Introduction
Adenomyosis, a common benign disease of women of
childbearing age, is caused by the presence of heterotopic
endometrial glands and stroma within the myometrium (1,2). The
primary clinical manifestations of adenomyosis are prolonged
menstruation, menorrhagia and secondary ingravescence dysmenorrhea.
It is common to observe increased uterine volume in adenomyosis. A
number of patients have high levels of serum CA125. CA125 modified
by platelet count and neutrophil-lymphocyte ratio improves the
predictive accuracy of adenomyosis-derived pelvic dense adhesion
(3). Adenomyosis was considered to
be a chronic immune inflammatory disease. Lesions, peripheral blood
and/or peritoneal fluid in patients with adenomyosis are rich in a
variety of inflammatory immune cells and inflammatory cytokines,
and are closely associated with its clinicopathological features
(4–7). Guo et al (8) observed that the expression of
Toll-like receptor 4 (TLR4) in adenomyosis was increased compared
with the normal endometrium. An established cell model revealed
that bacterial lipopolysaccharide can promote the inflammatory
response and cell proliferation of endometrial stromal cells in
adenomyosis, and improved the ability of invasion via the TLR4
signaling pathway. Cyclooxygenase 2 (COX-2) is a key enzyme in the
process of synthesis of various endogenous prostaglandins with
arachidonic acid. It was called ‘stress gene’ and can be detected
only when its expression is stimulated by inflammatory factors, but
not in normal tissue. COX-2 has certain associations with
inflammation, cell mitosis and specific signaling transduction
pathways. COX-2 regulates the occurrence and development of a wide
variety of tumor pathophysiological processes alongside its
inflammatory metabolites (9). A
previous study demonstrated that targeting the COX-2 gene can
significantly inhibit the expression of vascular endothelial growth
factor (VEGF) and matrix metalloproteinase-9 in eutopic and ectopic
endometrial stromal cells, as well as increase the rate of
apoptosis (10,11). Lipoxygenase-5 (LOX-5) is the key
enzyme catalyzing the reaction of arachidonic acid to generate
leukotrienes. It is present only in a few cell types (including
granulocytes, mast cells, dendritic cells and B lymphocytes), and
its metabolic products are important inflammatory mediators. A
preliminary study revealed that LOX-5 was distributed in the
cytoplasm and nucleus of the glandular epithelium and mesenchymal
cells of endometriosis (12). The
expression of prostaglandins and leukotrienes in the peritoneal
fluid of patients with endometriosis or adenomyosis is
significantly increased compared with normal individuals, and is
positively associated with the degree of dysmenorrhea.
Non-steroidal anti-inflammatory drugs can effectively relieve the
pain (12). Overall, the evidence
suggests that LOX-5 and COX-2 may be involved in the inflammatory
pathological mechanism of adenomyosis, and may be associated with
its clinical features. As a result, the present study aimed to
investigate the expression of LOX-5 and COX-2 in eutopic and
ectopic endometrium of adenomyosis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting, and analyze its association with interleukin
(IL)-6, IL-8 and clinical features of adenomyosis. Understanding
the roles of LOX-5 and COX-2 in the inflammatory pathological
mechanism of adenomyosis may provide a theoretical basis for
anti-inflammatory therapy.
Materials and methods
Study cohort
A total of 20 patients with uterine adenomyosis who
received surgery at the Tenth People's Hospital, Tongji University
School of Medicine (Shanghai, China) from April 9, 2015 to July 11,
2016 were included in the present study. The age of the 20 patients
with adenomyosis ranged from 32 to 52 years, with a mean of
42.40±5.37 years and a mean body mass index (BMI) of 23.12±2.63
kg/m2 (range, 18.73–27.73 kg/m2). All cases
included were confirmed by postoperative pathology. Reviewed
detailed case data included a record of the patients' general
information, menstruation history, marital and reproductive
history, complications, and clinical symptoms. In total, 20 cases
of women who received placing intrauterine device (a type of ring
with drugs inserted in the uterine cavity that can replace the
contraceptive pill and condoms serving the role of contraception)
operation as the control group. little endometrium tissue (0.5
cm3) was scraped about during surgery. All control and
adenomyotic women had regular menstrual cycles (28–35 days) and had
not received hormonal therapy within the previous 3 months. The
exclusion criteria were patients who were diagnosed with tumor,
autoimmune or inflammatory diseases. The present study was approved
by the Ethics Committee of Tongji University School of Medicine and
was performed in accordance with the tenets and guidelines of the
Declaration of Helsinki. All patients provided written informed
consent.
Specimen collection
The phase of the menstrual cycle was the early
proliferative phase. Control endometrium (CE) was obtained from
patients who voluntarily had an intrauterine device implanted.
Endometrium (0.5 cm3) was collected prior to the
operation. Eutopic endometrium (EU) was obtained from patients with
adenomyosis. A little endometrium was collected prior to the
operation from the normal uterine wall, while ectopic endometrium
(EC) was obtained from intraoperative excised specimens. The
specimens were stored in liquid nitrogen (−196°C) for RNA and
protein extraction.
