Introduction
Glioblastoma (GBM) is the most malignant and
frequent brain tumor, which is characterized as having high
recurrence and poor outcome (1).
At present, specific therapeutic strategies, including
radiotherapy, surgery and chemotherapy, have been developed for GBM
treatment (2). However, the
outcomes of patients with GBM are very poor. At present, the 5-year
overall survival rate of patients with GBM remains less than 3%
(3). GBM has become a principal
public health problem (4).
Therefore, there it is imperative to search for novel therapeutic
targets and develop efficient therapeutic approaches for GBM
intervention.
MicroRNAs (miRNAs) are a class of short and
non-coding RNAs, which have a length of ~22 nucleotides (5). Previous findings demonstrated that
miRNAs are expressed in almost all types of cells and regulate gene
expression by binding to the 3′-untranslated region (3′-UTR) of
target mRNAs for degradation (6).
In past decades, accumulating evidence suggests that miRNAs are
involved in a diversity of biological processes, including cell
proliferation, migration and survival (7–9). Due
to their important physiological functions, dysregulation of miRNAs
usually leads to human cancer, including GBM (10). Previous studies suggest that miRNAs
may be promising biomarkers and therapeutic targets for tumor
diagnosis, prognosis and treatment (11,12).
Improved understanding regarding the function and mechanism of
miRNAs in GBM progression may contribute to the development of
novel strategies for GBM treatment.
MicroRNA-3666 (miR-3666) has been demonstrated to
inhibit the progression of non-small cell lung cancer (13) and thyroid carcinoma (14). Whether miR-3666 serves a role in
GBM remains largely unknown. In the present study, it was
identified that miR-3666 was significantly downregulated in GBM
tissues and cell lines. In addition, the expression of miR-3666
expression levels is associated with the prognosis of patients with
GBM. Furthermore, it was demonstrated that overexpression of
miR-3666 significantly suppressed the proliferation, migration and
invasion. Regarding the underlying mechanisms, it was observed that
miR-3666 targeted lysine-specific demethylase 2A (KDM2A). By
inhibition of KDM2A expression, miR-3666 decreased the
proliferation, migration and invasion of GBM cells. Collectively,
the present study, to the best of our knowledge, for the first time
identified the key function of the miR-3666/KDM2A axis in GBM,
which suggested that miR-3666 may be a promising therapeutic target
for GBM intervention.
Patients and methods
Patient samples
The protocol of the present study and acquisition of
tissue specimens was approved by the Biomedical Research Ethics
Committee of The Affiliated Huaian No. 1 Hospital of Nanjing
Medical University (Huai'an, China). A total of 38 GBM tissue
specimens (21 males and 17 females; age range, 31–61 years old;
median age, 46 years old) were obtained from patients who received
surgical treatment at The Affiliated Huai'an No. 1 Hospital of
Nanjing Medical University between September 2014 and October 2016.
Patients who had previously undergone chemotherapy and radiotherapy
were excluded. All enrolled patients signed a written informed
consent document. To analyze overall survival, these samples were
divided into two groups (miR-3666 low group and miR-3666 high
group) according to the median value of miR-3666 expression.
Cell culture
The human GBM cell lines (A172, U251 and T98G) were
obtained from the American Type Culture Collection (Manassas, VA,
USA). Human astrocyte cell line HA was from Lonza Group, Ltd.
(Basel, Switzerland). All cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin G and 100 µg/ml streptomycin (Sigma-Aldrich; Merck
KGaA). Normal human astrocytes (NHAs), obtained from Lonza Group,
Ltd., were cultured in the provided astrocyte growth media and 5%
FBS. Cells were incubated in a humidified atmosphere with 5%
CO2 at 37°C.
Transfection
The U251 cells (2×106) were seeded in
6-well plates and cultured for 18 h prior to transfection.
Transfection was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. A total of 48 h post-transfection,
subsequent experiments were performed. RNA with no homology to any
human genomic sequence was regarded as the negative control
(miR-NC). The miR-3666 mimics (5′-CAGUGCAAGUGUAGAUGCCGA-3′; 50 nM)
and small interfering (si)-KDM2A (5′-GCCAUCUUCCGUUGCAAAG-3′; 50 nM)
sequences were designed and synthesized by Shanghai GenePharma Co.,
Ltd. (Shanghai, China).
Cell proliferation
For the Cell Counting kit-8 (CCK-8) assay,
2×103 U251 cells per well were seeded in 96-well plates
and the cells were subsequently cultured for 24, 48 and 72 h prior
to performing the CCK-8 assay (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Following incubation with CCK-8 at 37°C,
absorbance (optical density value) at a wavelength of 450 nm was
detected and used to calculate cell viability.
Colony formation
A total of 2×103 U251 cells per well were
seeded into 6-well plates. Cells were cultured at 37°C for 14 days.
Formed clones were then fixed with 4% polyformaldehyde for 30 min
at 25°C, stained with 0.1% crystal violet for 20 min at 25°C. Cell
numbers in five random fields were counted using a light microscope
(magnification, ×100).
