Introduction
Liver cancer is the third leading cause of death
from cancer worldwide. To date, chemotherapy is the primary
treatment for liver cancer. However, most anticancer drugs cause
systemic toxicity and side effects to patients due to their poor
specificity (1,2). Recently, nano-sized drug delivery
systems have been widely applied for cancer treatment through
targeted delivery with reduced adverse effects (3,4).
Natural copolymers, such as chitosan (5,6),
hyaluronic acid (HA) (7,8), and other polysaccharides (9,10),
have been well-recognized as nanoparticles in drug delivery and
cancer therapy.
HA, a natural linear and negatively charged
polysaccharide present in extracellular matrices, has been used as
a potential tumor-targeting moiety because of its biocompatibility,
biodegradability and overexpression of HA-binding receptors on
tumor cells. Drug-loaded nanocarriers based on HA conjugates, such
as doxorubicin (11,12), paclitaxel (13,14)
and siRNAs (15,16), have been found to exhibit enhanced
targeting ability in various tumor cells.
HA can be modified by other moieties, such as
galactose (17), glycyrrhetinic
acid (GA) (18–20), and various ligands, to improve the
selectivity of nanoparticles based on HA copolymers. This strategy
considerably increases the accumulation of drugs in tumor cells and
results in lower toxicity and fewer side effects than traditional
chemotherapy (21). Meanwhile, GA
has attracted increased attention as it can specifically bind with
GA-receptors in hepatocyte membranes and is less expensive than
antibodies. GA-modified drug-loaded nanoparticles can improve
anti-hepatoma efficacy and reduce toxic side effects (22–24).
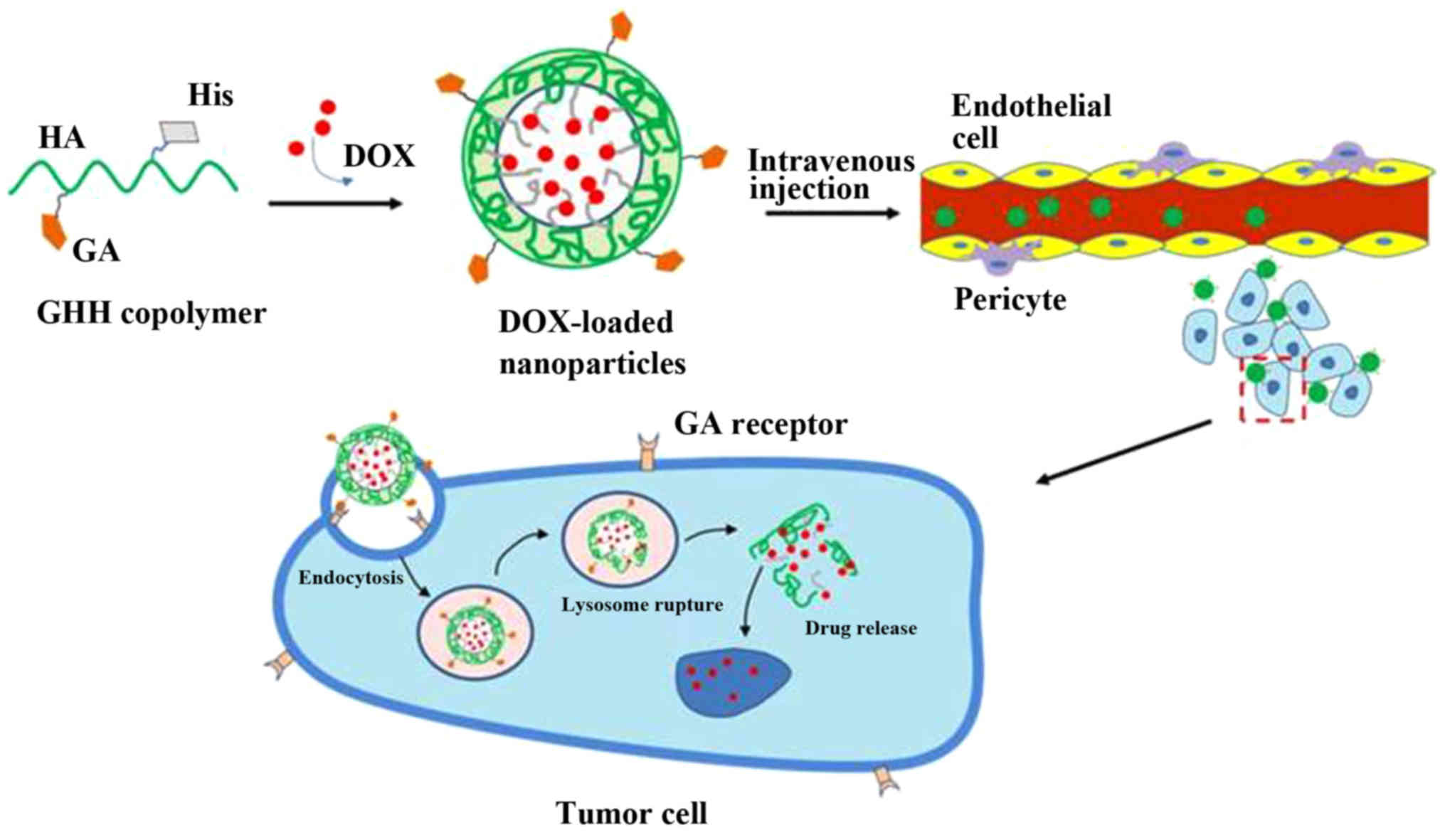
In the present study, we prepared a novel DOX/GHH
drug delivery system. The prepared DOX/GHH nanoparticles achieved
the dual-function of liver-targeted delivery via GA
receptor-mediated endocytosis and drug release from lysosomes via
protonation of the imidazole group of His (Fig. 1). First, HA polymers modified by GA
and His were synthesized. Then, the physicochemical characteristics
of the GHH nanoparticles were investigated. Finally, the
anti-hepatoma effect of DOX/GHH nanoparticles was evaluated in
vitro and in vivo.
Materials and methods
Materials
Hyaluronic acid (HA) (MW, 80 kDa) was purchased from
Bloomage Freda Biopharm Co., Ltd. (Jinan, China). L-histidine (His)
was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,
China). Glycyrrhetinic acid (GA) was acquired from Meheco Tianshan
Pharm Co., Ltd. (Beijing, China). DOX·HCl was purchased from
Shanghai Sangon Biomart Co., Ltd. (Shanghai, China).
4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride
(DMT-MM), pyrene and MTT was procured from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). RPMI-1640 medium was purchased from
Beijing BioDee Biotechnology Co., Ltd. (Beijing, China). All
chemicals were of analytical grade.
Cell cultures
Human hepatic cell line (HepG2) was obtained from
the China Center for Type Culture Collection (Wuhan, China), while
murine HCC cells (H22) were gifted by the Institute of
Immunopharmacology and Immunotherapy of Shandong University (Jinan,
China). Both cell lines were cultured in RPMI-1640 medium,
supplemented with 10% fetal bovine serum (FBS), 1% penicillin and
1% streptomycin at 37°C in an environment containing 5%
CO2.
Animals
Female BALB/c mice (weight: 18±2 g) were supplied by
the Experimental Animal Center of WeiFang Medical University
(Weifang, China). In total 36 mice were used for in vivo
imaging and antitumor efficacy experiments. The animals were fed at
25±2°C in the institutional animal house facility (relative
humidity: 40–70%, 12-h/d light dark cycle), with a standard diet
and allowed water ad libitum.
Synthesis of GHH copolymers
GHH copolymers were synthesized through a two-step
reaction. First, GA solution in methanol was activated to form an
active ester in the presence of DMT-MM. The active ester solution
was evaporated to remove methanol, and slowly added to an ethylene
diamine solution under stirring at room temperature for 24 h. Then,
the diamine-modified GA (GA-NH2) was obtained after
purification by column chromatography. The GA-HA conjugate was
synthesized by the chemical modification of GA–NH2 to
the backbone of HA (70 kDa). Second, the GA-HA conjugate was
dissolved in formylamine before DMT-MM was slowly added. Then, His
was slowly added to the GA-HA solution, followed by stirring at
room temperature for 24 h. After filtration, the solution was
freeze-dried to obtain GHH copolymers. The chemical structures of
the GHH conjugates were determined by 1H NMR (JNM
ECP-600, JEOL, Japan) by dissolving the conjugate in
D2O.
