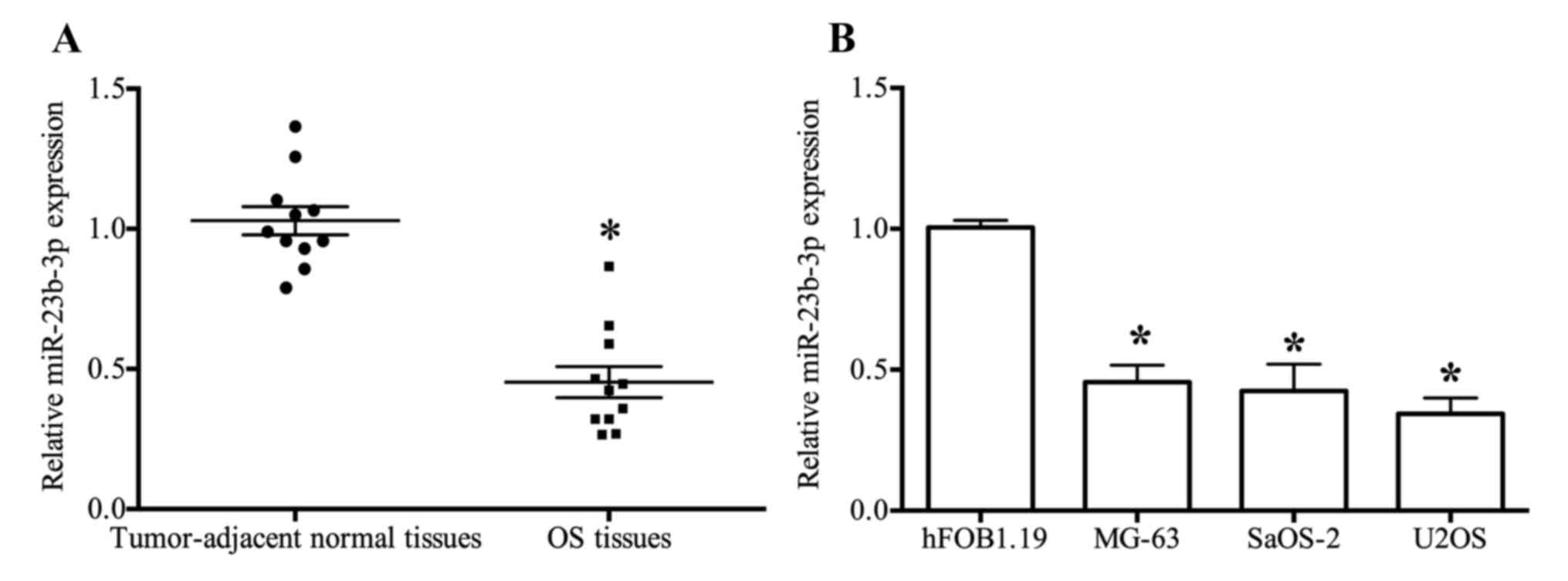
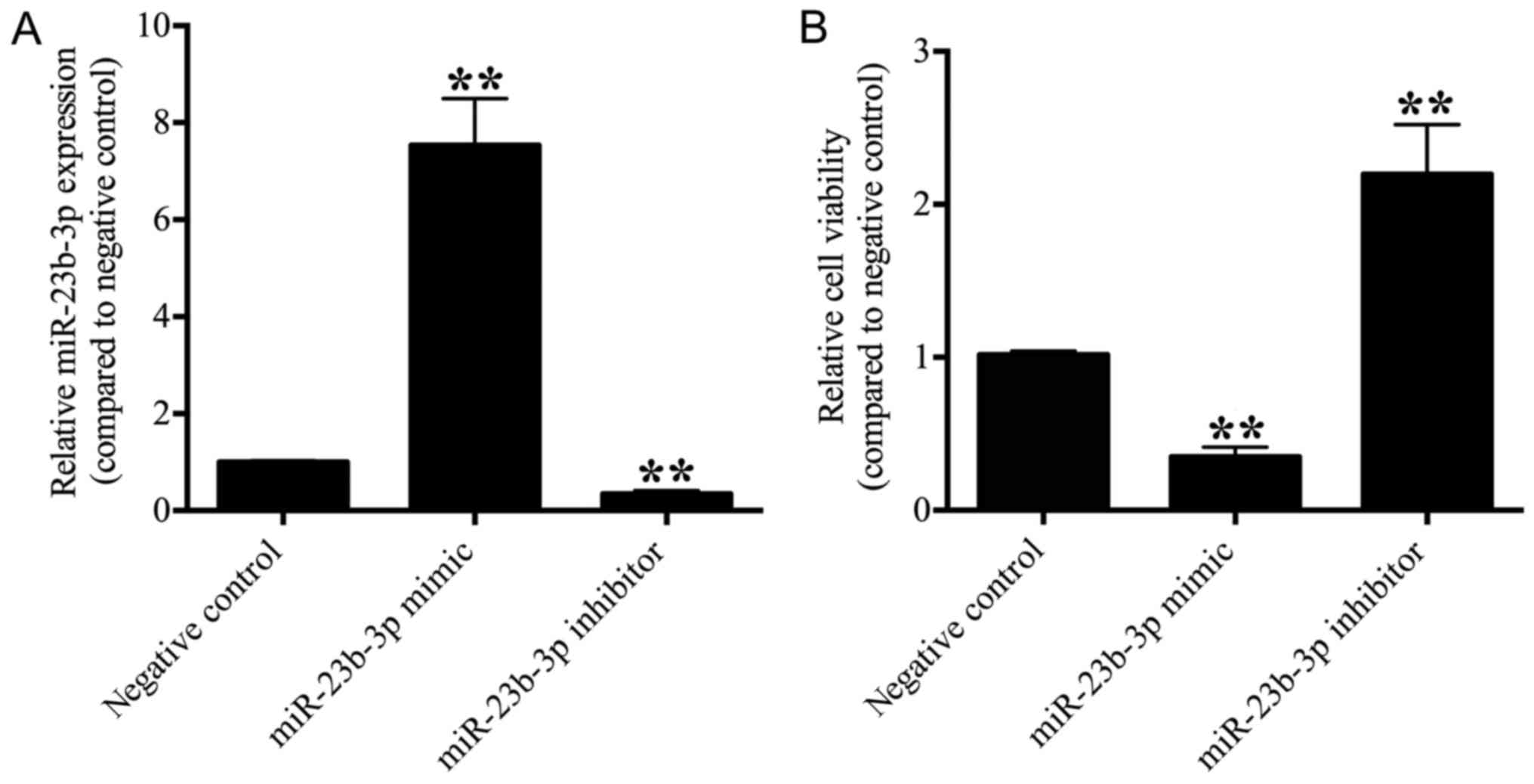
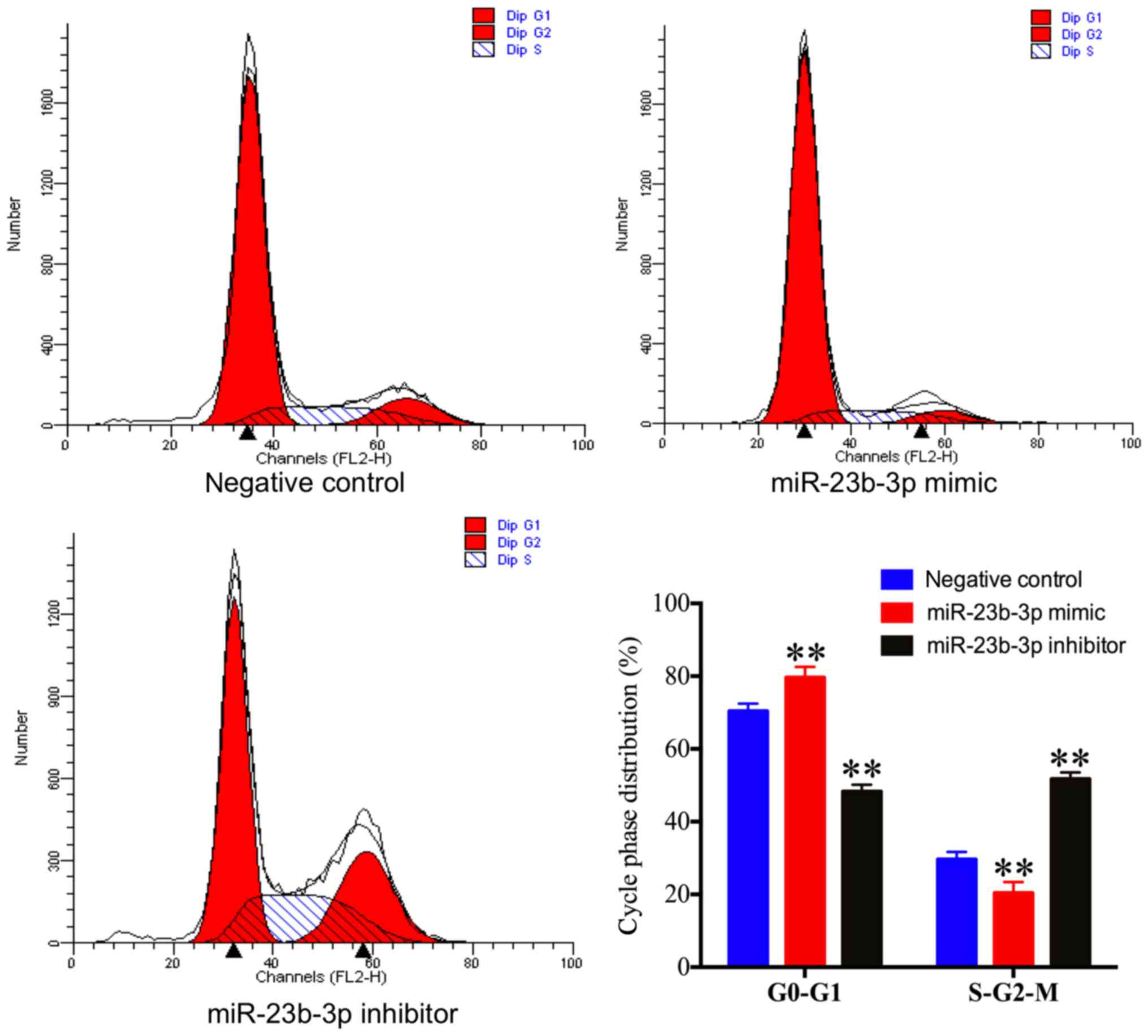
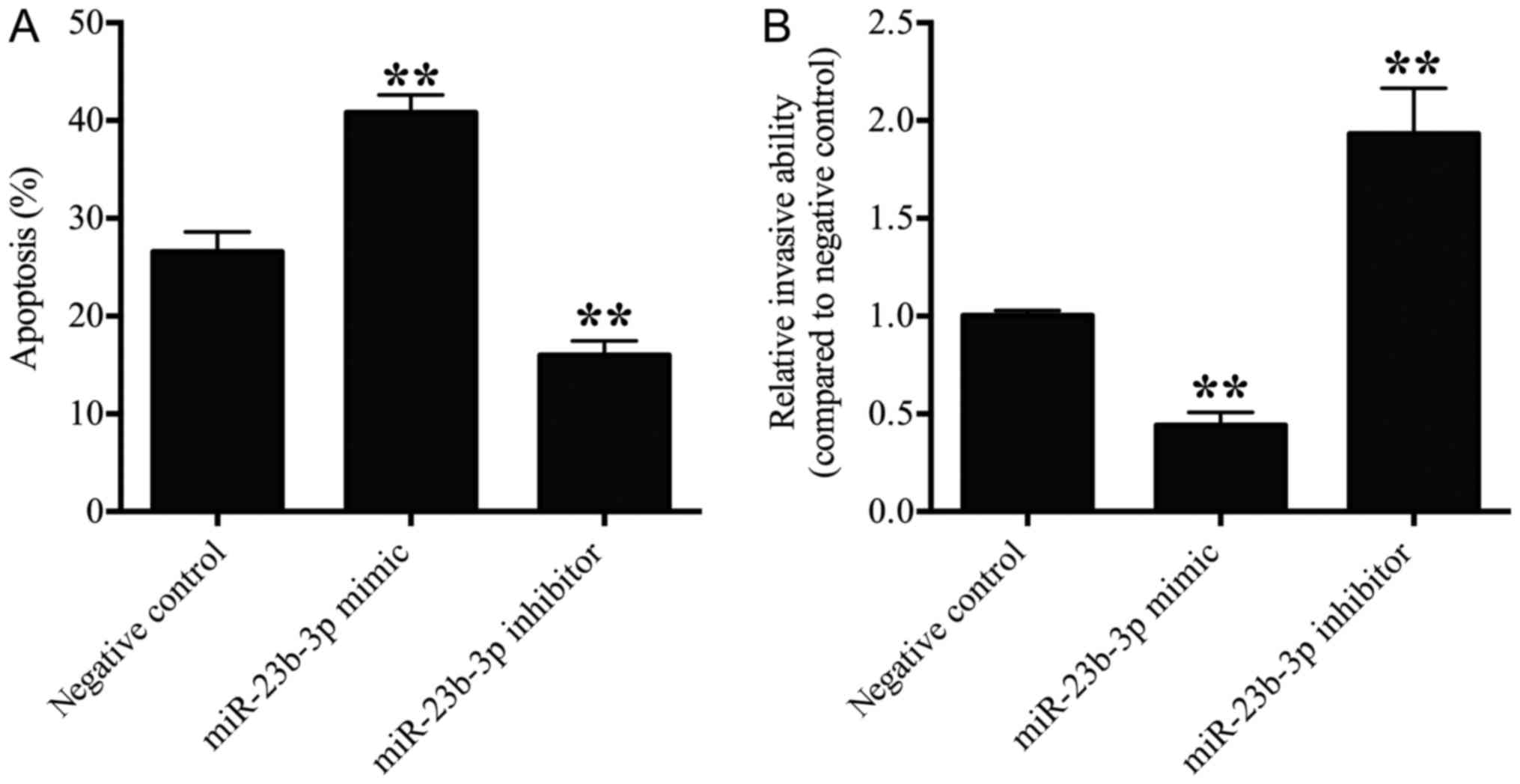
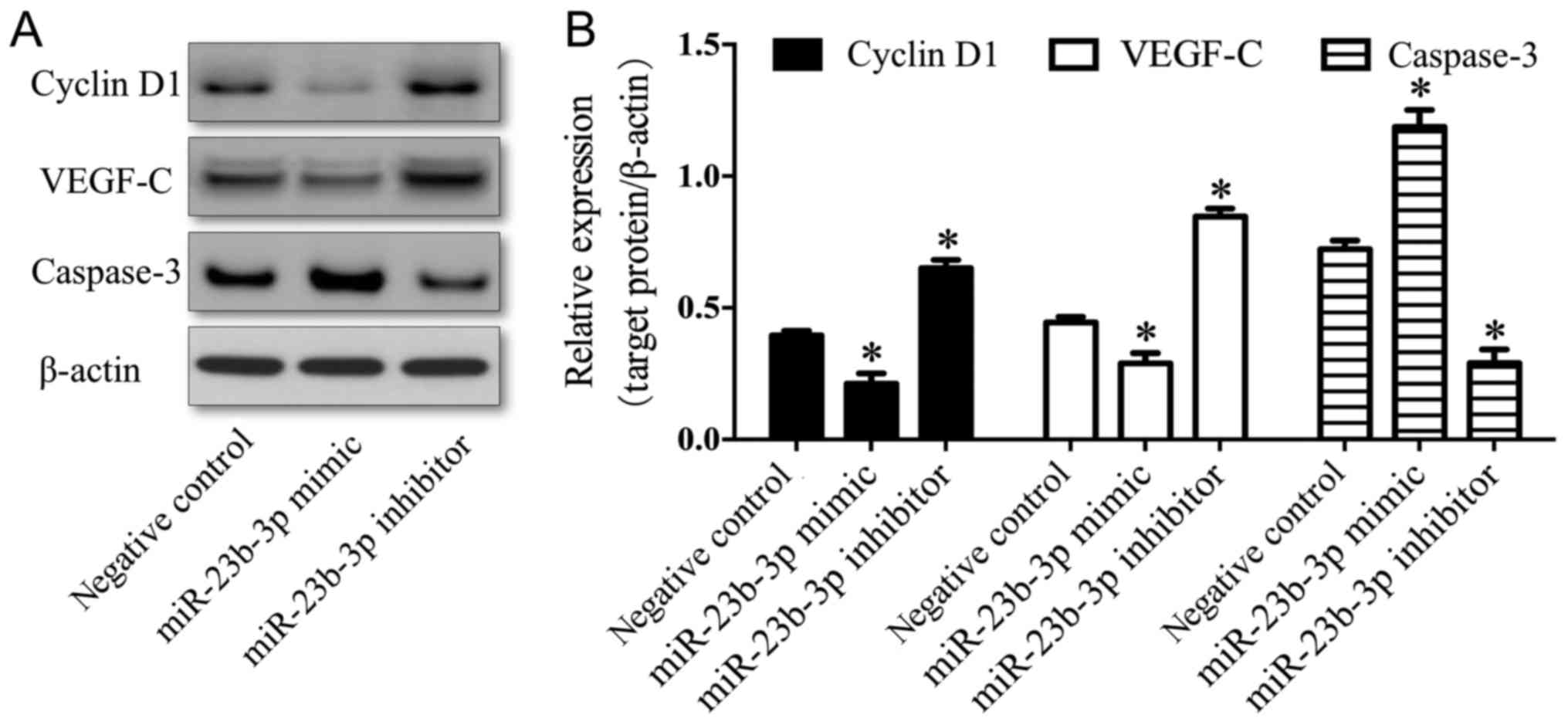
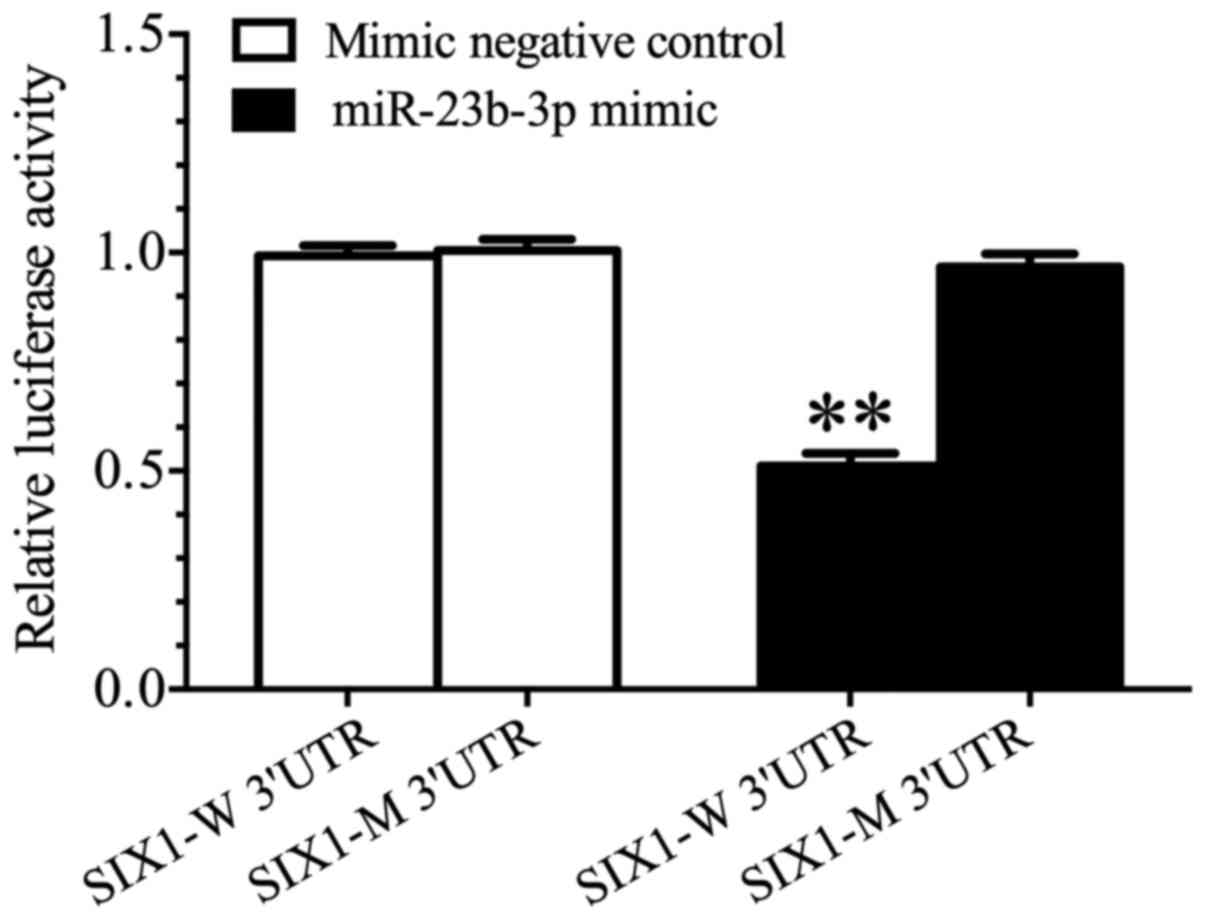
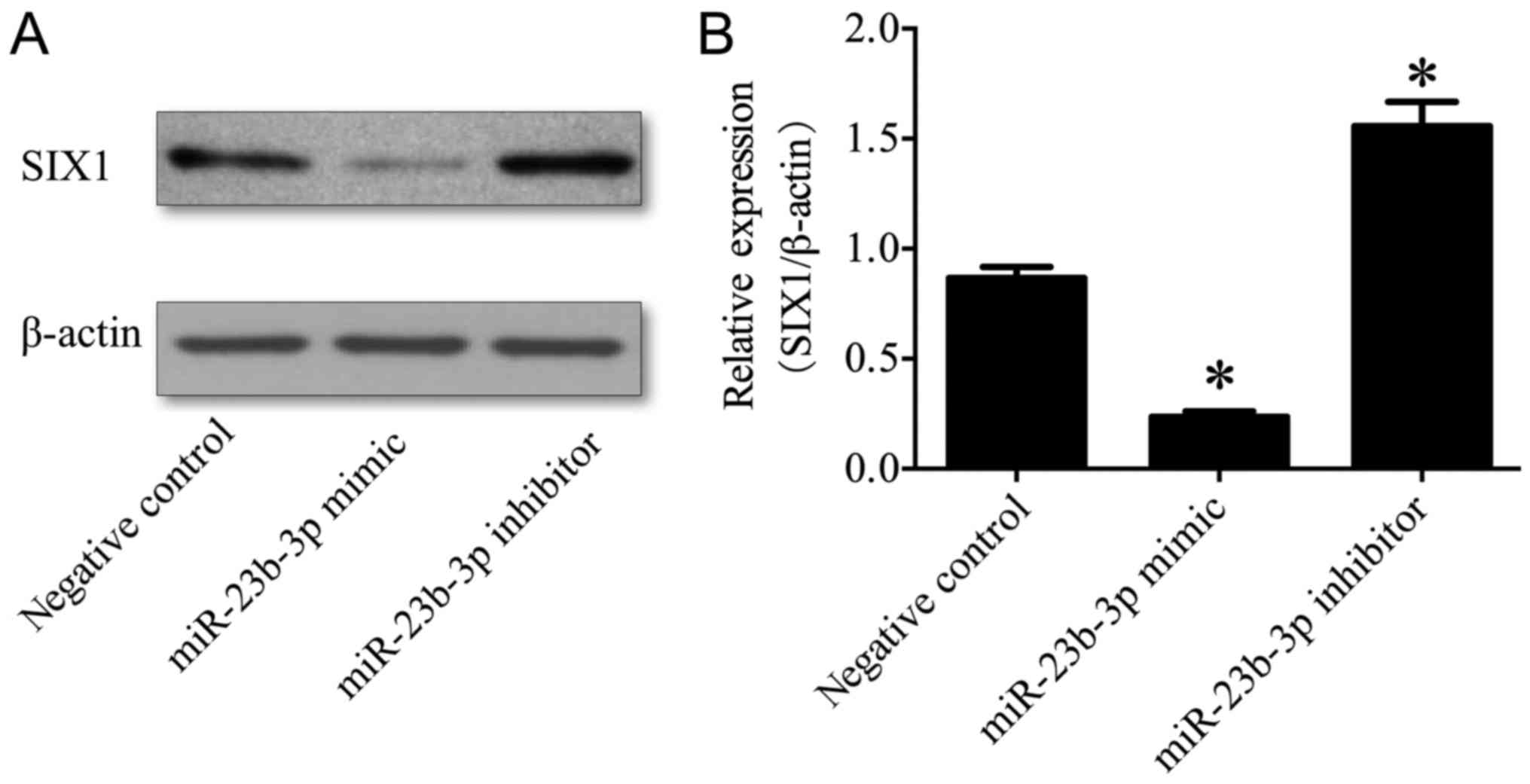
|
1
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hua L, Fan L, Aichun W, Yongjin Z,
