Introduction
Currently, diabetes is regarded as an epidemic
disease with an increasing incidence (1) and it has become the third major
disease after cardiovascular and cerebrovascular diseases and
cancer (2). Diabetes is divided
into type 1 and type 2, with type 2 diabetes accounting for 90% of
all cases, and caused a heavy burden on public health and
socioeconomic development of all nations. (3,4).
Diabetes is a chronic metabolic disease characterized by
hyperglycemia caused by a lack of insulin or impaired insulin
sensitivity (5). It has been
reported that the pathogenesis of diabetes may be associated with
an imbalance between the production of reactive oxygen species
(ROS) and antioxidant defense systems (6). Oxidative stress refers to the
imbalance between the production of ROS and reactive nitrogen
species and the clearance of antioxidant defense systems in the
body, which resulted in biological macromolecular damage including
tissue cells, proteins and nucleic acids (7). Currently, the main therapy for
diabetes aims to lower blood glucose with physical activities,
nutritional intervention, oral hypoglycemic agents, insulin and
glucagonlike peptide-1 receptor agonists (8). In addition, a number of studies have
revealed that 30–40% of patients with diabetes have kidney damage,
and there is no effective method for the prevention and treatment
of diabetic nephropathy (1,4,9).
Traditional Chinese Medicine (TCM) has been used to
treat various diseases in China for centuries, and natural products
extracted from herbs or plants are crucial resources for finding
novel candidate drugs with reliable effects and fewer side effects
(10,11). TCM has commonly been used to treat
diabetes since ancient times and Chinese medicine formulas with
antidiabetic activities are well developed (12). Rhizoma of Polygonatum
kingianum Coll. et Hemsl or Polygonatum sibiricum Red
(Polygonati rhizoma), a well-known herbal medicine in China,
has been used for the treatment of diabetes, cough, anorexia,
spleen deficiency and stomach diseases (13). In addition, according to various
Chinese traditional prescriptions, Polygonati rhizoma has
been widely used to treat diabetes (14).
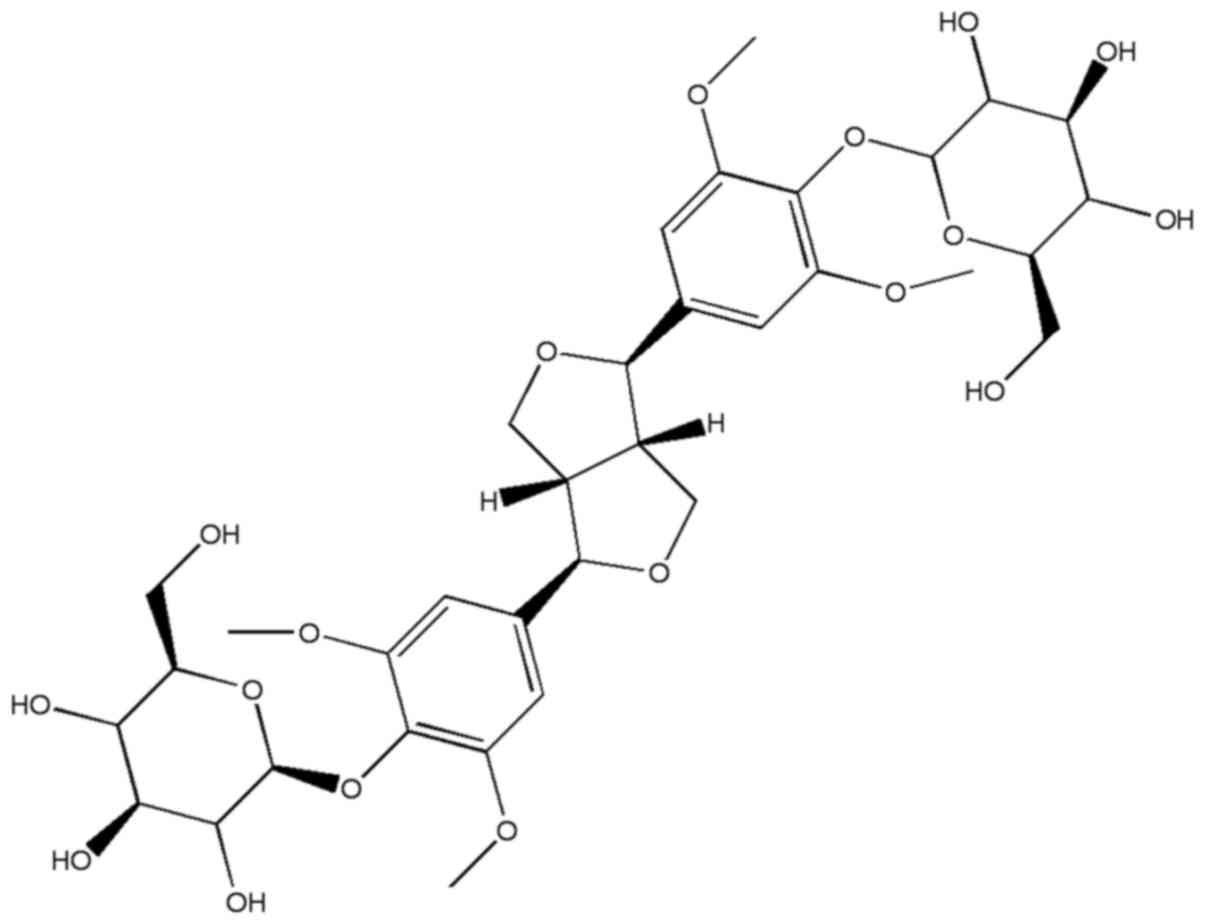
Syringaresinol-di-O-β-D-glucoside (SOG), whose
chemical structure presented in Fig.
1, is a phenolic compound isolated from Polygonati
rhizome. The activities of this compound have not been extensively
investigated (15). Therefore, the
present study aimed to elucidate the antidiabetic effect of SOG on
streptozocin (STZ)-induced experimental diabetic mice and determine
the potential underlying mechanisms.
Materials and methods
Chemicals
Syringaresinol-di-O-β-D-glucoside
(SOG; cat. no. E-0542; http://www.tautobiotech.com/BRM/ProductShow.asp?ArticleID=542)
was purchased from Shanghai TAUTO BIOTECH Co., Ltd. (Shanghai,
China). STZ (cat. no. S0130) and dimethyl sulfoxide (DMSO) were
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Nitrotyrosine (NT) antibody (cat. no. DMABT-Z60522) was purchased
from Creative Diagnostics, Shirley, NY, USA. Insulin (cat. no.
ab100578) and interleukin-6 (IL-6; cat. no. ab100712) ELISA kits,
and transforming growth factor-β1 (TGF-β1; cat. no. ab9758) and
GAPDH (cat. no. ab8245) antibodies, were purchased from Abcam
(Cambridge, MA, USA). Total cholesterol (TC; cat. no. CY81061),
triglyceride (TG; cat. no. CY81057), high-density lipoprotein
cholesterol (HDL-C; cat. no. HZL2329), total protein (cat. no.
HZL2206), low-density lipoprotein cholesterol (LDL-C; cat. no.
HZL2321), free fatty acid (FFA; cat. no. CY81055), malondialdehyde
(MDA; cat. no. EY2), superoxide dismutase (SOD; cat. no. FY1),
catalase (CAT; cat. no. HZL2444), total antioxidant capacity
(T-AOC; cat. no. FG5), aspartate transaminase (AST; cat. no.
HZL2382), alanine transaminase (ALT; cat. no. SEA207Ra) and
alkaline phosphatase (ALP; cat. no. HZL2351) kits were produced by
Shanghai Chaoyan Biological Technology Co., Ltd., (Shanghai, China;
http://biosis.biogo.net/sell/typeid-5638.shtml).