Characterizations of the GHH
copolymers
Pyrene was used as a probe to evaluate the
aggregation behavior of the GHH copolymer via fluorescence
spectrophotometry (25). In brief,
pyrene was dissolved in ethanol at a concentration of
6.0×105 M, and the solution was shaken for 24 h to
evaporate the ethanol at 60°C. Different concentrations of GHH
solutions were added to each tube, and the pyrene concentration was
maintained at 6.0×10−7 M. The fluorescence spectra of
pyrene were measured with an RF-5301PC fluorescence
spectrophotometer (Shimadzu Co., Kyoto, Japan). The variation in
intensity ratios from the first peak (372 nm) to the third peak
(383 nm) was sensitive to the polarity of the microenvironments
where pyrene was located. The I372/I383
fluorescence ratio of pyrene was analyzed for critical micelle
concentration (CMC) calculation. The CMC legend was used to
estimate the threshold concentration of the self-aggregated
nanoparticle formation, which was important for investigating the
self-aggregation behavior and structural stability of micelles.
The stability of the GHH micelles was tested by
dynamic light scattering spectrophotometry (Malvern Instruments
Ltd., Malvern, UK). In brief, the solution containing the GHH
micelles was mixed with RPMI-1640 medium containing 10% FBS. Then,
the mixture solution was maintained in a shaking water bath at 100
rpm and 37°C. All measurements were conducted at a wavelength of
635 nm at 25°C. The experiment was repeated for three samples.
pH-responsive behavior of the GHH
nanoparticles
The GHH nanoparticles were dissolved in PBS
solutions with different pH values (7.4, 7.0, 6.8, 6.4, 6.0 and
5.0). The concentration of the GHH nanoparticles was maintained at
1 mg/ml. pH-induced changes in particle size were examined by
Malvern Zetasizer Nano ZS90. All measurements were conducted in
triplicate.
Preparation of DOX-loaded
nanoparticles
DOX/GHH nanoparticles were prepared through a
modified dialysis method as described previously (26). In brief, GHH copolymers were
dissolved in formamide. DOX·HCl was dispersed in
N,N-dimethylformamide in the presence of triethylamine (TEA)
(MTEA:MDOX=1.3). Then, the latter was added
dropwise to the GHH solution by stirring. Then, a dialysis bag
[molecular weight cut-off (MWCO) 3,500 kDa] was used for the
dialysis of the mixed suspension against deionized water for the
removal of unloaded drugs. Additionally, DOX-loaded HA-GA
nanoparticles were prepared as control. DOX/HA-GA nanoparticles and
DOX/GHH nanoparticles were obtained by freeze-drying the dialysis
solution.
The drug loading capacity (DL) and entrapment
efficiency (EE) of the GHH nanoparticles were evaluated using a
UV-vis spectrophotometer at 479 nm. The DL and EE values were
calculated using the following equations:
DL=WS/WTx100%EE=WS/WAx100%
where WS is the DOX weight in the
nanoparticles, WT is the total weight of the
freeze-dried nanoparticles, and WA is the feeding
weight of DOX.
In vitro DOX release from the GHH
nanoparticles
The in vitro pH-responsive release behavior
of the DOX/GHH nanoparticles was investigated through a dialysis
method (cut-off=3.5 kDa). In brief, the DOX/GHH nanoparticles were
dissolved in PBS solution. Three solutions with different pH values
(7.4, 6.8, and 5.0) were prepared. The dialysis bags were dialyzed
against a fresh PBS solution (0.1 M; pH 7.4, 6.8 and 5.0) and
placed in a shaking incubator with a stirring speed of 100 rpm at
37°C.
At predetermined time intervals, the medium (4 ml)
was withdrawn, and the same volume of fresh PBS solution was added.
DOX concentration was measured with a UV-vis spectrophotometer at
479 nm. Cumulative DOX release percentage (Er) was calculated using
the following equation:
Er(%)=Ve∑1n-1Ci+V0CnmDOX×100%
Where mDOX is the amount of DOX in
the nanoparticles, V0 represents the whole volume
of the release medium, Ci is the concentration of
DOX in the medium, and Ve represents the volume
of the replaced medium. The in vitro DOX release measurement
was performed in triplicate at each pH value.
In vitro cytotoxicity of the DOX/GHH
nanoparticles
The cytotoxicity of the blank nanoparticles and the
DOX-loaded nanoparticles against HepG2 cells was tested by MTT
assay as previously described (27). In brief, the HepG2 cells were
seeded in 96-well plates (5×103 cells/well) and cultured
overnight at 37°C in a humidified atmosphere of 5% CO2.
Then, the cells were incubated with free DOX and DOX-loaded
nanoparticles for 48 h at equivalent DOX concentrations of 0.01,
0.1, 1.0, 5.0 and 10.0 µg/ml. Cell viability was determined through
MTT assay. The half maximal inhibitory concentration values
(IC50) of the different formulations were calculated in
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). All measurements were
performed in triplicate.
In vitro cellular uptake studies
To evaluate the targeting ability of the
nanoparticles, the in vitro cellular uptake of the GHH
nanoparticles was observed by fluorescence microscopy (IX51;
Olympus Corporation, Tokyo, Japan). A FITC-labeled GHH copolymer
was synthesized as previously reported (28). The HepG2 cells were seeded in
6-well plates (5×104 cells/ml) at 37°C. When the cells
reached 70–80% confluence, FITC-labeled GHH nanoparticles,
DOX/HA-GA nanoparticles, or DOX/GHH nanoparticles (5 µg/ml of DOX)
in serum-free medium were added and incubated at 37°C. After 2 h of
incubation, the cells were washed and fixed. DAPI staining (1:500;
Sigma-Aldrich) was performed to visualize the nuclei of the HepG2
cells. Finally, the cellular uptake and intracellular distribution
of the GHH nanoparticles were visualized by fluorescence
microscopy, and the merged images were created with Image Pro Plus
6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
In vivo near-infrared fluorescence
imaging
The in vivo biodistribution of the GHH
nanoparticles was monitored using DiR as a near-infrared
fluorescence agent. Imaging of the DiR-loaded GHH nanoparticles was
performed at pre-determined times (1, 2, 6 and 12 h), using the
Xenogen IVIS Spectrum from Caliper Life Sciences (Waltham, MA,
USA). The excitation and emission wavelengths selected were at 745
and 835 nm, respectively.
In vivo antitumor efficacy
H22 tumor-bearing mice were prepared to evaluate the
antitumor efficacy of the DOX/GHH nanoparticles. The mice were
subcutaneously injected at the right axillary space with 0.1 ml
cell suspensions containing 1×106 H22 cells. The mice
were divided into five groups and treated with: i) normal saline
(the control group), ii) blank GHH nanoparticles, iii) DOX, iv)
DOX/HA-GA nanoparticles, and v) DOX/GHH nanoparticles. When the
tumor volume reached 100 mm3, each treatment was
administered in an equivalent volume of 0.2 ml every other day. The
three drug formulations were injected at a dose of 5 mg/kg body
weight. Tumor volumes were observed for 14 days once per day. The
individual tumor volume (V) was calculated by
V=(W2xL)/2, where the width (W) is the shortest tumor
diameter, and the length (L) is the longest tumor diameter. The
values are presented as the mean ± standard deviation (SD) for
groups of at least five animals. Finally, the mice were sacrificed
by cervical vertebra dislocation after anesthesia using 10% chloral
hydrate, and the tumors were removed.
