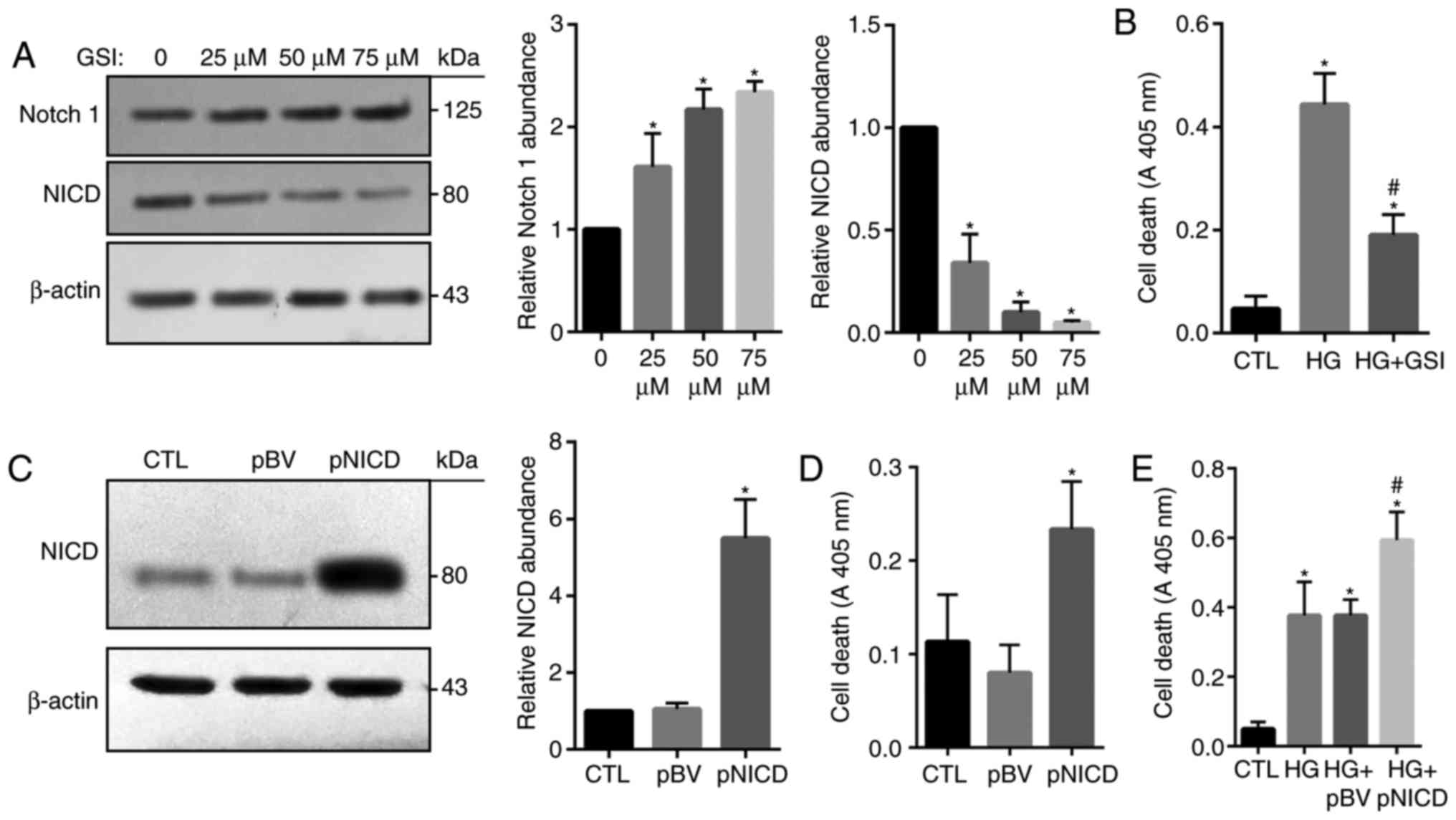
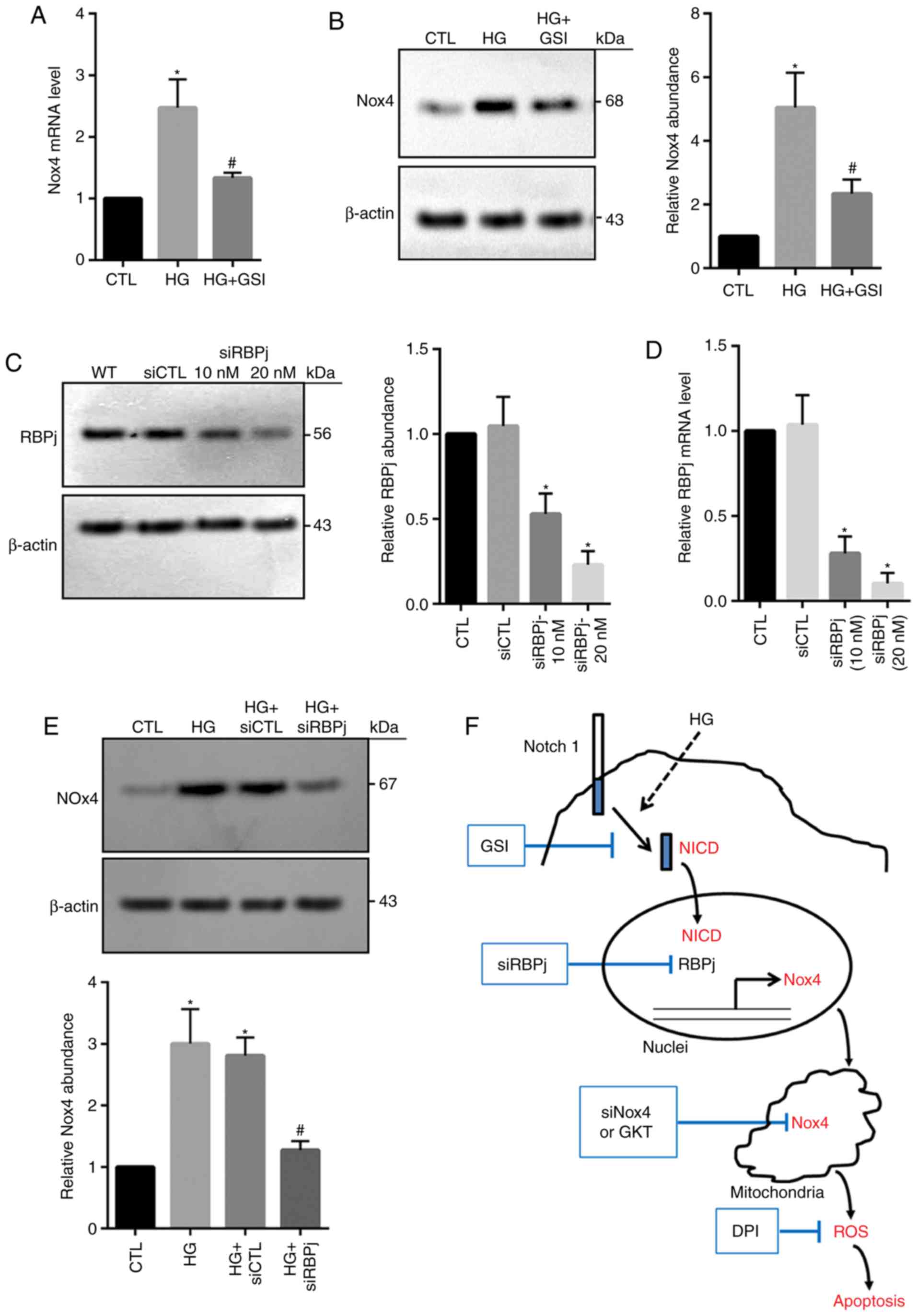
|
1
|
Yau JW, Rogers SL, Kawasaki R, Lamoureux
EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund
J, et al: Global prevalence and major risk factors of diabetic
retinopathy. Diabetes Care. 35:556–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanwar M, Chan PS, Kern TS and Kowluru RA:
Oxidative damage in the retinal mitochondria of diabetic mice:
Possible protection by superoxide dismutase. Invest Ophthalmol Vis
Sci. 48:3805–3811. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adamiec-Mroczek J, Oficjalska-Młyńczak J
and Misiuk-Hojło M: Roles of endothelin-1 and selected
proinflammatory cytokines in the pathogenesis of proliferative
diabetic retinopathy: Analysis of vitreous samples. Cytokine.
49:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Behl Y, Krothapalli P, Desta T, DiPiazza
A, Roy S and Graves DT: Diabetes-enhanced tumor necrosis
factor-alpha production promotes apoptosis and the loss of retinal
microvascular cells in type 1 and type 2 models of diabetic
retinopathy. Am J Pathol. 172:1411–1418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kowluru RA, Tang J and Kern TS:
Abnormalities of retinal metabolism in diabetes and experimental
galactosemia. VII. Effect of long-term administration of
antioxidants on the development of retinopathy. Diabetes.
50:1938–1942. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim J, Son JW, Lee JA, Oh YS and Shinn SH:
Methylglyoxal induces apoptosis mediated by reactive oxygen species
in bovine retinal pericytes. J Korean Med Sci. 19:95–100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saccà SC, Cutolo CA, Ferrari D, Corazza P
and Traverso CE: The eye, oxidative damage and polyunsaturated
fatty acids. Nutrients. 10(pii): E6682018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kowluru RA, Atasi L and Ho YS: Role of
mitochondrial superoxide dismutase in the development of diabetic
retinopathy. Invest Ophthalmol Vis Sci. 47:1594–1599. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coucha M, Elshaer SL, Eldahshan WS, Mysona
BA and El-Remessy AB: Molecular mechanisms of diabetic retinopathy:
Potential therapeutic targets. Middle East Afr J Ophthalmol.
22:135–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hatakeyama J and Kageyama R: Retinal cell
fate determination and bHLH factors. Semin Cell Dev Biol. 15:83–89.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hellström M, Phng LK, Hofmann JJ, Wallgard
E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N,
et al: Dll4 signalling through Notch1 regulates formation of tip
cells during angiogenesis. Nature. 445:776–780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borggrefe T and Oswald F: The Notch
signaling pathway: Transcriptional regulation at Notch target
genes. Cell Mol Life Sci. 66:1631–1646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng M, Zhang Z, Zhao X, Ding Y and Han
H: The Notch signaling pathway in retinal dysplasia and retina
vascular homeostasis. J Genet Genomics. 37:573–582. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin X, Zhang Z, Xu H and Wu Y: Notch
signaling protects retina from nuclear factor-κB- and
poly-ADP-ribose-polymerase-mediated apoptosis under high-glucose
stimulation. Acta Biochim Biophys Sin (Shanghai). 43:703–711. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dehdashtian E, Mehrzadi S, Yousefi B,
Hosseinzadeh A, Reiter RJ, Safa M, Ghaznavi H and Naseripour M:
Diabetic retinopathy pathogenesis and the ameliorating effects of
melatonin; involvement of autophagy, inflammation and oxidative
stress. Life Sci. 193:20–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JE, Cho KE, Lee KE, Kim J and Bae YS:
Nox4-mediated cell signaling regulates differentiation and survival
of neural crest stem cells. Mol Cells. 37:907–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Remessy AB, Rajesh M, Mukhopadhyay P,
Horváth B, Patel V, Al-Gayyar MM, Pillai BA and Pacher P:
Cannabinoid 1 receptor activation contributes to vascular
inflammation and cell death in a mouse model of diabetic
retinopathy and a human retinal cell line. Diabetologia.
54:1567–1578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian DY, Jin XR, Zeng X and Wang Y: Notch
signaling in endothelial cells: Is it the therapeutic target for
vascular neointimal hyperplasia? Int J Mol Sci. 18(pii): E16152017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanigaki K and Honjo T: Two opposing roles
of RBP-J in Notch signaling. Curr Top Dev Biol. 92:231–252. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Liu H, Al-Shabrawey M, Caldwell
RW and Caldwell RB: Inflammation and diabetic retinal microvascular
complications. J Cardiovasc Dis Res. 2:96–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tarr JM, Kaul K, Chopra M, Kohner EM and
Chibber R: Pathophysiology of diabetic retinopathy. ISRN
Ophthalmol. 2013:3435602013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caldwell RB, Bartoli M, Behzadian MA,
El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI and Caldwell RW:
Vascular endothelial growth factor and diabetic retinopathy: Role
of oxidative stress. Curr Drug Targets. 6:511–524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ali TK and El-Remessy AB: Diabetic
retinopathy: Current management and experimental therapeutic
targets. Pharmacotherapy. 29:182–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radak D, Resanovic I and Isenovic ER: Link
between oxidative stress and acute brain ischemia. Angiology.
