Introduction
Diabetes mellitus is a metabolic disease caused by a
variety of factors and is characterized by elevated blood sugar. In
previous years, the incidence of diabetes has consistently
increased year upon year, with the number of patients globally
predicted to reach 300 million by 2030 (1,2). The
resulting fundamental harm is caused by a variety of issues
(3–5), with cardiovascular complications
being the most prevalent (6).
Compared with non-diabetic patients with heart failure, diabetic
patients with heart failure have demonstrated worse cardiac
function and higher mortality rates (7).
Diabetic cardiomyopathy exhibits a number of notable
structural features in the myocardium, with myocardial hypertrophy
being the most prominent and typified by an increased cell surface
area and the upregulation of atrial natriuretic peptide (ANP)
(2). Myocardial hypertrophy is not
only a chronic compensatory process but also the main mechanism
resulting in the worsening of diabetic cardiomyopathy and risk of
heart failure (7).
The pathogenesis of diabetic cardiomyopathy involves
multiple signaling cascades, including the c-myc, 5′ AMP-activated
protein kinase (AMPK) and mechanistic target of rapamycin (mTOR)
pathways (1,8–10).
Thus, diabetic cardiomyopathy cannot be controlled through the
regulation of a single pathway (11). Methylation and histone
modifications serve an important role in the process of cell damage
in the cardiovascular and central nervous systems (1,12,13).
Histone acetylation induces the opening of the chromatin to
activate the transcription of MYC and nuclear factor-κβ,
which are crucial in the process of cardiac hypertrophy (1,12–17).
Based on these theoretical foundations, the present study aimed to
investigate the potential underlying mechanisms of epigenetics in
the development of diabetic cardiomyopathy.
Bromodomain-containing protein 4 (BRD4) is a protein
that recognizes and binds acetylated lysine, and promotes
transcription by binding to the transcription initiation regions of
relevant genes (18). At the same
time, it has been reported that the high expression of BRD4, as the
upstream gene of c-myc, is involved in cardiac hypertrophy
(1,18,19).
JQ1, a specific BRD4 inhibitor, has a notable effect in tumor
therapy and transverse aortic constriction (1,20–22).
The aim of the present study was to investigate the effect of this
inhibitor on the hypertrophy of cardiomyocytes during high glucose
(HG)-induced cardiac hypertrophy, to clarify whether epigenetic
regulation involving BRD4 is the main mechanism of cardiac
hypertrophy (1,23–25).
Materials and methods
Cell culture, treatments and
reagents
H9C2, an embryonic rat heart-derived cell line
(Shanghai Bioleaf Biotech Co., Ltd., Shanghai, China) was cultured
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 5.5 mmol/l D-glucose
and supplemented with 10% fetal bovine serum (Clark Bioscience,
Richmond, VA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin.
In the HG-treated group (HG), cells were incubated in DMEM
containing 20 and 30 mmol/l glucose. Control cells were incubated
in DMEM containing 5.5 mmol/l D-glucose. JQ1 was purchased from
Selleck Chemicals (Houston, TX, USA). Following the pretreatment of
cardiomyocytes with 5.5 or 30 mM glucose in a 37°C incubator
supplied with 5% CO2 for 48 h, H9C2 cells were treated
with 250 nM JQ1 or DMSO and incubated for 48 h under the same
conditions. Experimental groups consisted of: i) Normal glucose
(NG), ii) HG (30 mM glucose) and iii) HG (30 mM glucose) + JQ1 (250
nM). After 48 h of treatment, the cells were photographed under a
light microscope (Olympus Corporation, Tokyo, Japan), and the image
analysis software Image J version 1.6.0 (National Institutes of
Health, Bethesda, MD, USA) was used for the objective analysis of
the cell area.
Animal studies
Wistar rats (n=16, male, 160–180 g) were obtained
from the College of Pharmacy of Jilin University (Changchun,
China). Animal experiments were ethically approved by the Animal
Ethics Committee of Jilin University and were in accordance with
the regulations of the Institutional Committee for the Care and Use
of Laboratory Animals of the Experimental Animal Center of Jilin
University. Rats were housed under a 12-h light/dark cycle in an
air-conditioned room at 22±2°C and 45–55% humidity with free access
to food and water. Rats were randomly divided into a normal group
(n=8) and a diabetes model group (DM; n=8) following adaptive
feeding with standard experimental rat chow for one week.
Subsequently, the DM group was intraperitoneally injected with
streptozotocin (STZ; 30 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) dissolved in citric acid buffer, while the
control group was intraperitoneally injected with the same volume
of buffer. After 1 week, blood was collected from the tail vein to
test blood glucose levels using a blood glucose meter (BeneCheck,
Taiwan, China) and the body weight of the rats was recorded every
week. After 8 weeks, the rats were euthanized by excessive
intraperitoneal injection of barbiturates (Pentobarbital; 40 mg/kg;
Spofadental A.S, Prague) and excised heart tissue was prepared for
analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from H9C2 cells and rat
myocardial tissues using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA samples were converted to cDNA using a
Reverse Transcription kit (Vazyme, Piscataway, NJ, USA) according
to the manufacturer's protocol. RT-qPCR was performed on an EDC-800
Instrument (Dong Sheng Biotech Co., Ltd., Gunagzhou, China).
Briefly, the PCR reaction mix (final volume, 25 µl) consisted of 2
µl cDNA, 2 µl primers, 12.5 µl 2X Power Tap PCR Master Mix and 6.5
µl ddH2O. The PCR thermocycling conditions and primer
sequences are presented in Tables
I and II, respectively. PCR
was performed on PCR amplifiers (Takara Bio, Inc., Otsu, Japan) and
the relative quantities of each mRNA were calculated using agarose
gel electrophoresis and a Gel Imaging System (Tanon Science and
Technology Co., Ltd., Shanghai, China), and relative gene
expression data were analyzed using qPCR and the 2−ΔΔCq
method with GAPDH used as an internal control (26).
 | Table I.Polymerase chain reaction
thermocycling conditions. |
Table I.
