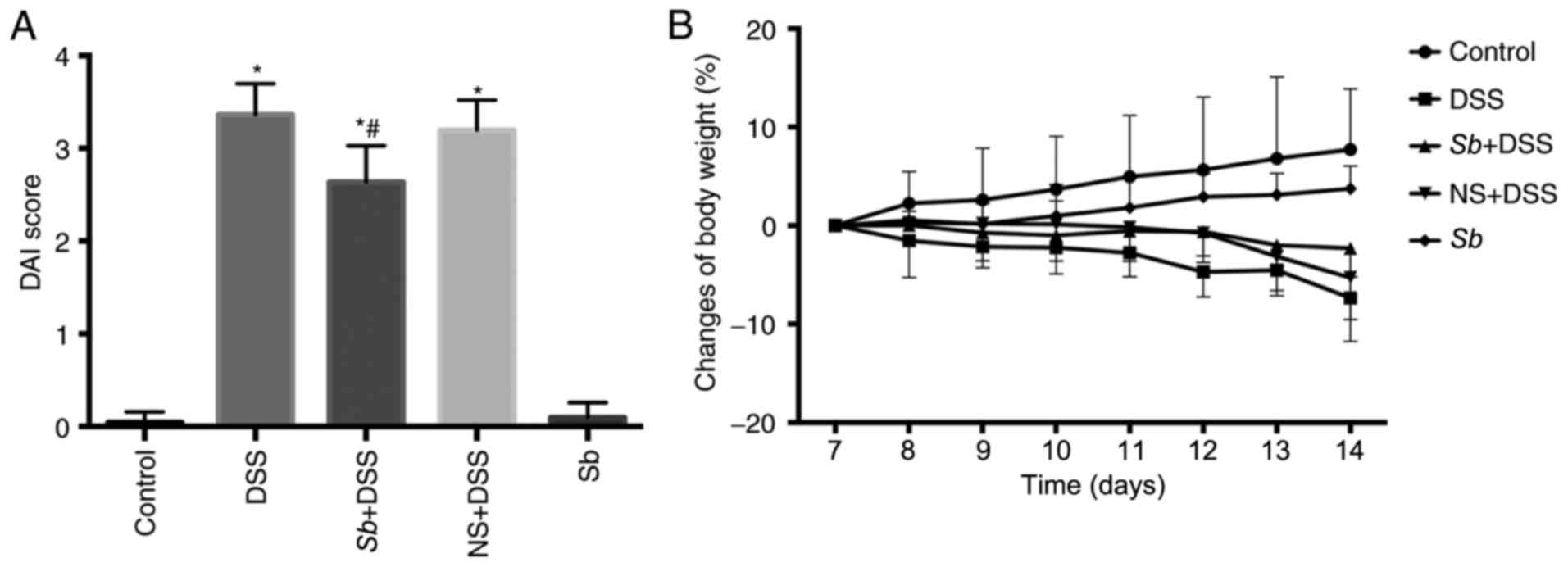
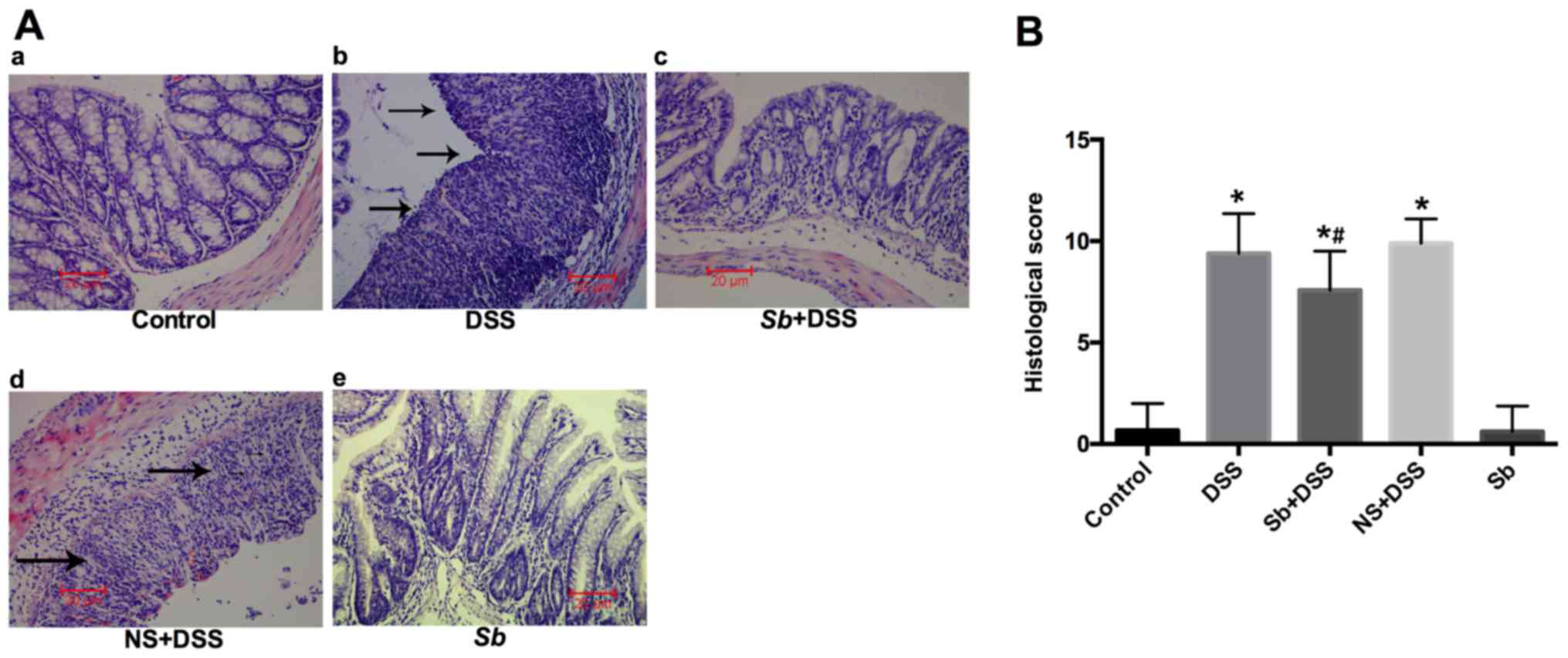
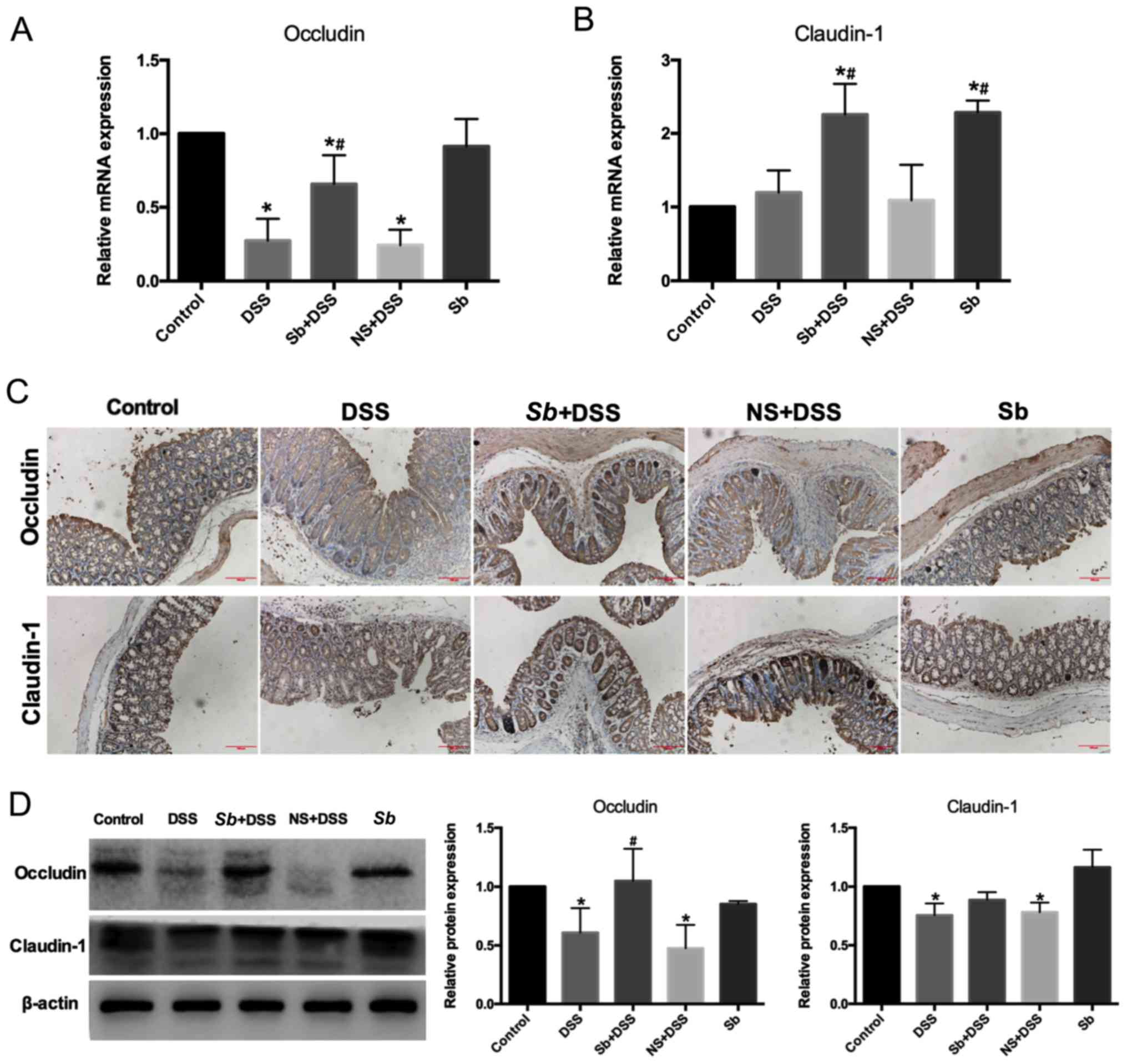