Qingqing C and Xiaojian W: Inhibition of Six1 promotes apoptosis,
suppresses proliferation, and migration of osteosarcoma cells.
Tumour Biol. 35:1925–1931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jianwei Z, Fan L, Xiancheng L, Enzhong B,
Shuai L and Can L: MicroRNA 181a improves proliferation and
invasion, suppresses apoptosis of osteosarcoma cell. Tumour Biol.
34:3331–3337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hughes DP: Strategies for the targeted
delivery of therapeutics for osteosarcoma. Expert Opin Drug Deliv.
6:1311–1321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Y, Feng Y, Shen JK, Lin M, Choy E,
Cote GM, Harmon DC, Mankin HJ, Hornicek FJ and Duan Z: CD44 is a
direct target of miR-199a-3p and contributes to aggressive
progression in osteosarcoma. Sci Rep. 5:113652015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
7
|
Poletajew S, Fus L and Wasiutynski A:
Current concepts on pathogenesis and biology of metastatic
osteosarcoma tumors. Ortop Traumatol Rehabil. 13:537–545. 2011.(In
English, Polish). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hurley C, McCarville MB, Shulkin BL, Mao
S, Wu J, Navid F, Daw NC, Pappo AS and Bishop MW: Comparison of
(18) F-FDG-PET-CT and bone scintigraphy for evaluation of osseous
metastases in newly diagnosed and recurrent osteosarcoma. Pediatr
Blood Cancer. 63:1381–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang Q, Xu T, Wu C, Zhou S and Sun H:
Biotargets in neural regeneration. Biotarget. 1:6–10. 2017.
View Article : Google Scholar
|
|
11
|
Wu Y and Jiang M: The revolution of lung
cancer treatment: From vaccines, to immune checkpoint inhibitors,
to chimeric antigen receptor T therapy. Biotarget. 1:72017.
View Article : Google Scholar
|
|
12
|
Ohtsuka M, Tanemura M and Akamatsu H: Long
noncoding RNAs regulate malignant phenotypes in colorectal cancer.
