Introduction
Osteosarcoma (OS) represents the most common primary
malignant bone tumor, and most frequently occurs in individuals
<20 years of age (1). Pulmonary
metastases occurring during OS account for nearly all associated
cases of mortality (2,3). The 5 year survival rate for patients
with localized OS remains at 60–70% when receiving multimodality
therapy, including surgery and radio- and chemotherapy (4). By contrast, the 5 year survival rate
for patients with aggressive metastases is only 10–30%, thereby
indicating an unsatisfactory response rate to multimodality therapy
(5). However, as the mechanisms
underlying the regulation of OS progression have not yet been
clearly characterized, it remains difficult for currently available
therapeutic strategies to effectively treat OS (6). Therefore, previous studies have
focused on broadening the understanding of biological targets
associated with OS cell malignant biological behavior, which has
important theoretical and clinical significance (7,8).
MicroRNAs (miRs/miRNAs) are comprised of short
non-coding RNA with a length of 22 nucleotides that typically
inhibit the translation and stability of mRNAs, as well as regulate
genes involved in cellular processes, including inflammation,
apoptosis, cell-cycle, invasion and migration (9–12).
As post-transcriptional regulators of developmental processes,
miRNAs have an important role in almost all biological processes,
and aberrant miRNA expression is associated with numerous diseases,
including cancer (13). Emerging
evidence has revealed that miRNAs are involved in human
carcinogenesis by functioning as tumor suppressors or oncogenes,
and thus have prognostic value for patients with cancer (14). In addition, previous studies have
also demonstrated that the expression of miR-23 is dysregulated in
angiogenesis, coronary artery disease, immune responses and cancer
(15–19); the dysregulation of miR-23 is
associated with numerous of cancer (20–22).
The miR-23 protein family has two members: mir-23a
and mir-23b (23). Cai et
al (20) demonstrated that the
levels of miR-23a were decreased in prostate cancer and that low
levels of miR-23a were associated with poor prognosis. Furthermore,
the expression of miR-23a has been demonstrated to be decreased in
OS due to hypermethylation of its promoter (21). Aghaee-Bakhtiari et al
(24) further demonstrated that
miR-23a and miR-23b were significantly downregulated in prostate
cancer, whereas their overexpression in prostate cell lines was
revealed to decrease interleukin-6R expression, which has an
important role in the mitogen-activated protein kinase (MAPK) and
Janus kinase/signal transducer and activator of transcription
signaling pathways, and in suppressing cell proliferation and
neoplastic transformation. Campos-Viguri et al (25) demonstrated that the expression of
miR-23b was also downregulated via methylation of its promoter,
which has been suggested to inversely regulate the expression of
c-Met, zinc finger E-box binding homeobox 1 and plasminogen
activator, urokinase in cervical cancer cell lines.
In addition, Begum et al (26) demonstrated that inhibition of
miR-23b in non-small cell lung carcinoma (NSCLC) cell lines (H1437
and H1944) significantly decreases cell doubling time (27), which is consistent with the
findings of Yan et al (27)
that miR-23b may inhibit ovarian cancer tumorigenesis and
progression via downregulation of cyclin G1 (27). MiR-23b-3p may also inhibit
autophagy mediated by autophagy related 12 and high mobility group
box 2, and sensitize gastric cancer cells to chemotherapy (28). By contrast, Jin et al
(29) determined that miR-23b-3p
is highly upregulated in human breast cancer, whereas knocking down
miR-23b-3p significantly decreased cell proliferation and
migration. Therefore, it remains unclear whether miR-23b-3p
functions as a tumor suppressor or as an oncogene. Dysregulation of
miR-23b-3p is currently considered to be associated with various
human cancers; however, the effects of miR-23b-3p on the biological
behavior of OS cells are largely unknown.
Sine oculis homeobox homolog 1 (SIX1), a
developmental transcription factor, represents a member of the Six
gene family, which contains six members (SIX1-6) in vertebrates
(30). At present, SIX1 is the
most widely studied gene from this family, a previous study
demonstrating that SIX1 is associated with the development of
tissues and organs (31). Previous
studies have indicated that SIX1 may regulate cell growth,
proliferation, differentiation, migration and apoptosis (2,32).
Furthermore, overexpression of SIX1 may induce
epithelial-to-mesenchymal transition (EMT) and metastasis of
colorectal cancer cells, whereas SIX1 knockdown may markedly
suppress cell proliferation, invasion and migration (33). Similarly, SIX1 overexpression in
human breast cancer cells has been demonstrated to promote EMT and
metastatic dissemination (34).
Consistently, SIX1 has been revealed to be highly overexpressed in
breast cancer cell lines derived from the metastatic site (35). A further study observed that SIX1
induces lymphangiogenesis and metastasis via the upregulation of
vascular endothelial growth factor (VEGF)-C in mouse models of
breast cancer (36). Notably, it
has been suggested that nuclear factor-κB may be involved in VEGF-C
expression via the p38 MAPK signaling pathway in cancer (37–39).
Furthermore, our previous study demonstrated that SIX1 is
upregulated in OS cell lines when compared with the human
osteoblastic cell line, hFOB1.19 (2). Preliminary analyses using online
software (www.targetscan.org) suggested that
SIX1 may represent a target gene of miR-23b-3p, therefore we
hypothesized that miR-23b-3p may be downregulated and inversely
regulate the expression of SIX1 in OS cells and thereby have an
important role in the occurrence and development of OS.
The aims of the present study were to investigate
the expression of miR-23b-3p and SIX1 in OS tissues and cell lines
by reverse transcription-quantitative polymerase chain (RT-qPCR)
and western blotting analysis, and to observe changes in the cell
viability, cell cycle, apoptosis and invasive abilities of U2OS
cells following enhanced and suppressed expression of miR-23b-3p.
These results may provide an experimental basis for the future
application of miR-23b-3p and SIX1 in the treatment of OS.
Materials and methods
Samples
The present study was granted ethical approval by
the Clinical Research Ethics Committee of the Haian Hospital of
Traditional Chinese Medicine (Nantong, China), and written informed
consent was obtained from all patients prior to enrollment. Fresh
OS tissues and matched adjacent non-tumor tissues were collected
from 11 patients who underwent resection surgery between August
2011 and July 2016 (Table I). None
of the included patients had undergone preoperative chemotherapy,
radiotherapy, other treatment history for OS or suffered from
additional inflammatory diseases. All tissue samples were
immediately frozen in liquid nitrogen and then stored at −80°C for
subsequent experimentation.
 | Table I.microRNA-23b-3p expression in
osteosarcoma tissues and its association with clinical pathological
factors. |
Table I.
microRNA-23b-3p expression in
osteosarcoma tissues and its association with clinical pathological
factors.
| Pathological
parameters | No. of
patients | miR-23b-3p
expression negative rate n (%) | χ2 | P-value |
|---|
| Tissue type |
|
|
|
|
|
Adjacent healthy tissue | 11 | 1 (9.09) | 5.30 | 0.02 |
|
Carcinoma tissue | 11 | 10 (90.91) |
|
|
| Sex |
|
|
|
|
|
Male | 4 | 4 (100.00) | 0.03 | 0.86 |
|
Female | 7 | 6 (85.71) |
|
|
| Age (years
old) |
|
|
|
|
|
<20 | 9 | 8 (88.89) | 0.01 | 0.92 |
|
≥20 | 2 | 2 (100.00) |
|
|
| Size of primary
carcinoma (cm) |
|
|
|
|
|
<5 | 5 | 4 (80.00) | 0.06 | 0.80 |
| ≥5 | 6 | 6 (100.00) |
|
|
| Enneking stage |
|
|
|
|
| I | 4 | 3 (75.00) | 0.10 | 0.95 |
| II | 3 | 3 (100.00) |
|
|
|
III | 4 | 4 (100.00) |
|
|
| Lung
metastasis |
|
|
|
|
|
Negative | 5 | 4 (80.00) | 0.06 | 0.80 |
|
Positive | 6 | 6 (100.00) |
|
|
Reagents
Cell culture reagents were obtained from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). OS cell lines
(MG-63, SaOS-2 and U2OS) and a non-cancerous human fetal
osteoblastic cell line (hFOB1.19) were obtained from the American
Type Culture Collection (Manassas, VA, USA). The following primer
sequences were purchased from Shanghai Generay Biotech Co., Ltd.
