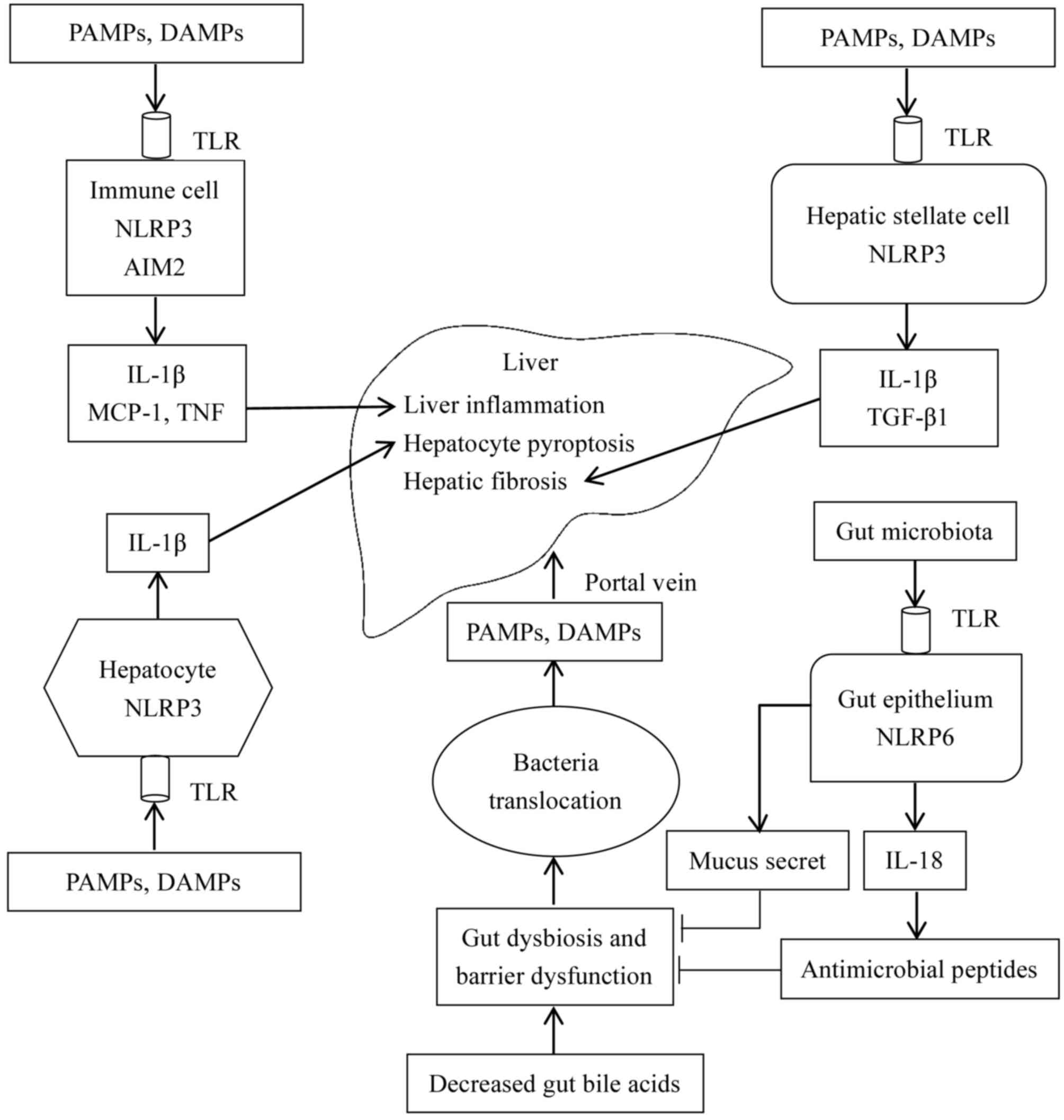
|
1
|
Visschers RG, Luyer MD, Schaap FG, Olde
Damink SW and Soeters PB: The gut-liver axis. Curr Opin Clin Nutr
Metab Care. 16:576–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Islam KB, Fukiya S, Hagio M, Fujii N,
Ishizuka S, Ooka T, Ogura Y, Hayashi T and Yokota A: Bile acid is a
host factor that regulates the composition of the cecal microbiota
in rats. Gastroenterology. 141:1773–1781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yokota A, Fukiya S, Islam KB, Ooka T,
Ogura Y, Hayashi T, Hagio M and Ishizuka S: Is bile acid a
determinant of the gut microbiota on a high-fat diet? Gut Microbes.