RT-qPCR
Liquid nitrogen-preserved tissues were homogenized
and total RNA was extracted using the TRIzol method (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Quantscript RT
Kit (KR-103, Tiangen Biotech Co., Ltd., Beijing, China) was used to
prepare complementary DNA, which was used as template for PCR
amplification. The PCR primer sets used are presented in Table I. QuantiFast SYBR Green PCR Kit
(cat. no. 204054, Qiagen, Inc., Valencia, CA, USA) was used and
β-actin served as an internal control.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer, annealing temperature and
product. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer, annealing temperature and
product.
| Name | Primer sequence
(5′-3′) | Annealing temperature
(°C) | Product (bp) |
|---|
| LOX-5 | F:
TGGCAGTCACATCTCTTCC | 61 | 111 |
|
| R:
GTGTAGAATGGGTCCCTATG |
|
|
| COX-2 | F:
TGAAACCCACTCCAAACACA | 61 | 187 |
|
| R:
GAGAAGGCTTCCCAGCTTTT |
|
|
| IL-6 | F:
AGCCACTCACCTCTTCAGAAC | 60 | 118 |
|
| R:
GCCTCTTTGCTGCTTTCACAC |
|
|
| IL-8 | F:
CAAGAGCCAGGAAGAAAC | 60 | 227 |
|
| R:
TGGTCCACTCTCAATCAC |
|
|
The amplification program was as follows: 95°C for 5
min and 40 cycles of 95°C for 15 sec and 62°C for 35 sec. A melting
curve was represented to confirm the specificity of the amplified
products. The mRNA expression levels of LOX-5, COX-2, IL-6 and IL-8
in CE, EU and EC tissues were detected by RT-qPCR. The
2−ΔΔCq method was used to calculate the mRNA levels
(13).
Western blotting
Liquid nitrogen-preserved tissues were homogenized
and total protein was extracted. Cell lysates buffer (Cell
Signaling Technology Inc., Danvers, MA, USA) were added to the
tissues collected from all groups, and homogenized for 1 h at 4°C.
Total protein extracts were quantified by Bicinchoninic acid
Protein Assay kit (Thermo Fisher Scientific, Inc.). Proteins (20
µg) were separated by 10% SDS-PAGE and then transferred to a
nitrocellulose membrane. The membranes were blocked with 5%
fat-free dry milk at room temperature for 1 h. The blots were
incubated with human anti-COX-2 (cat. no. ab15191, Abcam,
Cambridge, UK; 1:1,000), anti-IL-6 (cat. no. ab6672, Abcam, 1:500),
anti-IL-8 (cat. no. ab7747, Abcam, 1:500), anti-LOX-5 (cat. no.
ab169755, Abcam, 1:1,000) and anti-GAPDH (cat. no. 2118S, Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. The
membranes were again washed with PBS and incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary
antibodies (cat. no. P0448; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA; 1:2,000) at room temperature for 1 h. The proteins
were finally examined by an enhanced chemiluminescence system (ECL;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). Band intensities
were quantified by densitometry using ImageJ Software version 1.6
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All statistical analyses were performed with SPSS
version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Normally
distributed data are presented as the mean ± standard deviation of
three independent experiments and intragroup differences were
investigated using analysis of variance, followed by the Bonferroni
method to correct for errors. Categorical variables were expressed
as the number of cases and percentages. Correlations between
variables for each group were determined using the Pearson's
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference. Statistics diagrams were
represented with GraphPad Prism 5 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
General data of patients
The age of the 20 patients with adenomyosis ranged
from 32 to 52 years, with a mean of 42.40±5.37 years and a mean
body mass index (BMI) of 23.12±2.63 kg/m2 (range,
18.73–27.73 kg/m2). The 20 non-adenomyosis subjects were
all multiparas who ranged in age from 28 to 46 years, with a mean
of 39.46±7.13 years and a mean BMI of 22.49±4.01 kg/m2
(range, 18.49–26.28 kg/m2). There were no statistically
significant differences in age or BMI between the groups
(P>0.05). All the patients with adenomyosis were married and 18
of them had a history of childbirth with an average parity of
1.15±0.59 children (range, 0–2 children). A total of 16 cases had a
history of abortion, with a mean of 1.45±1.28 times (range, 0–5
times). Two cases had internal diseases (one case of high blood
pressure and one case of diabetes).