Bicinchoninic acid (BCA) protein assay reagent was purchased from
Beyotime Institute of Biotechnology (Haimen, China). All other
chemicals used in the present study were of analytical reagent
grade.
Animals
A total of 50 male specific pathogen-free mice (6–8
weeks old; weight, 18–23 g) were purchased from the Laboratory
Animal Center of Fujian Medical University (Fuzhou, China). The
animals were maintained under controlled conditions (22±2°C, 30–70%
humidity and <0.03% CO2) with a 12-h light/dark cycle
and free access to food and water. All animal treatments were
strictly in accordance with international ethical guidelines and
the National Institutes of Health Guide concerning the Care and Use
of Laboratory Animals (16), and
the experiments were performed with the approval of the Animal
Experimentation Ethics Committee of the Affiliated Hospital of
Nanjing University of Chinese Medicine (Nanjing, China).
Animal model establishment and
grouping
A diabetic animal model was established according to
the previously reported method with minor modifications (17). A total 50 male mice were divided
into five groups (n=10/group): Normal group, model group, 25 mg/kg
SOG-treated group, 50 mg/kg SOG-treated group and 75 mg/kg
SOG-treated group. All mice excluding the normal group were
injected with STZ dissolved in 0.1 mol/l citric acid buffer
solution (Chengdu Chemical Co., Ltd., Chengdu, China; 100 mg/kg)
intravenously. Mice in the normal group were treated with an equal
volume of 0.01 mol/l citric acid buffer solution. After 3 days, the
fasting blood glucose (FBG) of mice treated with STZ was measured
using an Accu-Chek Performa monitor. STZ-treated mice with FBG
>11.1 mmol/l were considered to be diabetic. Following
successful establishment of the diabetic model, mice in the normal
and the model group were treated with normal saline (containing
0.5% DMSO) and mice in SOG-treated groups were treated with SOG at
25, 50 or 75 mg/kg by means of intragastric administration daily
for 2 weeks. SOG was dissolved in normal saline (containing 0.5%
DMSO).
Effects of SOG on FBG, body weight,
water intake, food intake and organ indexes (kidney, pancreas,
spleen and liver) of diabetic mice induced by STZ
During administration of SOG for 2 weeks, the water
and food intake of mice were recorded each day. On days 0 (a day
prior to the administration of the first dose of SOG) and 15, the
body weight of mice was measured and the weight gain was
calculated. On days 0, 7 and 15, the FBG of mice was determined
using glucose testing strips. Blood samples were collected from the
caudal vein of mice. Following the final administration of SOG,
mice were sacrificed the next day by cervical dislocation. The
organs were separated and the organ index was calculated according
to the following formula: Organ index (g/100
g)=WOrgan/WBody, where W stands for
weight.
Effect of SOG on serum fasting
insulin, pancreatic insulin and pancreatic IL-6 levels in diabetic
mice induced by STZ
Following treatment with SOG for 2 weeks, the mice
were fasted for 12 h with free access to water. A total of 50 µl
serum was obtained from whole blood by centrifugation for 5 min at
10,000 × g at 4°C and serum fasting insulin of mice was measured
using a commercial insulin ELISA kit (Abcam; cat. no. ab100578),
according to manufacturer's protocol. In addition, the pancreas
were treated with lysis buffer (Abcam; cat. no. ab156035) for 10
min and centrifuged at 4°C for 5 min (10,000 × g) and then the
pancreatic levels of insulin and IL-6 in were also detected using
insulin and IL-6 ELISA kits (Abcam; cat. no. ab100712),
respectively.
Effect of SOG on TC, TG, HDL-C, LDL-C,
very low-density lipoprotein cholesterol (VLDL-C) and FFA in the
serum of diabetic mice induced by STZ
The levels of TC, TG, HDL-C, LDL-C and FFA in the
serum of STZ-induced diabetic mice after 12 h of fasting were
measured using TC, TG, HDL-C, LDL-C and FFA kits, respectively. The
value of VLDL-C in the serum was calculated according to the
Friedewald formula reported by previous study (17): VLDL-C=TG/5.
Effect of SOG on kidney TC, TG and
total protein levels in diabetic mice induced by STZ
Kidneys were treated with lysis buffer (Abcam; cat.
no. ab156035) for 10 min and centrifuged at 4°C for 5 min (10,000 ×
g) and then the levels of kidney TC and TG in STZ-induced diabetic
mice were determined using TC and TG kits, according to the
manufacturer's protocols. The content of kidney protein in
STZ-induced diabetic mice was measured using a BCA protein assay
reagent.
Effect of SOG on MDA, SOD, CAT, T-AOC,
AST, ALT and ALP in the kidneys of diabetic mice induced by
STZ
Following the final administration of SOG, mice were
fasted for 12 h and the blood samples were collected from the
eyeballs and centrifuged for 10 min (3,500 × g) at 4°C to obtain
the serum. The levels of MDA, SOD, CAT, T-AOC, AST, ALT and ALP in
the serum of STZ-induced diabetic mice were measured using
commercial ELISA kits for MDA (cat. no. EY2), SOD (cat. no. FY1),
CAT (cat. no. HZL2444), T-AOC (cat. no. FG5), AST (cat. no.
HZL2382), ALT (cat. no. SEA207Ra) and ALP (cat. no. HZL2351) kits,
respectively following the manufacturers' protocol.
Western blot analysis
The kidney tissues of mice were cut into small
pieces and treated with RIPA lysis buffer (Abcam; cat. no.
ab156035). The supernatant was obtained by centrifugation at 12,000
× g for 15 min at 4°C. Protein concentration was determined using
BCA protein assay reagent (Beyotime Institute of Biotechnology;
cat. no. P0010). Subsequently, 35 µg proteins were separated by 12%
SDS-PAGE and transferred to polyvinylidene difluoride membranes.
Membranes were blocked with 5% fat-free dry milk in 1X TBS-Tween-20
(containing 0.1% Tween-20) for 2 h at room temperature. Membranes
were subsequently incubated with primary antibodies specific to NT
(1:1,000), TGF-β1 (1:1,000) and GAPDH (1:2,000) at 4°C overnight.
Membranes were further incubated with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; Beyotime
Institute of Biotechnology; cat. no. A0286) at room temperature for
1 h. The proteins were detected using the Beyo ECL Star reagents
(Beyotime Institute of Biotechnology; cat. no. P0018A). Signals
were quantified using ImageQuant LAS 4000 Imaging system (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA). To normalize for
protein loading, an antibody against GAPDH was used and protein
expression levels were expressed relative to GAPDH.