Polymerase chain reaction
thermocycling conditions.
| Gene | Denaturation | Annealing | Extension | Cycle no. |
|---|
| GAPDH | 94°C for 30
sec | 55°C for 45
sec | 72°C for 30
sec | 32 |
| Atrial natriuretic
peptide | 94°C for 30
sec | 60°C for 30
sec | 72°C for 30
sec | 35 |
|
Bromodomain-containing protein 4 | 94°C for 30
sec | 60°C for 30
sec | 72°C for 45
sec | 40 |
| c-myc | 94°C for 30
sec | 55°C for 45
sec | 72°C for 30
sec | 32 |
| Transforming growth
factor-β | 94°C for 30
sec | 55°C for 45
sec | 72°C for 30
sec | 32 |
| Collagen, type 1,
α1 | 94°C for 30
sec | 55°C for 45
sec | 72°C for 30
sec | 32 |
| Connective tissue
growth factor | 94°C for 30
sec | 55°C for 45
sec | 72°C for 30
sec | 32 |
| SMAD family member
3 | 94°C for 30
sec | 58°C for 45
sec | 72°C for 30
sec | 32 |
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Forward primer
sequence | Reverse primer
sequence |
|---|
| GAPDH |
5′-AGAAGGCTGGGGCTCATTTG-3′ |
5′-AGGGGCCATCCACAGTCTTC-3′ |
| Atrial natriuretic
peptide |
5′-GCCGGTAGAAGATGAGGTCA-3′ |
5′-GGGCTCCAATCCTGTCAATC-3′ |
|
Bromodomain-containing protein 4 |
5′-ACAGCCCCAACAGAACAAAC-3′ |
5′-GCTGGTTCCTTCTTGCTCAC-3′ |
| c-myc |
5′-AAGGGAAGACGATGACGG-3′ |
3′-TGAGCGGGTAGGGAAAGA-5′ |
| Transforming growth
factor-β |
5′-GCAACAACGCAATCTATGAC-3′ |
3′-CCTGTATTCCGTCTCCTT-5′ |
| Collagen, type 1,
α1 |
5′-GTACATCAGCCCAAACCCCAAG-3′ |
3′-CGGAACCTTCGCTTCCATACTC-5′ |
| Connective tissue
growth factor |
5′-ACTATGATGCGAGCCAACTGC-3′ |
3′-TGTCCGGATGCACTTTTTGC-5′ |
| SMAD family member
3 |
5′-CACGCAGAACGTGAACACC-3′ |
3′-GGCAGTAGATAACGTGAGGGA-5′ |
Western blot analysis
Following treatment, cells and tissues, which were
frozen in liquid nitrogen, were harvested and lysed using RIPA
buffer (Boston BioProducts, Inc., Worcester, MA, USA) for 30 min at
4–8°C. Proteins were detected and quantified using a Bio-Rad
Protein assay and microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Protein samples (30–60 µg) were separated using
12% SDS-PAGE and transferred to polyvinylidene fluoride membranes.
Nonspecific binding was blocked using 5% low-fat milk in
Tris-buffered saline with Tween-20 (10 mmol/l Tris-HCl, pH 7.5, 200
mmol/l NaCl and 0.05% Tween-20) at room temperature (RT) for 2 h.
Subsequently, the membranes were incubated overnight at 4°C with
the following primary antibodies: Rabbit monoclonal anti-ANP
(1:500; cat no. C0917; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), rabbit monoclonal anti-BRD4 (1:1,000; cat no. ab128874;
Abcam, Cambridge, UK), rabbit polyclonal anti-c-myc (1:1,000; cat
no. 10828-I-AP; ProteinTech Group, Inc., Chicago, IL, USA), rabbit
monoclonal anti-protein kinase B (AKT; 1:1,000; cat no. 60203-I-Ig;
ProteinTech Group, Inc.), rabbit polyclonal anti-phosphorylated-AKT
(1:1,000; cat no. ARG51559; Arigo biolaboratories Corp., Taiwan)
and mouse monoclonal anti-β-actin (1:5,000; cat no. 66009-I-Ig;
ProteinTech Group, Inc.). Then the membranes were incubated for 1 h
at RT with secondary antibodies including goat anti-rabbit
(1:1,000; cat no. 10828-I-AP; ProteinTech Group, Inc., Chicago, IL,
USA) and goat anti-mouse (1:1,000; cat no. 10828-I-AP; ProteinTech
Group, Inc.). Electrochemiluminesence color liquid (1:1; Thermo
Fisher Scientific, Inc.) was used on a gel image processing system
with (Tanon Science and Technology Co., Ltd.) to scan images for
statistical analysis using SPSS version 17.0 software (SPSS, Inc.,
Chicago, IL, USA).
Immunocytochemistry
The hearts of the rats (euthanized as mentioned
above) were excised prior to washing with cold phosphate buffered
saline (PBS) using filter paper suction to remove residual blood,
fat and other non-myocardial tissues. They were weighed and then
immediately fixed in 4% paraformaldehyde solution for at least 4 h
at RT, followed by dehydration, dipping in wax and paraffin
embedding for the preparation of paraffin sections. Excess sections
were prepared for subsequent Masson's trichrome staining and
haemotoxylin and eosin (H&E) staining. The sections (0.2 mm)
were incubated with 2% bovine serum albumin (BSA) for 30 min at RT
to block nonspecific binding, and then incubated with rabbit
polyclonal anti-c-myc (1:400; cat no. 10828-I-AP; ProteinTech
Group, Inc.) for 2 h at RT, followed by incubation with goat
anti-rat immunoglobulin G (IgG; 1:400; cat no. SA0000I-I;
ProteinTech Group, Inc.) for c-myc visualization for 1 h at RT.
Between each incubation step, sections were washed three times with
Tris-buffered saline withTween-20 (12.5 mM Tris/HCl, pH 7.6, 137 mM
NaCl and 0.1% Tween-20).
Determination of collagen contents by
Masson's trichrome staining
Excised hearts were prepared as described above and
the sections cut from the resultant paraffin blocks were stained
using Masson's trichrome stain (0.01 g/ml) for 3–5 min at RT. The
sections were observed under an inverted fluorescence microscope
(IX71; Olympus Corporation, Tokyo, Japan).
H&E staining
For H&E staining, H9C2 cells were grown on glass
coverslips in the presence of HG with or without JQ1, and then
fixed in 4% paraformaldehyde for 30 min at RT. Then, these glass
coverslips, in addition to those prepared from the aforementioned
heart tissue paraffin blocks, were treated with a H&E stain
(0.002 g/ml) for 3–5 min at RT. The sections were observed under an
inverted fluorescence microscope (IX71; Olympus Corporation).
Immunofluorescence
Subsequent to counting, cells were grown on glass
coverslips in the presence of HG with or without JQ1 for 48 h, and
then fixed in 4% paraformaldehyde for 30 min at RT. Slides were
then washed three times for 3 min each time with PBS. Then, cells
were perforated with 0.5% Triton X-100 (in PBS) for 20 min at RT.
Subsequent to washing with PBS three times, serum or BSA was added
to the slides for 30 min at RT, and then each slide was dipped in
diluted primary anti-α-actin (1:500; cat no. 23660-I-AP;
ProteinTech Group, Inc.) and placed in a wet box at 4°C overnight.