Statistical analysis
All results are presented as mean ± SD, n=3 parallel
samples. One-way analysis of variance was used to make comparison
of several groups, and SNK-q test was used to make post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Synthesis and characterization of the
GHH conjugates
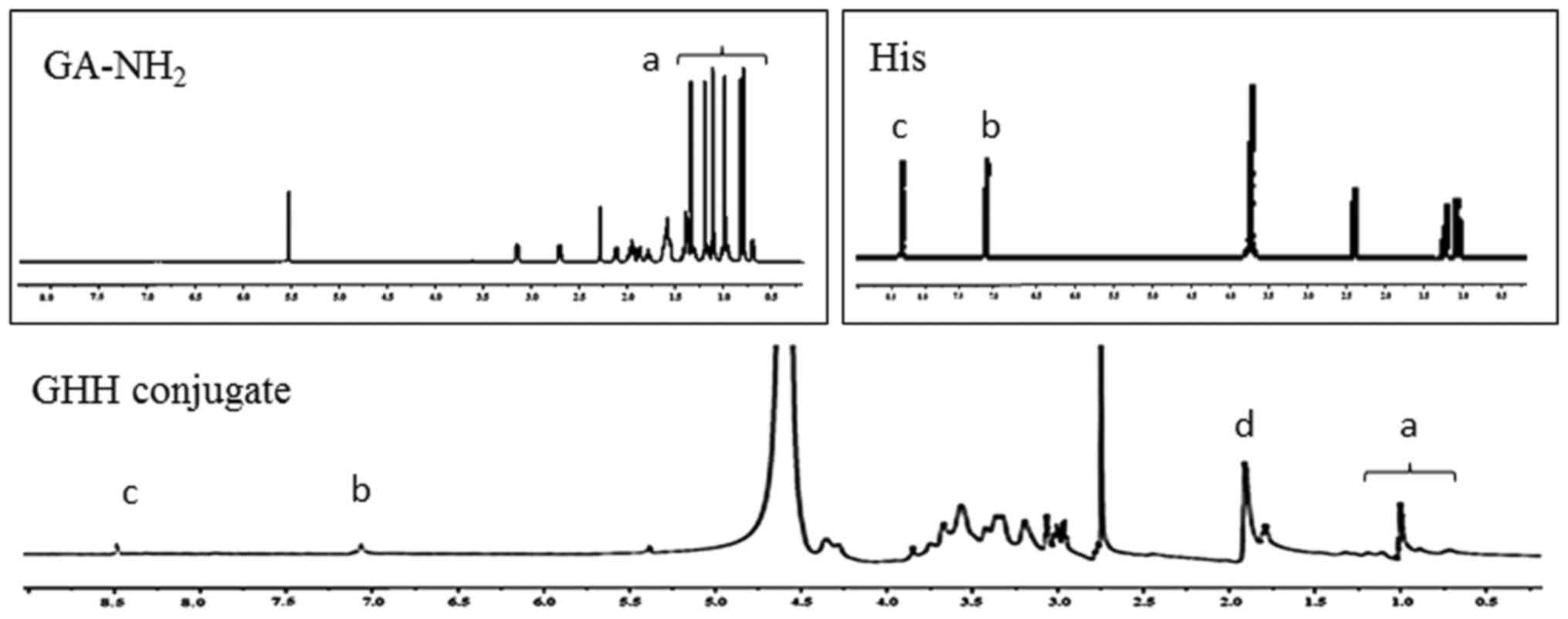
The GHH copolymer was synthesized by coupling
aminated GA and His to the HA backbone. The characteristic peaks of
HA, GA-NH2 and His were confirmed (Fig. 2). In this investigation, the
characteristic peaks of the methyl and methylene groups (0.7–1.5
ppm) of GA, the N-acetyl group (1.91 ppm) of HA, and the
imidazole ring (7.11 and 8.44 ppm) of His were confirmed. These
results indicated that the GA-NH2 and His groups were
successfully introduced into HA copolymers owing to the presence of
peaks at 0.6–1.5 ppm (peaks of GA-NH2), 7.11 and 8.44
ppm (peaks of His) in the GHH conjugates.
The degree of substitution (DS) was estimated by UV
measurement (λ=260 nm). The HA-GA conjugate (DS=5.8%) was selected
as the candidate for further research because of its low particles
size. His, a pH-responsive group, was successfully introduced to
the HA backbone in the presence of DMT-MM. When the molar ratios
between HA-GA and His were 1:3, 1:6 and 1:9, the DS values of His
were 4.6, 8.6 and 10.2%, respectively, and the copolymers were
designated as GHH-4, GHH-8 and GHH-10.
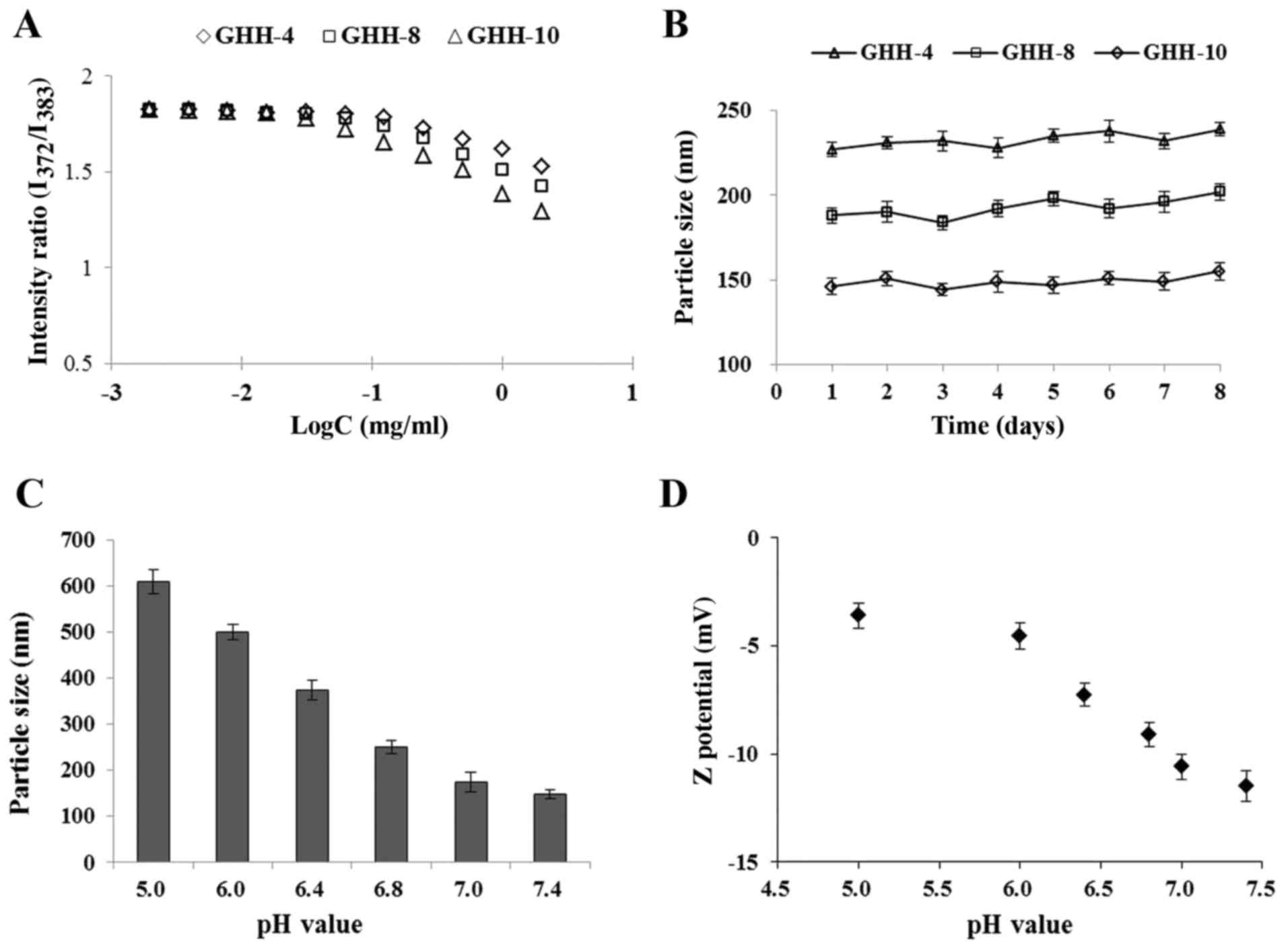
The CMC value is widely used to monitor the
self-aggregation behavior of amphiphilic polymers and the
structural stability of micelles in vitro and in
vivo. The CMC values of the GHH conjugates with different DS
values were measured with pyrene as the hydrophobic molecule. As
shown in Fig. 3A, the fluorescence
intensity ratio (I373/I383) was plotted, and
the CMC was measured from the threshold concentration of the GHH
copolymer. The CMC values of the GHH conjugate ranged from 0.024 to
0.089 mg/ml.