Statistical analysis
All tests were conducted in triplicate and all data
are presented as the mean ± standard deviation. The significance of
differences between groups was analyzed by one-way analysis of
variance followed by a Dunnett's multiple comparisons post-hoc test
using SPSS software (SPSS for Windows version 19.0; IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of SOG on FBG, body weight,
water intake, food intake and organ indexes of diabetic mice
induced by STZ
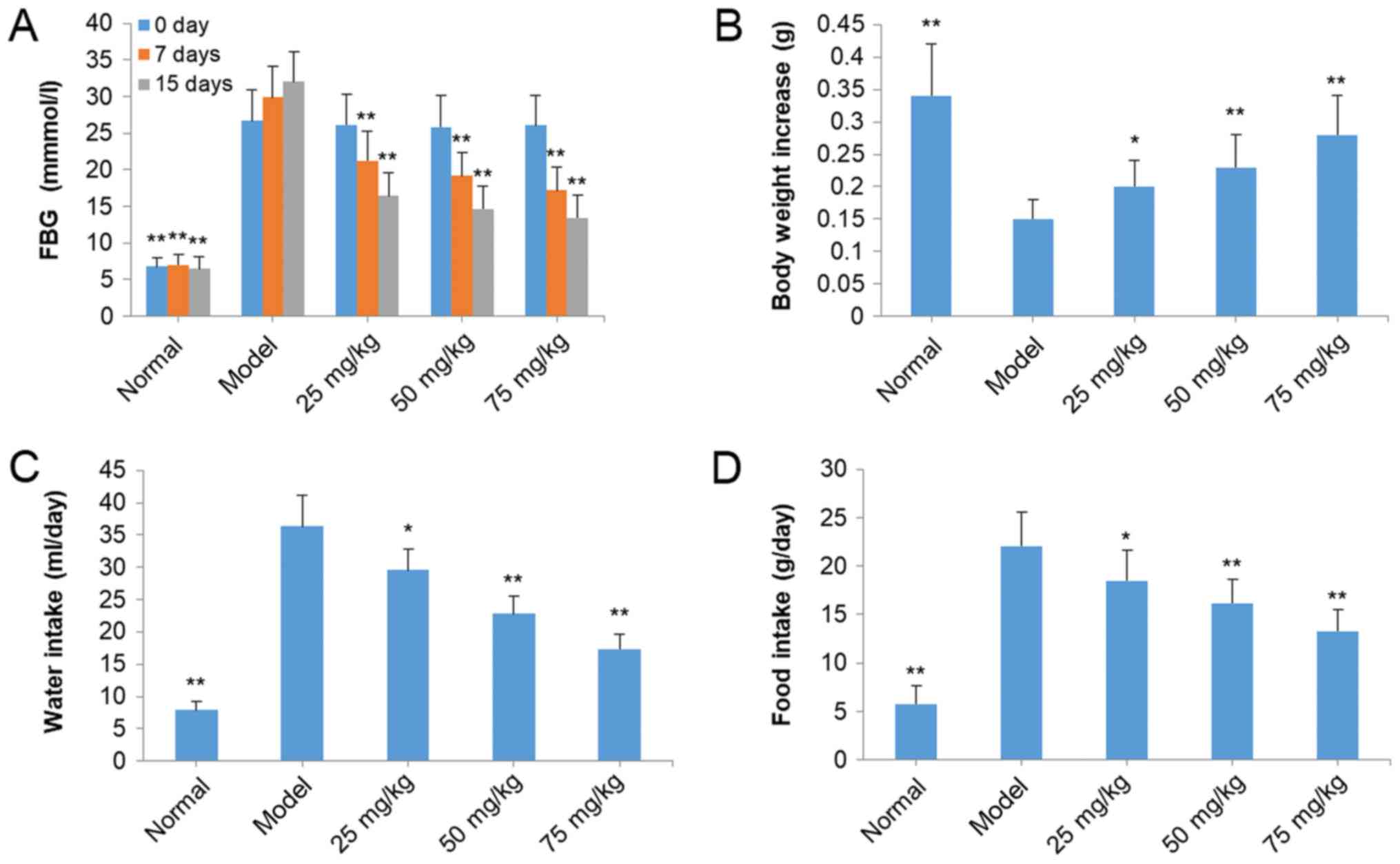
The FBG levels in model mice were higher compared
with mice in other groups, indicating that the diabetic animal
model was established successfully (Fig. 2A). However, treatment with SOG at
25, 50 and 75 mg/kg significantly decreased the levels of FBG in
STZ-induced diabetic mice in a dose-dependent and time-dependent
manner compared with the model group (P<0.01; Fig. 2A). In addition, the body weight of
the model significantly decreased compared with the normal mice
(P<0.01; Fig. 2B). The body
weight of mice in SOG-treated groups (25, 50 and 75 mg/kg) was
significantly increased compared with model group mice (P<0.05;
Fig. 2B). Overeating is a primary
characteristic of mice with diabetes (16). Water and food intake of model mice
with diabetes was increased compared with normal mice (Fig. 2C and D). Following treatment with
SOG (25, 50 and 75 mg/kg), the water and food intake of mice with
diabetes was significantly decreased compared with the model group
(P<0.05; Fig. 2C and D). The
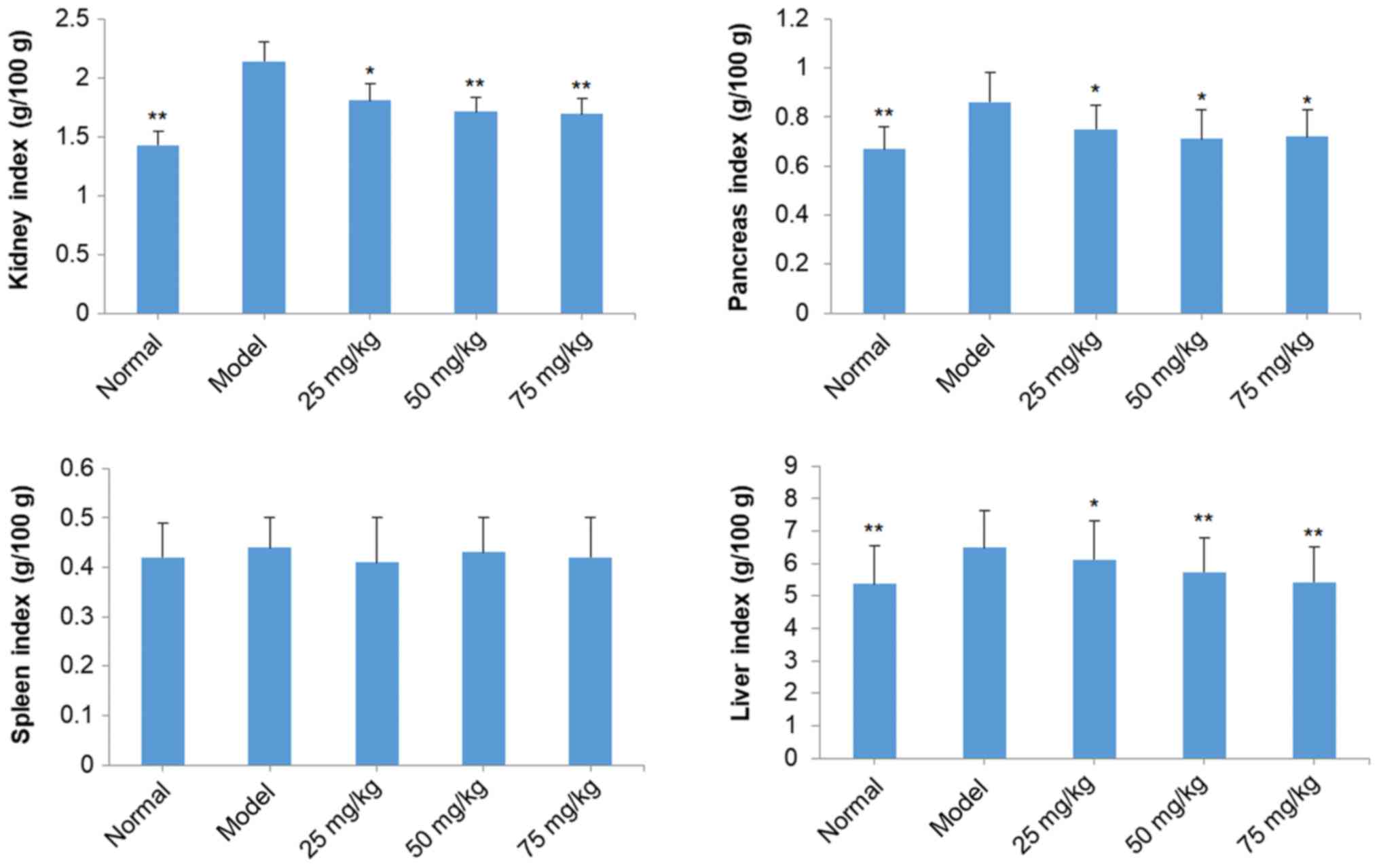
effect of treatment with SOG on organ indexes (kidney, pancreas,
spleen and liver) of STZ-induced diabetic mice are presented in
Fig. 3. Kidney, pancreas and liver
indexes of diabetic mice induced by STZ were increased compared
with mice in the normal group. SOG treatment (25, 50 and 75 mg/kg)
significantly reduced the kidney, pancreas and liver indexes,
compared with the model group, to levels similar to that in the
normal group. However, no significant effects of diabetes induction
or SOG treatment were observed on the spleen index.