The next day, following washing with PBS three times, a diluted
green Alexa Fluor 488-conjugated donkey anti-rabbit IgG secondary
antibody (1:200; cat no. AS035; ABclonal Biotech Co., Ltd., Woburn,
MA, USA) was added to the slides in the wet box and incubated at RT
for 1 h. Following washing, Hoechst 33258 at a concentration of 0.5
µg/ml (cat no. 1225A038; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) was added and slides were incubated at
RT in the dark for 5 min to stain the nuclei. Following another
wash with PBS, the samples were sealed with glycerol and observed
under a fluorescence microscope (Olympus Corporation).
Statistical analysis
Results were expressed as the mean ± standard error
of the mean of three independent experiments. The statistical
significance of differences between the mean values of groups were
determined using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA),
and Student's t-tests were used to compare the differences between
two groups. To compare >2 sets, one-way analysis of variance
analysis with a Newman-Keuls multiple comparison post-hoc test was
conducted. P<0.05 was considered to indicate a statistically
significant difference.
Results
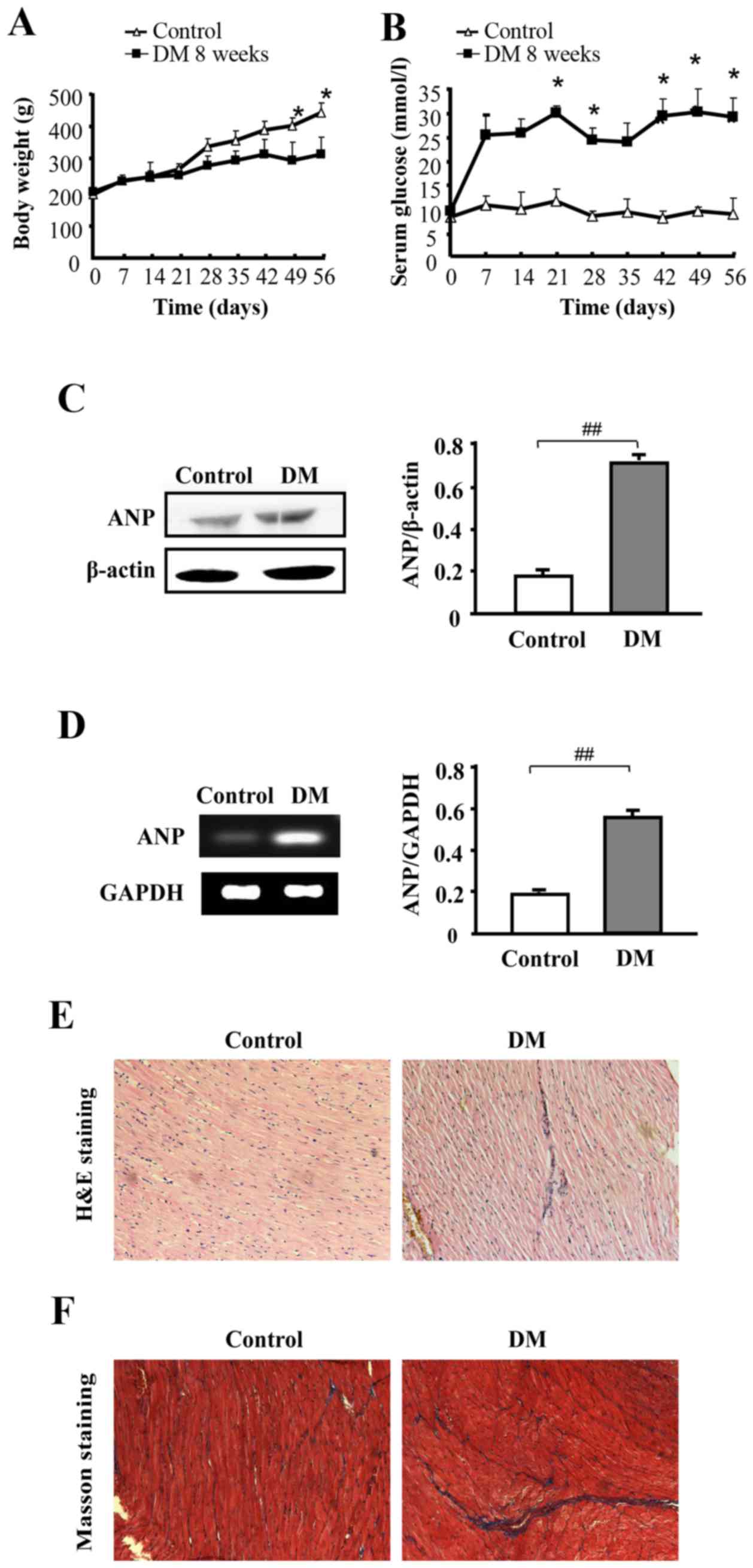
Diabetic rats present with substantial
cardiac hypertrophy and fibrosis
A diabetic model was established by injecting STZ
into rats for eight weeks, and then the blood glucose levels of
STZ-induced diabetic rats were assessed to determine whether the
diabetic model was created successfully. The model group
demonstrated significantly elevated blood glucose levels compared
with the control group, in addition to a relatively reduced body
weight (P<0.05; Fig. 1A and B).
Furthermore, the heart tissue of the diabetic rats demonstrated
significantly increased protein and mRNA expression levels of ANP
compared with the control (P<0.01; Fig. 1C and D, respectively). Next, the
morphology of the heart tissue from the DM group was examined using
H&E staining. Relative to the control group, hearts from the DM
group displayed a different degree of structural abnormalities,
including broken fibers, disturbed cellular structures, foci with
necrotic myocytes and increased cardiomyocyte transverse
cross-sectional areas (Fig. 1E).
Myocardial fibrosis was observed using Masson's staining (Fig. 1F) and fibrosis in the DM group was
enhanced compared with control group.