GHH nanoparticles were prepared by ultrasonic
dispersion. The mean diameters of the GHH nanoparticles exhibited
no significant changes over 7 days when stored under physiological
conditions (RPMI-1640 medium, 37°C), suggesting that the GHH
nanoparticles were highly stable (Fig.
3B).
The pH-responsive behavior of the GHH copolymers was
tested on the basis of particle size and zeta (ζ) potential at
different pH values (Fig. 3C and
D). At pH 7.0–7.4, the average particle size was nearly
unchanged (148.7–158.6 nm), suggesting that the GHH nanoparticles
were stable under physiological condition. The abrupt increases in
mean particle size and particle diameter distribution were caused
by a stepwise shift from pH 6.8 to 5.0. Fig. 3D demonstrates that the ζ potential
increased when the pH was changed from 7.4 to 5.0 and remained
negatively charged.
Formation and characterization of the
DOX/GHH nanoparticles
DOX-loaded nanoparticles based on GHH copolymers
were prepared through a simple ultrasonic method. When DOX was
mixed with GHH nanoparticles at an initial ratio of 1:10, DOX was
physically encapsulated in the GHH-4, GHH-8 and GHH-10 copolymers,
and the resulting complexes were named DOX/GHH-4, DOX/GHH-8 and
DOX/GHH-10, respectively. The mean particle sizes, ζ potential,
EEs, and DLs of the different DOX-loaded nanoparticles are shown in
Table I. The mean particle sizes
and absolute values of the ζ potential decreased when the DS values
of His increased. The DL and EE values of the DOX-loaded
nanoparticles decreased when the DS of His increased. DOX/GHH-10
was chosen as the nanocarrier for further research due to its low
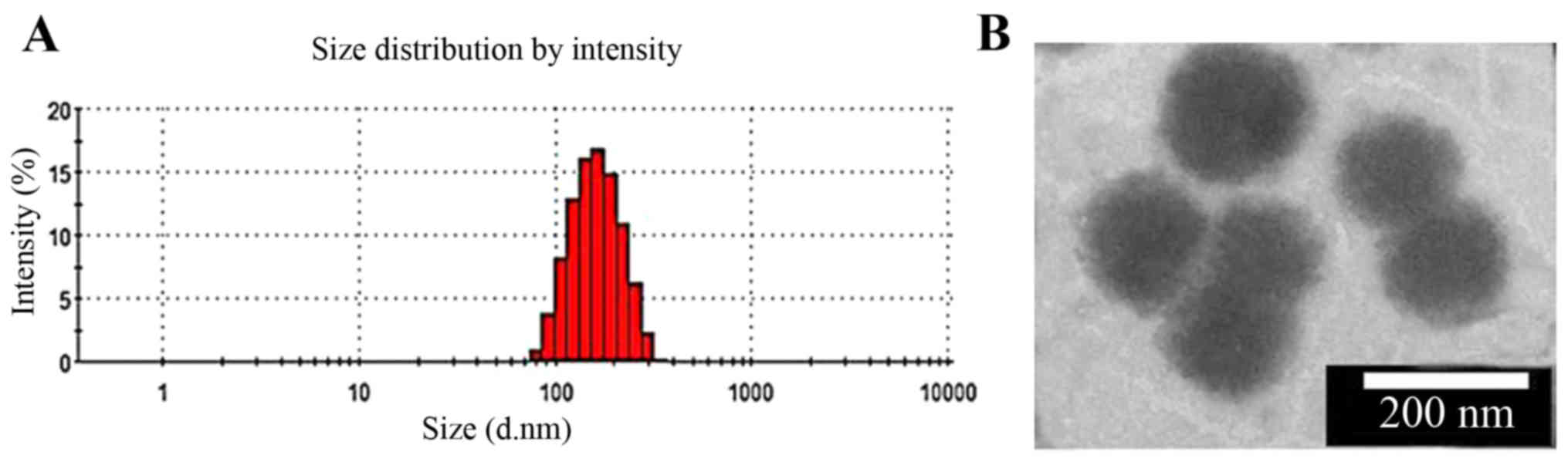
particle size. As shown in Fig.
4A, DOX/GHH-10 has well-separated particles with a rather
narrow size distribution. TEM micrograph shows that it was nearly
spherical (Fig. 4B).
 | Table I.Characterization of the DOX/GHH
nanoparticles at pH 7.4 (n=3). |
Table I.
Characterization of the DOX/GHH
nanoparticles at pH 7.4 (n=3).
| Nanoparticles | Diameter (nm) | PDI | ζ potential
(mV) | EE (%) | DL (%) |
|---|
| DOX/GHH-4 | 238.1±9.4 | 0.197 | −13.7±1.2 | 91.3±1.8 | 9.21±0.52 |
| DOX/GHH-8 | 172.7±5.7 | 0.159 | −11.2±0.9 | 88.7±2.1 | 8.92±0.47 |
| DOX/GHH-10 | 156.7±8.6 | 0.137 | −10.4±1.1 | 87.4±1.5 | 8.84±0.39 |
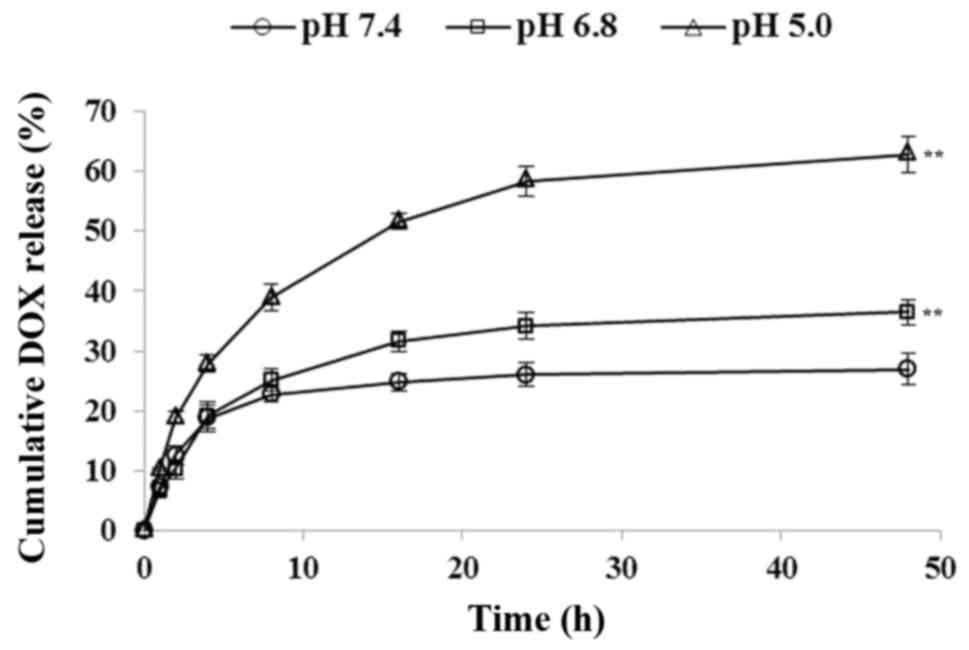
pH-responsive DOX release from GHH
nanoparticles in vitro
In vitro DOX release from the DOX/GHH
nanoparticles was measured at 37°C. As shown in Fig. 5, a pH-responsive release profile
was found in DOX release at the different pH values. The DOX-loaded
nanoparticles were stable at pH 7.4 and released only 21.4% of DOX
after 24 h. Under an extracellular tumoral condition (pH 6.8),
29.8% cumulative DOX was determined. However, at an intralysosomal
pH of 5.0, the DOX release rate was much faster, with 58.9% of DOX
released after 24 h.