3:455–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai SY and Boyer JL: Studies on the
mechanisms of bile acid initiated hepatic inflammation in
cholestatic liver injury. Inflamm Cell Signal.
4:e15612017.PubMed/NCBI
|
|
5
|
Cai SY, Ouyang X, Chen Y, Soroka CJ, Wang
J, Mennone A, Wang Y, Mehal WZ, Jain D and Boyer JL: Bile acids
initiate cholestatic liver injury by triggering a
hepatocyte-specific inflammatory response. JCI Insight.
2:e907802017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perez MJ and Briz O: Bile-acid-induced
cell injury and protection. World J Gastroenterol. 15:1677–1689.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allen K, Jaeschke H and Copple BL: Bile
acids induce inflammatory genes in hepatocytes: A novel mechanism
of inflammation during obstructive cholestasis. Am J Pathol.
178:175–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Minicis S, Rychlicki C, Agostinelli L,
Saccomanno S, Candelaresi C, Trozzi L, Mingarelli E, Facinelli B,
Magi G, Palmieri C, et al: Dysbiosis contributes to fibrogenesis in
the course of chronic liver injury in mice. Hepatology.
59:1738–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sabino J, Vieira-Silva S, Machiels K,
Joossens M, Falony G, Ballet V, Ferrante M, Van Assche G, Van der
Merwe S, Vermeire S and Raes J: Primary sclerosing cholangitis is
characterised by intestinal dysbiosis independent from IBD. Gut.
65:1681–1689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiest R, Albillos A, Trauner M, Bajaj JS
and Jalan R: Intestinal hepatic axis for liver disease. J Hepatol.
67:1084–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tripathi A, Debelius J, Brenner DA, Karin
M, Loomba R, Schnabl B and Knight R: The gut-liver axis and the
intersection with the microbiome. Nat Rev Gastroenterol Hepatol.
15:397–411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Toole A, Alakkari A, Keegan D, Doherty
G, Mulcahy H and O'Donoghue D: Primary sclerosing cholangitis and
disease distribution in inflammatory bowel disease. Clin
Gastroenterol Hepatol. 10:439–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weismüller TJ, Trivedi PJ, Bergquist A,
Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA,
Marschall HU, et al: Patient age, sex, and inflammatory bowel
disease phenotype associate with course of primary sclerosing
cholangitis. Gastroenterology. 152:1975–1984, e1978. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu R, Li X, Huang Z, Zhao D, Ganesh BS,
Lai G, Pandak WM, Hylemon PB, Bajaj JS, Sanyal AJ and Zhou H: C/EBP
homologous protein-induced loss of intestinal epithelial stemness
contributes to bile duct ligation-induced cholestatic liver injury
in mice. Hepatology. 67:1441–1457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jee J, Mourya R, Shivakumar P, Fei L,
Wagner M and Bezerra JA: Cxcr2 signaling and the microbiome
suppress inflammation, bile duct injury, and the phenotype of
experimental biliary atresia. PLoS One. 12:e01820892017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrasek J, Bala S, Csak T, Lippai D,
Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA and Szabo G:
IL-1 receptor antagonist ameliorates inflammasome-dependent
alcoholic steatohepatitis in mice. J Clin Invest. 122:3476–3489.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Henao-Mejia J, Elinav E, Jin C, Hao L,
Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ,
et al: Inflammasome-mediated dysbiosis regulates progression of
NAFLD and obesity. Nature. 482:179–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wlodarska M, Thaiss CA, Nowarski R,
Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN,
Philbrick WM, et al: NLRP6 inflammasome orchestrates the colonic
host-microbial interface by regulating goblet cell mucus secretion.
Cell. 156:1045–1059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levy M, Thaiss CA, Zeevi D, Dohnalová L,
Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig
Y, et al: Microbiota-modulated metabolites shape the intestinal
microenvironment by regulating NLRP6 inflammasome signaling. Cell.
163:1428–1443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu
C, Chen Y, Cai W and Wu J: Chenodeoxycholic acid activates NLRP3
inflammasome and contributes to cholestatic liver fibrosis.