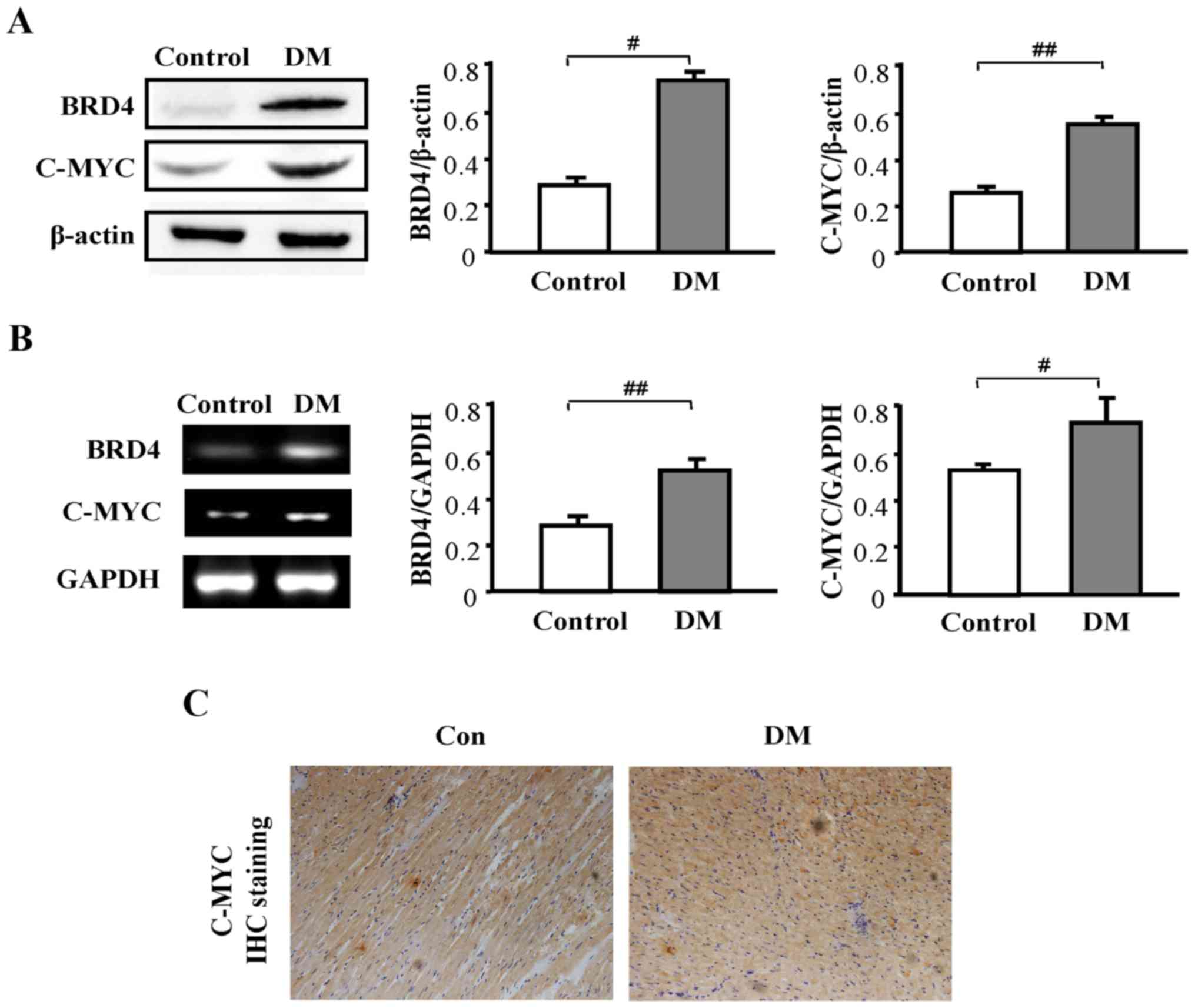
Expression of BRD4 and c-myc is higher
in STZ-induced diabetic rats
Next, the expression levels of BRD4 and c-myc were
examined. The results revealed a significantly higher protein
expression level of BRD4 (P<0.05) and c-myc (P<0.01) in the
DM group compared with the control group (Fig. 2A). Additionally, a significantly
higher expression level of BRD4 (P<0.01) and c-myc (P<0.05)
in the DM group compared with the control group was observed at the
mRNA level (Fig. 2B). Next,
immunohistochemistry was used to examine the expression of c-myc in
heart tissue. c-myc expression in the DM group was notably higher
compared with that in the control group (Fig. 2C).
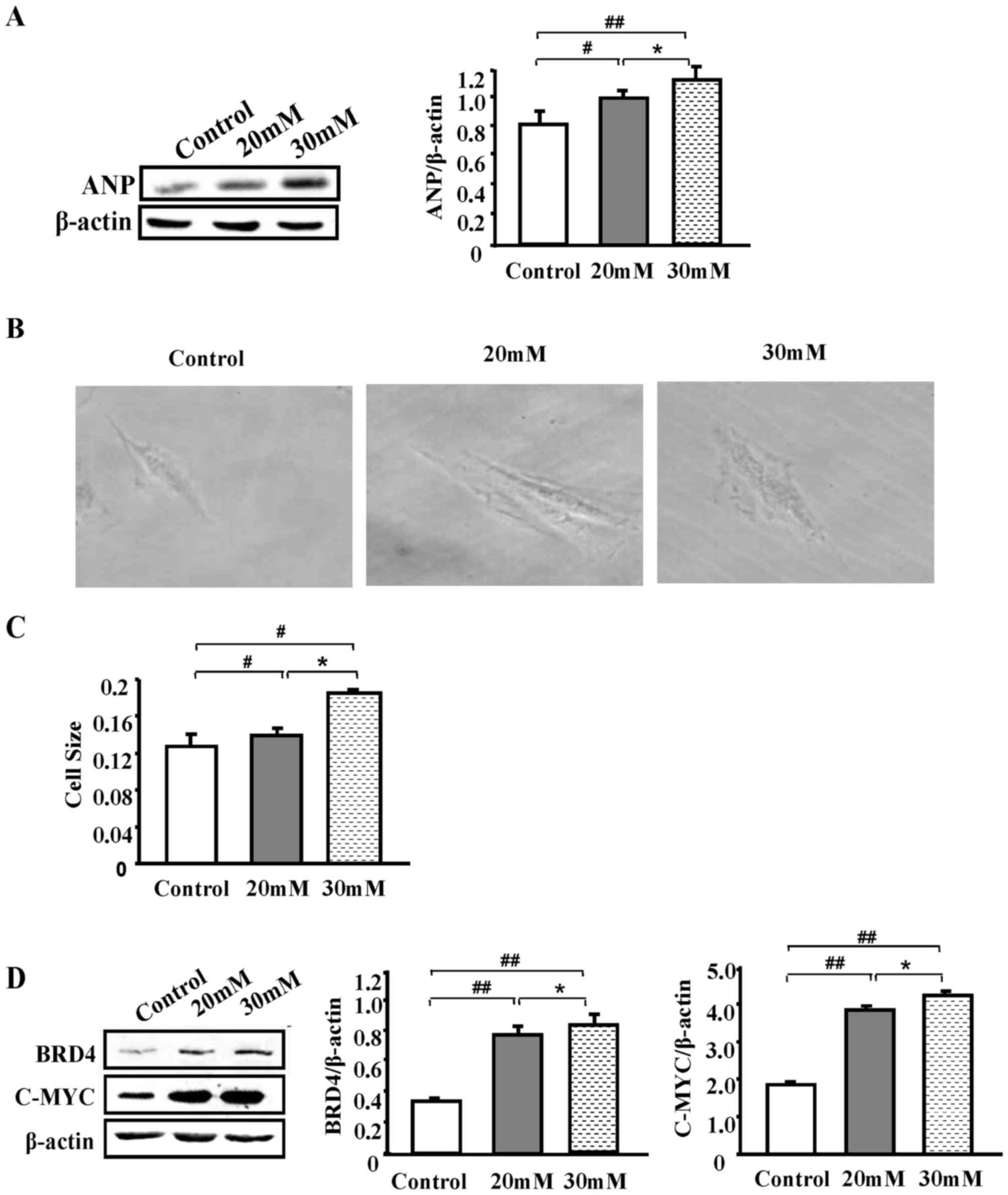
High glucose induces H9C2 cell
hypertrophy and BRD4 upregulation
Given that the DM animals displayed changes in the
expression of BRD4 and c-myc, in addition to changes in the
structure of the heart tissue, the present study then attempted to
elucidate whether the same phenomenon occurs in vitro. The
H9C2 cell line was used for this purpose, which is a rat
cardiomyocyte line. Compared with normal H9C2 cells, H9C2 cells
exposed to different glucose concentrations (20 and 30 mmol/l)
demonstrated significantly increased ANP protein expression levels
in a dose-dependent manner (P<0.05; Fig. 3A). Furthermore, morphological
changes in H9C2 cells were induced by high glucose concentrations,
as observed by light microscopy (Fig.