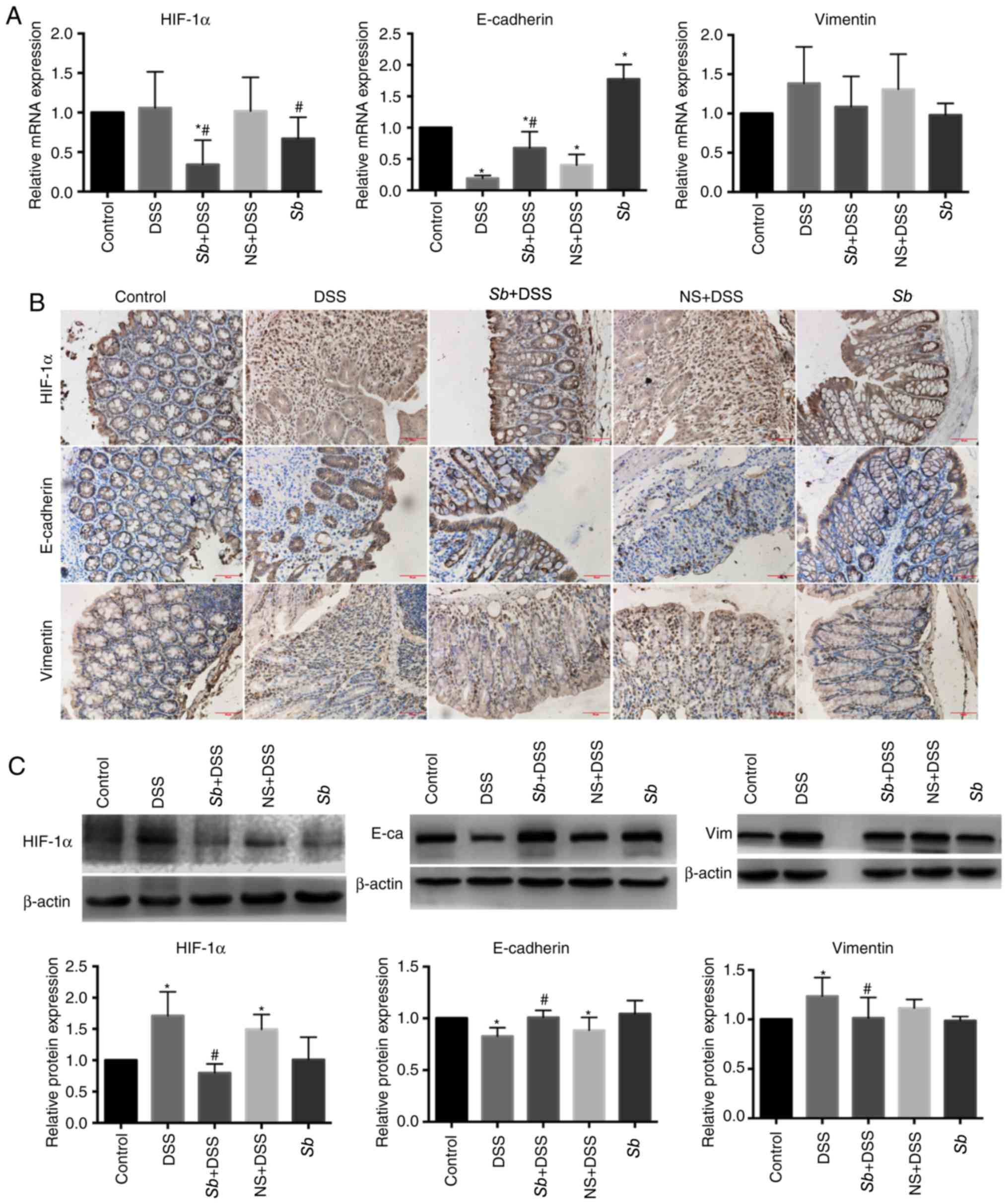
|
1
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema
HW, et al: Increasing incidence and prevalence of the inflammatory
bowel diseases with time, based on systematic review.