Expression of LOX-5, COX-2, IL-6 and
IL-8 mRNA in CE, EU and EC
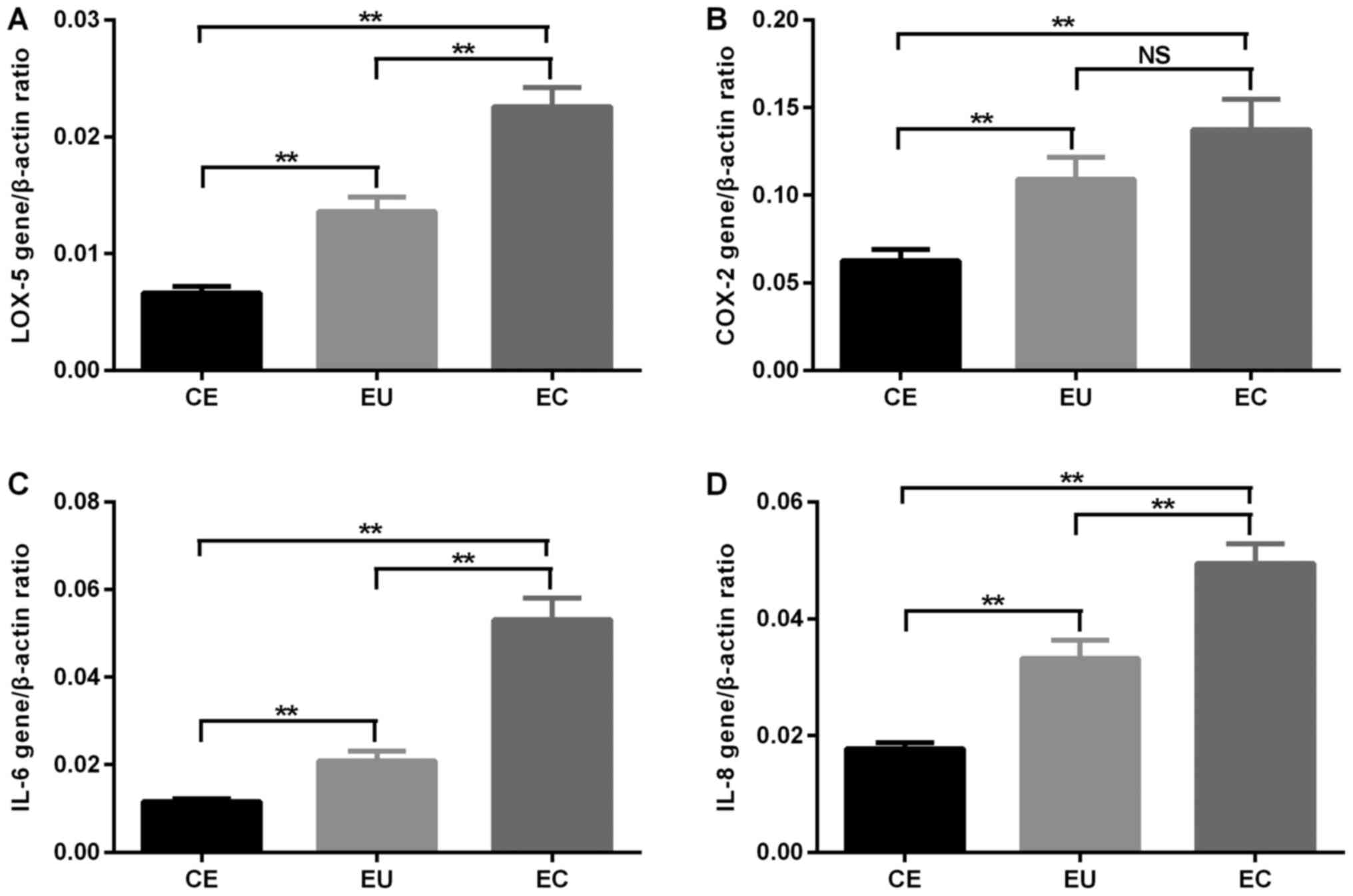
RT-qPCR analysis revealed that the mRNA expression
of LOX-5 in EC and EU was significantly increased compared with in
CE, and its expression in EC was significantly increased compared
with in EU (P<0.01). The mRNA expression of COX-2 in EC and EU
was significantly increased compared with in CE (P<0.01), and
there was no significant difference in COX-2 mRNA expression levels
between EC and EU. The mRNA expression of IL-6 and IL-8 in EC and
EU was significantly increased compared with in CE, and it was
significantly increased in EC compared with that in EU (P<0.01;
Fig. 1 and Table II).
 | Table II.The mRNA expression of LOX-5, COX-2,
IL-6 and IL-8 in CE, EU and EC. |
Table II.
The mRNA expression of LOX-5, COX-2,
IL-6 and IL-8 in CE, EU and EC.