3B). Differences in cell size following data analysis with
Image J were most pronounced when the glucose concentration was 30
mmol/l, and were significantly larger compared with the control
group (P<0.01; Fig. 3C).
Subsequently, western blot analysis was used to examine the
expression of BRD4 in the control group and HG group. The results
revealed that BRD4 and c-myc expression significantly increased
with glucose concentration (P<0.05), and the most significant
increase was observed with 30 mmol/l glucose compared with the
control group (P<0.01; Fig.
3D). These results indicate that BRD4 may participate in
cardiomyocyte hypertrophy.
JQ1 significantly inhibits HG-induced
cardiac hypertrophy, BRD4 and c-myc expression levels and cardiac
fibrosis
A total of 30 mM HG was selected as a suitable
concentration for the stimulation of H9C2 cells, and administered
JQ1 to observe its effect on the control and HG groups. The results
revealed that, compared with the HG group, the expression levels of
ANP, BRD4 and c-myc were significantly decreased following JQ1
treatment (250 nM) for 48 h (P<0.01; Fig. 4A). RT-qPCR analysis demonstrated a
similar significant reduction in mRNA expression levels (P<0.01;
Fig. 4B). Representative H&E
staining and immunofluorescence images of samples stained with
α-actin antibody from the HG group in the absence or presence of
JQ1 are presented in Fig. 4C.
These results indicated that BRD4 served a function in the process
of glucose-induced hypertrophy. As cardiac fibrosis was observed in
a large number of diabetic rats, whether HG-induced cardiomyocytes
also exhibit this phenomenon was further examined using RT-qPCR.
The analysis revealed a significant increase in the expression of
the pro-fibrotic genes transforming growth factor-β (TGF-β),
connective tissue growth factor, collagen, type 1, α1 and the
critical TGF-β mediating factor, SMAD family member 3, in the HG
group compared with the control group (P<0.05; Fig. 4D). However, treatment with JQ1
significantly attenuated this HG-induced expression (P<0.05). In
general, JQ1 displayed a strong effect on fibrosis induced by high
glucose.