(Shanghai, China): miR-23b-3p forward, 5′-CGGGCATCACATTGCCAGG-3′
and reverse, 5′-CAGCCACAAAAGAGCACAAT-3′; U6 (GI:161087014) forward,
5′-CTCGCTTCGGCAGCACA-3′, and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
SIX1 (NM_005982.3) forward, 5′-TGTCCTGCGGGAGTGGTA-3′, and
reverse, 5′-TGATGCTGGTGGGTCTGC-3′; and β-actin (NM_001101.3)
forward, 5′-GTGGACATCCGCAAAGAC-3′, and reverse,
5′-AAAGGGTGTAACGCAACTAA-3′. The following stem loop sequence was
also purchased from Shanghai Generay Biotech Co., Ltd.:
5′-CCTGTTGTCTCCAGCCACAAAAGAGCACAATATTTCAGGAGACAACAGGGGTAATC-3′. A
bicinchoninic acid (BCA) protein concentration assay kit,
TRIzol® reagent, propidium iodide, MTT, Annexin
V-fluorescein isothiocyanate (FITC) kit, Cell Counting Kit-8
(CCK-8), radioimmunoprecipitation assay (RIPA) buffer and RNase A
were all purchased from Beyotime Institute of Biotechnology
(Haimen, China). Polyvinylidene fluoride (PVDF) membranes were
purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). A
Transwell chamber was obtained from Corning Costar Corp.
(Cambridge, MA, USA). Rabbit anti-SIX1 (ab211359)), rabbit
anti-caspase-3 (ab44976) and goat anti-VEGF-C (ab18883) polyclonal
antibodies were purchased from Abcam (Cambridge, MA, USA). Mouse
anti-human cyclin D1 (556470) monoclonal antibodies (BD
Biosciences, San Jose, CA, USA), rabbit anti β-actin (250920)
polyclonal antibodies (Abbiotec, San Diego, CA, USA), as well as
horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG
(81–1620), rabbit anti-mouse IgG (61–6520) and goat anti-rabbit IgG
(32460) (Invitrogen; Thermo Fisher Scientific, Inc.) antibodies,
were all purchased for use in the present study.
Electrochemiluminescence (ECL) solution was purchased from Pierce
(Thermo Fisher Scientific, Inc.).
RT-qPCR to determine SIX1 and
miR-23b-3p expression levels
Total RNA was extracted from OS tissue (100 mg) or
OS cells (5×107) using either 1 ml TRIzol®
reagent or TaqMan miRNA isolation kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
cDNA was then synthesized at 42°C for 45 min using the first Strand
cDNA Synthesis kit (Qiagen GmbH, Hilden, Germany), and the PCR
reaction was subsequently performed using the Applied Biosystems
7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The RT-qPCR reaction was carried out at a final
volume of 25 µl with the addition of 5 µl cDNA, and the
thermocycling conditions used were as follows: an initial
denaturation at 94°C for 3 min, followed by 30 cycles of
denaturation at 94°C for 30 sec, annealing at 56°C for 30 sec and
extension at 72°C for 30 sec. Following this, the reaction mixture
was incubated at 72°C for 10 min. β-actin was used as an internal
reference gene. TaqMan miRNA Assay and TaqMan Universal PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) were used
to detect the expression of mature miR-23b-3p in normal human
osteoblasts (hFOB 1.19) and OS cell lines (MG-63, SaOS −2 and
U2OS), and U6 was used as the internal reference gene. The PCR
reaction conditions were as those already provided. All reactions
were performed in triplicate. Relative mRNA and miRNA expression
levels were calculated using the 2−ΔΔCq method (40).
Western blotting
Total protein was extracted from OS tissues and OS
cell lines using RIPA buffer [150 mM NaCl, 1% NP40, 0.5% sodium
deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris (pH 7.9), 10
mM NaF, phenylmethylsulfonyl fluoride, and 1X protease inhibitors
(complete cocktail tablets; Roche Diagnostics GmbH, Mannheim,
Germany)], and quantitative analysis of the protein concentration
was subsequently performed using a BCA kit. Total protein (50 µg
per lane) was separated by 12% SDS-PAGE, and then transferred to
PVDF membranes. Following this, PVDF membranes were blocked with 5%
non-fat milk in Tris-buffered saline containing Tween-20 (TBST; 10
mM Tris HCl, 150 mM NaCl, and 0.1% Tween-20, pH 7.5) at room
temperature for 1 h. The PVDF membranes were subsequently incubated
at 4°C overnight with the following primary antibodies: Rabbit
anti-SIX1 polyclonal antibodies (1:1,000), rabbit anti-caspase-3
polyclonal antibodies (1:2,000), goat anti-VEGF-C polyclonal
antibodies (1:2,000), monoclonal mouse anti-cyclin D1 antibodies
(1:2,000) and rabbit anti β-actin antibodies (1:2,000). Following
this, PVDF membranes were washed with TBST and then incubated with
HRP-conjugated secondary antibodies (1:2,000) at 37°C for 1 h.
Finally, PVDF membranes were washed again with TBST and the bands
were subsequently visualized using ECL reagent. Densitometric
analysis was performed using QuantityOne software version 4.62
(Bio-Rad Laboratories, Inc.).
Cell culture
Human OS MG-63, SaOS-2 and U2OS cells were cultured
in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin at
37°C in a humidified atmosphere containing 5% CO2.
hFOB1.19 cells were cultured in Dulbecco's modified Eagle medium
F-12 (Gibco; Thermo Fisher Scientific, Inc.) containing 0.5 mM
sodium pyruvate, 2.5 mM L-glutamine, 15 mM HEPES, 1%
penicillin/streptomycin and 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) at 34°C in a humidified atmosphere containing 5%
CO2. Inverted microscopes were used to observe the
growth rate of OS cells. When cells reached 70–80% confluence, they
were digested using 0.25% trypsin. Cells in the logarithmic growth
phase were isolated for further experimentation.
Cell treatment
U2OS cells were selected for the performance of
subsequent experiments. Normal cultured U2OS cells
(3×105) were inoculated into 6-well plates. Following
cell adherence, miR-23b-3p mimics (50 nM; cat. no. miR10000418-1-5;
Guangzhou RiboBio Co., Ltd., Guangzhou, China), miR-23b-3p
inhibitors (100 nM; cat. no. miR20000418-1-5; Guangzhou RiboBio
Co., Ltd.) and scrambled miRNA mimic (50 nM; cat. no. miR01201-1-5;
Guangzhou RiboBio Co., Ltd.) were transfected into U2OS cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol. miR-23b-3p
mimics, miR-23b-3p inhibitors and scrambled miRNA mimic were
diluted with RPMI-1640 medium in the absence of FBS. Diluted
Lipofectamine® 2000 was then separately incubated with
miR-23b-3p mimics, miR-23b-3p inhibitors and scrambled miRNA mimic
at room temperature for 20 min in order for the complexes to form.
Following this, U2OS cells were incubated with the complex mixture
at 37°C for 5 h in a humidified atmosphere of 5% CO2.
Finally, the culture medium was replaced with RPMI-1640 medium
containing 10% FBS and further incubated at 37°C for 48 h.
CCK-8 assays
Normal cultured U2OS cells (1×105) were
inoculated into 96-well plates. Following cell adherence, according
to the Lipofectamine® 2000 transfection reagent
protocol, the transfection of miR-23b-3p mimics, miR-23b-3p
inhibitors and negative controls was performed. At 24, 48 and 72 h
time intervals post-transfection, 10 µl CCK-8 solution (Beyotime
Institute of Biotechnology) was added to each well, the plates were
then incubated for 48 h at 37°C. Optical density (OD) values at 450
nm were determined using an ELx808 absorbance microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Flow cytometry analysis
Normal cultured U2OS cells (1×105) were
inoculated into 96-well plates. Following transfection, the U2OS
cells were fixed with 70% cold ethanol at 4°C for 18 h and then
stained with propidium iodide (30 µg/ml) at 37°C for 30 min. The
percentage of U2OS cells in each phase of the cell cycle were
determined using a BD FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) and BD CellQuest™ Pro software
version 5.1 (BD Biosciences). Following this, U2OS cells were
digested via 0.25% trypsin, washed using PBS and then resuspended
in 195 µl of Annexin V-FITC binding buffer. Subsequently, Annexin
V-FITC solution (2.5 µg/ml) was added and the solution was then
incubated for 10 min at room temperature in the dark. Propidium
iodide staining solution (5 µg/ml) was then added and the solution
was incubated for 15 min at room temperature. Finally, the
percentage of spontaneously apoptotic U2OS cells was determined
using a BD FACSCalibur flow cytometer using BD
CellQuest™ Pro software version 5.1 (BD
Biosciences).