|
| CE (mRNA) | EU (mRNA) | EC (mRNA) | Pa | Pb | Pc |
|---|
| LOX-5 | 0.007±0.003 | 0.014±0.005 | 0.023±0.007 | <0.01 | <0.01 | <0.01 |
| COX-2 | 0.063±0.029 | 0.109±0.056 | 0.137±0.077 | <0.01 | <0.01 | >0.05 |
| IL-6 | 0.012±0.003 | 0.021±0.010 | 0.053±0.023 | <0.01 | <0.01 | <0.01 |
| IL-8 | 0.018±0.004 | 0.033±0.014 | 0.049±0.015 | <0.01 | <0.01 | <0.01 |
Protein expression levels of LOX-5 and
COX-2 in CE, EU and EC
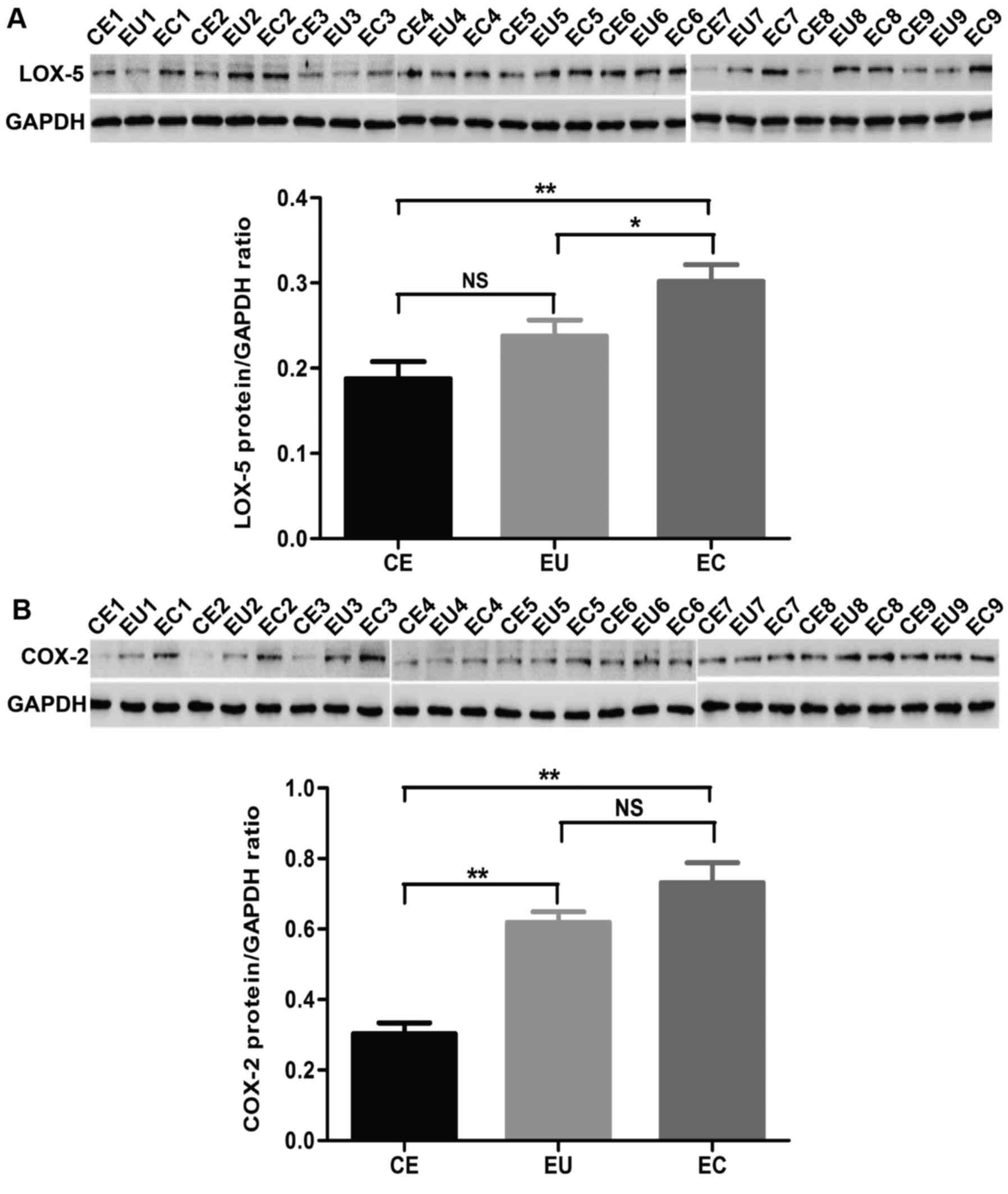
Western blot analysis was performed to detect the
protein expression of LOX-5 and COX-2 in CE, EU and EC. All 20
adenomyosis patients and 20 non-adenomyosis subjects were tested by
western blotting. In this study, 9 samples were chosen as typical
as demonstrated in Fig. 2. The
total results are presented as a histogram. The protein expression
level of LOX-5 in EC was significantly increased compared with in
CE and EU (P<0.01 and P<0.05, respectively), and there was no
significant difference between CE and EU. The protein expression
level of COX-2 in EC and EU was significantly increased compared
with CE (P<0.01), and there was no significant difference
between CE and EU (Fig. 2 and
Table III).
 | Table III.The protein expression of LOX-5 and
COX-2 in CE, EU, EC. |
Table III.
The protein expression of LOX-5 and
COX-2 in CE, EU, EC.
|
| CE | EU | EC | Pa | Pb | Pc |
|---|
| LOX-5 | 0.192±0.088 | 0.238±0.081 | 0.299±0.089 | >0.05 | <0.01 | <0.05 |
| COX-2 | 0.303±0.135 | 0.619±0.135 | 0.731±0.253 | <0.01 | <0.01 | >0.05 |
Correlations between the mRNA
expression levels of LOX-5 and COX-2 with IL-6 and IL-8 in CE, EU
and EC
There was a significant positive correlation between
the mRNA expression levels of LOX-5 and COX-2 and the levels of
IL-6 and IL-8 in CE (P<0.05), and the mRNA expression levels of
LOX-5 and COX-2 were also positively correlated with those of IL-6
and IL-8 in EU (P<0.01). There was also a significant positive
correlation between the mRNA expression levels of LOX-5 and COX-2
and the levels of IL-6 and IL-8 in EC (P<0.01 and P<0.05,
respectively; Fig. 3). The
correlation coefficient (r) values are presented in Table IV.