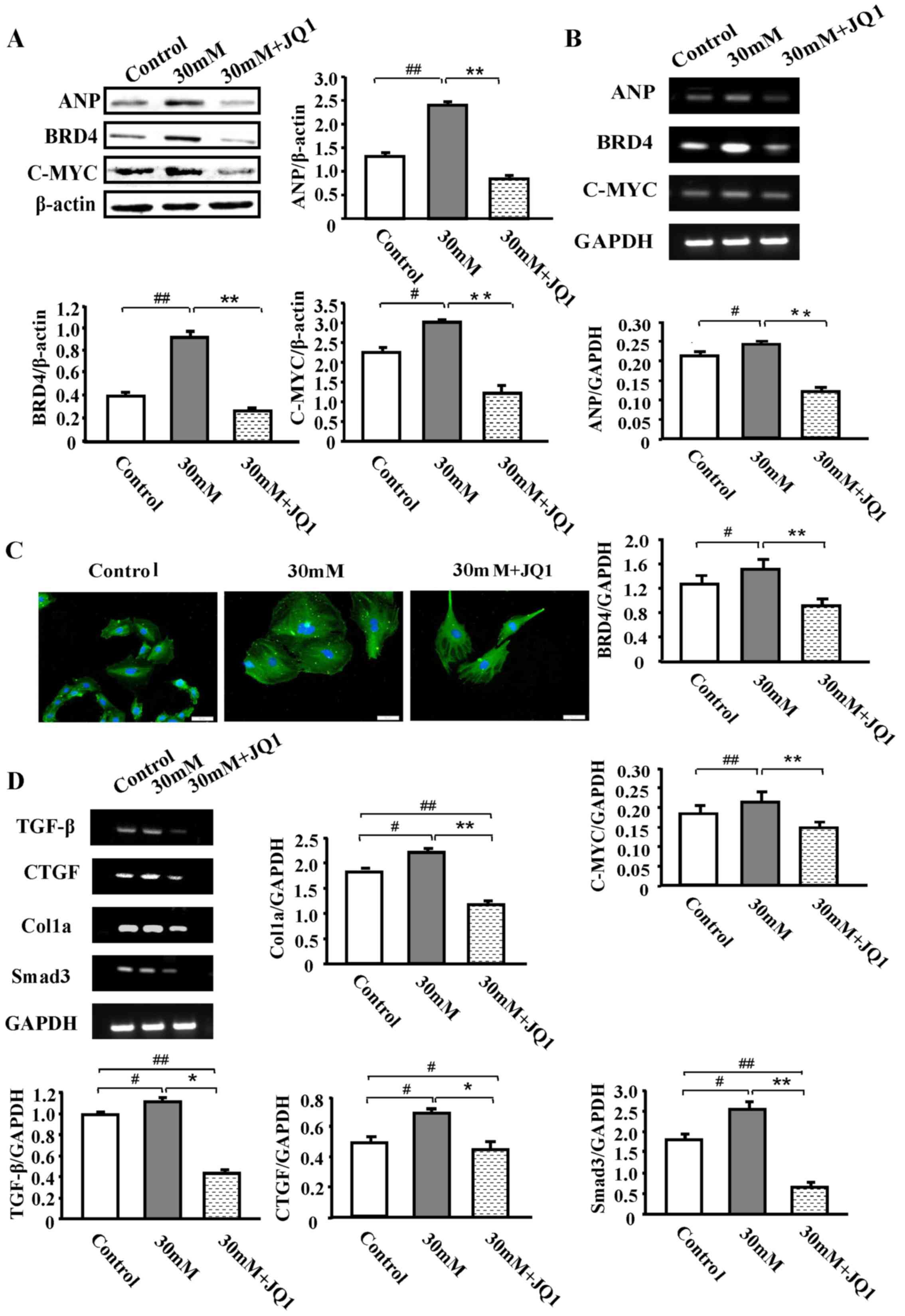 | Figure 4.JQ1 significantly inhibits HG-induced
cardiac hypertrophy, BRD4 and c-myc expression and cardiac
fibrosis. H9C2 cells were treated with 250 nM JQ1 for 48 h after
pretreatment with or without HG (30 mmol/l) for 48 h. (A) Western
blot analysis of ANP, BRD4 and c-myc protein expression in normal
glucose, HG (30 mM glucose) and HG (30 mM glucose) + JQ1 (250 nM)
groups for 48 h. (B) RT-qPCR analysis of ANP, BRD4 and c-myc mRNA
expression. (C) Images of α-actin staining of untreated H9C2 cells
and HG-induced H9C2 cells with or without 250 nM JQ1 treatment for
48 h. (D) mRNA expression levels of fibrosis-associated genes
including TGF-β, SMAD3, CTGF and COL1A1 were examined using
RT-qPCR. Scale bar, 50 µm. *P<0.05, **P<0.01,
#P<0.05 and ##P<0.01 with comparisons
shown by lines. BRD4, bromodomain-containing protein 4; ANP, atrial
natriuretic peptide; HG, high glucose; TGF-β, tumor growth factor
β; SMAD3, SMAD family member 3; CTGF, connective tissue growth
factor; COL1A1, collagen, type 1, α1; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
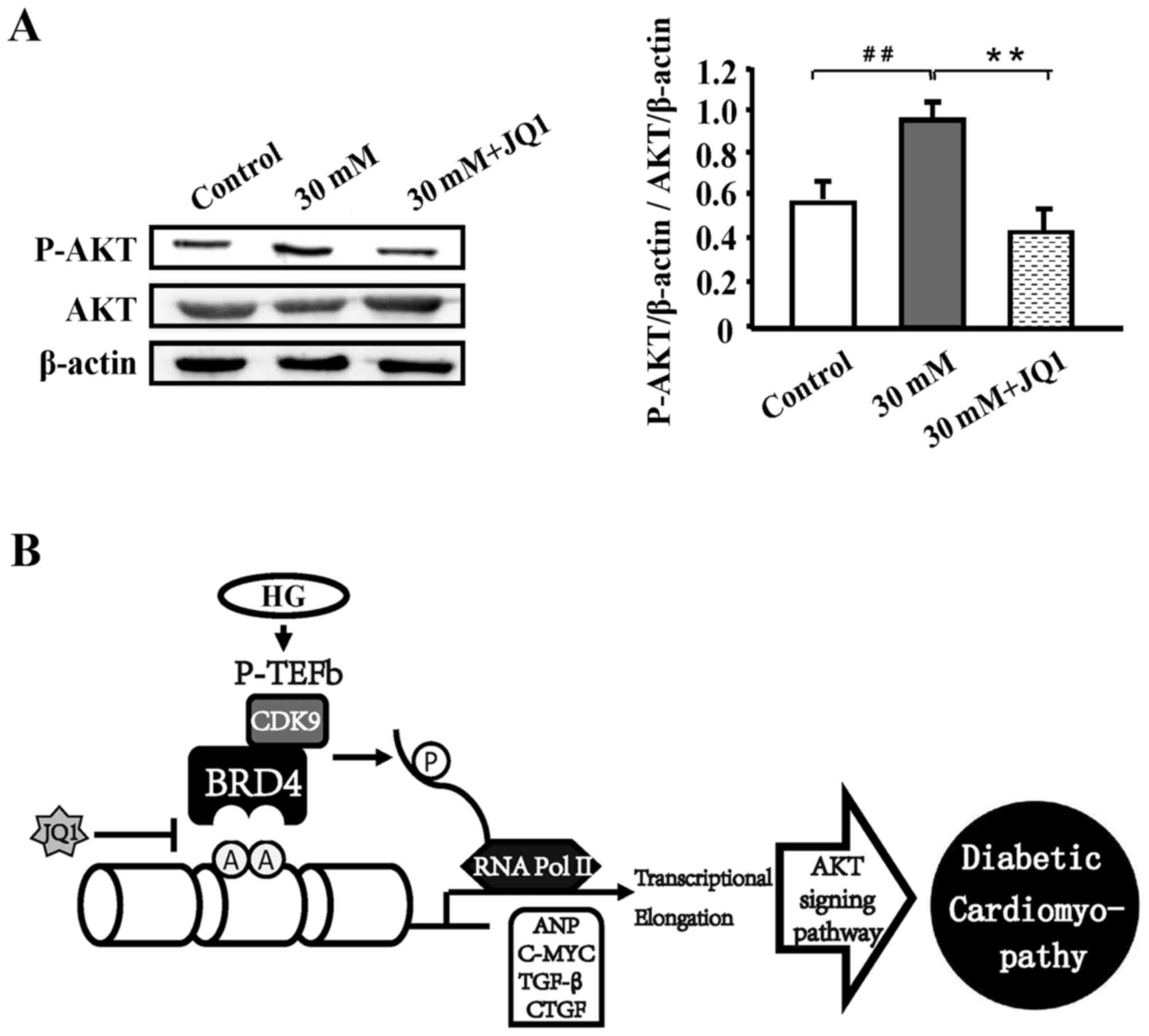
HG-induced diabetic cardiomyopathy is
mediated through the AKT signaling pathway
To evaluate the underlying mechanism of HG-induced
diabetic cardiomyopathy, western blot analysis was performed. As
presented in Fig. 5, the AKT
pathway was significantly activated under HG stimulation compared
with the control (P<0.01); however, JQ1 significantly suppressed
this activation (P<0.01).
Discussion
A characteristic of diabetes is high blood sugar,
which occurs due to insulin secretion defects, damage from
biological effects, or the two combined. Long-term hyperglycemia
results in various organ dysfunctions, including that of the eyes,
kidneys, heart and blood vessels, in addition to chronic nerve
damage, and is associated with the development of heart failure;
therefore, diabetes is a major public health problem (7). Diabetic cardiomyopathy has been
recognized as a major complication with a characteristic impairment
in diastolic function, accompanied by the development of
cardiomyocyte hypertrophy, myocardial fibrosis and apoptosis
(6,8,10,27,28).