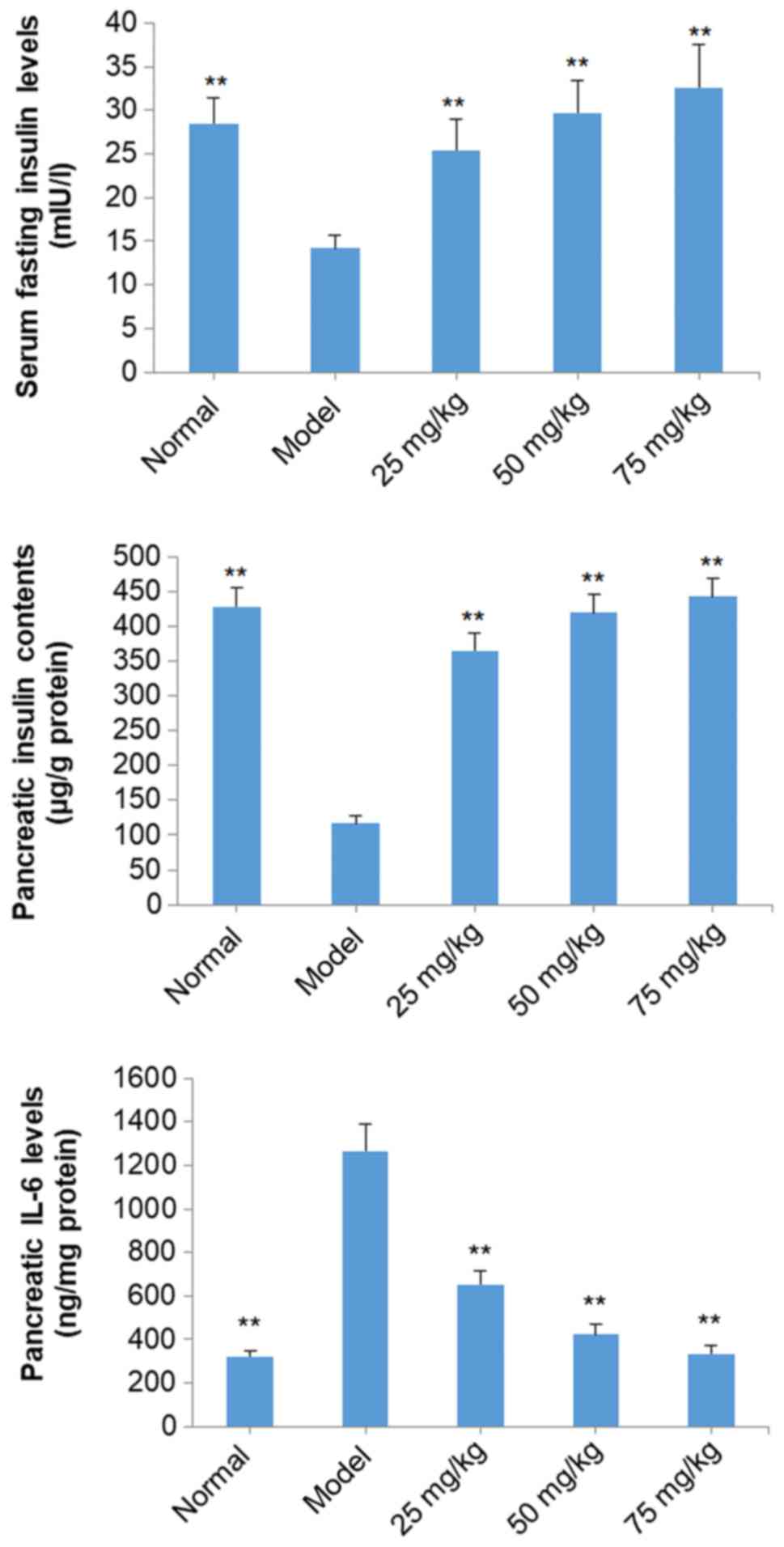
Effects of SOG on serum fasting
insulin, pancreatic insulin and pancreatic IL-6 of diabetic mice
induced by STZ
Serum fasting insulin and pancreatic insulin levels
of STZ-induced diabetic mice in the model group were significantly
decreased compared with normal mice (P<0.01; Fig. 4). However, treatment with SOG (25,
50 and 75 mg/kg) significantly increased serum fasting insulin and
pancreatic insulin levels in STZ-induced diabetic model mice
dose-dependently (P<0.01; Fig.
4). The pancreatic levels of IL-6 in diabetic mice in the model
group were significantly increased compared with the normal group
(P<0.01); however, following treatment with SOG at doses of 25,
50 and 75 mg/kg, the level of pancreatic IL-6 in diabetic mice was
significantly reduced compared with mice in the model group
(P<0.01; Fig. 4).
Effects of SOG on TC, TG, HDL-C,
LDL-C, VLDL-C and FFA in the serum of diabetic mice induced by
STZ
TC, TG, LDL-C, VLDL-C and FFA levels in the serum of
mice in the model group were significantly increased compared with
the normal mice (P<0.01; Fig.
5). However, HDL-C levels in the serum of model mice were
decreased compared with normal mice (P<0.01; Fig. 5). In all SOG-treated groups (25, 50
and 75 mg/kg), TC, TG, LDL-C and FFA levels in the serum of mice
were significantly decreased compared with model group mice
(P<0.05; Fig. 5). Similar
effects were observed for VLDL-C levels at 50 and 75 mg/kg SOG;
however, 25 mg/kg SOG did not induce a significant reduction in
VLDL-C levels in diabetic model mice (Fig. 5). By contrast, all three
concentrations of SOG led to significant increases in the HDL-C
levels in the serum of diabetic model mice (P<0.01; Fig. 5).