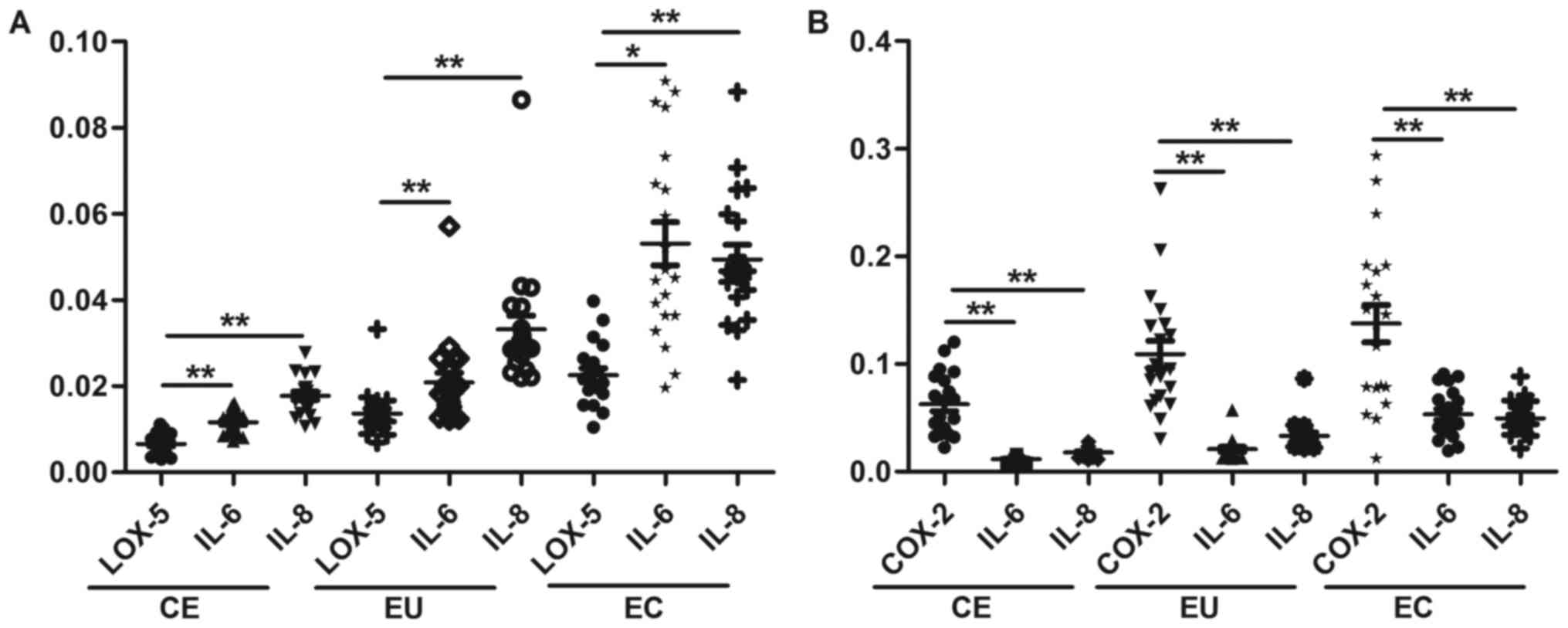 | Figure 3.Correlations between the mRNA
expression levels of (A) LOX-5 and (B) COX 2, and IL-6 and IL-8 in
CE, EU and EC. *P<0.05, **P<0.01. IL, interleukin, CE,
control endometrium; EU, eutopic endometrium; EC, ectopic
endometrium; COX-2, cyclooxygenase-2; LOX-5, lipoxygenase-5. |
 | Table IV.Correlations between the mRNA
expressions of LOX-5, COX-2 and IL-6, IL-8 in CE, EU and EC. |
Table IV.
Correlations between the mRNA
expressions of LOX-5, COX-2 and IL-6, IL-8 in CE, EU and EC.