Thus, there is an urgent need for novel therapeutic approaches.
However, present epidemiological studies of diabetes and treatment
management are remain unsatisfactory. Hence, the present research
is devoted to the study of diabetic cardiomyopathy.
Emerging data has demonstrated that epigenetic
molecules, particularly histone deacetylase, may provide an
important mechanism for controlling signaling and gene expression
in the heart and kidney. Furthermore, numerous studies have
demonstrated that histone deacetylase inhibitors affect
pathophysiological processes including myocyte hypertrophy,
fibrosis, inflammation and epithelial-to-mesenchymal transition
(1,12-15,17,29). Acetylated histones under the control of histone
acetyltransferases are recognized by bromodomain and extraterminal
domain (BET) proteins, enabling the coordinated regulation of genes
involved in cell proliferation, apoptosis and inflammation
(1,21,27,30).
BRD4, a histone acetylated reader protein, binds to acetylated
lysine residues on histone and non-histone proteins to recruit
transcriptional regulators, resulting in the activation or
repression of gene transcription. It has also been revealed to be
involved in various cancer types (20–22,31).
Furthermore, a number of studies support the idea that BET family
proteins are strongly associated with agonist-dependent cardiac
hypertrophy in a mouse model of pressure overload-induced left
ventricular hypertrophy (1,19).
These results suggest a role for acetyl lysine-binding proteins in
the control of pathological cardiac remodeling and lay a strong
theoretical basis for the hypothesis for the present study.
Understanding the epigenetic mechanisms of glucose-induced
myocardial hypertrophy may provide novel therapeutic strategies for
reducing heart damage.
Therefore, the aim of the present research was to
elucidate the function of BRD4 in diabetes mellitus. First, it was
revealed that BRD4 and c-myc expression in the DM group was
significantly higher compared with that in the control group
(Fig. 2A-C; P<0.05). This
suggests that BRD4 is associated with diabetes and confirms the
theory that this BET protein is closely associated with cardiac
hypertrophy. Next, BRD4 expression was examined in vitro.
H9C2 cells exposed to different glucose concentrations (20 and 30
mM) demonstrated high expression levels of ANP, BRD4 and c-myc,
similar to the results in the STZ-induced diabetic rats (Fig. 3A and B). BRD4 interacts with
positive transcription elongation factor (P-TEFb) in the nucleus
through its bromodomain. However, its overexpression results in
increased P-TEFb-dependent phosphorylation of the RNA polymerase II
(Pol II) C-terminal domain and stimulates transcription in
vivo (1,30,32).
Thus, it was speculated that BRD4, under hypertrophic stimuli,
promotes its own recruitment to the transcriptional start site of
the gene encoding ANP. The results of the present study validated
the idea that 30 mM glucose is an effective inducer of myocardial
hypertrophy.
JQ1 is a specific BRD4 inhibitor that binds the
bromodomain of BET proteins with high shape complementarity and
nanomolar affinity, resulting in the potent, competitive and
transient displacement of BRD4 from acetylated chromatin, which
causes the suppression of signaling events downstream of Pol II
(1,19,21).
The present study administered JQ1 to the control and HG groups to
examine its effect and to analyze the mechanism by which BRD4
functions. JQ1 significantly inhibited HG-induced cardiac
hypertrophy and cardiac fibrosis compared with the HG-alone groups
(Fig. 4A-D; P<0.05), in
addition to the expression of BRD4 and c-myc.
It has been reported that a single pathway cannot
achieve complete control of diabetic cardiomyopathy as it is
associated with multiple signaling pathways, including the c-myc,
AMPK and mTOR pathways (1,8–11).
In the present study, it was demonstrated that a significant
decrease in AKT phosphorylation occurred following the
administration of a high concentration of glucose (P<0.01),
which implies that HG-induced cardiac hypertrophy may be activated
through the AKT pathway. Further research of the specific
mechanisms and in vivo experiments are yet to be performed,
but the present study provides a theoretical basis for further
clinical research, making JQ1 a potential novel drug for diabetic
cardiomyopathy therapy.
The data presented here demonstrated that HG-induced
hypertrophy and cardiac fibrosis were associated with BRD4
upregulation. Under hypertrophic stimulus, BRD4 promoted its own
recruitment to the transcriptional start site of ANP. However, JQ1
blocked this process (Fig. 4).
Additionally, it was discovered that the AKT pathway was activated
subsequent to HG stimulation, suggesting that HG-induced cardiac
hypertrophy occurs through the AKT pathway, but the effects and
molecular mechanism of BRD4 upregulation in cardiac hypertrophy
have not been fully elucidated. In conclusion, these data revealed
that BRD4 serves a critical role in mediating HG-induced myocardial
hypertrophy and cardiac fibrosis, and provides a theoretical basis
for the further clinical research of diabetic cardiomyopathy.
Acknowledgements
The authors would like to thank Professor Yan Meng
and other teachers from Department of Pathophysiology, Prostate
Diseases Prevention and Treatment Research Center, College of Basic
Medical Science, Jilin University. The authors also thank H. Nikki
March, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text
of a draft of this manuscript.
Funding
This study was supported by the Research Fund for
the National Natural Science Foundation of China (grant nos.
81370240 and 30900518), the Science and Technology Natural Science
Foundation of Jilin Provincial (grant no. 20160101239JC) and the
Health Technology Innovation Project of Jilin Province (grant no.
2017J059).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW, YS and TL conceived and designed the project.
QW, YM and LZ participated in the idea of the manuscript. QW, LiaL,
YZ and LiyL conducted the cell experiments. QW and YM analysed the
data. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Jilin University (approval no. 2016-63).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spiltoir JI, Stratton MS, Cavasin MA,
Demos-Davies K, Reid BG, Qi J, Bradner JE and McKinsey TA: BET
acetyl-lysine binding proteins control pathological cardiac
hypertrophy. J Mol Cell Cardiol. 63:175–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roger VL, Go AS, Lloyd-Jones DM, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al:
Heart disease and stroke statistics-2012 update: A report from the
American Heart Association. Circulation. 125:e2–e220.
