Introduction
Acute myeloid leukemia (AML) is a highly
heterogeneous and malignant hematological tumor characterized by
the accumulation of undifferentiated myeloid progenitor cells in
the bone marrow and peripheral blood (1). At present, chemotherapy and stem cell
transplantation are the principal therapeutic approaches for AML
(2); however, the outcomes of
patients with AML at advanced stage with metastasis and recurrence
remain poor (3). Genetic mutations
and aberrant expression levels of cancer-associated genes may
induce AML (4,5). A previous study demonstrated that
impaired expression of Wilms tumor 1, high meningioma 1 and
ETS-related gene is associated with the clinical outcomes of AML
(6). In addition, a number of
microRNAs (miRNAs/miRs) have been reported to be involved in the
progression of AML, including miR-19b and miR-375 (7,8);
however, the underlying molecular mechanisms remain unknown.
miRNAs are a group of short noncoding RNAs with a
length of ~21 nucleotides (9). A
recent study has identified that miRNAs serve essential roles in
regulating gene expression by binding to the target sequence and
promoting the degradation of specific mRNAs (10). miRNAs regulate various biological
processes in cancer cells, including cell differentiation,
proliferation, metastasis and apoptosis (11,12).
A variety of miRNAs are aberrantly expressed in various types of
cancer, including AML (13,14).
In AML, numerous miRNAs are dysregulated (15). Ding et al (16) identified that upregulated miR-130a
may induce cell death in AML. Zhao et al (17) demonstrated that miR-144 is a
potential non-invasive biomarker for patients with AML. Ma et
al (14) identified that
miR-362-5p is a novel prognostic predictor of cytogenically normal
AML. Although the importance of miRNAs in AML has been identified,
the underlying mechanisms remain unknown.
Recent studies have demonstrated that miR-1271-5p is
involved in the pathogenesis of colorectal cancer and
hepatocellular carcinoma (18,19);
however, whether miR-1271-5p may regulate the progression of AML
remains unknown. In the present study, miR-1271-5p inhibited the
proliferation and induced apoptosis in AML via targeting zinc
family member 2 (ZIC2), which suggested that miR-1271-5p may be a
promising therapeutic target for the treatment of patients with
AML.
Materials and methods
Clinical specimens
The peripheral blood samples (50 ml per sample) were
obtained from patients with AML (n=35) and healthy donors (n=35) at
The Third Affiliated Hospital of Wenzhou Medical University
(Wenzhou, China) between January 2014 and December 2016. The sex
ratio of the patients and controls was ~1:1 and the patients were
aged between 2 and 60 years old. Patients were diagnosed with AML
according to the pathological and clinic features of the disease
(20). Patients who received
chemotherapy or radiotherapy prior to collection were excluded; all
other patients with AML were included. Informed consent was
obtained from each patient, and the present study was approved by
the Institutional Ethics Committee of The Third Affiliated Hospital
of Wenzhou Medical University. The patients with AML were divided
into miR-1271-5p high expression and low expression groups
according to the median value of miR-1271-5p expression.
Cell culture and transfection
AML cell lines (HL-60, Kasumi-1, AML193 and
OCI-AML2) and a normal cell line derived from marrow stroma (HS-5)
were purchased from The American Type Culture Collection (Manassas,
VA, USA) and cultured in Eagle's minimum essential medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) and 1% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified incubator
containing 5% CO2.
The ZIC2 coding sequence was constructed in a pcDNA3
vector. For cell transfection, 5×105 AML cells were
seeded in 6-well plates and cultured with Eagle's minimum rssential
medium at 37°C overnight and subsequently transfected with 50 nM
miR-1271-5p mimics (5′-CUUGGCACCUAGCAAGCACUCA-3′), inhibitors
(5′-UGAGUGCUUGCUAGGUGCCAAG-3′) or the controls
(5′-UCACAACCUCCUAGAAAGAGUAGA-3′) or pcDNA3-ZIC2 vector (1 µg;
Invitrogen; Thermo Fisher Scientific, Inc.) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
miR-1271-5p mimics, inhibitors and the controls were obtained from
Applied Biosystems (Thermo Fisher Scientific, Inc.). At 48 h
post-transfection, the effects of gene silencing or overexpressing
were determined by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR).
Cell proliferation assay
In total, 2×103 AML193 and OCI-AML2 cells
were transfected for 24 h and seeded in 96-well plates followed by
incubation at 37°C for 1, 3 and 5 days. A total of 10 µl Cell
Counting kit-8 (CCK-8) reagent (Beyotime Institute of
Biotechnology, Haimen, China) was added to the medium and incubated
for 2 h; the absorbance at 450 nm was measured using an ELISA
reader (Molecular Devices, LLC; Sunnyvale, CA, USA).
Cell cycle and apoptosis analyses
For cell cycle analysis, 2×106 AML193 and
OCI-AML2 cells were washed and resuspended in PBS solution
containing 0.04 mg/ml propidium iodide (Invitrogen; Thermo Fisher
Scientific, Inc.) and 100 mg/ml RNase (Invitrogen; Thermo Fisher
Scientific, Inc.) for 15 min on ice. The samples were analyzed with
a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using
the CELLQuest program 5.1 (BD Biosciences) followed by incubation
at room temperature for 30 min. Modifit software 5.0 (Verity
Software House, Inc., Topsham, ME, USA) was used to generate a
histogram to determine the number of cells in each phase of the
cell cycle.