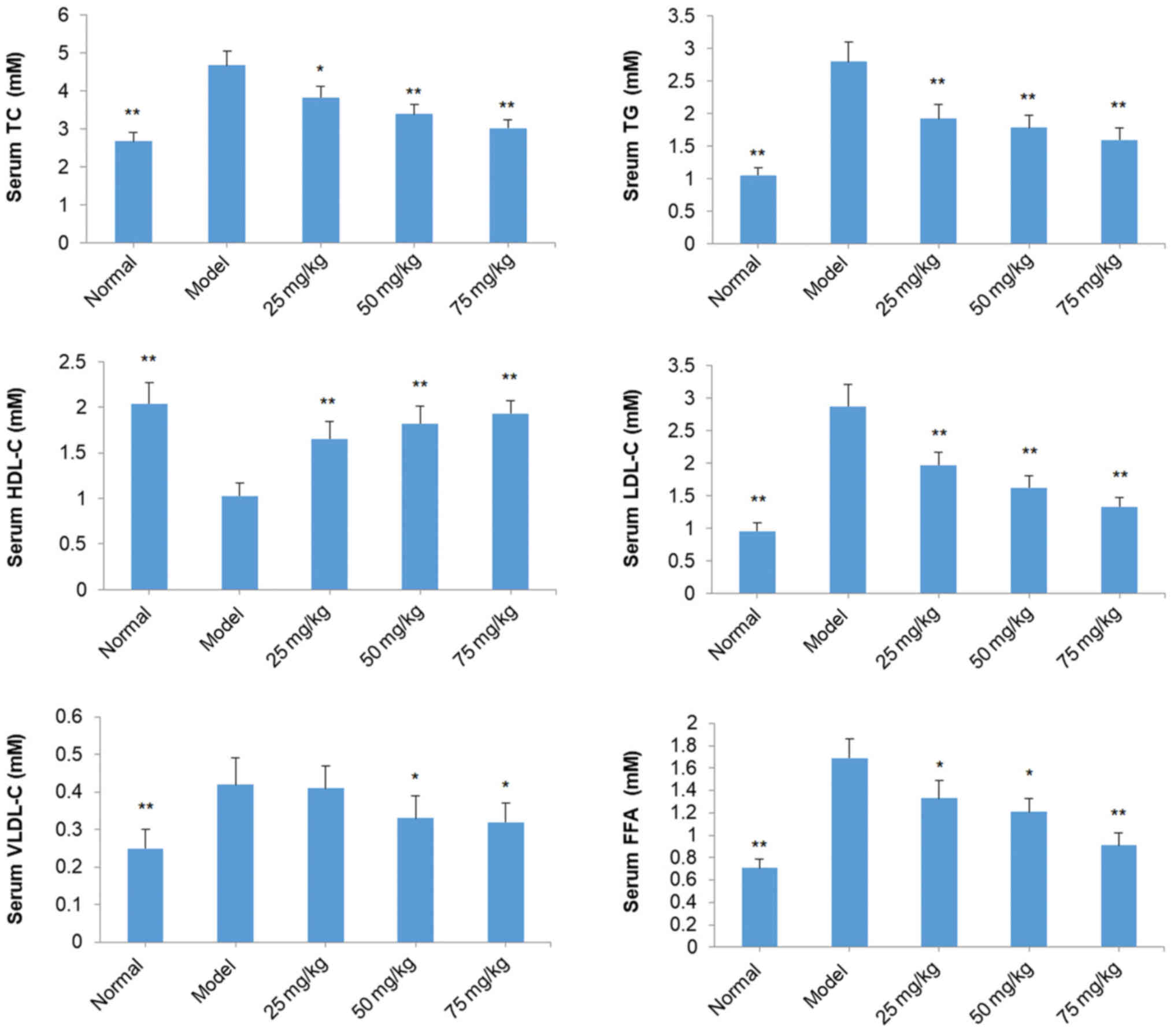 | Figure 5.Effect of SOG on the levels of TC, TG,
HDL-C, LDL-C, VLDL-C and FFA in the serum of experimental diabetic
mice induced by STZ. The normal group represents mice without STZ
injection and the model group represents mice with the induction of
diabetes by STZ injection. Data are presented as the mean ±
standard deviation (n=10 mice). *P<0.05 and **P<0.01 vs.
model group. SOG, syringaresinol-di-O-β-D-glucoside; TC, total
cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein
cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C,
very low-density lipoprotein cholesterol; FFA, free fatty acid;
STZ, streptozocin; 25 mg/kg, 25 mg/kg SOG; 50 mg/kg, 50 mg/kg SOG;
75 mg/kg, 75 mg/kg SOG. |
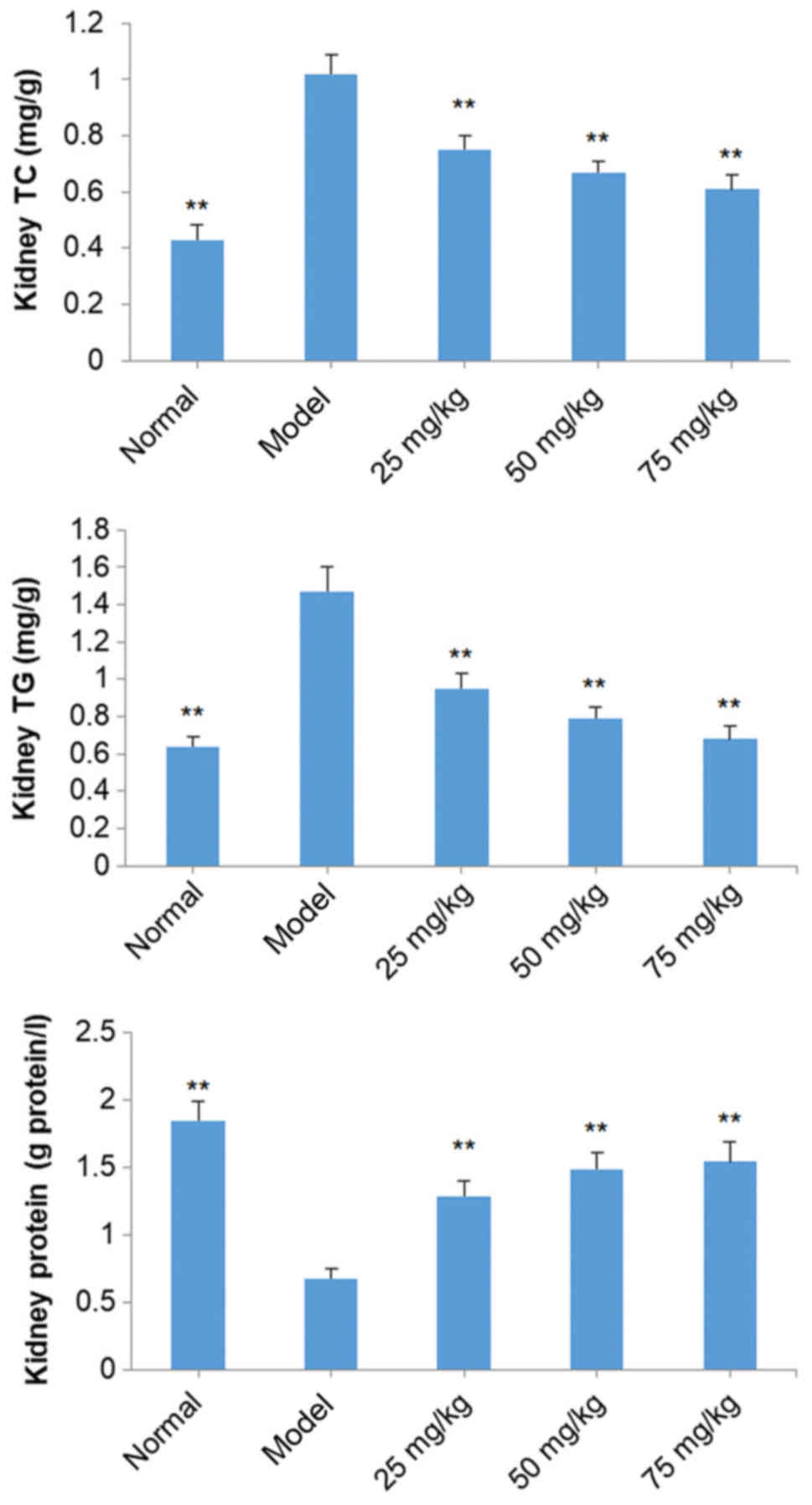
Effects of SOG on kidney TC, TG and
total protein levels in diabetic mice induced by STZ
TC and TG levels in the kidneys of model group mice
were markedly increased compared with mice in the normal group
(P<0.01), while total kidney protein levels were significantly
decreased (P<0.01; Fig. 6). SOG
(25, 50 and 75 mg/kg) significantly decreased kidney TC and TG
levels, and increased levels of total kidney protein, in diabetic
mice induced by STZ in a dose-dependent manner (P<0.01; Fig. 6).