Cell cycle analysis
A total of 1×106 cells were harvested,
washed twice with ice-cold PBS and then fixed in 70% ethanol for 24
h at 4°C. Cells were subsequently washed three times with ice cold
PBS and incubated with 1 mg/ml RNase A (cat. no. R6148; Sigma
Aldrich; Merck KGaA) for 30 min at 37°C. Following this, cells were
stained at 25°C for 10 min with 50 µg/ml propidium iodide (BD
Biosciences, Franklin Lakes, NJ, USA) in 0.5% Tween-20 with PBS and
subjected to analysis of cell cycle distribution using a BD FACScan
flow cytometer (BD Biosciences) coupled with Cell Quest acquisition
and analysis programs (version 2; BD Biosciences).
Bioinformatics analysis
The Target Scan tool (version 7.1; http://www.targetscan.org/index.html)
was used to predict the potential targets of miR-3666.
Western blot analysis
U251 cells were lysed in cold
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.), and the protein concentration was determined using a
Bicinchoninic Acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein (40 µg/lane) was separated via 10%
SDS-PAGE and then transferred to a polyvinylidene difluoride (PVDF)
membrane (Thermo Fisher Scientific, Inc.). Following this, the
membrane was blocked using 5% non-fat milk in PBS (Thermo Fisher
Scientific, Inc.) containing 0.1% Tween-20 (Sigma-Aldrich; Merck
KGaA) at room temperature for 3 h. Subsequently, the PVDF membrane
was incubated with rabbit anti-human polyclonal KDM2A (1:1,000;
cat. no. ab191387; Abcam, Cambridge, MA, USA) and rabbit anti-human
GAPDH (1:1,000; cat. no. ab9485; Abcam) primary antibodies at room
temperature for 2 h. Following washing with PBS for 10 min, the
PVDF membrane was incubated with horseradish peroxidase-tagged goat
anti-rabbit secondary antibodies (1:5,000; cat. no. ab7090; Abcam)
at room temperature for 1 h. Membranes were then washed with PBS
for 10 min, and the protein bands were visualized using an Enhanced
Chemiluminescence Western Blotting kit (Pierce; Thermo Fisher
Scientific, Inc.), in accordance with the manufacturer's protocol.
Protein densitometry was performed using ImageJ software (version
1.41; National Institutes of Health, Bethesda, MD, USA).
Transwell migration and Matrigel
invasion assays
U251 cells were seeded in 24-well culture plates at
a density of 1×105 cells/well and subsequently cultured
at 37°C for 18 h prior to transfection. Lipofectamine®
2000 RNAiMAX was added at a density of 1.5 µl/well and either
miR-3666 mimics or si-KDM2A was subsequently added (15 pM/well).
Cells were harvested 48 h post-transfection. Following the
manufacturer's protocol, 2×104 cells with 100 µl
serum-free DMEM (Thermo Fisher Scientific, Inc.) were seeded into
the upper chamber of the Transwell plates (Costar; Corning
Incorporated, Corning, NY, USA) for the migration assays, whereas,
cells with 100 µl serum-free DMEM were plated into the upper
chamber of an insert coated with Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA) for the invasion assays. The lower chambers were
filled with 600 µl DMEM containing 10% FBS. Following 48 h of
incubation, the cells remaining on the upper membrane were removed
with cotton swabs, whereas, those that had migrated or invaded
through the membrane were fixed in 4% polyformaldehyde for 30 min
at 25°C and stained with 0.1% crystal violet for 20 min at 25°C.
The number of cells was calculated by imaging five random
fields/filters using a fluorescence inversion microscope system
(Nikon Corporation, Tokyo, Japan) at a magnification of ×200. All
the experiments were performed at least three times
independently.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of GBM samples and cultured cells were
extracted with TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
cDNA was synthesized from isolated RNA using a TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. qPCR
was performed with a TaqMan MicroRNA Assay kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) on an ABI7300 PCR detection system.
The thermocycling conditions were as follows: Denaturation at 95°C
for 10 min; followed by 40 cycles of denaturation at 95°C for 15
sec and elongation at 60°C for 1 min. The expression levels of
miR-3666 and KDM2A were normalized with U6 or GAPDH. The RT-qPCR
primer sequences were as follows: miR-3666 forward,
A5′-ACGAGACGACGACAGAC-3′ and reverse, 5′-CAGTGCAAGTGTAGATGCCGA-3′;
U6 forward, 5′-AACGAGACGACGACAGAC-3′ and reverse,
5′-GCAAATTCGTGAAGCGTTCCATA-3′; KDM2A forward,
5′-GTGACGCAGCAGCATTGTTC-3′; and reverse,
5′-CAGACTACCCAGAGGGAGCA-3′; and GAPDH forward,
5′-ATGTTGCAACCGGGAAGGAA-3′ and reverse, 5′-AGGAAAAGCATCACCCGGAG-3′.