For apoptosis analysis, 2×106 AML cells
were transfected with miR-1271-5p mimics, inhibitors or controls
for 24 h. Cells were collected by centrifugation at 2,500 × g for 2
min at 4°C. Apoptosis analysis was performed using an Annexin
V-FITC Apoptosis Detection kit II (BD Biosciences) according to the
manufacturer's protocol. Cells were analyzed by flow cytometry
using a FACSCalibur flow cytometer (BD Biosciences). The data were
analyzed using Flowjo 7.6.1 (BD Biosciences).
RT-qPCR
Total RNA was extracted from cultured cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol, and cDNA was
synthesized from total RNA with a PrimerScript RT Reagent kit
(Takara Bio., Inc., Ostu, Japan) according to the manufacturer's
protocol. The conditions were as follows: Incubation for 1 h at
42°C and inactivation at 70°C for 15 min. miRNA from total RNA was
reverse transcribed using the Prime-Script miRNA cDNA Synthesis kit
(Takara Bio., Inc.) according to the manufacturer's protocol. qPCR
was performed using SYBR green Premix Ex Taq II (Takara Bio., Inc.)
on a Step One Plus Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The PCR thermocycling conditions were as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec. GAPDH
and U6 were used as the endogenous controls for mRNA and miRNA
expression levels irrespectively. Relative gene expression was
calculated using the 2−∆∆Cq method (21). A total of three repeats were
performed. The primer sequences were: U6 (forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′), GAPDH (forward,
5′-TCAACGACCACTTTGTCAAGCTCA-3′ and reverse,
5′-GCTGGTGGTCCAGGGGTCTTACT-3′), miR-1271-5p (forward,
5′-CTTGGCACCTAGCAAGCACTCA-3′ and reverse,
5′-GCGAGCACAGAATTAATACGAC-3′) and ZIC2 (forward,
5′-GTCCACACCTCCGATAAGCC-3′ and reverse,
5′-CTCATGGACCTTCATGTGCT-3′).
RNA pulldown assay
Purified synthesized RNAs (Invitrogen; Thermo Fisher
Scientific, Inc.) were labeled using biotin with Pierce RNA 3′ End
Desthiobiotinylation kit (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. A positive control
(biotin-labeled wild-type miR-1271-5p, biotin-miR-1271-5p) or
negative controls (5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were incubated
with AML cell lysates from 1×106 AML cells, isolated
using lysis buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
at 4°C overnight. The Streptavidin magnetic beads (Sigma-Aldrich;
Merck KGaA, Darmstadt) were added to the lysate and incubated at
4°C for 2 h. The RNA-enriched magnetic beads (Sigma-Aldrich; Merck
KGaA) were collected and precipitated RNAs were eluted using
elution buffer (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol, followed by RT-qPCR analysis as described
above. Forkhead box protein K2 (FOXK2) was the positive
control.
Western blotting
Proteins were extracted from AML cells using
radioimmunoprecipitation lysis buffer (0.15 mM NaCl, 0.05 mM
Tris-HCl, pH 7.5, 1% Triton, 0.1% SDS, 0.1% sodium deoxycholate and
1% NP40). The concentration of proteins was measured using the
Bradford method. A total of 20 µg total protein extract was
electrophoresed on 12% SDS-PAGE and transferred by electroblotting
onto polyvinylidene fluoride membranes. The membranes were blocked
for 1 h at 37°C in 5% (w/v) nonfat milk prior to incubation with
appropriate dilutions of the primary antibodies against ZIC2
(1:2,000; cat. no. Z4525; Sigma-Aldrich; Merck KGaA) and GAPDH
(1:2,000; cat. no. 5174; Cell Signaling Technology, Inc., Danvers,
MA, USA) at 4°C overnight. The membranes were subsequently
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. ab7090; Abcam, Cambridge, UK) at 25°C for 1 h.
The signals were visualized using an enhanced chemiluminescence kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol.
Luciferase reporter assay
The potential targets of miR-1271-5p were predicted
by TargetScan 7.0 (http://www.targetscan.org/vert_71/) and miRBase
(http://www.mirbase.org/search.shtml).
The sequence of ZIC3-3′UTR containing the wild-type or mutant
potential binding site for miR1271-5p were inserted into the
SpeI and HindIII sites in the pMIR-Report vector
(Ambion; Thermo Fisher Scientific, Inc.) to generate the reporter
plasmid. miR-1271-5p mimic (50 nM) or inhibitor, reporter plasmid
(1 µg) and pRL-CMV Renilla luciferase reporter (1 ng;
Ambion; Thermo Fisher Scientific, Inc.) were co-transfected into
the cells using Lipofectamine® 2000 according to the
manufacturer's protocol. Luciferase activity was assessed at 48 h
post-transfection using a Dual-Luciferase® Reporter
Assay (Promega Corporation, Madison, WI, USA), and the levels of
firefly luciferase activity were normalized to that of
Renilla luciferase.