Effects of SOG on MDA, SOD, CAT,
T-AOC, AST, ALT and ALP in the kidneys of diabetic mice induced by
STZ
Levels of MDA, SOD, CAT, AST, ALT and ALP in the
kidneys of model group mice were increased significantly, and T-AOC
was decreased significantly, compared with normal mice (P<0.01;
Fig. 7). Following treatment with
SOG (25, 50 and 75 mg/kg), the levels of MDA, SOD, CAT, AST, ALT
and ALP in the kidneys of diabetic mice were significantly
decreased, and T-AOC was significantly increased, compared with
model group mice (P<0.05; Fig.
7).
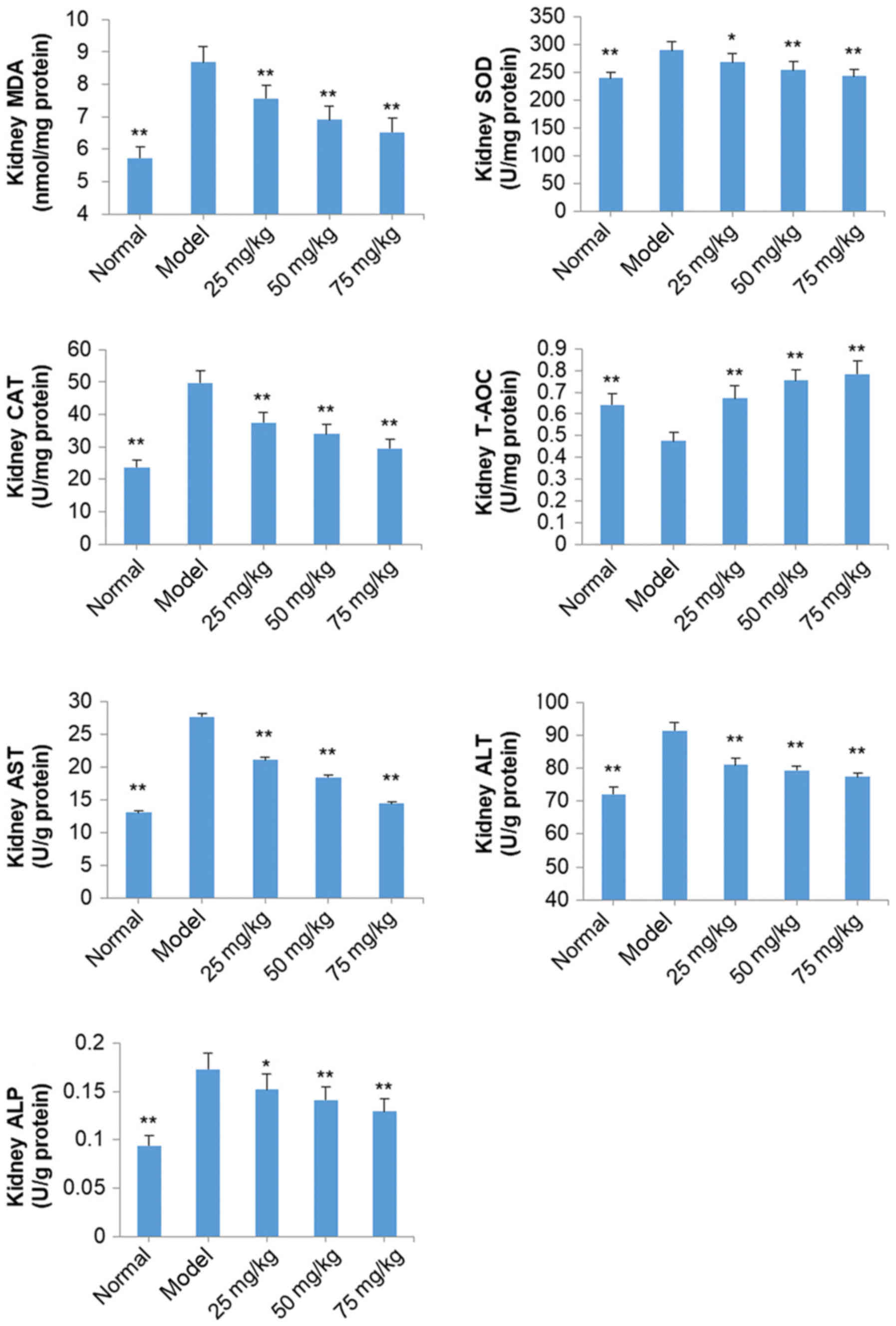 | Figure 7.Effect of SOG on MDA, SOD, CAT, T-AOC,
AST, ALT and ALP levels in the kidneys of experimental
diabetic mice induced by STZ. The normal group represents mice
without STZ injection and the model group represents mice with the
induction of diabetes by STZ injection. Data are presented as the
mean ± standard deviation (n=10 mice). *P<0.05 and **P<0.01
vs. model group. SOG, syringaresinol-di-O-β-D-glucoside; MDA,
malondialdehyde; SOD, superoxide dismutase; CAT, catalase; T-AOC;
total antioxidant capacity, AST; aspartate transaminase, ALT;
alanine transaminase, ALP; alkaline phosphatase; STZ, streptozocin;
25 mg/kg, 25 mg/kg SOG; 50 mg/kg, 50 mg/kg SOG; 75 mg/kg, 75 mg/kg
SOG. |
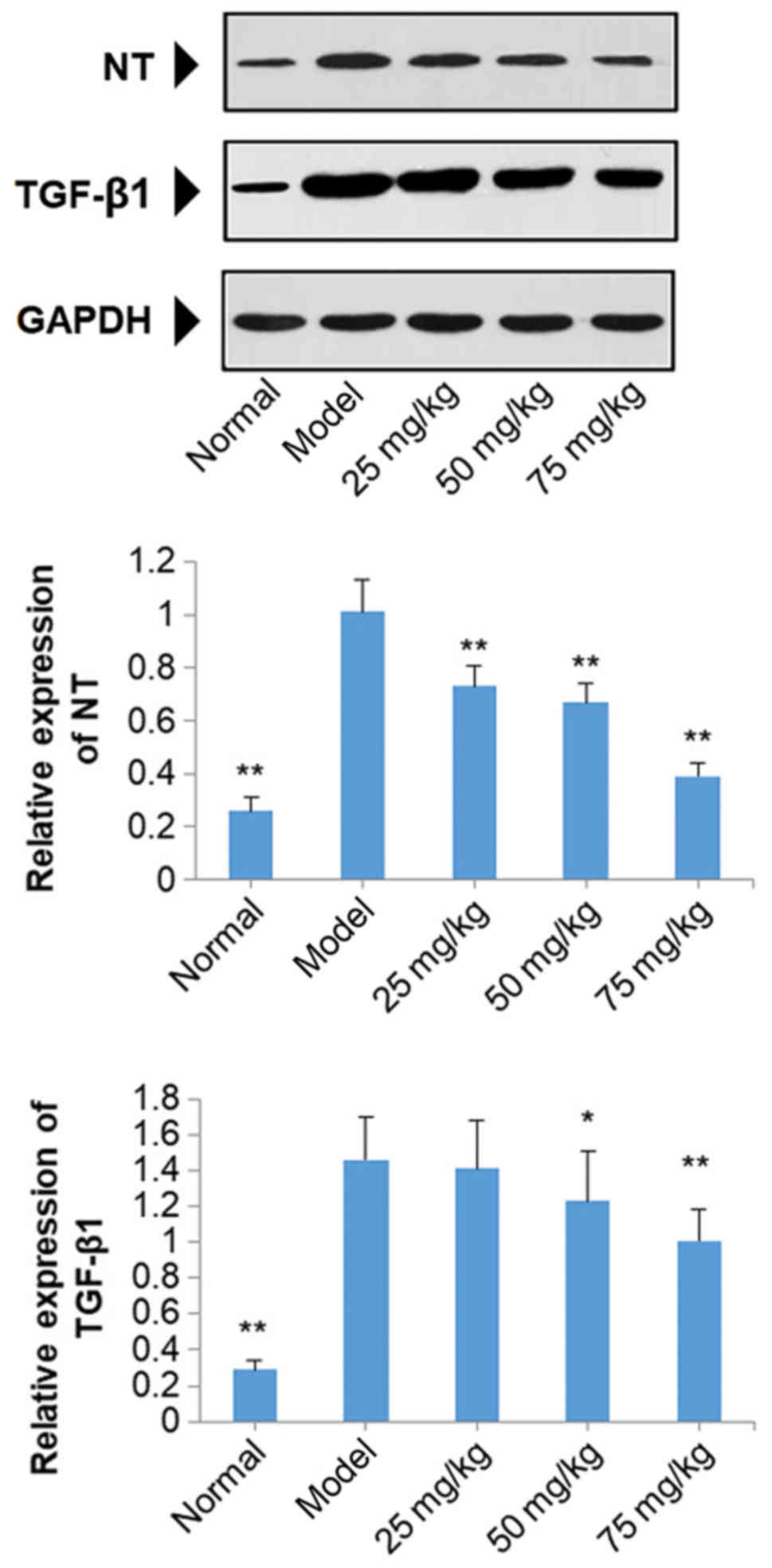
Effects of SOG on the protein
expression of NT and TGF-β1 in the kidneys of diabetic mice induced
by STZ
The results of western blot analysis demonstrated
that the protein expression of NT and TGF-β1 were significantly
increased in diabetic model mice compared with normal mice
(P<0.01; Fig. 8). Treatment
with SOG at 25, 50 and 75 mg/kg in diabetic model mice resulted in
a significant decrease in the protein expression of NT, compared
with the model group (P<0.01; Fig.