Transwell invasion analysis
The invasive capability of U2OS cells was
investigated using Transwell invasion assays. miR-23b-3p mimics,
miR-23b-3p inhibitors and negative controls were transfected into
U2OS cells. RPMI-1640 medium (200 µl) containing 1×103
U2OS cells was subsequently added to the Matrigel-coated, upper
chamber (Corning Incorporated, Corning, NY, USA). The lower
Transwell chamber was filled with 500 µl of RPMI-1640 medium
containing 10% FBS. Following a 48 h incubation period, U2OS cells
in the upper chamber were removed using a cotton swab. U2OS cells
that invaded into the Matrigel and through the filter into the
bottom of the transwell chamber were collected and stained with 100
µl of 0.5 mg/ml MTT for 4 h at 37°C. The reaction was terminated by
adding 200 µl/well dimethyl sulfoxide and absorbance at 570 nm was
subsequently determined using an ELx808 absorbance microplate
reader (BioTek Instruments, Inc.). All experiments were performed
in triplicate.
Dual-luciferase reporter assay
In preliminary analyses, TargetScan tools (release
7.2; www.targetscan.org/) were used to
predict the potential targets of miR-23b-3p, and the SIX1 3′-UTR
was identified as a candidate region. Firstly, the 3′-untranslated
region (UTR) fragment of the SIX1 gene was amplified via PCR
using genomic DNA isolated from hFOB1.19 cells, which was performed
in accordance with the protocol outlined in the RT-qPCR subsection.
Specific point mutations were then introduced into the 3′-UTR of
the SIX1 gene by overlap extension PCR cloning (41). Following this, PCR products were
cloned into the pmiR-RB-Report™ reporter vector
(Guangzhou RiboBio Co., Ltd.). A luciferase reporter plasmid
(Guangzhou RiboBio Co., Ltd.) was constructed, which included
either the wild type (SIX1-W) 3′-UTR or mutated (SIX1-M) 3′-UTR.
293 cells (3×105/ml) were inoculated into 6-well plates,
and then the luciferase plasmid containing either SIX1-W 3′-UTR or
SIX1-M 3′-UTR, and miR-23b-3p mimics or mimic negative controls
(Genepharma Co., Ltd.), were cotransfected into 293 cells using
Lipofectamine® 2000. At 48 h post-transfection, 293
cells were collected and luciferase activity was determined using a
luciferase assay kit (Promega Corporation, Madison, WI, USA) with a
microplate reader. The reporter luciferase was normalized to
Renilla luciferase activity.
Statistical analysis
All data are presented as the mean ± standard error
of the mean from at least three independent experiments. SPSS
statistical software version 17.0 (SPSS Inc., Chicago, IL, USA) was
used to perform statistical analyses. To determine statistically
significant differences, the Student's t-test was used for
comparisons between two groups, and one-way analysis of variance
followed by Tukey's post hoc test was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. GraphPad Prism software version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA) was used to plot
graphs.
Results
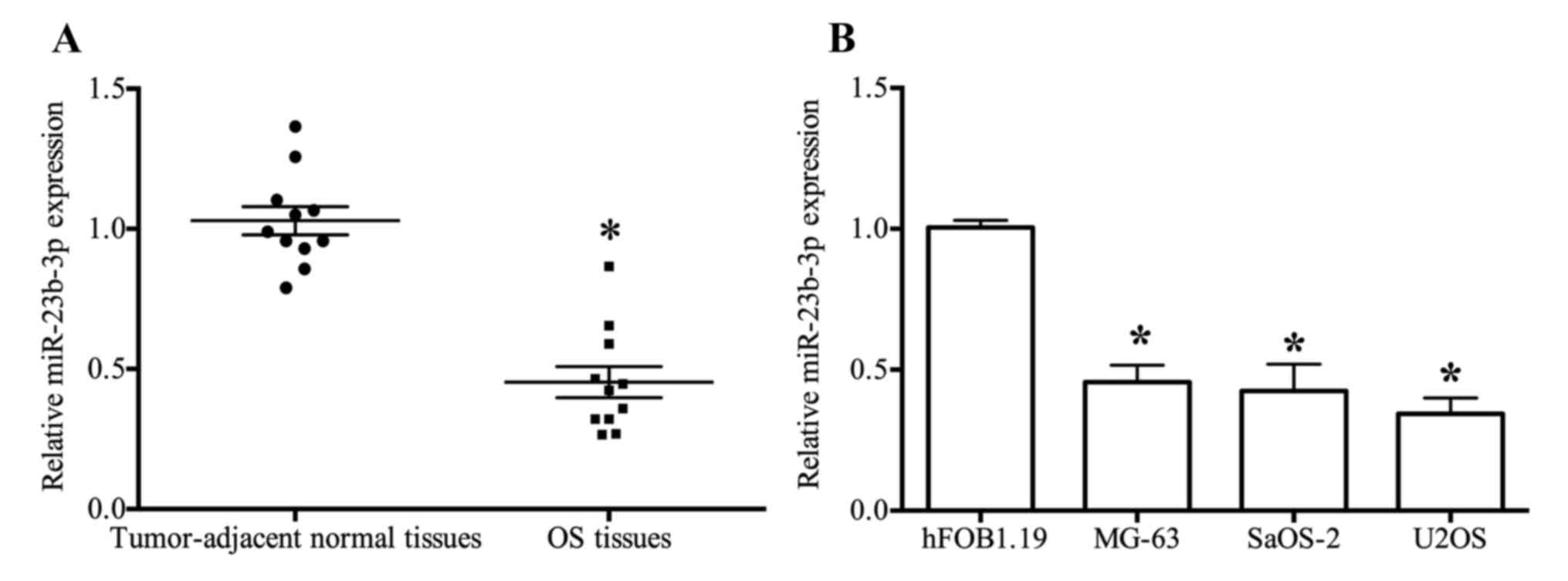
miR-23b-3p expression levels are
downregulated in OS tissues and cells
The results of RT-qPCR analyses demonstrated that
the expression of miR-23b-3p was significantly suppressed in OS
tissues when compared with tumor-adjacent normal tissues
(P<0.05; Table I and Fig. 1A). However, the expression of
miR-23b-3p in patients with OS was not associated with
clinicopathological characteristics (P>0.05). The expression
profile of miR-23b-3p in the three OS cell lines (MG-63, SaOS-2 and
U2OS) was similar to that exhibited by OS tissues, and was
significantly decreased when compared with the expression level
observed in normal osteoblast hFOB1.19 cells (Fig. 1B).