2012.PubMed/NCBI
|
|
3
|
Gao Q, Guan L, Hu S, Yao Y, Ren X, Zhang
Z, Cheng C, Liu Y, Zhang C, Huang J, et al: Study on the mechanism
of HIF1a-SOX9 in glucose-induced cardiomyocyte hypertrophy. Biomed
Pharmacother. 74:57–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manyari DE: Prognostic implications of
echocardiographically determined left ventricular mass in the
Framingham Heart Study. N Engl J Med. 323:1706–1707. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rababa'h A, Singh S, Suryavanshi SV,
Altarabsheh SE, Deo SV and McConnell BK: Compartmentalization role
of A-kinase anchoring proteins (AKAPs) in mediating protein kinase
A (PKA) signaling and cardiomyocyte hypertrophy. Int J Mol Sci.
16:218–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang D, Zhong P, Hu J, Lin F, Qian Y, Xu
Z, Wang J, Zeng C, Li X and Liang G: EGFR inhibition protects
cardiac damage and remodeling through attenuating oxidative stress
in STZ-induced diabetic mouse model. J Mol Cell Cardiol. 82:63–74.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukushima A and Lopaschuk GD: Cardiac
fatty acid oxidation in heart failure associated with obesity and
diabetes. Biochim Biophys Acta. 1861:1525–1534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Zhang J, Zhang L, Gao P and Wu X:
Adiponectin attenuates high glucose-induced apoptosis through the
AMPK/p38 MAPK signaling pathway in NRK-52E cells. PLoS One.
12:e01782152017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CY, Hsu YJ, Hsu SC, Chen Y, Lee HS,
Lin SH, Huang SM, Tsai CS and Shih CC: CB1 cannabinoid receptor
antagonist attenuates left ventricular hypertrophy and Akt-mediated
cardiac fibrosis in experimental uremia. J Mol Cell Cardiol.
85:249–261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Langer S, Kreutz R and Eisenreich A:
Metformin modulates apoptosis and cell signaling of human podocytes
under high glucose conditions. J Nephrol. 29:765–773. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SY, Morales CR, Gillette TG and Hill
JA: Epigenetic regulation in heart failure. Curr Opin Cardiol.
31:255–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dekker J and Mirny L: The 3D genome as
moderator of chromosomal communication. Cell. 164:1110–1121. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heinz S, Romanoski CE, Benner C and Glass
CK: The selection and function of cell type-specific enhancers. Nat
Rev Mol Cell Biol. 16:144–154. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
May D, Blow MJ, Kaplan T, McCulley DJ,
Jensen BC, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright
C, et al: Large-scale discovery of enhancers from human heart
tissue. Nat Genet. 44:89–93. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Phillips JE and Corces VG: CTCF: Master
weaver of the genome. Cell. 137:1194–1211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitt AD, Hu M, Jung I, Xu Z, Qiu Y, Tan
CL, Li Y, Lin S, Lin Y, Barr CL and Ren B: A compendium of
chromatin contact maps reveals spatially active regions in the
human genome. Cell Rep. 17:2042–2059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wamstad JA, Alexander JM, Truty RM,
Shrikumar A, Li F, Eilertson KE, Ding H, Wylie JN, Pico AR, Capra
JA, et al: Dynamic and coordinated epigenetic regulation of
developmental transitions in the cardiac lineage. Cell.
151:206–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Huang J and Song K: BET protein
inhibition mitigates acute myocardial infarction damage in rats via
the TLR4/TRAF6/NF-κB pathway. Exp Ther Med. 10:2319–2324. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mumby S, Gambaryan N, Meng C, Perros F,
Humbert M, Wort SJ and Adcock IM: Bromodomain and extra-terminal
protein mimic JQ1 decreases inflammation in human vascular
endothelial cells: Implications for pulmonary arterial
hypertension. Respirology. 22:157–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang HP, Li GQ, Guo WZ, Chen GH, Tang HW,
Yan B, Li J, Zhang JK, Wen PH, Wang ZH, et al: Oridonin
synergistically enhances JQ1-triggered apoptosis in hepatocellular
cancer cells through mitochondrial pathway. Oncotarget.
8:106833–106843. 2017.PubMed/NCBI
|
|
21
|
Mio C, Conzatti K, Baldan F, Allegri L,
Sponziello M, Rosignolo F, Russo D, Filetti S and Damante G: BET
bromodomain inhibitor JQ1 modulates microRNA expression in thyroid
cancer cells. Oncol Rep. 39:582–588. 2018.PubMed/NCBI
|
|
22
|
Wang H, Hong B, Li X, Deng K, Li H, Yan
Lui VW and Lin W: JQ1 synergizes with the Bcl-2 inhibitor ABT-263
against MYCN-amplified small cell lung cancer. Oncotarget.
8:86312–86324. 2017.PubMed/NCBI
|
|
23
|
Huynh K, Bernardo BC, McMullen JR and
Ritchie RH: Diabetic cardiomyopathy: Mechanisms and new treatment
strategies targeting antioxidant signaling pathways. Pharmacol
Ther. 142:375–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans
PR, Ballario P, Neuhaus D, Filetici P and Travers AA: The
structural basis for the recognition of acetylated histone H4 by
the bromodomain of histone acetyltransferase gcn5p. EMBO J.
19:6141–6149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bennett RL and Licht JD: Targeting
epigenetics in cancer. Annu Rev Pharmacol Toxicol. 58:187–207.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perry MM, Durham AL, Austin PJ, Adcock IM
and Chung KF: BET bromodomains regulate transforming growth
factor-β-induced proliferation and cytokine release in asthmatic
airway smooth muscle. J Biol Chem. 290:9111–9121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnson R, Dludla P, Joubert E, February
F, Mazibuko S, Ghoor S, Muller C and Louw J: Aspalathin, a
dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against
high glucose induced shifts in substrate preference and apoptosis.
Mol Nutr Food Res. 60:922–934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Babcock SA, Hu N, Maris JR, Wang
H and Ren J: Mitochondrial aldehyde dehydrogenase (ALDH2) protects
against streptozotocin-induced diabetic cardiomyopathy: Role of
GSK3β and mitochondrial function. BMC Med. 10:402012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan Q, McMahon S, Anand P, Shah H, Thomas
S, Salunga HT, Huang Y, Zhang R, Sahadevan A, Lemieux ME, et al:
BET bromodomain inhibition suppresses innate inflammatory and
profibrotic transcriptional networks in heart failure. Sci Transl
Med. 9(pii): eaah50842017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ning B, Li W, Zhao W and Wang R: Targeting
epigenetic regulations in cancer. Acta Biochim Biophys Sin
(Shanghai). 48:97–109. 2016.PubMed/NCBI
|
|
32
|
Itzen F, Greifenberg AK, Bösken CA and
Geyer M: Brd4 activates P-TEFb for RNA polymerase II CTD
phosphorylation. Nucleic Acids Res. 42:7577–7590. 2014. View Article : Google Scholar : PubMed/NCBI
|