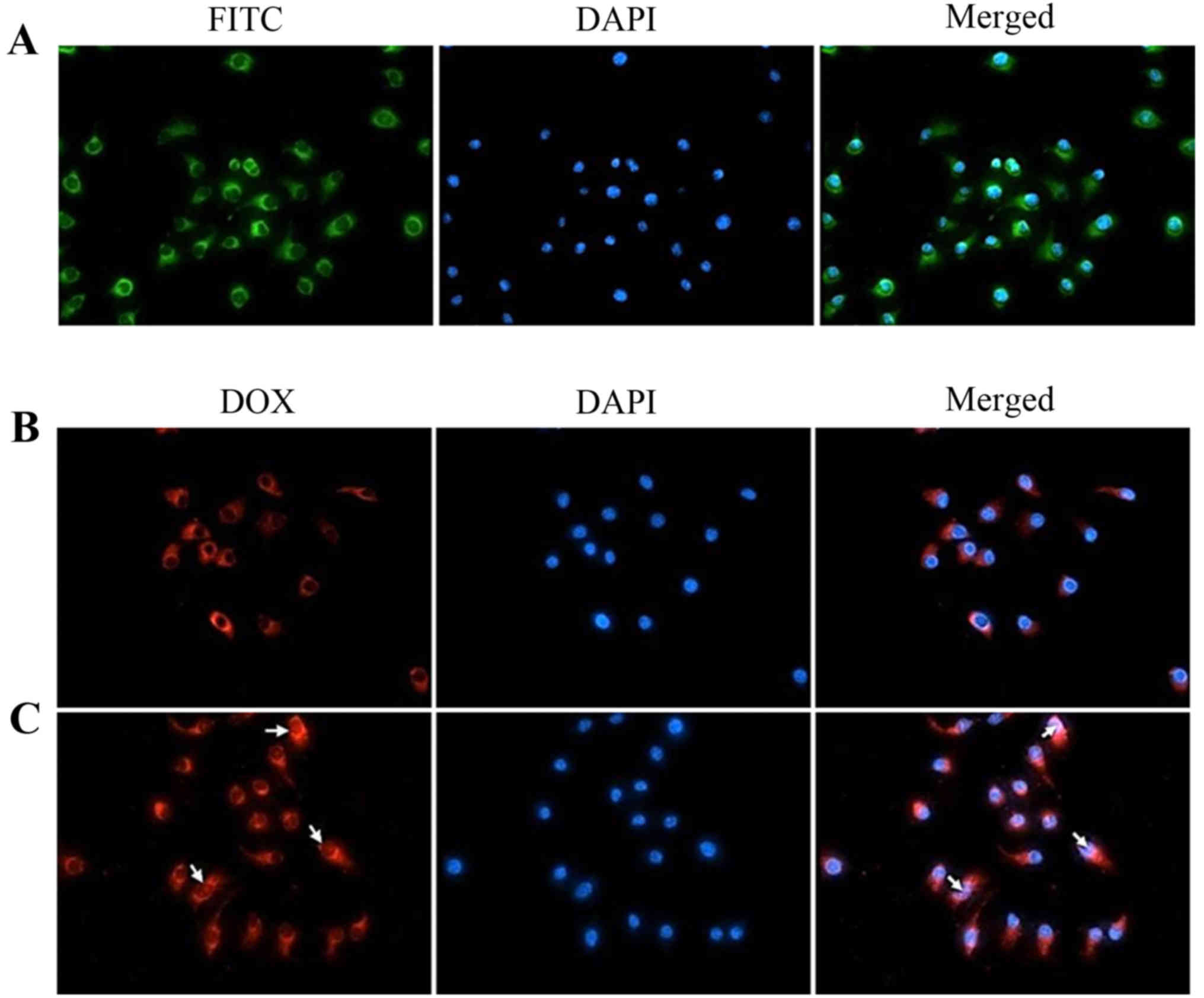
In vitro cellular uptake of the
DOX/GHH nanoparticles
The intracellular uptake of the GHH nanoparticles
was evaluated by fluorescence microscopy. FITC was used as a
fluorescence probe for tracking the distribution of GHH
nanoparticles in the HepG2 cells. DAPI was regarded as a
fluorescence marker for the visualization of the HepG2 cell nuclei.
In Fig. 6A, green spots were
observed in the cytoplasm after the cells were incubated with
FITC-labeled nanoparticles, suggesting that the GHH nanoparticles
were taken up by endocytosis of the HepG2 cells.
The cellular uptake of DOX from the GHH
nanoparticles was analyzed with the autofluorescence of DOX. The
distribution of DOX in the HepG2 cells was determined by obtaining
the overlay of the fluorescent images. The results of cellular
uptake after 1.5 h of incubation with the DOX/HA-GA or DOX/GHH
nanoparticles are showed in Fig. 6B
and C. Red spots (DOX) were observed in the HepG2 cells,
indicating that DOX was released from the HA-GA nanoparticles or
GHH nanoparticles. However, compared with the DOX/HA-GA
nanoparticles, a larger amount of DOX from the GHH nanoparticles
was distributed in the cytoplasm and nuclear regions.
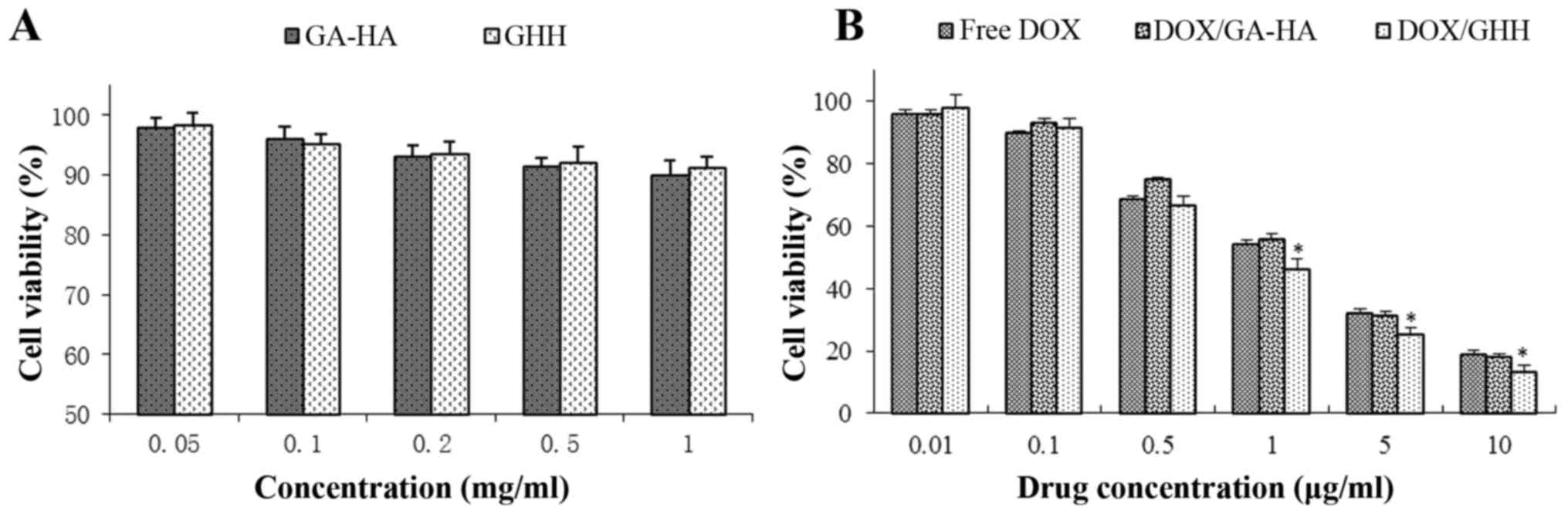
In vitro cytotoxicity of the DOX/GHH
nanoparticles
The cellular viability of blank GHH nanoparticles
was investigated by MTT assay. The results demonstrated that
cellular viability was over 85% after incubation with the blank
nanoparticles for 48 h, indicating that the GHH conjugate exhibited
no significant cytotoxicity with a concentration of up to 1 mg/ml,
and could be used as carriers of antitumor drugs (Fig. 7A). The in vitro cytotoxicity
levels of the DOX formulations were evaluated against the HepG2
cells. As demonstrated in Fig. 7B,
free DOX, DOX/GA-HA nanoparticles and DOX/GHH nanoparticles
exhibited dose-dependent cytotoxic effects after incubation for 48
h. The IC50 values of free DOX, DOX/GA-HA nanoparticles,
and DOX/GHH nanoparticles were 1.32, 1.41 and 1.07 µg DOX equiv/ml,
respectively.