| Tissue | IL-6 | IL-8 |
|---|
| LOX-5 |
| CE | 0.856b | 0.619b |
| EU | 0.923b | 0.940b |
| EC | 0.509a | 0.578b |
| CE | 0.861b | 0.821b |
| COX-2 |
| EU | 0.889b | 0.847b |
| EC | 0.890b | 0.890b |
Clinical features of the adenomyosis
group
Menstruation
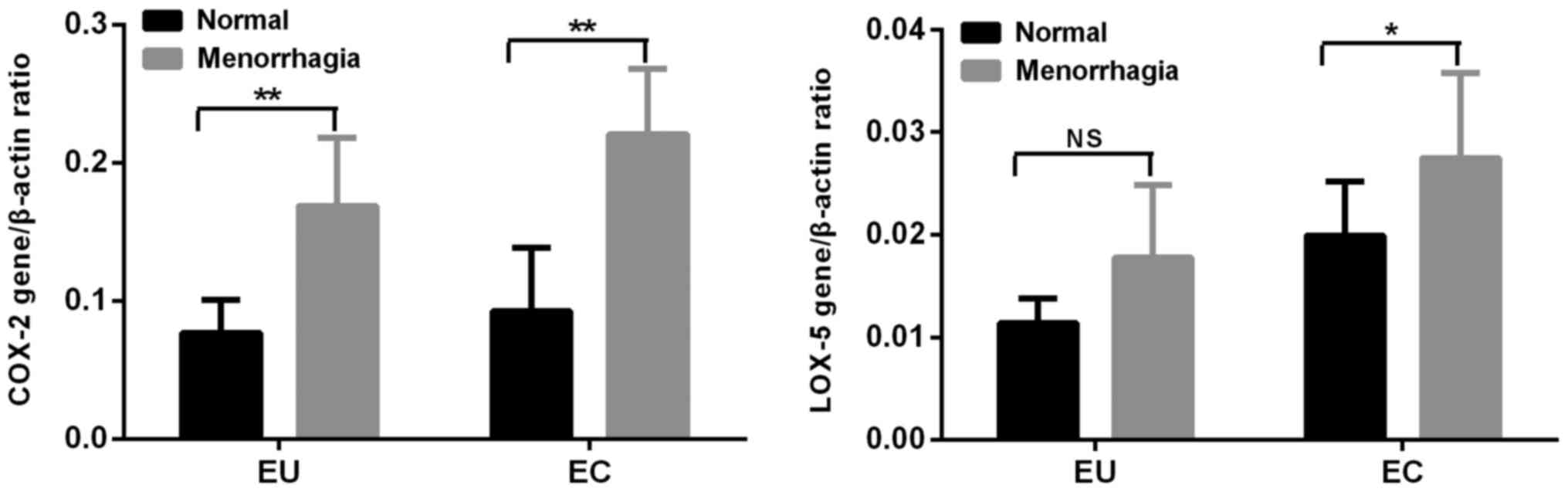
According to the pictorial blood loss assessment
chart the adenomyosis patients were divided into two groups. There
were 13 (65%) cases of normal menstrual capacity and 7 (35%) cases
of menorrhagia. The mRNA expression levels of COX-2 and LOX-5 in
the menorrhagia group were significantly increased compared with in
the normal group (P<0.01 and P<0.05, respectively) in EC. The
mRNA expression of COX-2 in the group of menorrhagia was increased
compared with in the normal group (P<0.05) in EU, but the mRNA
expression of LOX-5 had no significant difference between the two
groups (Fig. 4).
Dysmenorrhea
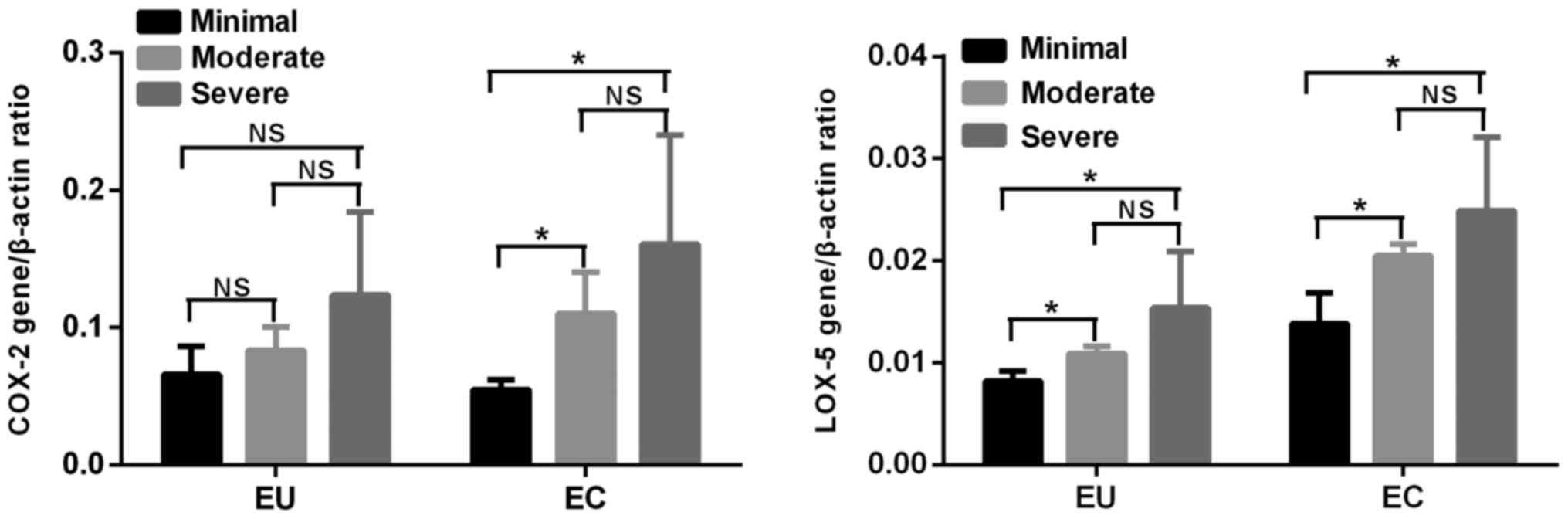
According to the visual analog scale system
(12), the patients with
adenomyosis were divided into 3 groups. There were 3 (15%) cases of
minimal dysmenorrhea, 5 (25%) cases of moderate dysmenorrhea and 12
(60%) cases of severe dysmenorrhea. There was no difference in the
mRNA expression of COX-2 among the three groups in EU. The mRNA
expression of LOX-5 in EU in the group of moderate and severe
dysmenorrhagia was increased in the minimal group (P<0.05), but
there was no difference between the moderate and severe
dysmenorrhagia groups. The mRNA expression of LOX-5 and COX-2 in EC
in the groups of moderate and severe dysmenorrhagia was increased
compared with in the minimal group (P<0.05), but not in the
moderate or severe dysmenorrhagia groups (Fig. 5).