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM Corp., Armonk, NY, USA). The results are expressed as the
mean ± standard deviation. A Student's t-test or one-way analysis
of variance followed by Tukey's post hoc test was used to analyze
the difference in two or multiple groups. Pearson's correlation
coefficient analysis was used to determine the correlations. The
effect of miR-1271-5p expression on overall survival was analyzed
by Kaplan-Meier analysis and a log-rank test. The experiments were
repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
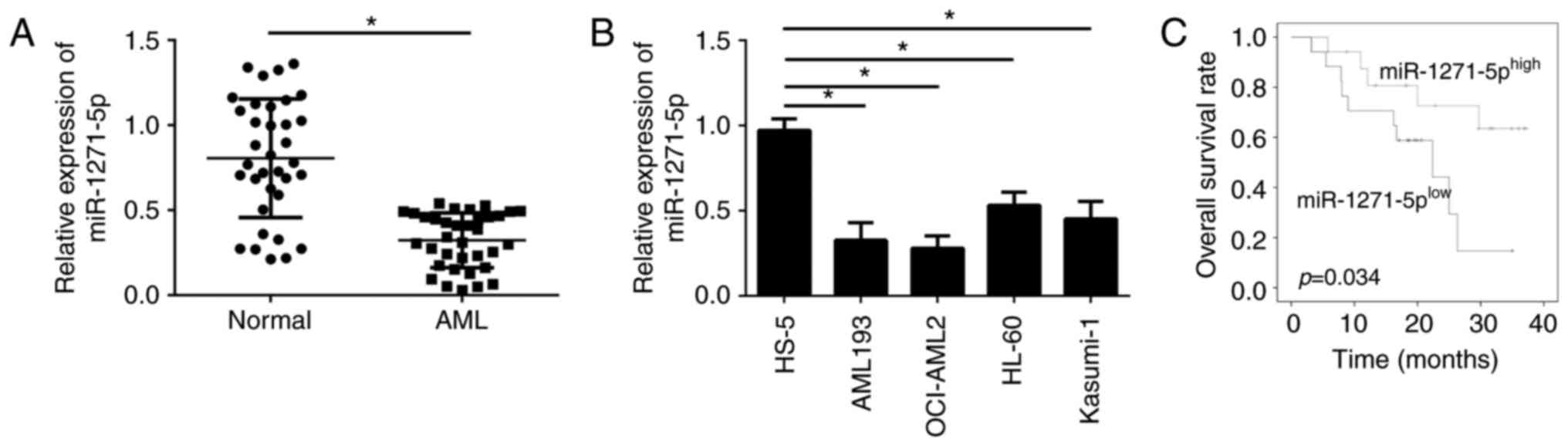
miR-1271-5p is downregulated in AML
samples
To examine the cellular functions of miR-1271-5p in
AML, the expression levels of miR-1271-5p in the blood of patients
with AML were evaluated by RT-qPCR. The expression levels of
miR-1271-5p were significantly downregulated in AML samples
compared with normal samples (P<0.05; Fig. 1A). In addition, miR-1271-5p
expression levels were significantly reduced in AML cell lines
compared with in the normal cell line HS-5 (P<0.05; Fig. 1B). The downregulation of
miR-1271-5p in AML cells may be associated with the clinical
outcomes of patients with AML. Furthermore, the AML samples were
divided into two groups according to the mean value of miR-1271-5p
expression levels. MiR-1271-5p expression above or below the mean
value were considered as high or low expression, respectively.
Kaplan-Meier analysis demonstrated that patients with AML with high
miR-1271-5p expression exhibited improved survival rates (Fig. 1C). These data suggested that
miR-1271-5p may be involved in the progression of AML.
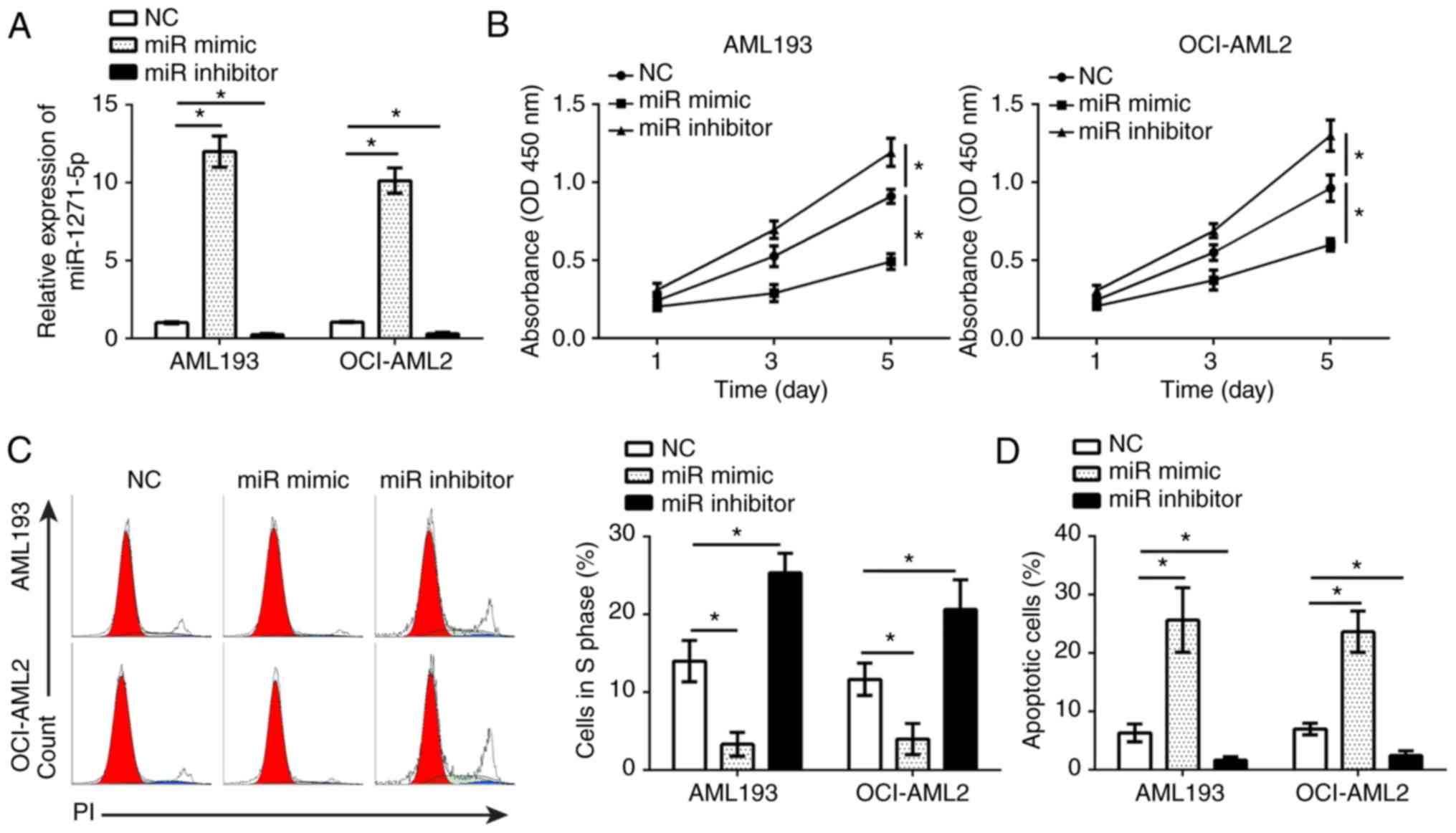
miR-1271-5p inhibits AML cell
proliferation and induces apoptosis