Oncotarget. 7:83951–83963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han J, Bae J, Choi CY, Choi SP, Kang HS,
Jo EK, Park J, Lee YS, Moon HS, Park CG, et al: Autophagy induced
by AXL receptor tyrosine kinase alleviates acute liver injury via
inhibition of NLRP3 inflammasome activation in mice. Autophagy.
12:2326–2343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wree A, McGeough MD, Inzaugarat ME, Eguchi
A, Schuster S, Johnson CD, Peña CA, Geisler LJ, Papouchado BG,
Hoffman HM and Feldstein AE: NLRP3 inflammasome driven liver injury
and fibrosis. Roles of IL-17 and TNF. Hepatology. 2017.
|
|
24
|
Barreyro FJ, Holod S, Finocchietto PV,
Camino AM, Aquino JB, Avagnina A, Carreras MC, Poderoso JJ and
Gores GJ: The pan-caspase inhibitor Emricasan (IDN-6556) decreases
liver injury and fibrosis in a murine model of non-alcoholic
steatohepatitis. Liver Int. 35:953–966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alaish SM, Smith AD, Timmons J, Greenspon
J, Eyvazzadeh D, Murphy E, Shea-Donahue T, Cirimotich S, Mongodin
E, Zhao A, et al: Gut microbiota, tight junction protein
expression, intestinal resistance, bacterial translocation and
mortality following cholestasis depend on the genetic background of
the host. Gut Microbes. 4:292–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pierantonelli I, Rychlicki C, Agostinelli
L, Giordano DM, Gaggini M, Fraumene C, Saponaro C, Manghina V,
Sartini L, Mingarelli E, et al: Lack of NLRP3-inflammasome leads to
gut-liver axis derangement, gut dysbiosis and a worsened phenotype
in a mouse model of NAFLD. Sci Rep. 7:122002017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang
L, Zheng M, Zhang X, Xia D, Ke Y, et al: Bile acids control
inflammation and metabolic disorder through inhibition of NLRP3
inflammasome. Immunity. 45:802–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hao H, Cao L, Jiang C, Che Y, Zhang S,
Takahashi S, Wang G and Gonzalez FJ: Farnesoid X receptor
regulation of the NLRP3 inflammasome underlies
cholestasis-associated sepsis. Cell Metab. 25:856–867, e855. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie S, Guo C, Chi Z, Huang B, Wu Y, Wang D
and Xia D: A rapid administration of GW4064 inhibits the NLRP3
inflammasome activation independent of farnesoid X receptor
agonism. FEBS Lett. 591:2836–2847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giebeler A, Brandenburg LO, Kaldenbach M,
Erschfeld S, Wasmuth H, Wruck C, Trautwein C and Streetz KL: Lack
of hepatic c-Met and gp130 expression is associated with an
impaired antibacterial response and higher lethality after bile
duct ligation. Lab Invest. 92:1726–1737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsushita H, Miyake Y, Takaki A, Yasunaka
T, Koike K, Ikeda F, Shiraha H, Nouso K and Yamamoto K: TLR4, TLR9,
and NLRP3 in biliary epithelial cells of primary sclerosing
cholangitis: Relationship with clinical characteristics. J
Gastroenterol Hepatol. 30:600–608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szabo G and Petrasek J: Inflammasome
activation and function in liver disease. Nat Rev Gastroenterol
Hepatol. 12:387–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gross O, Thomas CJ, Guarda G and Tschopp
J: The inflammasome: An integrated view. Immunol Rev. 243:136–151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ting JP, Lovering RC, Alnemri ES, Bertin
J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA,
et al: The NLR gene family: A standard nomenclature. Immunity.
28:285–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bauernfeind FG, Horvath G, Stutz A,
Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks
BG, Fitzgerald KA, et al: Cutting edge: NF-kappaB activating
pattern recognition and cytokine receptors license NLRP3
inflammasome activation by regulating NLRP3 expression. J Immunol.
183:787–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boaru SG, Borkham-Kamphorst E, Van de Leur
E, Lehnen E, Liedtke C and Weiskirchen R: NLRP3 inflammasome
expression is driven by NF-κB in cultured hepatocytes. Biochem
Biophys Res Commun. 458:700–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kahlenberg JM and Dubyak GR: Mechanisms of
caspase-1 activation by P2X7 receptor-mediated K+
release. Am J Physiol Cell Physiol. 286:C1100–C1108. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kanneganti TD, Lamkanfi M, Kim YG, Chen G,
Park JH, Franchi L, Vandenabeele P and Núñez G: Pannexin-1-mediated
recognition of bacterial molecules activates the cryopyrin
inflammasome independent of Toll-like receptor signaling. Immunity.