Biotarget. 2:42018. View Article : Google Scholar
|
|
13
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin S, Pan L, Guo S, Wu J, Jin L, Wang JC
and Wang S: Prognostic role of microRNA-181a/b in hematological
malignancies: A meta-analysis. PLoS one. 8:e595322013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Q, Gallagher R, Ufret-Vincenty R, Li
X, Olson EN and Wang S: Regulation of angiogenesis and choroidal
neovascularization by members of microRNA-23~27~24 clusters. Proc
Natl Acad Sci U S A. 108:8287–8292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho S, Wu CJ, Yasuda T, Cruz LO, Khan AA,
Lin LL, Nguyen DT, Miller M, Lee HM, Kuo ML, et al: miR-23
approximately 27 approximately 24 clusters control effector T cell
differentiation and function. J Exp Med. 213:235–249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Y, Zhang D, Hu T and Li D: miR-23
regulate the pathogenesis of patients with coronary artery disease.
Int J Clin Exp Med. 8:11759–11769. 2015.PubMed/NCBI
|
|
18
|
Wang Y, Liang H, Zhou G, Hu X, Liu Z, Jin
F, Yu M, Sang J, Zhou Y, Fu Z, et al: HIC1 and miR-23~27~24
clusters form a double-negative feedback loop in breast cancer.
Cell Death Differ. 24:421–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noma D, Inamura K, Matsuura Y, Ninomiya H,
Ichinose J, Nakao M, Mun M, Ishikawa Y and Okumura S:
ALK-rearranged lung adenocarcinoma showing intra-bronchial
protrusion: A case of actually peripheral origin with a rare
spreading pattern. Biotarget. 1:152017. View Article : Google Scholar
|
|
20
|
Cai S, Chen R, Li X, Cai Y, Ye Z, Li S, Li
J, Huang H, Peng S, Wang J, et al: Downregulation of microRNA-23a
suppresses prostate cancer metastasis by targeting the PAK6-LIMK1
signaling pathway. Oncotarget. 6:3904–3917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He Y, Meng C, Shao Z, Wang H and Yang S:
MiR-23a functions as a tumor suppressor in osteosarcoma. Cell
Physiol Biochem. 34:1485–1496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haier J, Strose A, Matuszcak C and Hummel
R: miR clusters target cellular functional complexes by defining
their degree of regulatory freedom. Cancer Metastasis Rev.
35:289–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mercatelli N, Fittipaldi S, De Paola E,
Dimauro I, Paronetto MP, Jackson MJ and Caporossi D: MiR-23-TrxR1
as a novel molecular axis in skeletal muscle differentiation. Sci
Rep. 7:72192017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aghaee-Bakhtiari SH, Arefian E, Naderi M,
Noorbakhsh F, Nodouzi V, Asgari M, Fard-Esfahani P, Mahdian R and
Soleimani M: MAPK and JAK/STAT pathways targeted by miR-23a and
miR-23b in prostate cancer: Computational and in vitro approaches.
Tumour Biol. 36:4203–4212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campos-Viguri GE, Jimenez-Wences H,
Peralta-Zaragoza O, Torres-Altamirano G, Soto-Flores DG,
Hernández-Sotelo D, Ldel Alarcón-Romero C, Jiménez-López MA,
Illades-Aguiar B and Fernández-Tilapa G: miR-23b as a potential
tumor suppressor and its regulation by DNA methylation in cervical
cancer. Infect Agents Cancer. 10:422015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Begum S, Hayashi M, Ogawa T, Jabboure FJ,
Brait M, Izumchenko E, Tabak S, Ahrendt SA, Westra WH, Koch W, et
al: An integrated genome-wide approach to discover deregulated
microRNAs in non-small cell lung cancer: Clinical significance of
miR-23b-3p deregulation. Sci Rep. 5:132362015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan J, Jiang JY, Meng XN, Xiu YL and Zong
ZH: MiR-23b targets cyclin G1 and suppresses ovarian cancer
tumorigenesis and progression. J Exp Clin Cancer Res. 35:312016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An Y, Zhang Z, Shang Y, Jiang X, Dong J,
Yu P, Nie Y and Zhao Q: miR-23b-3p regulates the chemoresistance of
gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis.
6:e17662015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin L, Wessely O, Marcusson EG, Ivan C,
Calin GA and Alahari SK: Prooncogenic factors miR-23b and miR-27b
are regulated by Her2/Neu, EGF, and TNF-alpha in breast cancer.
Cancer Res. 73:2884–2896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bricaud O and Collazo A: The transcription
factor six1 inhibits neuronal and promotes hair cell fate in the
developing zebrafish (Danio rerio) inner ear. J Neurosci.
26:10438–10451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Christensen KL, Patrick AN, McCoy EL and
Ford HL: The six family of homeobox genes in development and
cancer. Adv Cancer Res. 101:93–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu C, Zhang B, Li YL and Yu XR: SIX1
reduces the expression of PTEN via activating PI3K/AKT signal to
promote cell proliferation and tumorigenesis in osteosarcoma.