To determine the effects of miR-1271-5p on AML
cells, gain- and loss-of-function studies were performed in AML193
and OCI-AML2 cells transfected with miR-1271-5p mimics or
inhibitors. RT-qPCR analysis demonstrated that, compared with the
control, miR-1271-5p was significantly up- or downregulated in
AML193 and OCI-AML2 cells following transfection with mimics and
inhibitors, respectively (P<0.05; Fig. 2A). A CCK-8 assay and flow cytometry
were used to analyze the effects of miR-1271-5p on cell
proliferation and the cell cycle. The results indicated that,
compared with the control, miR-1271-5p overexpression significantly
inhibited the proliferation of AML cells and reduced the number of
cells in S phase, and vice versa (P<0.05; Fig. 2B and C). Additionally, flow
cytometry indicated that, compared with the control, miR-1271-5p
overexpression significantly promoted the apoptosis of AML cells,
and vice versa (P<0.05; Fig.
2D). These results demonstrated that miR-1271-5p serves as a
tumor suppressor in the progression of AML.
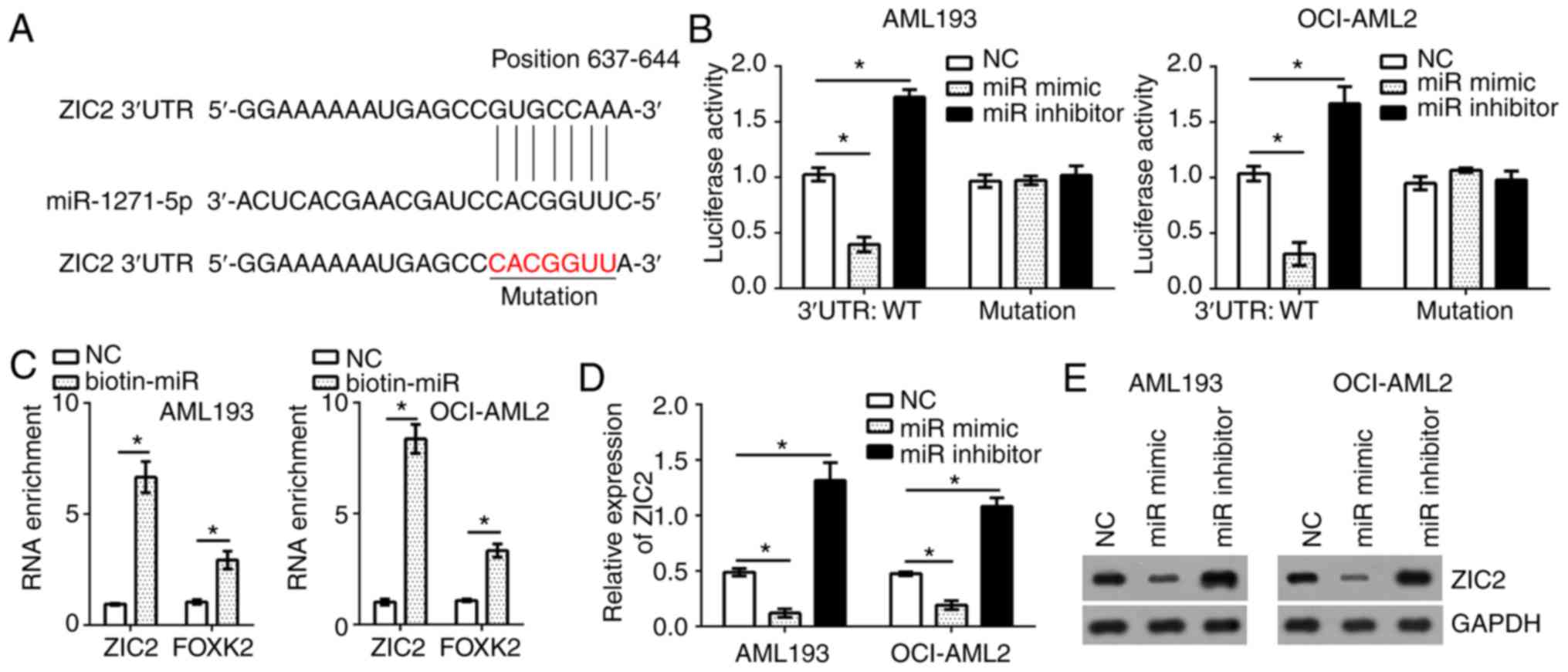
ZIC2 is a target of miR-1271-5p in AML
cells
To identify the target genes of miR-1271-5p in AML
cells, bioinformatics analysis was conducted using online software
(TargetScan 7.0 and miRBase). The results identified that
transcription factor ZIC2 was a possible target gene of
miR-1271-5p. There was a potential binding site of miR-1271-5p in
the 3′-untranslated region of ZIC2 mRNA (Fig. 3A). To determine the interaction
between miR-1271-5p and ZIC2, a luciferase reporter assay was
performed. The results indicated that, compared with the control,
the luciferase activity of ZIC2 3′-UTR (wild-type, WT) in AML193
and OCI-AML2 cells were significantly reduced and increased by