26:433–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hornung V, Bauernfeind F, Halle A, Samstad
EO, Kono H, Rock KL, Fitzgerald KA and Latz E: Silica crystals and
aluminum salts activate the NALP3 inflammasome through phagosomal
destabilization. Nat Immunol. 9:847–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou R, Tardivel A, Thorens B, Choi I and
Tschopp J: Thioredoxin-interacting protein links oxidative stress
to inflammasome activation. Nat Immunol. 11:136–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Elinav E, Strowig T, Kau AL, Henao-Mejia
J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon
JI and Flavell RA: NLRP6 inflammasome regulates colonic microbial
ecology and risk for colitis. Cell. 145:745–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gremel G, Wanders A, Cedernaes J,
Fagerberg L, Hallström B, Edlund K, Sjöstedt E, Uhlén M and Pontén
F: The human gastrointestinal tract-specific transcriptome and
proteome as defined by RNA sequencing and antibody-based profiling.
J Gastroenterol. 50:46–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Del Chierico F, Vernocchi P, Petrucca A,
Paci P, Fuentes S, Praticò G, Capuani G, Masotti A, Reddel S, Russo
A, et al: Phylogenetic and metabolic tracking of gut microbiota
during perinatal development. PLoS One. 10:e01373472015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kempster SL, Belteki G, Forhead AJ, Fowden
AL, Catalano RD, Lam BY, McFarlane I, Charnock-Jones DS and Smith
GC: Developmental control of the Nlrp6 inflammasome and a
substrate, IL-18, in mammalian intestine. Am J Physiol Gastrointest
Liver Physiol. 300:G253–G263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chudnovskiy A, Mortha A, Kana V, Kennard
A, Ramirez JD, Rahman A, Remark R, Mogno I, Ng R, Gnjatic S, et al:
Host-Protozoan interactions protect from mucosal infections through
activation of the inflammasome. Cell. 167:444–456, e414. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun Y, Zhang M, Chen CC, Gillilland M III,
Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC, et
al: Stress-induced corticotropin-releasing hormone-mediated NLRP6
inflammasome inhibition and transmissible enteritis in mice.
Gastroenterology. 144:1478–1487, e1471-1487.e1-e8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Birchenough GM, Nyström EE, Johansson ME
and Hansson GC: A sentinel goblet cell guards the colonic crypt by
triggering Nlrp6-dependent Muc2 secretion. Science. 352:1535–1542.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huber S, Gagliani N, Zenewicz LA, Huber
FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W Jr, Murphy AJ, et
al: IL-22BP is regulated by the inflammasome and modulates
tumorigenesis in the intestine. Nature. 491:259–263. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang P, Zhu S, Yang L, Cui S, Pan W,
Jackson R, Zheng Y, Rongvaux A, Sun Q, Yang G, et al: Nlrp6
regulates intestinal antiviral innate immunity. Science.
350:826–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Elinav E, Thaiss CA and Flavell RA:
Analysis of microbiota alterations in inflammasome-deficient mice.
Methods Mol Biol. 1040:185–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen GY, Liu M, Wang F, Bertin J and Núñez
G: A functional role for Nlrp6 in intestinal inflammation and
tumorigenesis. J Immunol. 186:7187–7194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Normand S, Delanoye-Crespin A, Bressenot
A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D and
Chamaillard M: Nod-like receptor pyrin domain-containing protein 6
(NLRP6) controls epithelial self-renewal and colorectal
carcinogenesis upon injury. Proc Natl Acad Sci USA. 108:9601–9606.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu B, Elinav E, Huber S, Strowig T, Hao L,
Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC and
Flavell RA: Microbiota-induced activation of epithelial IL-6
signaling links inflammasome-driven inflammation with transmissible
cancer. Proc Natl Acad Sci USA. 110:9862–9867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Seo SU, Kamada N, Muñoz-Planillo R, Kim
YG, Kim D, Koizumi Y, Hasegawa M, Himpsl SD, Browne HP, Lawley TD,
et al: Distinct commensals induce interleukin-1β via NLRP3
inflammasome in inflammatory monocytes to promote intestinal
inflammation in response to injury. Immunity. 42:744–755. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Filardy AA, He J, Bennink J, Yewdell J and
Kelsall BL: Posttranscriptional control of NLRP3 inflammasome
activation in colonic macrophages. Mucosal Immunol. 9:850–858.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Allen IC, TeKippe EM, Woodford RM, Uronis
JM, Holl EK, Rogers AB, Herfarth HH, Jobin C and Ting JP: The NLRP3
inflammasome functions as a negative regulator of tumorigenesis
during colitis-associated cancer. J Exp Med. 207:1045–1056. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hu B, Elinav E, Huber S, Booth CJ, Strowig
T, Jin C, Eisenbarth SC and Flavell RA: Inflammation-induced
tumorigenesis in the colon is regulated by caspase-1 and NLRC4.