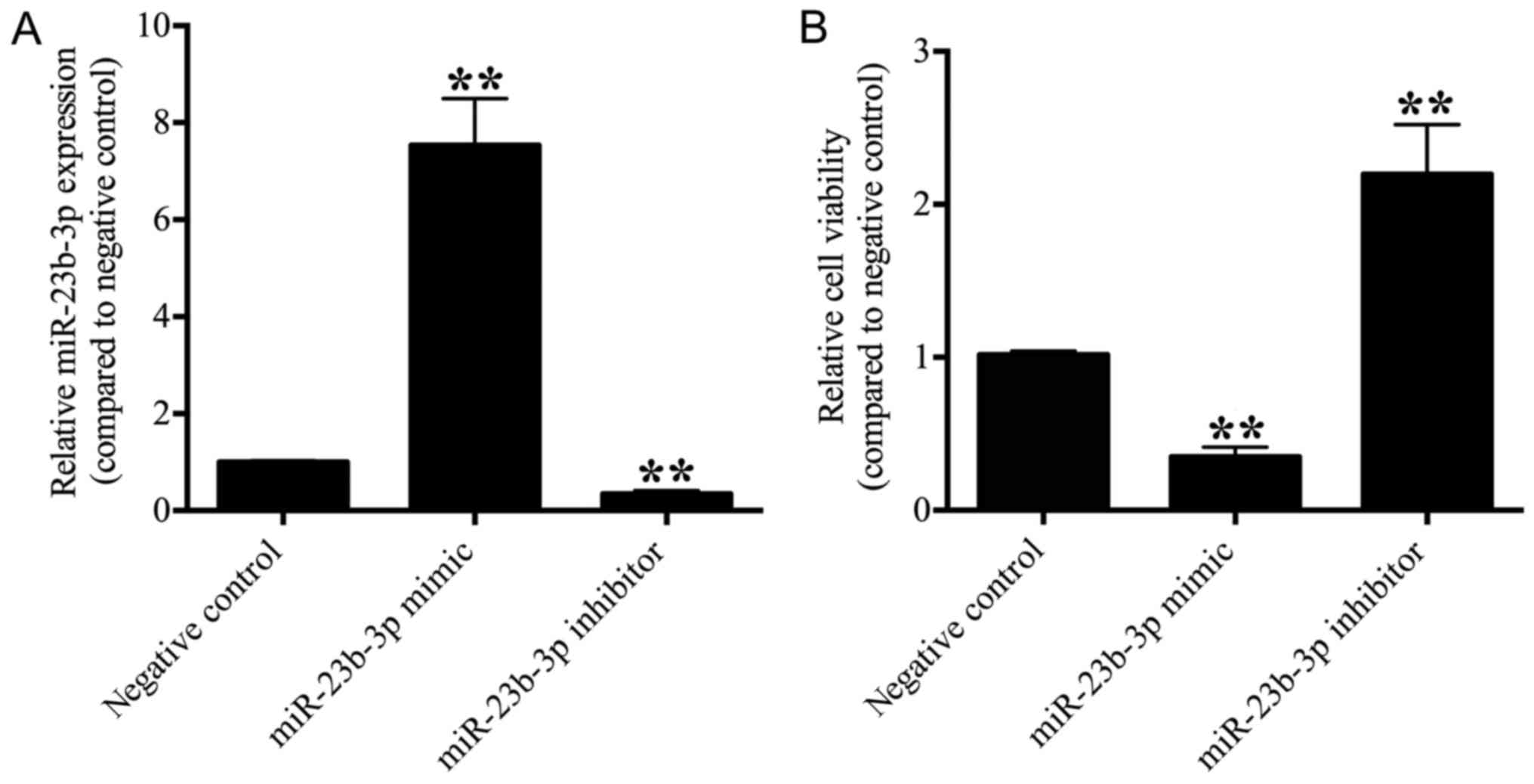
miR-23b-3p suppresses OS cell
viability and proliferation
To investigate the effects of miR-23b-3p on OS
cells, miR-23b-3p mimics, miR-23b-3p inhibitors and negative
controls were transfected into U2OS cells, and the expression of
miR-23b-3p was detected by RT-qPCR. The results demonstrated that
the expression of miR-23b-3p was significantly suppressed in the
miR-23b-3p inhibitor transfection group and significantly enhanced
in the miR-23b-3p mimic transfection group when compared with the
negative control group (P<0.01; Fig. 2A), which confirmed that human OS
U2OS cells, in which miR-23b-3p was either overexpressed or
depleted, were successfully established.
The effect of miR-23b-3p on U2OS cell viability was
determined using a CCK-8 kit. The OD value was significantly
decreased in the miR-23b-3p mimic transfection group and
significantly increased in the miR-23b-3p inhibitor transfection
group when compared with the negative control group (P<0.01;
Fig. 2B), which revealed that
miR-23b-3p suppressed the cell viability of U2OS cells.
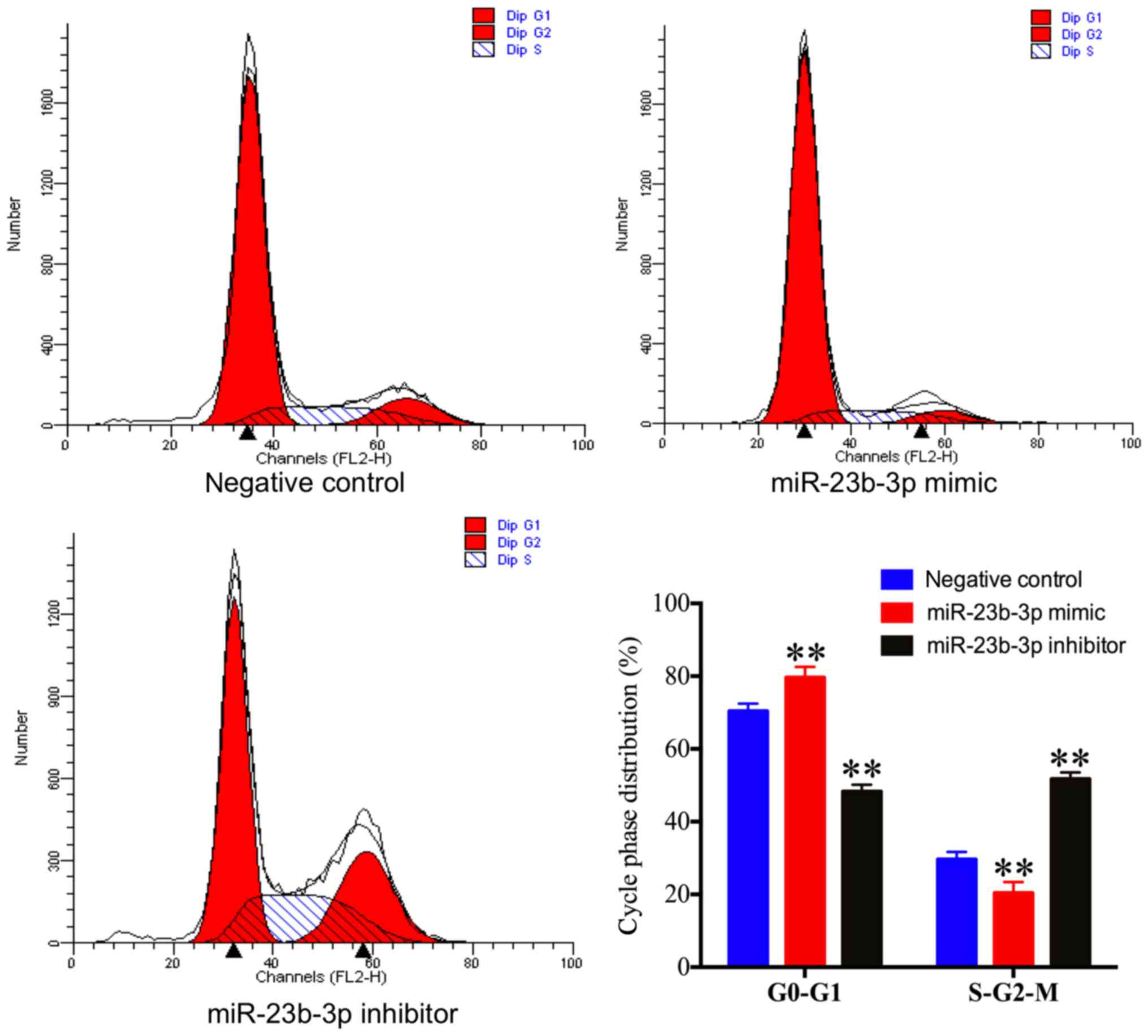
The effect of miR-23b-3p on U2OS cell cycle phase
distribution was investigated via flow cytometry. The percentage of
U2OS cells in the S and G2-M phases were significantly decreased in
the miR-23b-3p mimic transfection group and significantly increased
in the miR-23b-3p inhibitor transfection group when compared with
the negative control group (Fig.
3). By contrast, the percentage of U2OS cells at the G0-G1
phase was significantly increased in the miR-23b-3p mimic
transfection group and significantly decreased in the miR-23b-3p
inhibitor transfection group when compared with the negative
control group (Fig. 3). These
results demonstrated that miR-23b-3p inhibited OS cell cycle
progression.