transfection with miR-1271-5p mimics and inhibitors, respectively
(Fig. 3B); however, a mutation in
the binding site of miR-1271-5p in ZIC2 3′-UTR abrogated the
effects of miR-1271-5p on the luciferase activity (Fig. 3B). Furthermore, an RNA pulldown
assay was performed using FOXK2 as a positive control (19). The results indicated that
biotin-miR-1271-5p significantly promoted the precipitation of ZIC2
and FOXK2 mRNA in AML cells compared with the control (P<0.05;
Fig. 3C), suggesting the direct
interaction between miR-1271-5p and ZIC2. To further confirm
whether ZIC2 was a target of miR-1271-5p in AML cells, the mRNA and
protein expression levels of ZIC2 were evaluated by RT-qPCR and
western blotting, respectively. Overexpression of miR-1271-5p
significantly suppressed the mRNA expression levels of ZIC2 in
AML193 and OCI-AML2 cells, and vice versa, compared with the
control; a notable decrease in ZIC2 protein expression was observed
in response to miR-1271-5p overexpression (P<0.05; Fig. 3D and E).
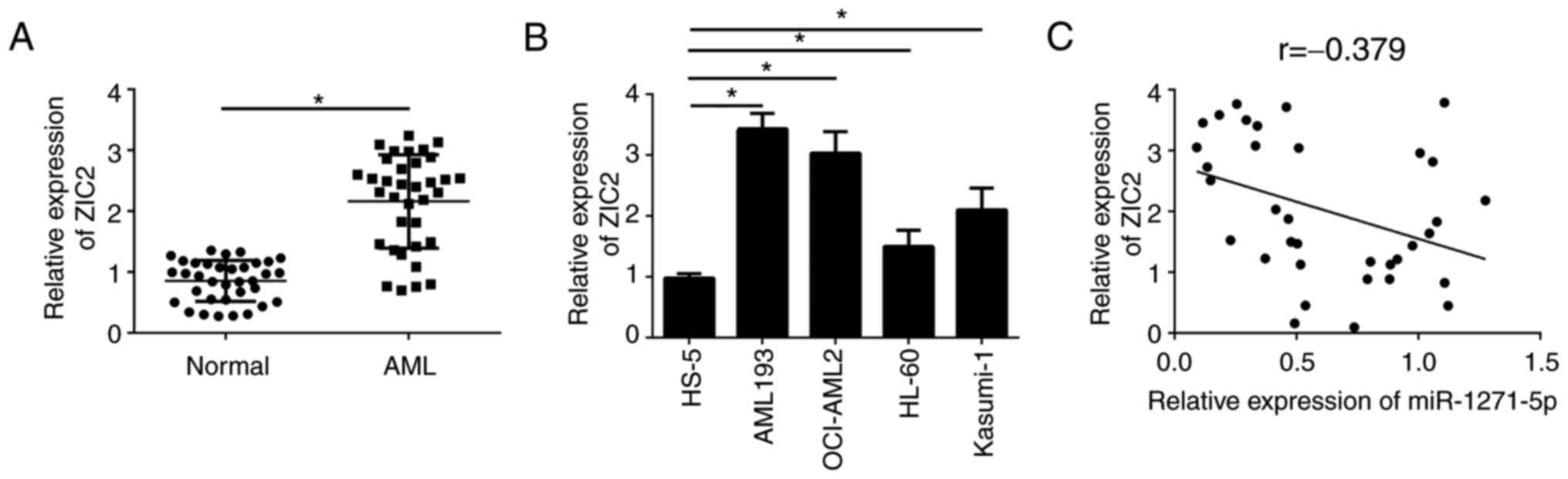
miR-1271-5p is negatively correlated
with the expression levels of ZIC2 in AML samples
To further investigate the association between
miR-1271-5p and ZIC2 in AML samples, the expression patterns of
ZIC2 were analyzed by RT-qPCR. ZIC2 was significantly upregulated
in AML tissues compared with normal samples (P<0.05; Fig. 4A). Consistently, ZIC2 was
additionally upregulated in AML cell lines compared with the normal
cell line, HS-5 (Fig. 4B).
Furthermore, RT-qPCR analysis indicated that ZIC2 was negatively
correlated with miR-1271-5p in AML samples (Fig. 4C). These results suggested that
ZIC2 may be regulated by miR-1271-5p and serve an important role in
AML.