Proc Natl Acad Sci USA. 107:21635–21640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ruiz PA, Morón B, Becker HM, Lang S,
Atrott K, Spalinger MR, Scharl M, Wojtal KA, Fischbeck-Terhalle A,
Frey-Wagner I, et al: Titanium dioxide nanoparticles exacerbate
DSS-induced colitis: Role of the NLRP3 inflammasome. Gut.
66:1216–1224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zherebiatiev A and Kamyshnyi A: Expression
levels of proinflammatory cytokines and NLRP3 inflammasome in an
experimental model of Oxazolone-induced colitis. Iran J Allergy
Asthma Immunol. 15:39–45. 2016.PubMed/NCBI
|
|
61
|
De la Fuente M, Franchi L, Araya D,
Díaz-Jiménez D, Olivares M, Álvarez-Lobos M, Golenbock D, González
MJ, López-Kostner F, Quera R, et al: Escherichia coli isolates from
inflammatory bowel diseases patients survive in macrophages and
activate NLRP3 inflammasome. Int J Med Microbiol. 304:384–392.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bauer C, Duewell P, Lehr HA, Endres S and
Schnurr M: Protective and aggravating effects of Nlrp3 inflammasome
activation in IBD models: Influence of genetic and environmental
factors. Dig Dis. 30 Suppl 1:S82–S90. 2012. View Article : Google Scholar
|
|
63
|
Hirota SA, Ng J, Lueng A, Khajah M, Parhar
K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, et al: NLRP3
inflammasome plays a key role in the regulation of intestinal
homeostasis. Inflamm Bowel Dis. 17:1359–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zaki MH, Boyd KL, Vogel P, Kastan MB,
Lamkanfi M and Kanneganti TD: The NLRP3 inflammasome protects
against loss of epithelial integrity and mortality during
experimental colitis. Immunity. 32:379–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Szabo G and Csak T: Inflammasomes in liver
diseases. J Hepatol. 57:642–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mandrekar P, Ambade A, Lim A, Szabo G and
Catalano D: An essential role for monocyte chemoattractant
protein-1 in alcoholic liver injury: Regulation of proinflammatory
cytokines and hepatic steatosis in mice. Hepatology. 54:2185–2197.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Miura K, Kodama Y, Inokuchi S, Schnabl B,
Aoyama T, Ohnishi H, Olefsky JM, Brenner DA and Seki E: Toll-like
receptor 9 promotes steatohepatitis by induction of
interleukin-1beta in mice. Gastroenterology. 139:323–334.e327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kubes P and Mehal WZ: Sterile inflammation
in the liver. Gastroenterology. 143:1158–1172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Csak T, Pillai A, Ganz M, Lippai D,
Petrasek J, Park JK, Kodys K, Dolganiuc A, Kurt-Jones EA and Szabo
G: Both bone marrow-derived and non-bone marrow-derived cells
contribute to AIM2 and NLRP3 inflammasome activation in a
MyD88-dependent manner in dietary steatohepatitis. Liver Int.
34:1402–1413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rao RK and Samak G: Bile duct epithelial
tight junctions and barrier function. Tissue Barriers.
1:e257182013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fickert P, Fuchsbichler A, Wagner M,
Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C,
Zatloukal K, et al: Regurgitation of bile acids from leaky bile
ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice.
Gastroenterology. 127:261–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Maroni L, Agostinelli L, Saccomanno S,
Pinto C, Giordano DM, Rychlicki C, De Minicis S, Trozzi L, Banales
JM, Melum E, et al: Nlrp3 activation induces Il-18 synthesis and
affects the epithelial barrier function in reactive cholangiocytes.