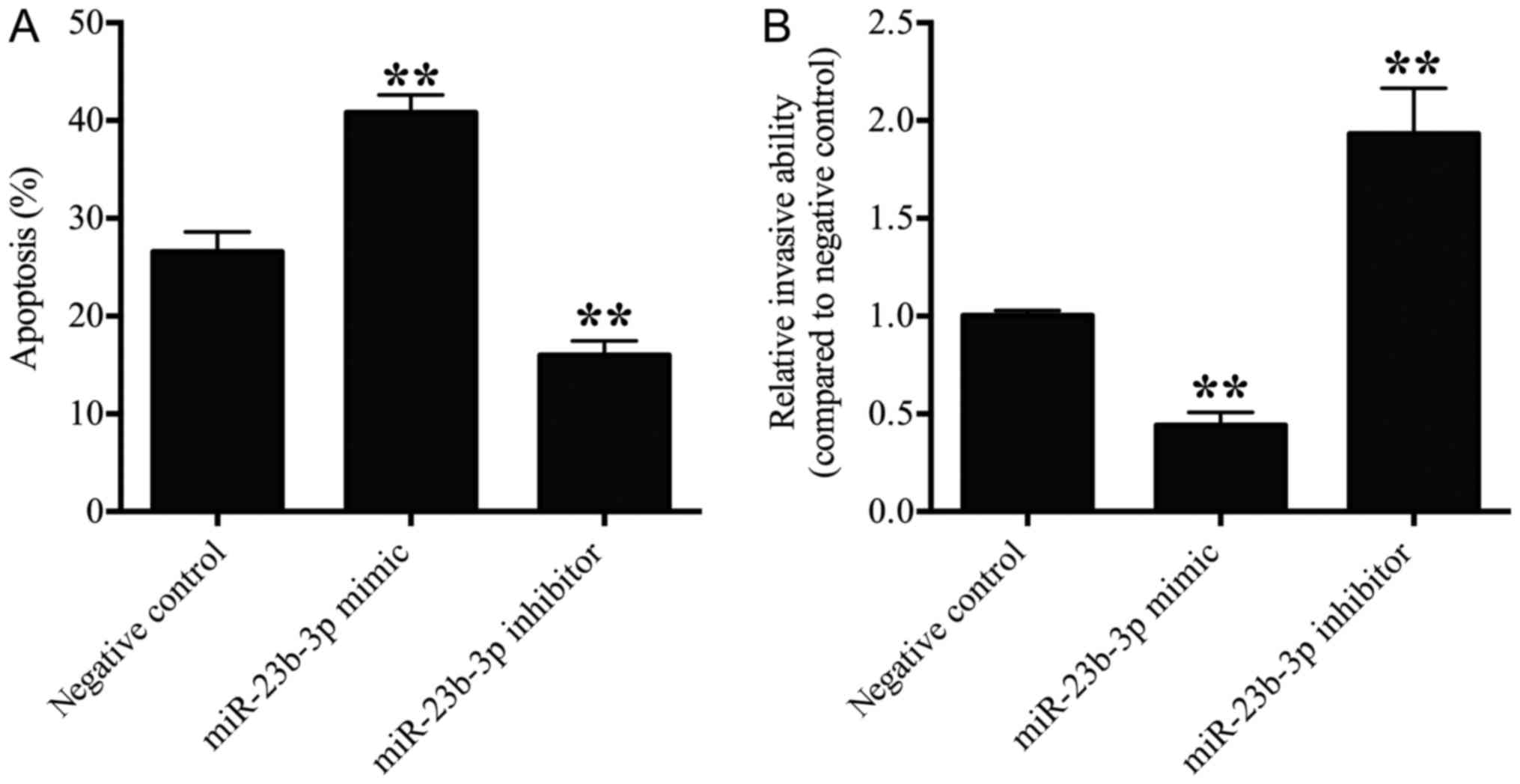
miR-23b-3p promotes OS cell apoptosis
and suppresses OS cell invasion
The effect of miR-23b-3p on the apoptosis of U2OS
cells was detected by flow cytometry analysis. The number of
apoptotic cells in the miR-23b-3p mimic transfection group was
significantly increased (P<0.01), and number of apoptotic cells
in the miR-23b-3p inhibitor transfection group was significantly
decreased when compared with the negative control group (P<0.01;
Fig. 4A), which suggested that
miR-23b-3p promoted OS cell apoptosis.
The effect of miR-23b-3p on the invasion of U2OS
cells was detected via transwell invasion assays. The number of
invasive cells was significantly suppressed in the miR-23b-3p mimic
transfection group (P<0.01), and significantly increased in the
miR-23b-3p inhibitor transfection group when compared with the
negative control group (P<0.01; Fig. 4B). These results indicated that
miR-23b-3p suppressed the invasive ability of OS cells.
Effects of miR-23b-3p on the
expression levels of cyclin D1, caspase-3 and VEGF-C in U2OS
cells
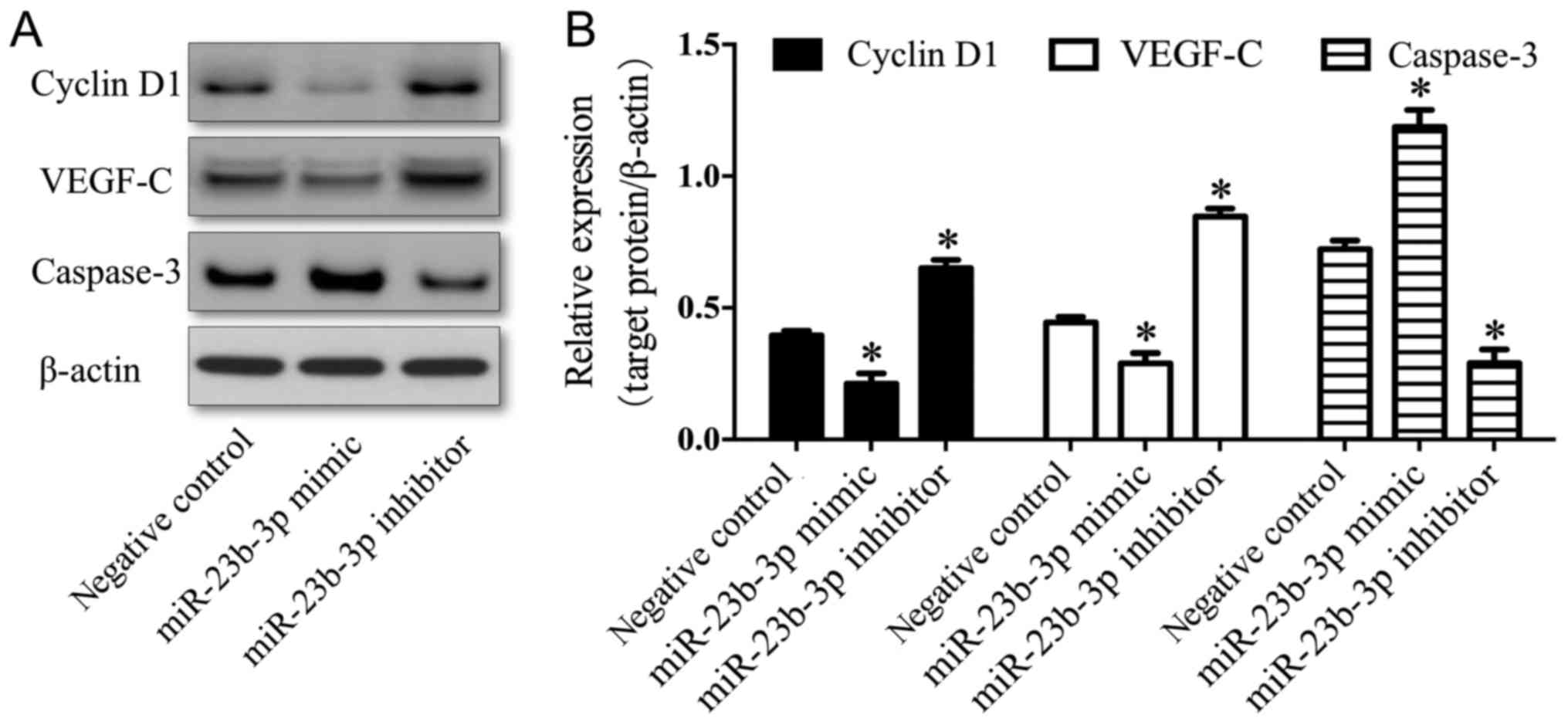
To investigate the mechanisms underlying the effects
of miR-23b-3p on the biological behavior of OS cells, the
expression levels of cyclin D1, caspase-3 and VEGF-C were
determined by western blotting. The expression levels of cyclin D1
and VEGF-C were downregulated in the miR-23b-3p mimic transfection
group (P<0.01), and significantly upregulated in the miR-23b-3p
inhibitor transfection group, compared with the negative control
group (P<0.01; Fig. 5). By
contrast, the expression of caspase-3 was upregulated in the
miR-23b-3p mimic transfection group (P<0.01), and downregulated
in the miR-23b-3p inhibitor transfection group, compared with the
negative control group (P<0.01; Fig. 5). These results suggested that
miR-23b-3p may be associated with decreased expression levels of
cyclin D1 and VEGF-C, and increased expression levels of caspase-3,
in U2OS cells.
