–50. 2018.(In Chinese).
|
|
14
|
Xiao Y, Lin M, Jiang X, Ye J, Guo T, Shi Y
and Bian X: The recent advance on liver cancer stem cells:
Biomarkers, separation, and therapy. Anal Cell Pathol (Amst).
2017:51086532017.PubMed/NCBI
|
|
15
|
Wang W, Ou HY, Xiao BQ, Huang YJ, Yang W
and Wang QS: The study of abirritation of a first type of new drug,
felbinac trometamol injection. Chin J Med Guide. 11:1327–1332.
2009.
|
|
16
|
Wang W, Ou H and Liang H: Preliminary
pharmacodynamics and safety studies of a first type of new drug,
felbinac trometamol injection. Chin J Ethnomedicine Ethnopharmacy.
12:3–4. 2009.(In Chinese).
|
|
17
|
Zhang C, Cui X, Yang Y, Gao F, Sun Y, Gu
J, Fawcett JP, Yang W and Wang W: Pharmacokinetics of felbinac
after intravenous administration of felbinac trometamol in rats.
Xenobiotica. 41:340–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
AVMA Guidelines for the Euthanasia of
Animals: 2013 Edition. Version 2013.0.1. American Veterinary
Medical Association; Schaumburg, IL: 2013
|
|
19
|
Riera TV, Wigle TJ and Copeland RA:
Characterization of inhibitor binding through multiple inhibitor
analysis: A novel local fitting method. Methods Mol Biol.
1439:33–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagendra S, Vanlalhmuaka, Verma S, Tuteja
U and Thavachelvam K: Recombinant expression of bacillus
anthracis lethal toxin components of indian isolate in
escherichia coli and determination of its acute toxicity
level in mouse model. Toxicon. 108:108–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao CC, Day YJ, Lee HC, Liou JT, Chou AH
and Liu FC: ERK signaling pathway plays a key role in baicalin
protection against acetaminophen-induced liver injury. Am J Chin
Med. 45:105–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knodell RG, Ishak KG, Black WC, Chen TS,
Craig R, Kaplowitz N, Kiernan TW and Wollman J: Formulation and
application of a numerical scoring system for assessing
histological activity in asymptomatic chronic active hepatitis.
Hepatology. 1:431–435. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chevallier M, Guerret S, Chossegros P,
Gerard F and Grimaud JA: A histological semiqunatitative scoring
system for evaluation of hepatic fibrosis in needle liver biopsy
specimens: Comparison with morphometric studies. Hepatology.
20:349–355. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar M, Sarin SK, Hissar S, Pande C,
Sakhuja P, Sharma BC, Chauhan R and Bose S: Virologic and
histologic features of chronic hepatitis B virus-infected
asymptomatic patients with persistently normal ALT.
Gastroenterology. 134:1376–1384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malinoski FJ: Strenuous exercise
simulating hepatic injury during vaccine trials. Vaccine. 10:39–42.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith JE, Garbutt G, Lopes P and Pedoe
Tunstall D: Effects of prolonged strenuous exercise (marathon
running) on biochemical and haematological markers used in the
investigation of patients in the emergency department. Br J Sports
Med. 38:292–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen J, Zhang J, Wen J, Ming Q, Zhang J
and Xu Y: Correlation of serum alanine aminotransferase and
aspartate aminotransferase with coronary heart disease. Int J Clin
Exp Med. 8:4399–4404. 2015.PubMed/NCBI
|
|
28
|
Halkes S, van den Berg A, Hoekstra M, du
Pont J and Kreis R: Transaminase and alkaline phosphatase activity
in the serum of burn patients treated with highly purified tannic
acid. Burns. 28:449–453. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moss DW and Henderson AR: EnzymesTietz
Fundamentals of Clinical Chemistry. Burtis CA and Ashwood ER: 5th
edition. WB Saunders; Philadelphia, PA: pp. 735–896. 1999
|
|
30
|
Hu GH and Lü XS: Effect of normothermic
liver ischemic preconditioning on the expression of
apoptosis-regulating genes C-jun and Bcl-XL in rats. World J
Gastroenterol. 11:2579–2582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ashamiss F, Wierzbicki Z, Chrzanowska A,
Scibior D, Pacholczyk M, Kosieradzki M, Lagiewska B, Porembska Z
and Rowiński W: Clinical significance of arginase after liver
transplantation. Ann Transplant. 9:58–60. 2004.PubMed/NCBI
|
|
32
|
Chen GF and Baylis C: In vivo renal
arginine release is impaired throughout development of chronic
kidney disease. Am J Physiol Renal Physiol. 298:F95–F102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stéphenne X, Najimi M, Sibille C, Nassogne
MC, Smets F and Sokal EM: Sustained engraftment and tissue enzyme
activity after liver cell transplantation for argininosuccinate
lyase deficiency. Gastroenterology. 130:1317–1323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murayama H, Ikemoto M, Fukuda Y, Tsunekawa
S and Nagata A: Advantage of serum type-I arginase and ornithine
carbamoyltransferase in the evaluation of acute and chronic liver
damage induced by thioacetamide in rats. Clin Chim Acta. 375:63–68.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ray DC, Robbins AG, Howie AF, Beckett GJ
and Drummond GB: Effect of spinal anaesthesia on plasma
concentrations of glutathione S-transferase. Br J Anaesth.