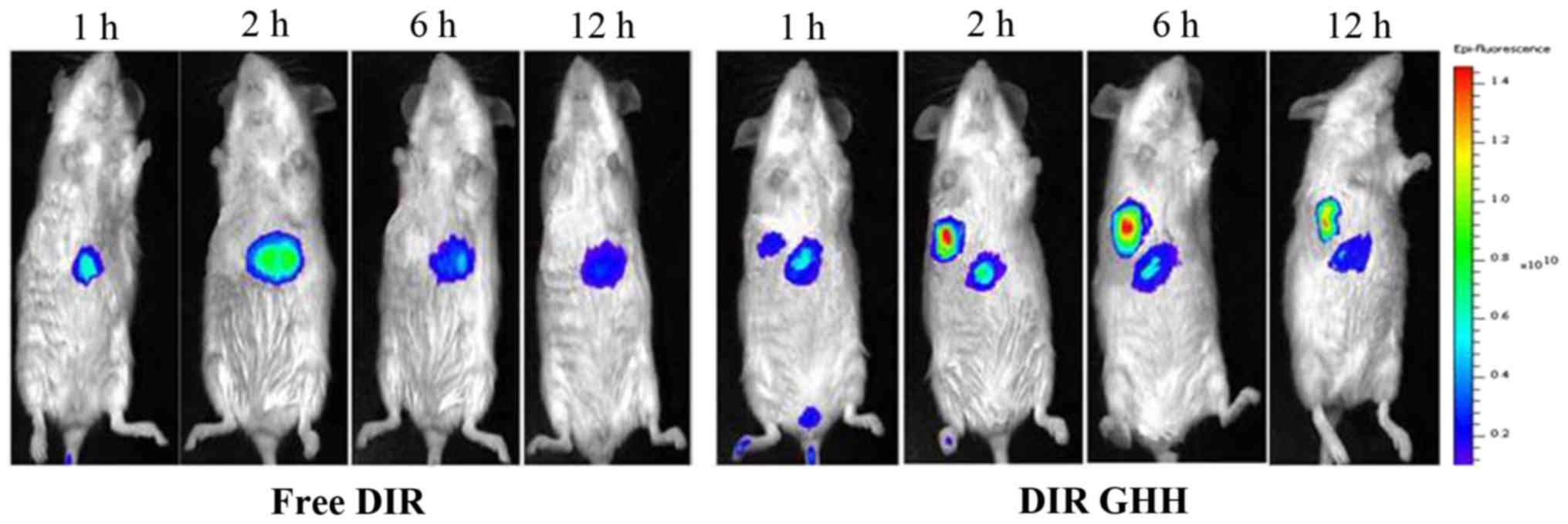
In vivo imaging analysis
To investigate the liver-targeting capacity of the
GHH nanoparticles, DiR-loaded micelles were prepared to analyze the
biodistribution of GHH nanoparticles in mice by fluorescence
imaging. As presented in Fig. 8,
DIR was obviously accumulated in the liver and tumor. DiR-loaded
GHH nanoparticles began to accumulate in the tumor at 1 h, reached
the maximum fluorescent intensity at 6 h, and then declined
gradually but was still detectable until 12 h.
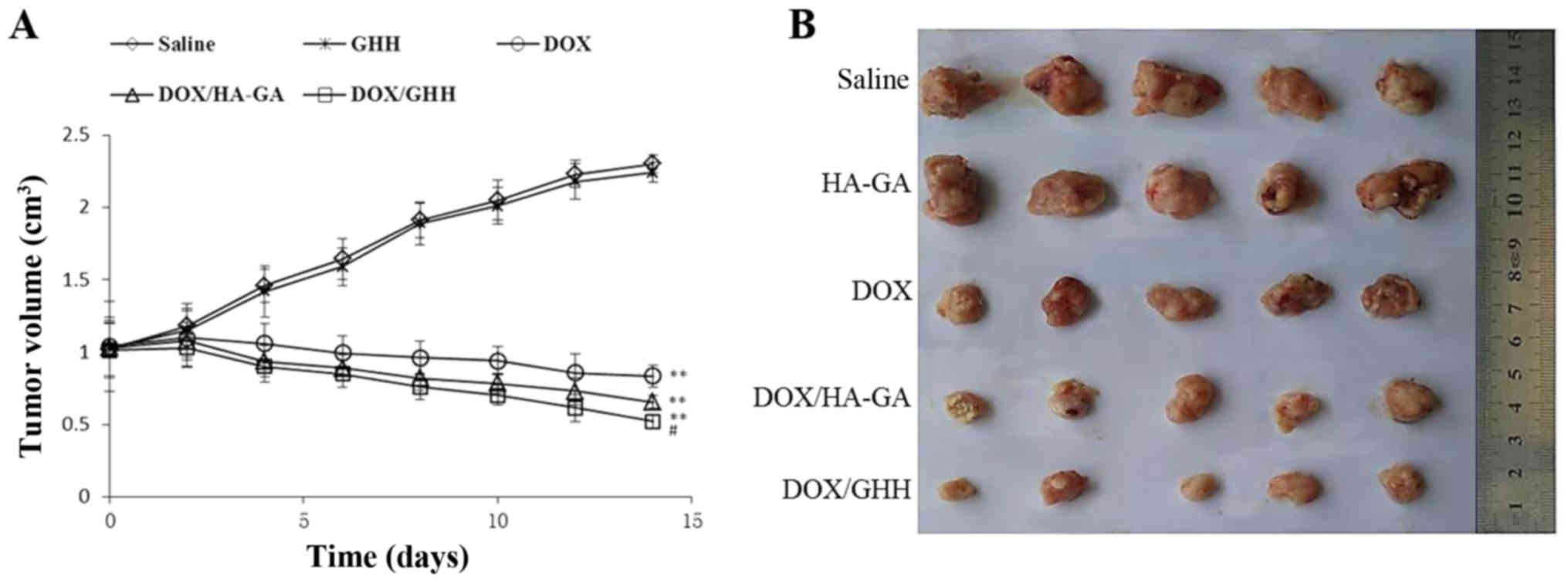
In vivo antitumor efficacy
The in vivo anti-hepatoma efficacy of the
DOX/GHH nanoparticles for H22 tumor-bearing mice was tested for 14
days. In Fig. 9, the blank GHH
nanoparticle treatment results showed an equivalent increase in
tumor size with the control group. This result suggested that the
blank nanoparticles had no antitumor efficacy. As expected, the
tumor sizes of the three DOX formation groups were significantly
smaller than that of the saline group. Notably, compared with the
free DOX group, the groups containing the DOX/HA-GA and DOX/GHH
nanoparticles had considerably higher antitumor efficacy. To
investigate the in vivo antitumor activity, we extracted the
tumors from the five groups of H22 cell-bearing mice (Fig. 9). The results demonstrated that the
tumor sizes from the three DOX treatments were considerably smaller
than those in the control group, indicating significant antitumor
effect. Notably, the DOX/GHH nanoparticle groups showed higher
inhibition efficiency than the two other DOX treatment groups.
Discussion
Liver-targeting nanoparticles can deliver antitumor
drugs to liver cancer tissues, reducing drug side effects.
Glycyrrhetinic acid (GA), an aglycone of glycyrrhizin, can
specifically bind to receptors on the membrane of liver cancer
cells. This characteristic makes GA a suitable candidate for the
development of a liver-targeted delivery nanocarrier (29,30).
In our previous study, pH-responsive nanoparticles based on
His-modified HA polymers were prepared and used as nanocarrier for
doxorubicin (DOX) delivery against MCF-7 cells (28). In the present study, we prepared
dual-functional GHH nanoparticles that were expected to achieve the
liver-targeted delivery of DOX and efficient escape from lysosomes.
The critical micelle concentration (CMC) value is widely used to
monitor the self-aggregation behavior of amphiphilic polymers and
structural stability of micelles in vitro and in vivo
(31). The CMC values of the GHH
conjugate ranged from 0.024 to 0.089 mg/ml, indicating that the
structural integrity of the conjugate was improved because of the
strong hydrophobic interactions in the inner core of the GHH
conjugate at a low copolymer concentration. At a low CMC value, the
stability of the self-assembled micelles in the bloodstream may be
retained as dissociation is prevented under highly diluted
conditions (32).