8). SOG treatment at 50 and 75 mg/kg also significantly
decreased the expression levels of TGF-β1 compared with mice in the
model group (P<0.05), but no significant reduction in TGF-β1
levels was observed at 25 mg/kg SOG in diabetic model mice
(Fig. 8).
Discussion
To the best of our knowledge, the present study is
the first to investigate the antidiabetic effect of SOG in a
diabetic mouse model induced by STZ. In the present study, SOG
exhibited an antidiabetic effect and its mechanism may be
associated with antioxidative effects.
STZ is a widely used antibiotic produced by
Streptomyces achromogenes. In 1963, Rakieten et al
(18) demonstrated that rats and
dogs exhibited symptoms of diabetes following intravenous injection
with STZ. STZ is formed by the coupling of a glucose molecule with
a highly active urea side chain, which is considered to contribute
to cytotoxicity. Therefore, a STZ-induced diabetic animal model was
used in the present study.
The primary symptoms of diabetes include polydipsia,
polyuria, emaciation, weakness and hyperglycemia (17). In the present study, FBG, body
weight, water intake, food intake and organ indexes (kidney,
pancreas, spleen and liver) of diabetic mice induced by STZ were
analyzed, and the results demonstrated that SOG ameliorated
symptoms of STZ-induced diabetes. In addition, diabetes primarily
results from insulin deficiency or insulin insensitivity (5). Therefore, the levels of serum fasting
insulin and pancreatic insulin may reflect the severity of diabetes
symptoms. In the process of insulin resistance, a series of
reactions occur, including increases in the levels of fatty acids,
inflammation and oxidative stress (19). The level of pancreatic IL-6 in
experimental mice was determined in the present study. The results
indicated that SOG exerted an antidiabetic effect through
increasing the serum fasting insulin and pancreatic insulin levels,
and decreasing the levels of pancreatic IL-6.
Lipids, including fat and fatty substances in the
blood, serve roles in energy balance, the physiological function of
reproduction and organs, and cell biology (20). The major components of fatty
substances in the blood are fatty acids, cholesterol, triglycerides
and phospholipids (21).
Additionally, increased concentrations of TC, TG, LDL-C and VLDL-C,
and reduced HDL-C levels, are characteristic of hyperlipidemia and
hypercholesterolemia (22,23), which are associated with diabetes
(21). In the present study, TC,
TG, HDL-C, LDL-C, VLDL-C and FFA in the serum of experimental mice
were analyzed. The results demonstrated that SOG significantly
decreased the levels of TC, TG, LDL-C, VLDL-C and FFA, and
increased the levels of HDL-C, in the serum of STZ-induced diabetic
mice, indicating that SOG may increase sensitivity to insulin.
Diabetic nephropathy is the most common chronic
complication of diabetes (24). It
was reported that 30–40% of diabetic patients suffer from kidney
damage (9). Rats with diabetic
nephropathy have specific symptoms, including high levels of TC and
TG, and low total kidney protein levels (25). In the present study, SOG reduced
levels of TC and TG in the kidney, and increased total kidney
protein levels, which indicate that SOG may ameliorate diabetic
nephropathy of STZ-induced diabetic mice.
Oxidative stress is a major mechanism contributing
to the development of diabetes mellitus, which it mediates via
alterations in the formation of free radicals (26,27).
Oxygen free radicals are associated with the pathogenesis of
diabetes mellitus (20). It has
been previously demonstrated that an imbalance between ROS and the
endogenous protective mechanisms in patients with diabetes is
associated with cell damage (25).
The level of MDA reflects the extent of lipid peroxidation, with
increases in MDA levels indicating an increase in the number of
free radicals. SOD catalyzes the dismutation of superoxide anion
radicals into hydrogen peroxide. Hydrogen peroxide may be changed
into peroxynitrite to weaken the interaction between the superoxide
anion and nitric oxide or be hydrolyzed by hydrogen peroxide to
water and oxygen (28–30). T-AOC is considered to be the
cumulative effect of all antioxidants in the blood and body fluids,
which is used to assess alterations in the antioxidant status,
particularly in patients with diabetes (31). It was reported that the levels of
AST and ALT were increased in the kidneys of STZ-induced diabetic
mice compared with normal mice (32). ALP is a membrane-bound glycoprotein
enzyme that is used as an indicator of biliary function and
cholestasis (33). In the present
study, SOG decreased the levels of SOD, CAT, AST, ALT and ALP in
the kidney of STZ-induced diabetic mice, and increased levels of
T-AOC, indicating that SOG may exhibit antidiabetic effects by
regulating oxidative stress.
Excessive amounts of ROS and NO are produced by
patients with diabetes, leading to the production of peroxynitrite
anions, which is an oxidant and leads to elevated oxidative stress
(34). Peroxynitrite anions have
been reported to activate nuclear factor-κB to upregulate the
expression of TGF-β and eventually promote the development of
diabetes. Furthermore, NT in the kidney is a specific marker of
peroxynitrite anions (35). The
present study investigated the protein expression of NT and TGF-β1.
The results demonstrated that SOG downregulated the expression
levels of NT and TGF-β1 in the kidneys of STZ-induced diabetic mice
in a dose-dependent manner.
In conclusion, the results of the present study
demonstrated that SOG may exert notable antidiabetic effects and
ameliorate diabetic nephropathy. The mechanism of SOG may be
associated with decreasing the levels of oxidative stress through
downregulation of the expression of NT and TGF-β1 in kidneys. In
addition, the present investigation can provide specific scientific
basis for the use of Polygonati rhizome. Further studies on
structure-bioactivity association of SOG should be performed in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Jiangsu Provincial Department of Science and
Technology (grant no. BK20151572).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
For preparation of the paper, XW conceived and
designed the study; LZ performed the experiments; XW analyzed the
data; LZ and XW wrote the manuscript.