Restoration of ZIC2 reverses the
effects of miR-1271-5p overexpression on the proliferation and
apoptosis of AML cells
The functions of ZIC2 in AML remain unknown. The
present study investigated the functions of ZIC2 and whether
miR-1271-5p inhibits the progression of AML by regulating ZIC2; the
expression of ZIC2 was restored in AML193 and OCI-AML2 cells
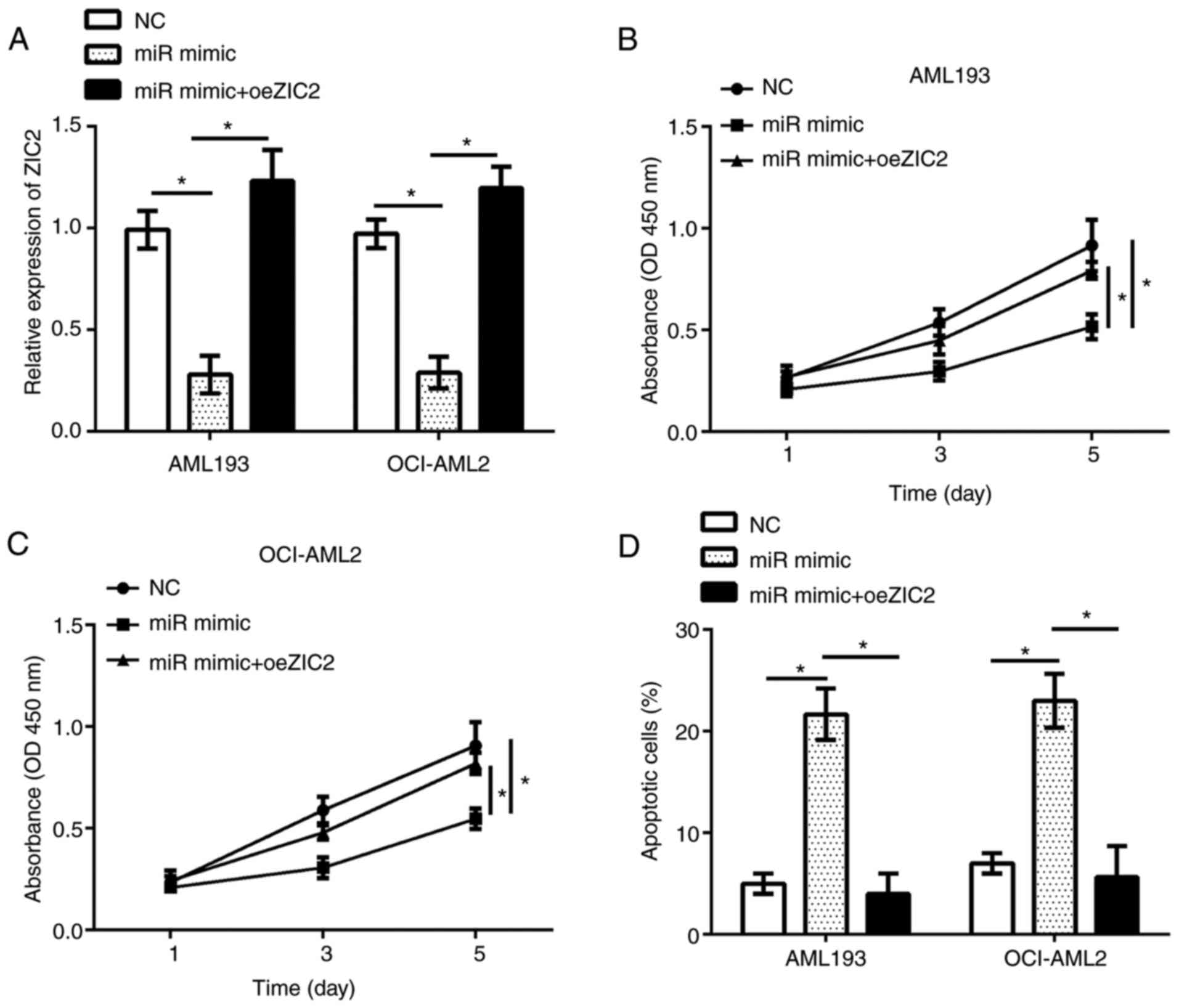
transfected with miR-1271-5p. RT-qPCR analysis demonstrated that
ZIC2 expression was significantly upregulated in AML193 and
OCI-AML2 cells following transfection with an overexpression
plasmid compared with the control (Fig. 5A). The results of CCK-8 and flow
cytometry analyses indicated that, compared with the control,
restoration of ZIC2 significantly rescued the proliferation and
reduced apoptosis in AML193 and OCI-AML2 cells transfected with
miR-1271-5p (Fig. 5B-D). In
summary, these data suggested that miR-1271-5p regulated the
progression of AML by targeting ZIC2.
Discussion
A recent study revealed that numerous miRNAs were
aberrantly expressed in AML (22).
For example, Tian et al (23) demonstrated that miR-494 was
downregulated, and suppresses bone marrow stromal cell-mediated
drug resistance in AML cells. Wang et al (12) demonstrated that miR-183 is
upregulated and promotes cell proliferation by regulating
programmed cell death 6 in pediatric AML. Yang et al
(24) identified that
downregulated miR-34c was associated with poor outcome in de
novo AML. Jiang et al (25) observed that miR-22 is downregulated
in AML and has a notable anti-tumor role with therapeutic
potential. A recent study identified that miR-1271-5p suppressed
the progression of colorectal cancer (18). Another previous study suggested
that miR-1271-5p inhibited cell growth by targeting FOXK2 in
hepatocellular carcinoma (19);
however, the functions of miR-1271-5p in AML remain unknown. In the
present study, the expression patterns of miR-1271-5p in AML
samples and cell lines were investigated. The data indicated that
miR-1271-5p was significantly downregulated in AML samples and cell
lines; miR-1271-5p overexpression suppressed AML cell proliferation
and induced apoptosis by directly targeting ZIC2.