Am J Pathol. 187:366–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ikegami T and Honda A: Reciprocal
interactions between bile acids and gut microbiota in human liver
diseases. Hepatol Res. 48:15–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hofmann AF: The enterohepatic circulation
of bile acids in mammals: Form and functions. Front Biosci
(Landmark Ed). 14:2584–2598. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dawson PA and Karpen SJ: Intestinal
transport and metabolism of bile acids. J Lipid Res. 56:1085–1099.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ridlon JM, Kang DJ and Hylemon PB: Bile
salt biotransformations by human intestinal bacteria. J Lipid Res.
47:241–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Halilbasic E, Claudel T and Trauner M:
Bile acid transporters and regulatory nuclear receptors in the
liver and beyond. J Hepatol. 58:155–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Makishima M, Okamoto AY, Repa JJ, Tu H,
Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ and Shan B:
Identification of a nuclear receptor for bile acids. Science.
284:1362–1365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Parks DJ, Blanchard SG, Bledsoe RK,
Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki
AM, Moore DD and Lehmann JM: Bile acids: Natural ligands for an
orphan nuclear receptor. Science. 284:1365–1368. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Potthoff MJ, Potts A, He T, Duarte JA,
Taussig R, Mangelsdorf DJ, Kliewer SA and Burgess SC: Colesevelam
suppresses hepatic glycogenolysis by TGR5-mediated induction of
GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol.
304:G371–G380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Schaap FG, Trauner M and Jansen PL: Bile
acid receptors as targets for drug development. Nat Rev
Gastroenterol Hepatol. 11:55–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Reich M, Klindt C, Deutschmann K, Spomer
L, Häussinger D and Keitel V: Role of the G protein-coupled bile
acid receptor TGR5 in liver damage. Dig Dis. 35:235–240. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kawamata Y, Fujii R, Hosoya M, Harada M,
Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, et al:
A G protein-coupled receptor responsive to bile acids. J Biol Chem.
278:9435–9440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ding JW, Andersson R, Soltesz V, Willén R
and Bengmark S: The role of bile and bile acids in bacterial
translocation in obstructive jaundice in rats. Eur Surg Res.
25:11–19. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Inagaki T, Moschetta A, Lee YK, Peng L,
Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, et al:
Regulation of antibacterial defense in the small intestine by the
nuclear bile acid receptor. Proc Natl Acad Sci USA. 103:3920–3925.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wahlström A, Sayin SI, Marschall HU and
Bäckhed F: Intestinal crosstalk between bile acids and microbiota
and its impact on host metabolism. Cell Metab. 24:41–50. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang YD, Chen WD, Wang M, Yu D, Forman BM
and Huang W: Farnesoid X receptor antagonizes nuclear factor kappaB
in hepatic inflammatory response. Hepatology. 48:1632–1643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wagner M, Zollner G and Trauner M: Nuclear
receptors in liver disease. Hepatology. 53:1023–1034. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhu C, Fuchs CD, Halilbasic E and Trauner
M: Bile acids in regulation of inflammation and immunity: Friend or
foe? Clin Exp Rheumatol. 34 (4 Suppl 98):S25–S31. 2016.PubMed/NCBI
|
|
90
|
Inagaki T, Choi M, Moschetta A, Peng L,
Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA,
et al: Fibroblast growth factor 15 functions as an enterohepatic
signal to regulate bile acid homeostasis. Cell Metab. 2:217–225.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Péan N, Doignon I, Garcin I, Besnard A,
Julien B, Liu B, Branchereau S, Spraul A, Guettier C, Humbert L, et
al: The receptor TGR5 protects the liver from bile acid overload
during liver regeneration in mice. Hepatology. 58:1451–1460. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Baghdasaryan A, Claudel T, Gumhold J,
Silbert D, Adorini L, Roda A, Vecchiotti S, Gonzalez FJ, Schoonjans
K, Strazzabosco M, et al: Dual farnesoid X receptor/TGR5 agonist
INT-767 reduces liver injury in the Mdr2-/- (Abcb4-/-) mouse
cholangiopathy model by promoting biliary HCO3 output.
Hepatology. 54:1303–1312. 2011. View Article : Google Scholar : PubMed/NCBI
|