Biomed Pharmacother. 105:10–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Tian T, Hu X, Zhang X, Li L, Nan F,
Chang Y, Wang X, Sun Z, Lv F and Zhang M: Targeting Six1 by
lentivirus-mediated RNA interference inhibits colorectal cancer
cell growth and invasion. Int J Clin Exp Pathol. 7:631–639.
2014.PubMed/NCBI
|
|
34
|
Iwanaga R, Wang CA, Micalizzi DS, Harrell
JC, Jedlicka P, Sartorius CA, Kabos P, Farabaugh SM, Bradford AP
and Ford HL: Expression of Six1 in luminal breast cancers predicts
poor prognosis and promotes increases in tumor initiating cells by
activation of extracellular signal-regulated kinase and
transforming growth factor-beta signaling pathways. Breast Cancer
Res. 14:R1002012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reichenberger KJ, Coletta RD, Schulte AP,
Varella-Garcia M and Ford HL: Gene amplification is a mechanism of
Six1 overexpression in breast cancer. Cancer Res. 65:2668–2675.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang CA, Jedlicka P, Patrick AN, Micalizzi
DS, Lemmer KC, Deitsch E, Casás-Selves M, Harrell JC and Ford HL:
SIX1 induces lymphangiogenesis and metastasis via upregulation of
VEGF-C in mouse models of breast cancer. J Clin Invest.
122:1895–1906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong YN, Wang X, Wang J, Yang Z, Li S,
Yang J, Liu L, Lei X and Shao F: Chemical probing reveals insights
into the signaling mechanism of inflammasome activation. Cell Res.
20:1289–1305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai PW, Shiah SG, Lin MT, Wu CW and Kuo
ML: Up-regulation of vascular endothelial growth factor C in breast
cancer cells by heregulin-beta 1. A critical role of p38/nuclear
factor-kappa B signaling pathway. J Biol Chem. 278:5750–5759. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takaesu G: Two types of TRAF6-dependent
TAK1 activation in the IL-1 signaling pathway. Biotarget. 2:22018.
View Article : Google Scholar
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bryksin A and Matsumura I: Overlap
extension PCR cloning. Methods Mol Biol. 1073:31–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin
S, Sun C, Ma M, Huang Y and Xi JJ: Genome-wide functional screening
of miR-23b as a pleiotropic modulator suppressing cancer
metastasis. Nat Commun. 2:5542011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pysz MA, Hao F, Hizli AA, Lum MA, Swetzig
WM, Black AR and Black JD: Differential regulation of cyclin D1
expression by protein kinase C alpha and signaling in intestinal
epithelial cells. J Biol Chem. 289:22268–22283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang H, Wang X, Archer TK, Zwaka TP and
Cooney AJ: GCNF-dependent activation of cyclin D1 expression via
repression of Mir302a during ESC differentiation. Stem Cells.
32:1527–1537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vagner T, Mouravlev A and Young D: A novel
bicistronic sensor vector for detecting caspase-3 activation. J
Pharmacol Toxicol Methods. 72:11–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang HC, Zhang ZT, Huang JS, Zhang P,
Xiong NA and Wang T: The contribution of Cdc2 in rotenone-induced
G2/M arrest and caspase-3-dependent apoptosis (vol 53, pg 31,
2014). J Mol Neurosci. 55:812–813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mirabello L, Koster R, Moriarity BS,
Spector LG, Meltzer PS, Gary J, Machiela MJ, Pankratz N, Panagiotou
OA, Largaespada D, et al: A genome-wide scan identifies variants in
NFIB associated with metastasis in patients with osteosarcoma.
Cancer Discov. 5:920–931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen H, Suo K, Cheng Y, Zheng B and Xu L:
Vascular endothelial growth factor C enhances cervical cancer
migration and invasion via activation of focal adhesion kinase.
Gynecol Endocrinol. 29:20–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Su CM, Su YH, Chiu CF, Chang YW, Hong CC,
Yu YH, Ho YS, Wu CH, Yen CS and Su JL: Vascular endothelial growth
factor-C upregulates cortactin and promotes metastasis of
esophageal squamous cell carcinoma. Ann Surg Oncol. 4 Suppl
21:S767–S775. 2014. View Article : Google Scholar
|
|
50
|
Lim J, Ryu JH, Kim EJ, Ham S and Kang D:
Inhibition of vascular endothelial growth factor receptor 3 reduces
migration of gastric cancer cells. Cancer Invest. 33:398–404. 2015.
View Article : Google Scholar : PubMed/NCBI
|