ZIC2 is a member of the ZIC family of C2H2-type zinc
finger proteins, which function as transcription factors and may
regulate tissue specific gene expression (26). A recent study indicated that ZIC2
served oncogenic roles in human cancer, and regulated P21
protein-activated kinase 4 to promote the growth and metastasis of
hepatocellular carcinoma (27).
Marchini et al (28)
demonstrated that ZIC2 exhibited features of an oncogene and its
overexpression strongly correlated with the clinical course of
epithelial ovarian cancer. Sakuma et al (29) identified that ZIC2 served as a
prognostic marker in oral squamous cell carcinoma. Chan et
al (30) demonstrated that
ZIC2 synergistically promoted Hedgehog signaling via the nuclear
retention of zinc finger protein GLI1 in cervical cancer cells. In
addition, recent studies demonstrated that ZIC2 regulated the
progression of nasopharyngeal carcinoma (31), liver cancer (32) and bladder cancer (33); however the functions of ZIC2 in AML
remain unknown. In the present study, ZIC2 was identified as a
direct target of miR-1271-5p in AML cells. Furthermore, ZIC2 was
upregulated in AML samples and cell lines, which suggested that
ZIC2 may serve oncogenic roles in AML. The expression level of
miR-1271-5p was negatively correlated with that of ZIC2 in AML
samples; however, ZIC2 mRNA may also be targeted by other miRNAs.
The mechanisms underlying ZIC2-regulated proliferation and
apoptosis in AML cell remain unknown. Wang et al (33) demonstrated that ZIC2 correlated
with the phosphoinositide 3-kinase/protein kinase B signaling
pathway to regulate cell proliferation and apoptosis in bladder
cancer. Whether ZIC2 regulates AML cells in a similar manner
requires further investigation.
In the present study, the interaction between
miR-1271-5p and ZIC2 was identified. Overexpression of miR-1271-5p
was associated with the reduced luciferase activity in AML cells
transfected with WT-ZIC2 3′-UTR. In addition, the overexpression of
miR-1271-5p markedly reduced the mRNA and protein expression levels
of ZIC2 in AML193 and OCI-AML2 cells. Furthermore, an inverse
correlation between the expression levels of miR-1271-5p and ZIC2
in AML samples was demonstrated by RT-qPCR. Additionally,
restoration of ZIC2 reversed the effects of miR-1271-5p
overexpression on the proliferation and apoptosis of AML cells.
In summary, the present study demonstrated that
miR-1271-5p was downregulated in AML cells, which may regulate cell
proliferation and apoptosis by targeting ZIC2. The findings
additionally indicated ZIC2 as an oncogene in AML progression,
suggesting that the miR-1271-5p/ZIC2 axis may be a promising
therapeutic target for the treatment of AML.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XC and SY collected all the acute myeloid leukemia
samples. XC, SY, JZ and MC performed the experiments. MC designed
the present study and drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the present study was
approved by the Institutional Ethics Committee of The Third
Affiliated Hospital of Wenzhou Medical University. Written informed
consent was obtained from all patients for the use of their
clinical tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Estey EH: Acute myeloid leukemia: 2013
update on risk-stratification and management. Am J Hematol.
88:318–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dombret H and Gardin C: An update of
current treatments for adult acute myeloid leukemia. Blood.
127:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khalaj M, Tavakkoli M, Stranahan AW and
Park CY: Pathogenic microRNA's in myeloid malignancies. Front
Genet. 5:3612014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marcucci G, Haferlach T and Döhner H:
Molecular genetics of adult acute myeloid leukemia: Prognostic and
therapeutic implications. J Clin Oncol. 29:475–486. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baldus CD, Mrózek K, Marcucci G and
Bloomfield CD: Clinical outcome of de novo acute myeloid leukaemia
patients with normal cytogenetics is affected by molecular genetic
alterations: A concise review. Br J Haematol. 137:387–400. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bi L, Zhou B, Li H, He L, Wang C, Wang Z,
Zhu L, Chen M and Gao S: A novel miR-375-HOXB3-CDCA3/DNMT3B
regulatory circuitry contributes to leukemogenesis in acute myeloid
leukemia. BMC Cancer. 18:1822018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang TJ, Lin J, Zhou JD, Li XX, Zhang W,
Guo H, Xu ZJ, Yan Y, Ma JC and Qian J: High bone marrow miR-19b
level predicts poor prognosis and disease recurrence in de novo
acute myeloid leukemia. Gene. 640:79–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Zhang Q, Shi G and Yin J:
MiR-182-5p regulates BCL2L12 and BCL2 expression in acute myeloid
leukemia as a potential therapeutic target. Biomed Pharmacother.
97:1189–1194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zebisch A, Hatzl S, Pichler M, Wölfler A
and Sill H: Therapeutic resistance in acute myeloid leukemia: The
role of non-coding RNAs. Int J Mol Sci. 17(pii): E20802016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan N, Zhang J, Cheng C, Zhang X, Feng J
and Kong R: MicroRNA-384 represses the growth and invasion of
non-small-cell lung cancer by targeting astrocyte elevated
gene-1/Wnt signaling. Biomed Pharmacother. 95:1331–1337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Xue J, Kuang H, Zhou X, Liao L and
Yin F: microRNA-1297 inhibits the growth and metastasis of
colorectal cancer by suppressing cyclin D2 expression. DNA Cell
Biol. 36:991–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You Y, Tan J, Gong Y, Dai H, Chen H, Xu X,
Yang A, Zhang Y and Bie P: MicroRNA-216b-5p Functions as a
Tumor-suppressive RNA by targeting TPT1 in pancreatic cancer cells.