88:285–287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahmed AM, Abdel-Tawab AH and Morsy AT:
Alpha-Glutathione S-transferase and serum aminotransferases in
schistosomiasis mansoni patients with or without hepatitis C
virus. J Egypt Soc Parasitol. 38:561–572. 2008.PubMed/NCBI
|
|
37
|
Ouaissi A, Ouaissi M and Sereno D:
Glutathione S-transferases and related proteins from pathogenic
human parasites behave as immunomodulatory factors. Immunol Lett.
81:159–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang X, Lei J, Zhang Y, Tang X, Zheng Y
and Chen J: The diagnostic value and limitation of total serum bile
acid determined enzymatically. Zhonghua Nei Ke Za Zhi. 40:16–18.
2001.(In Chinese). PubMed/NCBI
|
|
39
|
Negoro S, Tanaka A and Takikawa H: Urinary
bile acid sulfate levels in patients with hepatitis C virus related
chronic liver diseases. Hepatol Res. 39:760–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neale G, Lewis B, Weaver V and
Panveliwalla D: Serum total bile acids in liver diseases. Gut.
12:145–152. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Caccialanza R, Palladini G, Klersy C,
Cereda E, Bonardi C, Quarleri L, Vadacca G, Albertini R and Merlini
G: Serum prealbumin: An independent marker of short-term energy
intake in the presence of multiple-organ disease involvement.
Nutrition. 29:580–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aliyazicioglu Y, Dagdemir A, Dilber C and
Albayrak D: Serum prealbumin levels in hepatotoxicity of
chemotherapy in children with cancer. Bratisl Lek Listy.
113:368–371. 2012.PubMed/NCBI
|
|
43
|
Saito M, Seo Y, Yano Y, Miki A, Yoshida M
and Azuma T: Short-term reductions in non-protein respiratory
quotient and prealbumin can be associated with the long-term
deterioration of liver function after transcatheter arterial
chemoembolization in patients with hepatocellular carcinoma. J
Gastroenterol. 47:704–714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chung YH, Kim JA, Song BC, Song IH, Koh
MS, Lee HC, Yu E, Lee YS and Suh DJ: Isocitrate dehydrogenase as a
marker of centrilobular hepatic necrosis in the experimental model
of rats. J Gastroenterol Hepatol. 16:328–332. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Racine-Samson L, Scoazec JY, D'Errico A,
Fiorentino M, Christa L, Moreau A, Roda C, Grigioni WF and Feldman
G: The metabolic organization of the adult human liver: A
comparative tudy of normal, fibrotic, and cirrhotic liver tissue.
Hepatology. 24:104–113. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pathiratne A, Pathiratne KA and De Seram
PK: Assessment of biological effects of pollutants in a hyper
eutrophic tropical water body, Lake Beira, Sri Lanka using multiple
biomarker responses of resident fish, Nile tilapia (Oreochromis
niloticus). Ecotoxicology. 19:1019–1026. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Harrill AH, Roach J, Fier I, Eaddy JS,
Kurtz CL, Antoine DJ, Spencer DM, Kishimoto TK, Pisetsky DS, Park
BK and Watkins PB: The effects of heparins on the liver:
Application of mechanistic serum biomarkers in a randomized study
in healthy volunteers. Clin Pharmacol Ther. 92:214–220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Murayama H, Ikemoto M and Hamaoki M:
Ornithine carbamyltransferase is a sensitive marker for
alcohol-induced liver injury. Clin Chim Acta. 401:100–104. 2009.
View Article : Google Scholar : PubMed/NCBI
|