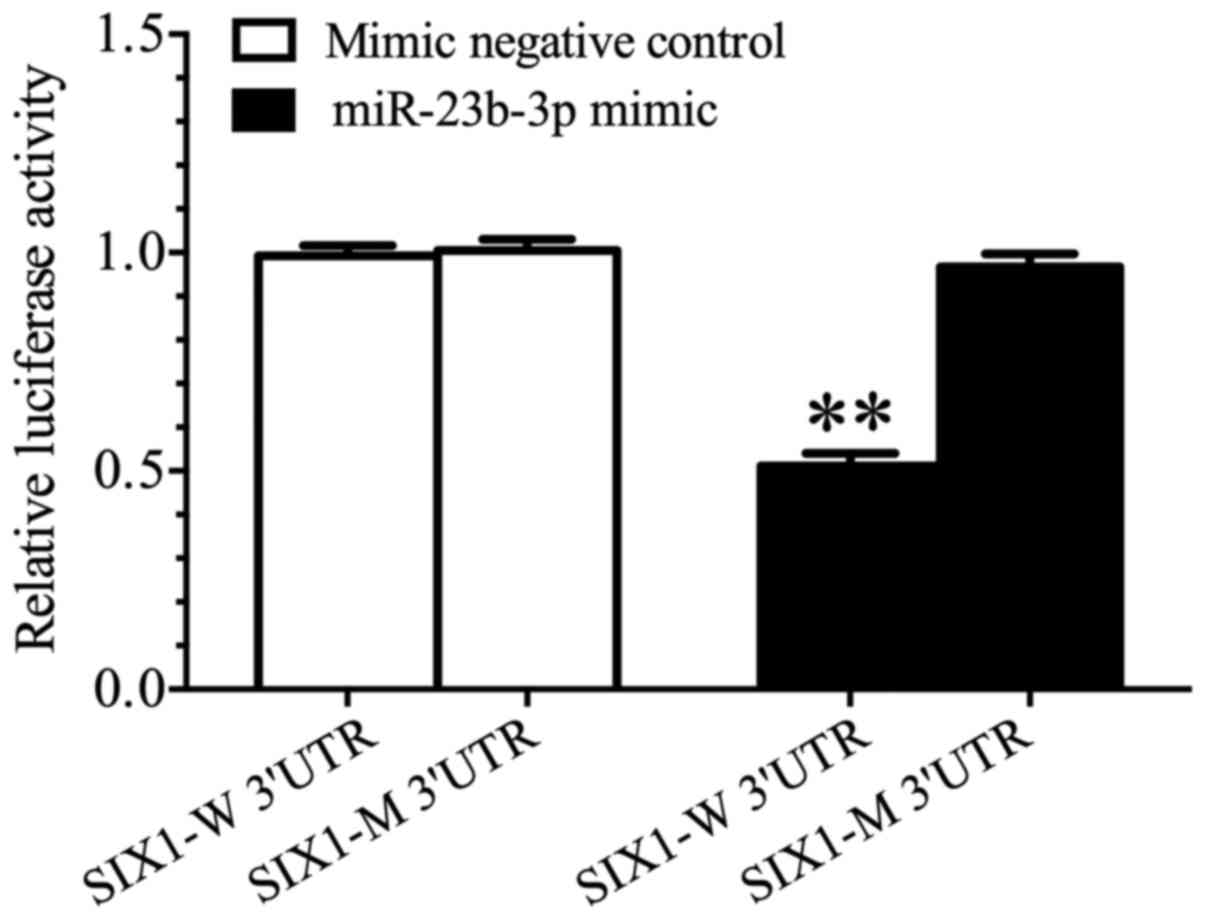
SIX1 constitutes a direct target gene
of miR-23b-3p
In the present study, luciferase reporter gene
analyses demonstrated that the activity of luciferase was
significantly decreased in 293 cells co-transfected with the
miR-23b-3p mimic and SIX1-W 3′-UTR when compared with cells
co-transfected with either the mimic negative control and SIX1-W
3′-UTR, or the miR-23b-3p mimic and SIX1-M 3′-UTR (P<0.01;
Fig. 6). Our previous study
revealed that the expression of SIX1 mRNA in OS cell lines
was significantly increased compared with normal human osteoblasts
(2), which indicated that there
was an inverse association between the expression of SIX1 and
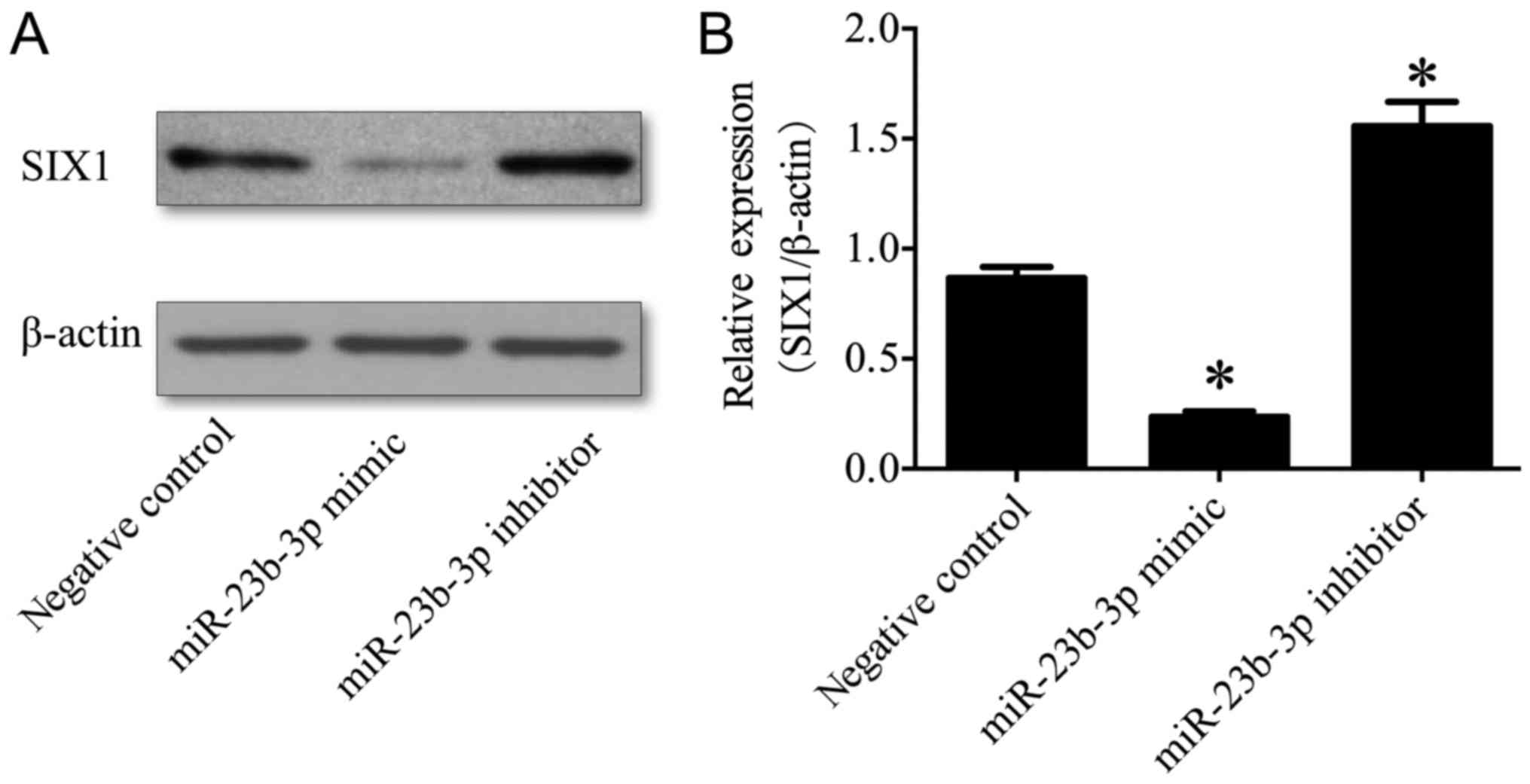
miR-23b-3p in OS. To investigate the role of miR-23b-3p in the
regulation of SIX1 expression, western blotting was performed to
determine the protein expression levels of SIX1 in U2OS cells
transfected with either miR-23b-3p mimics or inhibitors. The
results demonstrated that the expression levels of SIX1 protein
were significantly suppressed in the miR-23b-3p mimic transfection
group (P<0.01), and significantly enhanced in the miR-23b-3p
inhibitor transfection group, compared with the negative control
group (P<0.01; Fig. 7), which
demonstrated that miR-23b-3p may inhibit the expression of SIX1
protein in U2OS cells. Together, these results supported the
hypothesis that SIX1 represents a target gene of miR-23b-3p
in OS cells.