J Cancer. 8:2854–2865. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma QL, Wang JH, Yang M, Wang HP and Jin J:
MiR-362-5p as a novel prognostic predictor of cytogenetically
normal acute myeloid leukemia. J Transl Med. 16:682018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y, Zou Y, Lin L, Ma X and Chen H:
Identification of serum miR-34a as a potential biomarker in acute
myeloid leukemia. Cancer Biomark. 22:799–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding C, Chen SN, Macleod RAF, Drexler HG,
Nagel S, Wu DP, Sun AN and Dai HP: MiR-130a is aberrantly
overexpressed in adult acute myeloid leukemia with t(8;21) and its
suppression induces AML cell death. Ups J Med Sci. 123:19–27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Q, Li J, Chen S, Shen K, Ai G, Dai X,
Xie B, Shi Y, Jiang S, Feng J and Li W: Decreased miR-144
expression as a non-invasive biomarker for acute myeloid leukemia
patients. Pharmazie. 72:232–235. 2017.PubMed/NCBI
|
|
18
|
Wu Z, Hu Z, Han X, Li Z, Zhu Q, Wang Y,
Zheng Q and Yan J: The BET-Bromodomain Inhibitor JQ1 synergized
ABT-263 against colorectal cancer cells through suppressing
c-Myc-induced miR-1271-5p expression. Biomed Pharmacother.
95:1574–1579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin MF, Yang YF, Peng ZP, Zhang MF, Liang
JY, Chen W, Liu XH and Zheng YL: FOXK2, regulted by miR-1271-5p,
promotes cell growth and indicates unfavorable prognosis in
hepatocellular carcinoma. Int J Biochem Cell Biol. 88:155–161.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Li J, Hu J, Zheng J, Zheng Z, Liu
T, Lin Z and Lin M: Emodin enhances ATRA-induced differentiation
and induces apoptosis in acute myeloid leukemia cells. Int J Oncol.
45:2076–2084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao Q, Wang B, Li X and Jiang G: miRNAs
in acute myeloid leukemia. Oncotarget. 8:3666–3682. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian C, Zheng G, Zhuang H, Li X, Hu D, Zhu
L, Wang T, You MJ and Zhang Y: MicroRNA-494 activation suppresses
bone marrow stromal cell-mediated drug resistance in acute myeloid
leukemia cells. J Cell Physiol. 232:1387–1395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang DQ, Zhou JD, Wang YX, Deng ZQ, Yang
J, Yao DM, Qian Z, Yang L, Lin J and Qian J: Low miR-34c expression
is associated with poor outcome in de novo acute myeloid leukemia.
Int J Lab Hematol. 39:42–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang X, Hu C, Arnovitz S, Bugno J, Yu M,
Zuo Z, Chen P, Huang H, Ulrich B, Gurbuxani S, et al: miR-22 has a
potent anti-tumour role with therapeutic potential in acute myeloid
leukaemia. Nat Commun. 7:114522016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inaguma S, Ito H, Riku M, Ikeda H and
Kasai K: Addiction of pancreatic cancer cells to zinc-finger
transcription factor ZIC2. Oncotarget. 6:28257–28268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu SX, Zhang CZ, Luo RZ, Wang CH, Liu LL,
Fu J, Zhang L, Wang H, Xie D and Yun JP: Zic2 promotes tumor growth
and metastasis via PAK4 in hepatocellular carcinoma. Cancer Lett.
402:71–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marchini S, Poynor E, Barakat RR, Clivio
L, Cinquini M, Fruscio R, Porcu L, Bussani C, D'Incalci M, Erba E,
et al: The zinc finger gene ZIC2 has features of an oncogene and
its overexpression correlates strongly with the clinical course of
epithelial ovarian cancer. Clin Cancer Res. 18:4313–4324. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakuma K, Kasamatsu A, Yamatoji M, Yamano
Y, Fushimi K, Iyoda M, Ogoshi K, Shinozuka K, Ogawara K, Shiiba M,
et al: Expression status of Zic family member 2 as a prognostic
marker for oral squamous cell carcinoma. J Cancer Res Clin Oncol.
136:553–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan DW, Liu VW, Leung LY, Yao KM, Chan
KK, Cheung AN and Ngan HY: Zic2 synergistically enhances Hedgehog
signalling through nuclear retention of Gli1 in cervical cancer
cells. J Pathol. 225:525–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen ZH, Zhao KM and Du T: HOXA10 promotes
nasopharyngeal carcinoma cell proliferation and invasion via
inducing the expression of ZIC2. Eur Rev Med Pharmacol Sci.
21:945–952. 2017.PubMed/NCBI
|
|
32
|
Zhu P, Wang Y, He L, Huang G, Du Y, Zhang
G, Yan X, Xia P, Ye B, Wang S, et al: ZIC2-dependent OCT4
activation drives self-renewal of human liver cancer stem cells. J
Clin Invest. 125:3795–3808. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Ma W and Liu Y: Long non-coding
RNA HULC promotes bladder cancer cells proliferation but inhibits
apoptosis via regulation of ZIC2 and PI3K/AKT signaling pathway.
Cancer Biomark. 20:425–434. 2017. View Article : Google Scholar : PubMed/NCBI
|