65:667–676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Babior BM: The NADPH oxidase of
endothelial cells. IUBMB Life. 50:267–269. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frey RS, Ushio-Fukai M and Malik AB: NADPH
oxidase-dependent signaling in endothelial cells: Role in
physiology and pathophysiology. Antioxid Redox Signal. 11:791–810.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wingler K, Hermans JJ, Schiffers P, Moens
A, Paul M and Schmidt HH: NOX1, 2, 4, 5: Counting out oxidative
stress. Br J Pharmacol. 164:866–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kowluru RA, Kowluru A, Veluthakal R,
Mohammad G, Syed I, Santos JM and Mishra M: TIAM1-RAC1 signalling
axis-mediated activation of NADPH oxidase-2 initiates mitochondrial
damage in the development of diabetic retinopathy. Diabetologia.
57:1047–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Shabrawey M, Bartoli M, El-Remessy AB,
Ma G, Matragoon S, Lemtalsi T, Caldwell RW and Caldwell RB: Role of
NADPH oxidase and Stat3 in statin-mediated protection against
diabetic retinopathy. Invest Ophthalmol Vis Sci. 49:3231–3238.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Wang JJ and Zhang SX: NADPH oxidase
4-derived H2O2 promotes aberrant retinal neovascularization via
activation of VEGF receptor 2 pathway in oxygen-induced
retinopathy. J Diabetes Res. 2015:9632892015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Yang Z, Jiang Y and Hartnett ME:
Endothelial NADPH oxidase 4 mediates vascular endothelial growth
factor receptor 2-induced intravitreal neovascularization in a rat
model of retinopathy of prematurity. Mol Vis. 20:231–241.
2014.PubMed/NCBI
|
|
34
|
Yao M, Gao F, Wang X, Shi Y, Liu S and
Duan H: Nox4 is involved in high glucose-induced apoptosis in renal
tubular epithelial cells via Notch pathway. Mol Med Rep.
15:4319–4325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai WX, Liang L, Wang L, Han JT, Zhu XX,
Han H, Hu DH and Zhang P: Inhibition of Notch signaling leads to
increased intracellular ROS by up-regulating Nox4 expression in
primary HUVECs. Cell Immunol. 287:129–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hofmann JJ and Luisa Iruela-Arispe M:
Notch expression patterns in the retina: An eye on receptor-ligand
distribution during angiogenesis. Gene Expr Patterns. 7:461–470.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oswald F, Täuber B, Dobner T, Bourteele S,
Kostezka U, Adler G, Liptay S and Schmid RM: p300 acts as a
transcriptional coactivator for mammalian Notch-1. Mol Cell Biol.
21:7761–7774. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Zhou X, Kuang X, Long C, Liu W,
Tang Y, Liu H, He J, Huang Z, Fan Y, Zhang Q and Shen H: The
inhibition of NOTCH2 reduces UVB-induced damage in retinal pigment
epithelium cells. Mol Med Rep. 16:730–736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li H, Zhu Z, Liu J, Wang J and Qu C:
MicroRNA-137 regulates hypoxia-induced retinal ganglion cell
apoptosis through Notch1. Int J Mol Med. 41:1774–1782.
2018.PubMed/NCBI
|
|
41
|
Arboleda-Velasquez JF, Primo V, Graham M,
James A, Manent J and D'Amore PA: Notch signaling functions in
retinal pericyte survival. Invest Ophthalmol Vis Sci. 55:5191–5199.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hori K, Sen A and Artavanis-Tsakonas S:
Notch signaling at a glance. J Cell Sci. 126:2135–2140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Zang C, Taing L, Arnett KL, Wong
YJ, Pear WS, Blacklow SC, Liu XS and Aster JC: NOTCH1-RBPJ
complexes drive target gene expression through dynamic interactions
with superenhancers. Proc Natl Acad Sci USA. 111:705–710. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schouwey K, Aydin IT, Radtke F and
Beermann F: RBP-Jκ-dependent Notch signaling enhances retinal
pigment epithelial cell proliferation in transgenic mice. Oncogene.
30:313–322. 2011. View Article : Google Scholar : PubMed/NCBI
|