The particle size and ζ potential of the GHH
nanoparticles were increased as the pH values decreased from 7.4 to
5.0. This phenomenon might be explained by the introduction of the
ionizable imidazole ring of His. These imidazole groups are
protonated at an acidic pH, resulting in the increased size of the
GHH nanoparticles. Furthermore, the shells of the nanoparticles are
covered by negatively charged HA chains, and the protonated
imidazole groups of His increase at a low pH, resulting in change
in the surface charge of the GHH nanoparticles (33). Table
I shows that the mean particle sizes and absolute values of the
ζ potential of the DOX/GHH nanoparticles decreased with the
increase in the DS of the His group. This trend might be due to the
introduction of more His molecules, resulting in the formation of
more compact hydrophobic cores and the reduction in the number of
carboxyl groups in the GHH copolymers. Moreover, the modification
of more His molecules could form tighter cores in GHH
nanoparticles, resulting in a weak repulsion between DOX and the
hydrophobic core (34).
To investigate the release behavior of the
DOX-loaded nanoparticles under physiological conditions, a tumor
acidic microenvironment, and an intralysosomal pH, we measured the
in vitro DOX release of the DOX/GHH nanoparticles at pH 7.4,
6.8 and 5.0, respectively. The DOX release rates were significantly
differed at pH 7.4 and 5.0 (P<0.05). The results were due to the
protonated imidazole ring in the core of His at pH 5.0, which is
below the pKa of the histidyl imidazole ring (pH, 6.5). However, no
significant difference (P>0.05) was observed between pH 7.4 and
6.8. The pH-responsive drug release behavior showed that the rate
and amount of DOX release from the nanoparticles increased as the
pH was decreased from 7.4 to 5.0. Under physiological conditions
(pH 7.4), the micelles had a stable hydrophobic cores composed of
GA and His, and DOX was released slowly via a diffusion mechanism.
At pH 6.8, the release rates of DOX increased due to the slight
swelling of the micelles owing to the partial protonation of the
imidazole ring of His. Under an intralysosomal condition (pH 5.0),
the majority of the imidazole rings were protonated, and the
charged imidazole groups repelled each other and moved out of the
hydrophobic core, which caused the marked swelling and
demicellization of the GHH micelles. Luo and Jiang also reported
that drugs are released from pH-responsive nanoparticles/vesicles
through the swelling-demicellization–releasing mechanism (35).
MTT assay was used to evaluate the cytotoxicity of
the DOX/GHH nanoparticles. The IC50 value of the DOX/GHH
nanoparticles was lower than that of the DOX/HA-GA nanoparticles.
These results indicated that the DOX/GHH nanoparticles escaped
quickly from lysosomes and rapidly released DOX into the cytoplasm
through a proton sponge effect, which enhanced the cytotoxicity
levels (36,37). Meanwhile, compared with the free
DOX group, the DOX/GHH nanoparticle group showed higher antitumor
efficacy. A possible explanation is that GA-receptor-mediated
endocytosis inhibits P-glycoprotein-mediated drug efflux, resulting
in its high antitumor efficacy (38,39).
The in vivo antitumor efficacy of the DOX/GHH nanoparticles
was investigated against H22 tumor-bearing mice. Relative to the
control group, the three drug treatment groups had antitumor
efficacy. Notably, the DOX-loaded nanoparticles had considerably
higher antitumor efficacy than free DOX. The results might be due
to the fact that the nano-delivery system improves DOX accumulation
in tumor cells via the enhanced permeability and retention effect
(40,41). Importantly, the GHH nanoparticle
treatment group showed a higher antitumor effect than the DOX/HA-GA
nanoparticles. A possible explanation is that the DOX released from
the GHH nanoparticles easily escaped from the lysosomes after the
introduction of His, resulting in their higher antitumor efficacy
(42).
In conclusion, a novel GHH copolymer was
synthesized, and self-assembled dual-functional nanoparticles were
prepared for the liver-targeted delivery of DOX. In vitro
release studies showed that the GHH nanoparticles released DOX in a
pH-responsive manner. Cellular uptake results indicated that the
introduction of His to the HA backbone substantially increased the
release rate of DOX from the lysosomes of HepG2 cells. Moreover,
in vivo antitumor activity analysis showed that the GHH
nanoparticles exhibited higher antitumor efficacy than free DOX or
DOX/HA-GA nanoparticles. All of these results demonstrated that GHH
copolymers are biocompatible and exhibit great potential as
liver-targeted and pH-responsive delivery systems in the prevention
and treatment of liver cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81274093), the Higher
Education Science and Technology Project of Shandong Province
(grant no. J17KA141), the Medical and Health Technology Development
Program in Shandong province (grant no. 2016WS0673), the Project of
Traditional Chinese Medicine Technology Development Program in
Shandong Province (2017–212), and the Science and Technology
Development Program in Weifang (grant nos. 2017YX065 and
2016YX011).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JW and GT conceived and designed the study. GT, JB,
JD and BZ performed the experiments. ZG and XS analyzed and
interpreted the data. JW, ZG and BZ wrote the paper. XS, GT and BZ
reviewed and edited the manuscript.
Ethics approval and consent to
participate
All animal care and experimental protocols complied
with the Animal Management Rules of the Ministry of Health of China
and were approved by the Animal Research Ethics Committee (Weifang,
China), approval no. 2017-025.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakagawa H, Fujita M and Fujimoto A:
Genome sequencing analysis of liver cancer for precision medicine.
Semin Cancer Biol. Mar 29–2018;(Epub ahead of print). PubMed/NCBI
|
|
2
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karimi M, Ghasemi A, Sahandi Zangabad P,
Rahighi R, Moosavi Basri SM, Mirshekari H, Amiri M, Shafaei
Pishabad Z, Aslani A, Bozorgomid M, et al: Smart
micro/nanoparticles in stimulus-responsive drug/gene delivery
systems. Chem Soc Rev. 45:1457–1501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cervello M, Pitarresi G, Volpe AB, Porsio
B, Balasus D, Emma MR, Azzolina A, Puleio R, Loria GR, Puleo S and
Giammona G: Nanoparticles of a polyaspartamide-based brush
copolymer for modified release of sorafenib: In vitro and in vivo
evaluation. J Control Release. 266:47–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin Q, Bao C, Yang Y, Liang Q, Zhang D,
Cheng S and Zhu L: Highly discriminating photorelease of anticancer
drugs based on hypoxia activatable phototrigger conjugated chitosan
nanoparticles. Adv Mater. 25:1981–1986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castro F, Pinto ML, Silva AM, Pereira CL,
Teixeira GQ, Gomez-Lazaro M, Santos SG, Barbosa MA, Gonçalves RM
and Oliveira MJ: Pro-inflammatory chitosan/poly(gamma-glutamic
acid) nanoparticles modulate human antigen-presenting cells
phenotype and revert their pro-invasive capacity. Acta Biomater.
63:96–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang X, Fang L, Li X, Zhang X and Wang F:
Activatable near infrared dye conjugated hyaluronic acid based
nanoparticles as a targeted theranostic agent for enhanced
fluorescence/CT/photoacoustic imaging guided photothermal therapy.
Biomaterials. 132:72–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu JL, Tian GX, Yu WJ, Jia GT, Sun TY and
Gao ZQ: pH-Responsive Hyaluronic Acid-Based Mixed Micelles for the
Hepatoma-Targeting Delivery of Doxorubicin. Int J Mol Sci.
17:3642016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sui J, Cui Y, Cai H, Bian S, Xu Z, Zhou L,
Sun Y, Liang J, Fan Y and Zhang X: Synergistic chemotherapeutic
effect of sorafenib-loaded pullulan-Dox conjugate nanoparticles
against murine breast carcinoma. Nanoscale. 9:2755–2767. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamura R, Uemoto S and Tabata Y: Augmented
liver targeting of exosomes by surface modification with cationized
pullulan. Acta Biomater. 57:274–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong H, Du S, Ni J, Zhou J and Yao J:
Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite
nanoparticles for enhancing therapeutic efficacy of doxorubicin.