Discussion
Adenomyosis is a chronic immune inflammatory
disease. The inflammation microenvironment of local lesion tissue
serves an important role in the occurrence and development of
endometrium and adenomyosis, and is closely associated with the
patients' clinical symptoms (14).
In previous years, numerous studies have reported that there is not
only a large quantity of inflammatory cell infiltration, but also a
variety of inflammatory cytokines in adenomyosis lesions. IL-6 and
IL-8, as cytokines, are the most commonly used markers of
adenomyosis' inflammation pathological state (15,16).
Another report indicated that there was gathered neutrophils (NEU)
in the peritoneal fluid and ovary nidus of patients with
endometriosis. NEU chemokines (IL-8) and the formation of the
neutral extracellular traps when NEU was activated were
significantly increased in the peritoneal fluid, which was closely
associated with inflammation pathology in endometriosis (17). The present study indicated that the
mRNA expression of IL-6 and IL-8 in EC and EU was increased
compared with in CE in patients with adenomyosis, which was in
accordance with the majority of previous studies, and also verified
the inflammatory pathological state of adenomyosis.
As two key enzymes in the arachidonic acid (AA)
metabolic pathways, LOX-5, COX2 and their metabolic products
(prostaglandin E2 and leukotriene B4) not only are involved in the
regulation of pathophysiological processes but are also associated
with the occurrence and development of a wide variety of tumors.
The present study demonstrated that the COX-2 gene and protein
expression levels in cervical squamous cell carcinoma and
adenocarcinoma tissues were significantly higher than those in
normal cervical tissues (18). The
expression of COX-2 in ovarian cancer cells was also significantly
higher than that in benign ovarian cystadenoma cells (19). Fujiwaki et al (20) detected the expression of COX-2 and
VEGF in 63 patients with endometrial cancer by RT-qPCR and
immunohistochemistry, and observed that COX-2 had a significantly
positive correlation with VEGF. The above studies suggest that
COX-2 may serve a certain role in the occurrence and development of
gynecological tumors. Similar to COX-2, LOX-5 also serves an
important role in promoting tumor cell proliferation, angiogenesis,
invasion and metastasis, and inhibiting tumor cell apoptosis. The
present study further detected the expression of COX-2 and LOX-5 in
adenomyosis. The results revealed that the mRNA expression levels
of LOX-5 and COX-2 in the EC and EU groups were increased compared
with in the EC group, and LOX-5 expression in the EC group was
higher than in the EU group. Western blotting revealed that the
expression of LOX-5 in the EC group was higher than in the CE and
EU groups, while COX-2 expression in the EC and EU groups was
increased compared with in the CE group. However, the mRNA and
protein expression levels of LOX-5 and COX-2 between CE and EU were
slightly different.
There may be several reasons for this result. First,
there is a large amount of mRNA to be translated into protein and a
certain number of them remain. Next the translation or stability of
protein is regulated. Additionally, it was demonstrated that there
was no difference in COX-2 expression between the EC and EU groups.
In the present study it was hypothesized that this may be ascribed
to the small sample size used. Furthermore, COX-2 is an indicator
of inflammation; the endometria of the EC and EU groups are in a
state of inflammation, so there is no difference in COX-2
expression between the EC and EU groups. It is known that COX-2 and
LOX-5 can create an inflammatory microenvironment and promote the
development of cancer by promoting proliferation and angiogenesis
while inhibiting tumor cell apoptosis. Accordingly, the present
study hypothesized that COX-2 and LOX-5 may be involved in the
formation and development of the inflammatory pathology of
adenomyosis by regulating the inflammatory pathways.
Above all, adenomyosis affects women's physical and
mental health due to its symptoms including pain and low fertility.
The present results revealed that the expression levels of COX-2,
LOX-5, IL-6 and IL-8 were increased in normal, EU and EC. In
addition, the expression levels of COX-2 and LOX-5 were correlated
with the levels of IL-6 and IL-8, and the expression of COX-2 and
LOX-5 was associated with clinical symptoms (i.e., menstrual
quantity and/or degree of dysmenorrhea) according to analysis of
the clinical data of patients with adenomyosis. These results may
provide a theoretical basis for further investigation into the
development and progression of the inflammatory pathologies and the
underlying immune inflammatory pathogenesis of adenomyosis. Future
studies in cell or animal models are required to address the role
of COX-2 and LOX-5 in the inflammatory pathogenesis and molecular
mechanisms of adenomyosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Science and Technology Commission of Shanghai Municipality (grant
no. 14411972100).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CXL wrote the manuscript and performed statistical
analysis. RC contributed to conceptualization. CXJ performed
statistical analyses. LC contributed to data curation. ZPC was
responsible for the methodology and supervision of the project.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Tenth People's Hospital, Tongji University
School of Medicine (Shanghai, China). Written informed consent was
obtained from all the subjects participating in the study.