Discussion
In the present study, the results of RT-qPCR
analyses revealed that miR-23b-3p was significantly downregulated
in OS tissues and cell lines when compared with tumor-adjacent
normal tissues or normal osteoblast hFOB1.19 cells. The expression
profile of miR-23b-3p in OS was similar to that exhibited in
prostate cancer, cervical cancer and NSCLC (24–26),
which suggested that miR-23b-3p may function as a tumor suppressor
in OS. Despite the limited number of patients in the present study,
the results indicated that the downregulation of miR-23b-3p may be
associated with the tumorigenesis and progression of OS.
To further investigate the role of miR-23b-3p in OS,
miR-23b-3p mimics, miR-23b-3p inhibitors and negative controls were
transfected into U2OS cells to establish U2OS cells in which
miR-23b-3p was either overexpressed or depleted. Using these lines,
it was revealed that miR-23b-3p suppressed U2OS cell viability. The
effects of miR-23b-3p on the U2OS cell cycle were determined via
flow cytometry, and it was demonstrated that miR-23b-3p inhibited
OS cell proliferation. These results were consistent with those of
a previous study, which suggested that in human colon cancer,
downregulated miR-23b-3p regulates either frizzled class receptor 7
or MAPK kinase kinase 1, subsequently mediating cancer
proliferation as well as numerous stages of metastasis in
vivo (42). Considering that
cyclin D1 has been well established to represent an important
regulator of cellular proliferation and tumorigenesis (43,44),
the expression of cyclin D1 in U2OS lines was determined in the
present study. The results demonstrated that miR-23b-3p is involved
in the suppression of cyclin D1 expression in U2OS cells, which
suggested that miR-23b-3p may inhibit OS cell proliferation by
suppressing the levels of cyclin D1. In addition, flow cytometry
analyses demonstrated that miR-23b-3p also promotes OS cell
apoptosis. Furthermore, a previous study revealed that miR-23b-3p
promoted apoptosis and sensitized gastric cancer cells to
chemotherapy (31), which
indicated that miR-23b-3p may be applied for treatment for OS as
well as the prediction of drug resistance, thus supporting the
results of the present study. In addition, the results of the
present study indicated that miR-23b-3p may be associated with
increased caspase-3 expression levels, which represents one of the
most important biochemical markers of apoptosis (45) and a final effector of the apoptosis
process (46), and thus may induce
U2OS cell apoptosis.
Metastasis represents the leading cause of mortality
in patients with OS (47). In the
present study, the results of Transwell invasion assays revealed
that miR-23b-3p suppressed the invasive ability of OS cells, which
was consistent with previous findings in human colon cancer
(42). Furthermore, in our
previous study it was demonstrated that SIX1, a developmental
transcription factor predicted to represent a potential target gene
of miR-23b-3p, may enhance the expression of VEGF-C in OS (2). VEGF-C promotes cervical cancer cell
invasion and migration (48), and
enhances the metastatic ability of esophageal carcinoma (49), whereas knocking down VEGF-C
suppresses gastric cancer cell migration (50). Therefore, VEGF-C was investigated
in the present study, and the results demonstrated that miR-23b-3p
is associated with decreased expression levels of VEGF-C in U2OS
cells.
In addition, the results of our previous study
revealed that SIX1 is upregulated in OS cell lines and that
upregulated SIX1 may be associated with the promotion of the
growth, proliferation and migration of U2OS cells, as well as the
inhibition of U2OS cell apoptosis (2), which was in agreement with previous
studies suggesting that enhanced SIX1 expression is associated with
metastasis status or degree in breast cancer (35). Furthermore, our previous study also
revealed that transfection with SIX1 increased cyclin D1 and VEGF-C
expression levels, and decreased caspase-3 expression levels
(2). In the present study, it was
demonstrated that miR-23b-3p directly interacted with the
SIX1 3′UTR and suppressed the expression of SIX1 protein in
OS cells. Therefore, we hypothesized that miR-23b-3p downregulation
may inversely regulate the expression of SIX1 in OS cells, thereby
having an important role in the occurrence and development of
OS.
The results of the present study demonstrated that
miR-23b-3p was downregulated in OS, potentially because miR-23b-3p
suppressed the proliferation and invasion abilities of OS cells,
and enhanced OS cell apoptosis. In addition, the downregulated
miR-23b-3p subsequently upregulated SIX1 expression, which may have
induced the expression of cyclin D1 and VEGF-C, and inhibited the
expression of caspase-3. In conclusion, these results suggested
that OS exhibited downregulated miR-23b-3p and upregulated SIX1
expression levels, with the former inducing enhanced proliferation
and invasion in OS cells, as well as the suppression of cell
apoptosis via negative regulation of SIX1. Therefore, the
miR-23b-3p/SIX1 pathway may represent a potential target for the
treatment of OS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Nantong
Science and Technology Innovation Program (grant no. YYZ17012).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HL, WW and XW designed the study. HL, WW, XW, XG,
QC, ZP, XX and AW performed the experiments. HL, XG, QC, ZP and XX
collected and assembled the data. HL and WW performed the data
analysis. XW provided scientific expertise. HL wrote the
manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Clinical Research Ethics Committee of the Haian Hospital of
Traditional Chinese Medicine (Nantong, China), and written informed
consent was obtained from all patients prior to enrollment.
Patient consent for publication
All patients agreed to publication and provided
written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hua L, Fan L, Aichun W, Yongjin Z,
Qingqing C and Xiaojian W: Inhibition of Six1 promotes apoptosis,
suppresses proliferation, and migration of osteosarcoma cells.
Tumour Biol. 35:1925–1931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jianwei Z, Fan L, Xiancheng L, Enzhong B,
Shuai L and Can L: MicroRNA 181a improves proliferation and
invasion, suppresses apoptosis of osteosarcoma cell. Tumour Biol.
34:3331–3337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hughes DP: Strategies for the targeted
delivery of therapeutics for osteosarcoma. Expert Opin Drug Deliv.
6:1311–1321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Y, Feng Y, Shen JK, Lin M, Choy E,
Cote GM, Harmon DC, Mankin HJ, Hornicek FJ and Duan Z: CD44 is a
direct target of miR-199a-3p and contributes to aggressive
progression in osteosarcoma. Sci Rep. 5:113652015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
7
|
Poletajew S, Fus L and Wasiutynski A:
Current concepts on pathogenesis and biology of metastatic
osteosarcoma tumors. Ortop Traumatol Rehabil. 13:537–545. 2011.(In
English, Polish). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hurley C, McCarville MB, Shulkin BL, Mao
S, Wu J, Navid F, Daw NC, Pappo AS and Bishop MW: Comparison of
(18) F-FDG-PET-CT and bone scintigraphy for evaluation of osseous
metastases in newly diagnosed and recurrent osteosarcoma. Pediatr
Blood Cancer. 63:1381–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang Q, Xu T, Wu C, Zhou S and Sun H:
Biotargets in neural regeneration. Biotarget. 1:6–10. 2017.
View Article : Google Scholar
|
|
11
|
Wu Y and Jiang M: The revolution of lung
cancer treatment: From vaccines, to immune checkpoint inhibitors,
to chimeric antigen receptor T therapy. Biotarget. 1:72017.
View Article : Google Scholar
|
|
12
|
Ohtsuka M, Tanemura M and Akamatsu H: Long
noncoding RNAs regulate malignant phenotypes in colorectal cancer.
Biotarget. 2:42018. View Article : Google Scholar
|
|
13
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin S, Pan L, Guo S, Wu J, Jin L, Wang JC
and Wang S: Prognostic role of microRNA-181a/b in hematological
malignancies: A meta-analysis. PLoS one. 8:e595322013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Q, Gallagher R, Ufret-Vincenty R, Li
X, Olson EN and Wang S: Regulation of angiogenesis and choroidal
neovascularization by members of microRNA-23~27~24 clusters. Proc
Natl Acad Sci U S A. 108:8287–8292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho S, Wu CJ, Yasuda T, Cruz LO, Khan AA,
Lin LL, Nguyen DT, Miller M, Lee HM, Kuo ML, et al: miR-23
approximately 27 approximately 24 clusters control effector T cell
differentiation and function. J Exp Med. 213:235–249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Y, Zhang D, Hu T and Li D: miR-23
regulate the pathogenesis of patients with coronary artery disease.