Ethics approval and consent to
participate
Animal experiments were performed with the approval
of the Animal Experimentation Ethics Committee of The Affiliated
Hospital of Nanjing University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mrudula T, Suryanarayana P, Srinivas PN
and Reddy GB: Effect of curcumin on hyperglycemia-induced vascular
endothelial growth factor expression in streptozotocin-induced
diabetic rat retina. Biochem Biophys Res Commun. 361:528–532. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danaei G, Finucane MM, Lu Y, Singh GM,
Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA,
et al: National, regional, and global trends in fasting plasma
glucose and diabetes prevalence since 1980: Systematic analysis of
health examination surveys and epidemiological studies with 370
country-years and 2.7 million participants. Lancet. 378:31–40.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruiz-Ramos M, Escolar-Pujolar A,
Mayoral-Sánchez E, Laureano Corral-San F and Fernández-Fernández I:
Diabetes mellitus in Spain: Death rates, prevalence, impact, costs
and inequalities. Gac Sanit. 20 Suppl 1:S15–S24. 2006. View Article : Google Scholar
|
|
5
|
Kim SH, Hyun SH and Choung SY:
Anti-diabetic effect of cinnamon extract on blood glucose in db/db
mice. J Ethnopharmacol. 104:119–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ezeja MI, Anaga AO and Asuzu IU:
Antidiabetic, antilipidemic, and antioxidant activities of Gouania
longipetala methanol leaf extract in alloxan-induced diabetic rats.
Pharm Biol. 53:605–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reddy VP, Zhu X, Perry G and Smith MA:
Oxidative stress in diabetes and Alzheimer's disease. J Alzheimers
Dis. 16:763–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J, Yang Y, Hu R and Chen L:
Anti-interleukin-1 therapy has mild hypoglycaemic effect in type 2
diabetes. Diabetes Obes Metab. 20:1024–1028. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li JY, Liu XM, Li B and Li M: Research
progress in the symptom and treatment of diabetic nephropathy.
Diabetes New World. 20:70–72. 2015.(In Chinese).
|
|
10
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng W, Shen W, Lin B, Han P, Li C, Zhang
Q, Ye B, Rahman K, Xin H, Qin L and Han T: Docking study and
antiosteoporosis effects of a dibenzylbutane lignan isolated from
Litsea cubeba targeting Cathepsin K and MEK1. Med Chem Res.
27:2062–2070. 2018. View Article : Google Scholar
|
|
12
|
Xie W and Du L: Diabetes is an
inflammatory disease: Evidence from traditional Chinese medicines.
Diabetes Obes Metab. 13:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chinese Pharmacopoeia Commission, .
Pharmacopoeia of the People's Republic of China Part IPeople's
Medical Publishing House. Beijing: pp. 3062015
|
|
14
|
Liu J, Zhang H, Ji B, Cai S, Wang R, Zhou
F, Yang J and Liu H: A diet formula of Puerariae radix, Lycium
barbarum, Crataegus pinnatifida, and Polygonati rhizoma
alleviates insulin resistance and hepatic steatosis in CD-1 mice
and HepG2 cells. Food Funct. 5:1038–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Feng SS, Sun YJ, Hao ZY, Feng WS
and Zheng XK: Advances in studies on chemical constituents of three
medicinal plants from Polygonatum Mill. and their
pharmacological activities. Chin Tradit Herb Drugs. 46:2329–2338.
2015.
|
|
16
|
National Institute of Health, . USA:
Public health service policy on humane care and use of laboratory
animals; 2002
|
|
17
|
Jin DK: Antidiabetic effect of DMDD
Isolated from Averrhoa carambola L. roots on STZ-induced
diabetic mice and its potential mechanism (unpublished PhD thesis).
Guangxi Medical University. 2014.
|
|
18
|
Rakieten N, Rakieten ML and Nadkarni MV:
Studies on the diabetogenic action of streptozotocin (NSC-37917).
Cancer Chemother Rep. 29:91–98. 1963.
|
|
19
|
Kintoko K and Huang R: Mechanism of
insulin resistance in type 2 diabetes mellitus. Proceeding of
International Symposium on Progress in Pharmacology. 2013.
|
|
20
|
Russell DW: Cholesterol biosynthesis and
metabolism. Cardiovasc Drugs Ther. 6:103–110. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ibrahim SRM, Mohamed GA, Banjar ZM and
Kamal HKM: Natural antihyperlipidemic agents: Current status and
future persepctives. Phytopharmacology. 4:492–531. 2013.
|
|
22
|
Grundy SM, Cleeman JI, Rifkind BM and
Kuller LH: Cholesterol lowering in the elderly population.
Coordinating Committee of the National Cholesterol Education
Program. Arch Intern Med. 159:1670–1678. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahmood ZA, Ahmed SW, Sualeh M and Mahmood
SBZ: Hyperlipidemia development and consequences. Med Channel.
5:14–17. 2009.
|
|
24
|
Weng X: Research progress of treatment of
diabetic nephropathy in traditional Chinese medicine. Asia-Pacific
Tradit Med. 2:62–63. 2015.(In Chinese).
|
|
25
|
Lv SQ, Zhang SF, Wang ZQ, Song HL, Han ZQ,
Su XH, Liu AR, Wang YS, Yu WX and Chi XE: Protective effect of
jianpi gushen huayu decoction on diabetic nephropathy rats. Chin J
Exp Tradi Med Formulae. 24:142–147. 2018.
|
|
26
|
Kim HJ, Lee SG, Chae IG, Kim MJ, Im NK, Yu
MH, Lee EJ and Lee IS: Antioxidant effects of fermented red ginseng
extract in streptozotocin-induced diabetic rats. J Ginseng Res.
35:129–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kakkar R, Kalra J, Mantha SV and Prasad K:
Lipid peroxidation and activity of antioxidant enzymes in diabetic
rats. Mol Cell Biochem. 151:113–119. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manna P, Sinha M and Sil PC: Protective
role of arjunolic acid in response to streptozotocin-induced type-1
diabetes via the mitochondria dependent and independent pathways.
Toxicol. 257:53–63. 2009. View Article : Google Scholar
|
|
29
|
Maritim AC, Sanders RA and Watkins JB III:
Diabetes, oxidative stress, and antioxidant: A review. J Biochem
Mol Toxicol. 17:24–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Winterbourne CC: Superoxide as
intracellular radical sink. Free Radic Biol Med. 14:85–90. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sies H: Total antioxidant capacity:
Appraisal of a concept. J Nutr. 137:1493–1495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okada M, Murakami Y and Miyamoto E:
Glycation and inactivation of aspartate aminotransferase in
diabetic rat tissues. J Nutr Sci Vitaminol (Tokyo). 43:463–469.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leibovitch I, Ben-Chaim J, Ramon J and
Goldwasser B: Increased serum alkaline phosphatase activity: A
possible indicator of renal damage. J Clin Lab Anal. 5:406–409.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ceriello A, Mercuri F, Quagliaro L,
Assaloni R, Motz E, Tonutti L and Taboga C: Detection of
nitrotyrosine in the diabetic plasma: Evidence of oxidative stress.
Diabetologia. 44:834–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fang F, Wu YG, Dong J, Ren KJ, Qi XM,
Liang C and Zhang W: Effects of total glucosides of paeony on
oxidative stress in renal tissue of diabetic rats. Chin J Pharmacol
Toxicol. 22:199–204. 2008.(In Chinese).
|