Patient consent for publication
Written informed consent was provided by each
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Struble J, Reid S and Bedaiwy MA:
Adenomyosis: A clinical review of a challenging gynecologic
condition. J Minim Invasive Gynecol. 23:164–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benagiano G, Brosens I and Habiba M:
Adenomyosis: A life-cycle approach. Reprod Biomed Online.
30:220–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang C, Liu C, Guo J, Chen L, Luo N, Qu
X, Yang W, Ren Q and Cheng Z: CA125 modified by PLT and NLR
improves the predictive accuracy of adenomyosis-derived pelvic
dense adhesion. Medicine (Baltimore). 96:e68802017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan KN, Kitajima M, Inoue T, Tateishi S,
Fujishita A, Nakashima M and Masuzaki H: Additive effects of
inflammation and stress reaction on Toll-like receptor 4-mediated
growth of endometriotic stromal cells. Hum Reprod. 28:2794–2803.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Banu SK, Lee J, Speights VO Jr,
Starzinski-Powitz A and Arosh JA: Cyclooxygenase-2 regulates
survival, migration, and invasion of human endometriotic cells
through multiple mechanisms. Endocrinology. 149:1180–1189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nisenblat V, Bossuyt PM, Shaikh R,
Farquhar C, Jordan V, Scheffers CS, Mol BW, Johnson N and Hull ML:
Blood biomarkers for the non-invasive diagnosis of endometriosis.
Cochrane Database Syst Rev. CD0121792016.PubMed/NCBI
|
|
7
|
Rižner TL: Diagnostic potential of
peritoneal fluid biomarkers of endometriosis. Expert Rev Mol Diagn.
15:557–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Chen L, Luo N, Li C, Chen R, Qu X,
Liu M, Kang L and Cheng Z: LPS/TLR4-mediated stromal cells acquire
an invasive phenotype and are implicated in the pathogenesis of
adenomyosis. Sci Rep. 6:214162016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen J, Li WX, Xiao ZG, Zhang L, Li MX, Li
LF, Hu W, Lu L, Boudreau F and Cho CH: The Co-regulatory role of
5-lipoxygenase and cyclooxygenase-2 in the carcinogenesis and their
promotion by cigarette smoking in colons. Curr Med Chem.
23:1131–1138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SJ, Kim CE, Yun MR, Seo KW, Park HM,
Yun JW, Shin HK, Bae SS and Kim CD: 4-Hydroxynonenal enhances MMP-9
production in murine macrophages via 5-lipoxygenase-mediated
activation of ERK and p38 MAPK. Toxicol Appl Pharmacol.
242:191–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knab LM, Grippo PJ and Bentrem DJ:
Involvement of eicosanoids in the pathogenesis of pancreatic
cancer: The roles of cyclooxygenase-2 and 5-lipoxygenase. World J
Gastroenterol. 20:10729–10739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lousse JC, Defrère S, Colette S, Van
Langendonckt A and Donnez J: Expression of eicosanoid biosynthetic
and catabolic enzymes in peritoneal endometriosis. Hum Reprod.
25:734–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levy G, Dehaene A, Laurent N, Lernout M,
Collinet P, Lucot JP, Lions C and Poncelet E: An update on
adenomyosis. Diagn Interv Imaging. 94:3–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aboussahoud W, Aflatoonian R, Bruce C,
Elliott S, Ward J, Newton S, Hombach-Klonisch S, Klonisch T and
Fazeli A: Expression and function of Toll-like receptors in human
endometrial epithelial cell lines. J Reprod Immunol. 84:41–51.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan KN, Fujishita A, Kitajima M, Hiraki
K, Nakashima M and Masuzaki H: Occult microscopic endometriosis:
Undetectable by laparoscopy in normal peritoneum. Hum Reprod.
29:462–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berkes E, Oehmke F, Tinneberg HR,
Preissner KT and Saffarzadeh M: Association of neutrophil
extracellular traps with endometriosis-related chronic
inflammation. Eur J Obstet Gynecol Reprod Biol. 183:193–200. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marutha Muthu AK, Cheah PL, Koh CC, Chew
MF, Toh YF and Looi LM: Cyclooxygenase-2 (COX2) expression in
adenocarcinoma surpasses that of squamous cell carcinoma in the
uterine cervix. Malays J Pathol. 39:251–255. 2017.PubMed/NCBI
|
|
19
|
Masferrer JL, Leahy KM, Koki AT, Zweifel
BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ and
Seibert K: Antiangiogenic and antitumor activities of
cyclooxygenase-2 inhibitors. Cancer Res. 60:1306–1311.
2000.PubMed/NCBI
|
|
20
|
Fujiwaki R, Iida K, Kanasaki H, Ozaki T,
Hata K and Miyazaki K: Cyclooxygenase-2 expression in endometrial
cancer: Correlation with microvessel count and expression of
vascular endothelial growth factor and thymidine phosphorylase. Hum
Pathol. 33:213–219. 2002. View Article : Google Scholar : PubMed/NCBI
|