Gastroenterology. 142:46–54, e42; quiz e30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah YM: The role of hypoxia in intestinal
inflammation. Mol Cell Pediatr. 3:12016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fagundes RR and Taylor CT: Determinants of
hypoxia-inducible factor activity in the intestinal mucosa. J Appl
Physiol. 123:1328–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cummins EP and Crean D: Hypoxia and
inflammatory bowel disease. Microbes Infect. 19:210–221. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reiff C and Kelly D: Inflammatory bowel
disease, gut bacteria and probiotic therapy. Int J Med Microbiol.
300:25–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guandalini S: Update on the role of
probiotics in the therapy of pediatric inflammatory bowel disease.
Expert Rev Clin Immunol. 6:47–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu CG and Huang Q: Recent progress on the
role of gut microbiota in the pathogenesis of inflammatory bowel
disease. J Dig Dis. 14:513–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hold GL, Smith M, Grange C, Watt ER,
El-Omar EM and Mukhopadhya I: Role of the gut microbiota in
inflammatory bowel disease pathogenesis: What have we learnt in the
past 10 years? World J Gastroenterol. 20:1192–1210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatoum R, Labrie S and Fliss I:
Antimicrobial and probiotic properties of yeasts: From fundamental
to novel applications. Front Microbiol. 3:4212012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Generoso SV, Viana M, Santos R, Martins
FS, Machado JA, Arantes RM, Nicoli JR, Correia MI and Cardoso VN:
Saccharomyces cerevisiae strain UFMG 905 protects against bacterial
translocation, preserves gut barrier integrity and stimulates the
immune system in a murine intestinal obstruction model. Arch
Microbiol. 192:477–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buts JP and De Keyser N: Effects of
Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci.
51:1485–1492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelesidis T and Pothoulakis C: Efficacy
and safety of the probiotic Saccharomyces boulardii for the
prevention and therapy of gastrointestinal disorders. Therap Adv
Gastroenterol. 5:111–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guslandi M, Mezzi G, Sorghi M and Testoni
PA: Saccharomyces boulardii in maintenance treatment of
Crohn's disease. Dig Dis Sci. 45:1462–1464. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamamoto N, Maemura K, Hirata I, Murano M,
Sasaki S and Katsu K: Inhibition of dextran sulphate sodium
(DSS)-induced colitis in mice by intracolonically administered
antibodies against adhesion molecules (endothelial leucocyte
adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1
(ICAM-1)). Clin Exp Immunol. 117:462–468. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCole DF: IBD candidate genes and
intestinal barrier regulation. Inflamm Bowel Dis. 20:1829–1849.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YE, Lee M, Gu H, Kim J, Jeong S, Yeo
S, Lee YJ, Im SH, Sung YC, Kim HJ, et al: HIF-1α activation in
myeloid cells accelerates dextran sodium sulfate-induced colitis
progression in mice. Dis Model Mech. 11:112018. View Article : Google Scholar
|
|
19
|
Kim SL, Park YR, Lee ST and Kim SW:
Parthenolide suppresses hypoxia-inducible factor-1α signaling and
hypoxia induced epithelial-mesenchymal transition in colorectal
cancer. Int J Oncol. 51:1809–1820. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng N, Chen H, Fu S, Bian Z, Lin X, Yang
L, Gao Y, Fang J and Ge Z: HIF-1α and HIF-2α induced angiogenesis
in gastrointestinal vascular malformation and reversed by
thalidomide. Sci Rep. 6:272802016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hill C, Guarner F, Reid G, Gibson GR,
Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S,
et al: Expert consensus document. The International Scientific
Association for Probiotics and Prebiotics consensus statement on
the scope and appropriate use of the term probiotic. Nat Rev
Gastroenterol Hepatol. 11:506–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Generoso SV, Viana ML, Santos RG, Arantes
RM, Martins FS, Nicoli JR, Machado JA, Correia MI and Cardoso VN:
Protection against increased intestinal permeability and bacterial