Int J Clin Exp Med. 8:11759–11769. 2015.PubMed/NCBI
|
|
18
|
Wang Y, Liang H, Zhou G, Hu X, Liu Z, Jin
F, Yu M, Sang J, Zhou Y, Fu Z, et al: HIC1 and miR-23~27~24
clusters form a double-negative feedback loop in breast cancer.
Cell Death Differ. 24:421–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noma D, Inamura K, Matsuura Y, Ninomiya H,
Ichinose J, Nakao M, Mun M, Ishikawa Y and Okumura S:
ALK-rearranged lung adenocarcinoma showing intra-bronchial
protrusion: A case of actually peripheral origin with a rare
spreading pattern. Biotarget. 1:152017. View Article : Google Scholar
|
|
20
|
Cai S, Chen R, Li X, Cai Y, Ye Z, Li S, Li
J, Huang H, Peng S, Wang J, et al: Downregulation of microRNA-23a
suppresses prostate cancer metastasis by targeting the PAK6-LIMK1
signaling pathway. Oncotarget. 6:3904–3917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He Y, Meng C, Shao Z, Wang H and Yang S:
MiR-23a functions as a tumor suppressor in osteosarcoma. Cell
Physiol Biochem. 34:1485–1496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haier J, Strose A, Matuszcak C and Hummel
R: miR clusters target cellular functional complexes by defining
their degree of regulatory freedom. Cancer Metastasis Rev.
35:289–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mercatelli N, Fittipaldi S, De Paola E,
Dimauro I, Paronetto MP, Jackson MJ and Caporossi D: MiR-23-TrxR1
as a novel molecular axis in skeletal muscle differentiation. Sci
Rep. 7:72192017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aghaee-Bakhtiari SH, Arefian E, Naderi M,
Noorbakhsh F, Nodouzi V, Asgari M, Fard-Esfahani P, Mahdian R and
Soleimani M: MAPK and JAK/STAT pathways targeted by miR-23a and
miR-23b in prostate cancer: Computational and in vitro approaches.
Tumour Biol. 36:4203–4212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campos-Viguri GE, Jimenez-Wences H,
Peralta-Zaragoza O, Torres-Altamirano G, Soto-Flores DG,
Hernández-Sotelo D, Ldel Alarcón-Romero C, Jiménez-López MA,
Illades-Aguiar B and Fernández-Tilapa G: miR-23b as a potential
tumor suppressor and its regulation by DNA methylation in cervical
cancer. Infect Agents Cancer. 10:422015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Begum S, Hayashi M, Ogawa T, Jabboure FJ,
Brait M, Izumchenko E, Tabak S, Ahrendt SA, Westra WH, Koch W, et
al: An integrated genome-wide approach to discover deregulated
microRNAs in non-small cell lung cancer: Clinical significance of
miR-23b-3p deregulation. Sci Rep. 5:132362015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan J, Jiang JY, Meng XN, Xiu YL and Zong
ZH: MiR-23b targets cyclin G1 and suppresses ovarian cancer
tumorigenesis and progression. J Exp Clin Cancer Res. 35:312016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An Y, Zhang Z, Shang Y, Jiang X, Dong J,
Yu P, Nie Y and Zhao Q: miR-23b-3p regulates the chemoresistance of
gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis.
6:e17662015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin L, Wessely O, Marcusson EG, Ivan C,
Calin GA and Alahari SK: Prooncogenic factors miR-23b and miR-27b
are regulated by Her2/Neu, EGF, and TNF-alpha in breast cancer.
Cancer Res. 73:2884–2896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bricaud O and Collazo A: The transcription
factor six1 inhibits neuronal and promotes hair cell fate in the
developing zebrafish (Danio rerio) inner ear. J Neurosci.
26:10438–10451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Christensen KL, Patrick AN, McCoy EL and
Ford HL: The six family of homeobox genes in development and
cancer. Adv Cancer Res. 101:93–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu C, Zhang B, Li YL and Yu XR: SIX1
reduces the expression of PTEN via activating PI3K/AKT signal to
promote cell proliferation and tumorigenesis in osteosarcoma.
Biomed Pharmacother. 105:10–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Tian T, Hu X, Zhang X, Li L, Nan F,
Chang Y, Wang X, Sun Z, Lv F and Zhang M: Targeting Six1 by
lentivirus-mediated RNA interference inhibits colorectal cancer
cell growth and invasion. Int J Clin Exp Pathol. 7:631–639.
2014.PubMed/NCBI
|
|
34
|
Iwanaga R, Wang CA, Micalizzi DS, Harrell
JC, Jedlicka P, Sartorius CA, Kabos P, Farabaugh SM, Bradford AP
and Ford HL: Expression of Six1 in luminal breast cancers predicts
poor prognosis and promotes increases in tumor initiating cells by
activation of extracellular signal-regulated kinase and
transforming growth factor-beta signaling pathways. Breast Cancer
Res. 14:R1002012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reichenberger KJ, Coletta RD, Schulte AP,
Varella-Garcia M and Ford HL: Gene amplification is a mechanism of
Six1 overexpression in breast cancer. Cancer Res. 65:2668–2675.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang CA, Jedlicka P, Patrick AN, Micalizzi
DS, Lemmer KC, Deitsch E, Casás-Selves M, Harrell JC and Ford HL:
SIX1 induces lymphangiogenesis and metastasis via upregulation of
VEGF-C in mouse models of breast cancer. J Clin Invest.
122:1895–1906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong YN, Wang X, Wang J, Yang Z, Li S,
Yang J, Liu L, Lei X and Shao F: Chemical probing reveals insights
into the signaling mechanism of inflammasome activation. Cell Res.
20:1289–1305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai PW, Shiah SG, Lin MT, Wu CW and Kuo
ML: Up-regulation of vascular endothelial growth factor C in breast
cancer cells by heregulin-beta 1. A critical role of p38/nuclear
factor-kappa B signaling pathway. J Biol Chem. 278:5750–5759. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takaesu G: Two types of TRAF6-dependent
TAK1 activation in the IL-1 signaling pathway. Biotarget. 2:22018.
View Article : Google Scholar
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bryksin A and Matsumura I: Overlap
extension PCR cloning. Methods Mol Biol. 1073:31–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin
S, Sun C, Ma M, Huang Y and Xi JJ: Genome-wide functional screening
of miR-23b as a pleiotropic modulator suppressing cancer
metastasis. Nat Commun. 2:5542011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pysz MA, Hao F, Hizli AA, Lum MA, Swetzig
WM, Black AR and Black JD: Differential regulation of cyclin D1
expression by protein kinase C alpha and signaling in intestinal
epithelial cells. J Biol Chem. 289:22268–22283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang H, Wang X, Archer TK, Zwaka TP and
Cooney AJ: GCNF-dependent activation of cyclin D1 expression via
repression of Mir302a during ESC differentiation. Stem Cells.
32:1527–1537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vagner T, Mouravlev A and Young D: A novel
bicistronic sensor vector for detecting caspase-3 activation. J
Pharmacol Toxicol Methods. 72:11–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang HC, Zhang ZT, Huang JS, Zhang P,
Xiong NA and Wang T: The contribution of Cdc2 in rotenone-induced
G2/M arrest and caspase-3-dependent apoptosis (vol 53, pg 31,
2014). J Mol Neurosci. 55:812–813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mirabello L, Koster R, Moriarity BS,
Spector LG, Meltzer PS, Gary J, Machiela MJ, Pankratz N, Panagiotou
OA, Largaespada D, et al: A genome-wide scan identifies variants in
NFIB associated with metastasis in patients with osteosarcoma.
Cancer Discov. 5:920–931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen H, Suo K, Cheng Y, Zheng B and Xu L:
Vascular endothelial growth factor C enhances cervical cancer
migration and invasion via activation of focal adhesion kinase.
Gynecol Endocrinol. 29:20–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Su CM, Su YH, Chiu CF, Chang YW, Hong CC,
Yu YH, Ho YS, Wu CH, Yen CS and Su JL: Vascular endothelial growth
factor-C upregulates cortactin and promotes metastasis of
esophageal squamous cell carcinoma. Ann Surg Oncol. 4 Suppl
21:S767–S775. 2014. View Article : Google Scholar
|
|
50
|
Lim J, Ryu JH, Kim EJ, Ham S and Kang D:
Inhibition of vascular endothelial growth factor receptor 3 reduces
migration of gastric cancer cells. Cancer Invest. 33:398–404. 2015.
View Article : Google Scholar : PubMed/NCBI
|