Biomaterials. 94:70–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li K, Liu H, Gao W, Chen M, Zeng Y, Liu J,
Xu L and Wu D: Mulberry-like dual-drug complicated nanocarriers
assembled with apogossypolone amphiphilic starch micelles and
doxorubicin hyaluronic acid nanoparticles for tumor combination and
targeted therapy. Biomaterials. 39:131–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han X, Dong X, Li J, Wang M, Luo L, Li Z,
Lu X, He R, Xu R and Gong M: Free paclitaxel-loaded E-selectin
binding peptide modified micelle self-assembled from hyaluronic
acid-paclitaxel conjugate inhibit breast cancer metastasis in a
murine model. Int J Pharm. 528:33–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Li W, Guo X, Kong F, Wang Z, Zhu
C, Luo L, Li Q, Yang J, Du Y and You J: Specifically increased
paclitaxel release in tumor and synergetic therapy by a hyaluronic
acid-tocopherol nanomicelle. ACS Appl Mater Interfaces.
9:20385–20398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin L, Cai M, Deng S, Huang W, Huang J,
Huang X, Huang M, Wang Y, Shuai X and Zhu K: Amelioration of
cirrhotic portal hypertension by targeted cyclooxygenase-1 siRNA
delivery to liver sinusoidal endothelium with polyethylenimine
grafted hyaluronic acid. Nanomedicine. 13:2329–2339. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Z, Li H, Wang K, Guo Q, Li C, Jiang
H, Hu Y, Oupicky D and Sun M: Bioreducible cross-linked hyaluronic
acid/calcium phosphate hybrid nanoparticles for specific delivery
of siRNA in melanoma tumor therapy. ACS Appl Mater Interfaces.
9:14576–14589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan J and Yang J: Preparation and
characterization of a chitosan/galactosylated hyaluronic
acid/heparin scaffold for hepatic tissue engineering. J Biomater
Sci Polym Ed. 28:569–581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han X, Wang Z, Wang M, Li J, Xu Y, He R,
Guan H, Yue Z and Gong M: Liver-targeting self-assembled hyaluronic
acid-glycyrrhetinic acid micelles enhance hepato-protective effect
of silybin after oral administration. Drug Deliv. 23:1818–1829.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Yao J, Zhou J, Wang T and Zhang
Q: Glycyrrhetinic acid-graft-hyaluronic acid conjugate as a carrier
for synergistic targeted delivery of antitumor drugs. Int J Pharm.
441:654–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Gu X, Wang H, Sun Y, Wu H and Mao
S: Synthesis, characterization and liver targeting evaluation of
self-assembled hyaluronic acid nanoparticles functionalized with
glycyrrhetinic acid. Eur J Pharm Sci. 96:255–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dahlman JE, Kauffman KJ, Xing Y, Shaw TE,
Mir FF, Dlott CC, Langer R, Anderson DG and Wang ET: Barcoded
nanoparticles for high throughput in vivo discovery of targeted
therapeutics. Proc Natl Acad Sci USA. 114:2060–2065. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Chen T, Deng F, Wan J, Tang Y, Yuan
P and Zhang L: Synthesis, characterization, and in vitro evaluation
of curcumin-loaded albumin nanoparticles surface-functionalized
with glycyrrhetinic acid. Int J Nanomedicine. 10:5475–5487.
2015.PubMed/NCBI
|
|
23
|
Lv Y, Li J, Chen H, Bai Y and Zhang L:
Glycyrrhetinic acid-functionalized mesoporous silica nanoparticles
as hepatocellular carcinoma-targeted drug carrier. Int J
Nanomedicine. 12:4361–4370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi WW, Yu HY, Guo H, Lou J, Wang ZM, Liu
P, Sapin-Minet A, Maincent P, Hong XC, Hu XM and Xiao YL:
Doxorubicin-loaded glycyrrhetinic acid modified recombinant human
serum albumin nanoparticles for targeting liver tumor chemotherapy.
Mol Pharm. 12:675–683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spaeth JR, Kevrekidis IG and
Panagiotopoulos AZ: A comparison of implicit- and explicit-solvent
simulations of self-assembly in block copolymer and solute systems.
J Chem Phys. 134:1649022011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park K, Lee GY, Kim YS, Yu M, Park RW, Kim
IS, Kim SY and Byun Y: Heparin-deoxycholic acid chemical conjugate
as an anticancer drug carrier and its antitumor activity. J Control
Release. 114:300–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang W, Wang W, Wang P, Tian Q, Zhang C,
Wang C, Yuan Z, Liu M, Wan H and Tang H: Glycyrrhetinic
acid-modified poly(ethylene glycol)-b-poly(gamma-benzyl
l-glutamate) micelles for liver targeting therapy. Acta Biomater.
6:3927–3935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu JL, Liu CG, Wang XL and Huang ZH:
Preparation and characterization of nanoparticles based on
histidine-hyaluronic acid conjugates as doxorubicin carriers. J
Mater Sci Mater Med. 23:1921–1929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang C, Wang W, Liu T, Wu Y, Guo H, Wang
P, Tian Q, Wang Y and Yuan Z: Doxorubicin-loaded glycyrrhetinic
acid-modified alginate nanoparticles for liver tumor chemotherapy.
Biomaterials. 33:2187–2196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen H, Li M, Wan T, Zheng Q, Cheng M,
Huang S and Wang Y: Design and synthesis of dual-ligand modified
chitosan as a liver targeting vector. J Mater Sci Mater Med.
23:431–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee H, Mok H, Lee S, Oh YK and Park TG:
Target-specific intracellular delivery of siRNA using degradable
hyaluronic acid nanogels. J Control Release. 119:245–252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi KY, Chung H, Min KH, Yoon HY, Kim K,
Park JH, Kwon IC and Jeong SY: Self-assembled hyaluronic acid
nanoparticles for active tumor targeting. Biomaterials. 31:106–114.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiu L, Li Z, Qiao M, Long M, Wang M, Zhang
X, Tian C and Chen D: Self-assembled pH-responsive hyaluronic
acid-g-poly((L)-histidine) copolymer micelles for targeted
intracellular delivery of doxorubicin. Acta Biomater. 10:2024–2035.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bastakoti BP, Liao SH, Inoue M, Yusa SI,
Imura M, Nakashima K, Wu KC and Yamauchi Y: pH-responsive polymeric
micelles with core-shell-corona architectures as intracellular
anti-cancer drug carriers. Sci Technol Adv Mater. 14:0444022013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo Z and Jiang J: pH-sensitive drug
loading/releasing in amphiphilic copolymer PAE-PEG: Integrating
molecular dynamics and dissipative particle dynamics simulations. J
Control Release. 162:185–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiu L, Qiao M, Chen Q, Tian C, Long M,
Wang M, Li Z, Hu W, Li G, Cheng L, et al: Enhanced effect of
pH-sensitive mixed copolymer micelles for overcoming multidrug
resistance of doxorubicin. Biomaterials. 35:9877–9887. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Du H, Liu M, Yu A, Ji J and Zhai G:
Insight into the role of dual-ligand modification in low molecular
weight heparin based nanocarrier for targeted delivery of
doxorubicin. Int J Pharm. 523:427–438. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Di Y, Li T, Zhu Z, Chen F, Jia L, Liu W,
Gai X, Wang Y, Pan W and Yang X: pH-sensitive and folic
acid-targeted MPEG-PHIS/FA-PEG-VE mixed micelles for the delivery
of PTX-VE and their antitumor activity. Int J Nanomedicine.
12:5863–5877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kobayashi T, Ishida T, Okada Y, Ise S,
Harashima H and Kiwada H: Effect of transferrin receptor-targeted
liposomal doxorubicin in P-glycoprotein-mediated drug resistant
tumor cells. Int J Pharm. 329:94–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iyer AK, Khaled G, Fang J and Maeda H:
Exploiting the enhanced permeability and retention effect for tumor
targeting. Drug Discov Today. 11:812–818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Björnmalm M, Thurecht KJ, Michael M, Scott
AM and Caruso F: Bridging Bio-Nano Science and Cancer Nanomedicine.
ACS Nano. 11:9594–9613. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Ma W, Guo Q, Li Y, Hu Z, Zhu Z,
Wang X, Zhao Y, Chai X and Tu P: The effect of dual-functional
hyaluronic acid-vitamin E succinate micelles on targeting delivery
of doxorubicin. Int J Nanomedicine. 11:5851–5870. 2016. View Article : Google Scholar : PubMed/NCBI
|