translocation induced by intestinal obstruction in mice treated
with viable and heat-killed Saccharomyces boulardii. Eur J
Nutr. 50:261–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terciolo C, Dobric A, Ouaissi M, Siret C,
Breuzard G, Silvy F, Marchiori B, Germain S, Bonier R, Hama A, et
al: Saccharomyces boulardii CNCM I-745 Restores intestinal
Barrier Integrity by Regulation of E-cadherin Recycling. J Crohn's
Colitis. 11:999–1010. 2017. View Article : Google Scholar
|
|
24
|
Glover LE and Colgan SP: Hypoxia and
metabolic factors that influence inflammatory bowel disease
pathogenesis. Gastroenterology. 140:1748–1755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flück K and Fandrey J: Oxygen sensing in
intestinal mucosal inflammation. Pflugers Arch. 468:77–84. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colgan SP and Taylor CT: Hypoxia: An alarm
signal during intestinal inflammation. Nat Rev Gastroenterol
Hepatol. 7:281–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joseph JP, Harishankar MK, Pillai AA and
Devi A: Hypoxia induced EMT: A review on the mechanism of tumor
progression and metastasis in OSCC. Oral Oncol. 80:23–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scharl M, Weber A, Fürst A, Farkas S,
Jehle E, Pesch T, Kellermeier S, Fried M and Rogler G: Potential
role for SNAIL family transcription factors in the etiology of
Crohn's disease-associated fistulae. Inflamm Bowel Dis.
17:1907–1916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang SW, Zhang ZG, Hao YX, Zhao YL, Qian
F, Shi Y, Li PA, Liu CY and Yu PW: HIF-1α induces the
epithelial-mesenchymal transition in gastric cancer stem cells
through the Snail pathway. Oncotarget. 8:9535–9545. 2017.PubMed/NCBI
|
|
30
|
Chen S, Chen JZ, Zhang JQ, Chen HX, Yan
ML, Huang L, Tian YF, Chen YL and Wang YD: Hypoxia induces
TWIST-activated epithelial-mesenchymal transition and proliferation
of pancreatic cancer cells in vitro and in nude mice. Cancer Lett.
383:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karayiannakis AJ, Syrigos KN, Efstathiou
J, Valizadeh A, Noda M, Playford RJ, Kmiot W and Pignatelli M:
Expression of catenins and E-cadherin during epithelial restitution
in inflammatory bowel disease. J Pathol. 185:413–418. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mehta S, Nijhuis A, Kumagai T, Lindsay J
and Silver A: Defects in the adherens junction complex
(E-cadherin/β-catenin) in inflammatory bowel disease. Cell Tissue
Res. 360:749–760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ippolito C, Colucci R, Segnani C, Errede
M, Girolamo F, Virgintino D, Dolfi A, Tirotta E, Buccianti P, Di
Candio G, et al: Fibrotic and vascular remodelling of colonic wall
in patients with active ulcerative colitis. J Crohn's Colitis.
10:1194–1204. 2016. View Article : Google Scholar
|
|
34
|
Andoh A, Fujino S, Okuno T, Fujiyama Y and
Bamba T: Intestinal subepithelial myofibroblasts in inflammatory
bowel diseases. J Gastroenterol. 37 Suppl 14:S33–S37. 2002.
View Article : Google Scholar
|
|
35
|
Xue X, Ramakrishnan S, Anderson E, Taylor
M, Zimmermann EM, Spence JR, Huang S, Greenson JK and Shah YM:
Endothelial PAS domain protein 1 activates the inflammatory
response in the intestinal epithelium to promote colitis in mice.
Gastroenterology. 145:831–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang X, Yang Y, Yuan H, You J,
Burkatovskaya M and Amar S: Novel transcriptional regulation of
VEGF in inflammatory processes. J Cell Mol Med. 17:386–397. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Yang G, Song JH, Xu H, Li D,
Goldsmith J, Zeng H, Parsons-Wingerter PA, Reinecker HC and Kelly
CP: Probiotic yeast inhibits VEGFR signaling and angiogenesis in
intestinal inflammation. PLoS One. 8:e642272013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Skuli N, Majmundar AJ, Krock BL, Mesquita
RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, et al:
Endothelial HIF-2α regulates murine pathological angiogenesis and
revascularization processes. J Clin Invest. 122:1427–1443. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramakrishnan S, Anand V and Roy S:
Vascular endothelial growth factor signaling in hypoxia and
inflammation. J Neuroimmune Pharmacol. 9:142–160. 2014. View Article : Google Scholar : PubMed/NCBI
|