Quantification was performed using the 2−∆∆Cq method
(15).
Luciferase reporter assay
To investigate whether downregulation of KDM2A by
miR-3666 is caused by the direct interaction between the miRNA seed
sequence and the 3′UTR of the target mRNA, pmirGLO dual-luciferase
vectors (cat. no. E133A; Promega, Corporation, Madison, WI, USA)
containing the 3′UTR of KDM2A mRNA were constructed, pmirGLO-KDM2A
3′UTR. Two individual vectors were prepared, containing wild-type
(WT) or mutated seed region sequences of the miR-3666-binding site
in the 3′UTR of KDM2A mRNA. U251 cells were transfected with NC or
miR-3666 mimics in addition to either WT or mutant-type luciferase
vector using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). A total of 48 h post-transfection, the
effect of the miR-3666 on luciferase expression was assessed using
a Dual-GLO™ Luciferase Assay System (cat. no. E2940;
Promega, Corporation). Luciferase activity was normalized to
Renilla luciferase activity.
Statistical analysis
Each experiment was repeated at least three times.
Data are expressed as the mean ± standard deviation. All
statistical analyses were performed using SPSS 20.0 (IBM Corp.,
Armonk, NY, USA) and GraphPad Prism (version 6; GraphPad Software,
Inc., La Jolla, CA, USA). The Kaplan-Meier method was used to
calculate the survival curve, and log-rank test to determine
statistical significance. Student's t-test and one-way analysis of
variance followed by Tukey's post hoc test were used to analyze two
or multiple groups, respectively, for statistical significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-3666 is downregulated in GBM
tissues
miRNAs have been demonstrated to be important
regulators in human cancer. In order to examine the function of
miR-3666 in GBM, its expression patterns were analyzed by RT-qPCR.
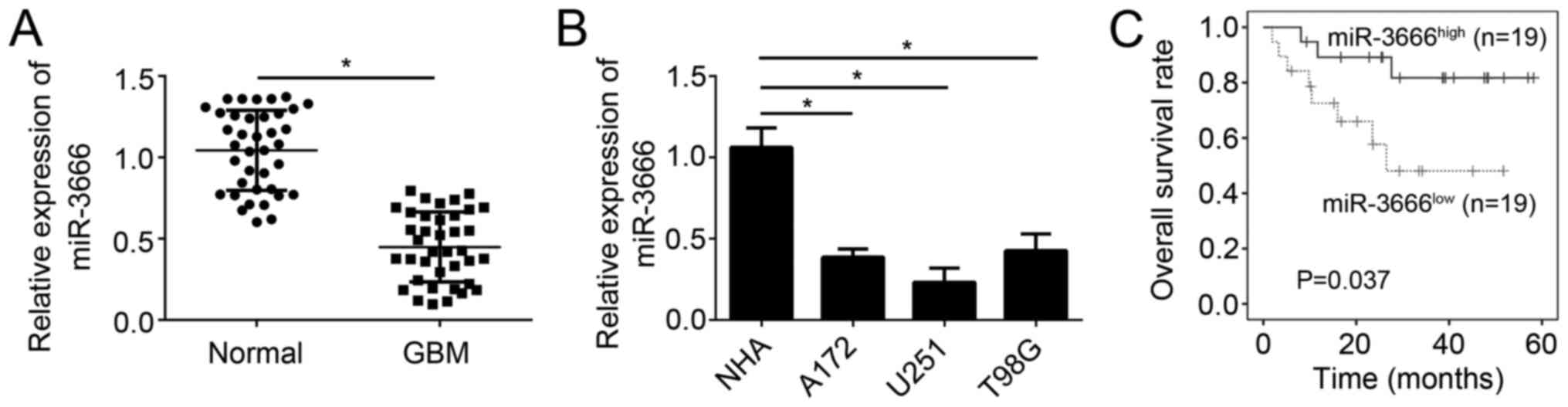
The results demonstrated that miR-3666 expression was significantly
decreased in GBM tissues (n=38) compared with adjacent normal
tissues (n=38; Fig. 1A;
P<0.05). The expression of miR-3666 was additionally assessed in
GBM cell lines. RT-qPCR analysis demonstrated that miR-3666
expression was significantly downregulated in U251, A172 and T98G
cells compared with NHAs (Fig. 1B;
P<0.05). Furthermore, to determine whether miR-3666 expression
is able to serve as a biomarker for GBM prognosis, these GBM
samples were divided into two groups based on miR-3666 expression
levels. Kaplan-Meier survival analysis revealed that higher
expression of miR-3666 in patients with GBM was associated with
higher survival rate (Fig. 1C).
Collectively, these results demonstrated miR-3666 was downregulated
in GBM tissues, which suggested its dysregulation may contribute to
GBM progression.
miR-3666 suppresses GBM cell
proliferation and cell cycle progression
To determine the physiological roles of miR-3666 in
GBM, miR-3666 was overexpressed in U251 cells by transfection with
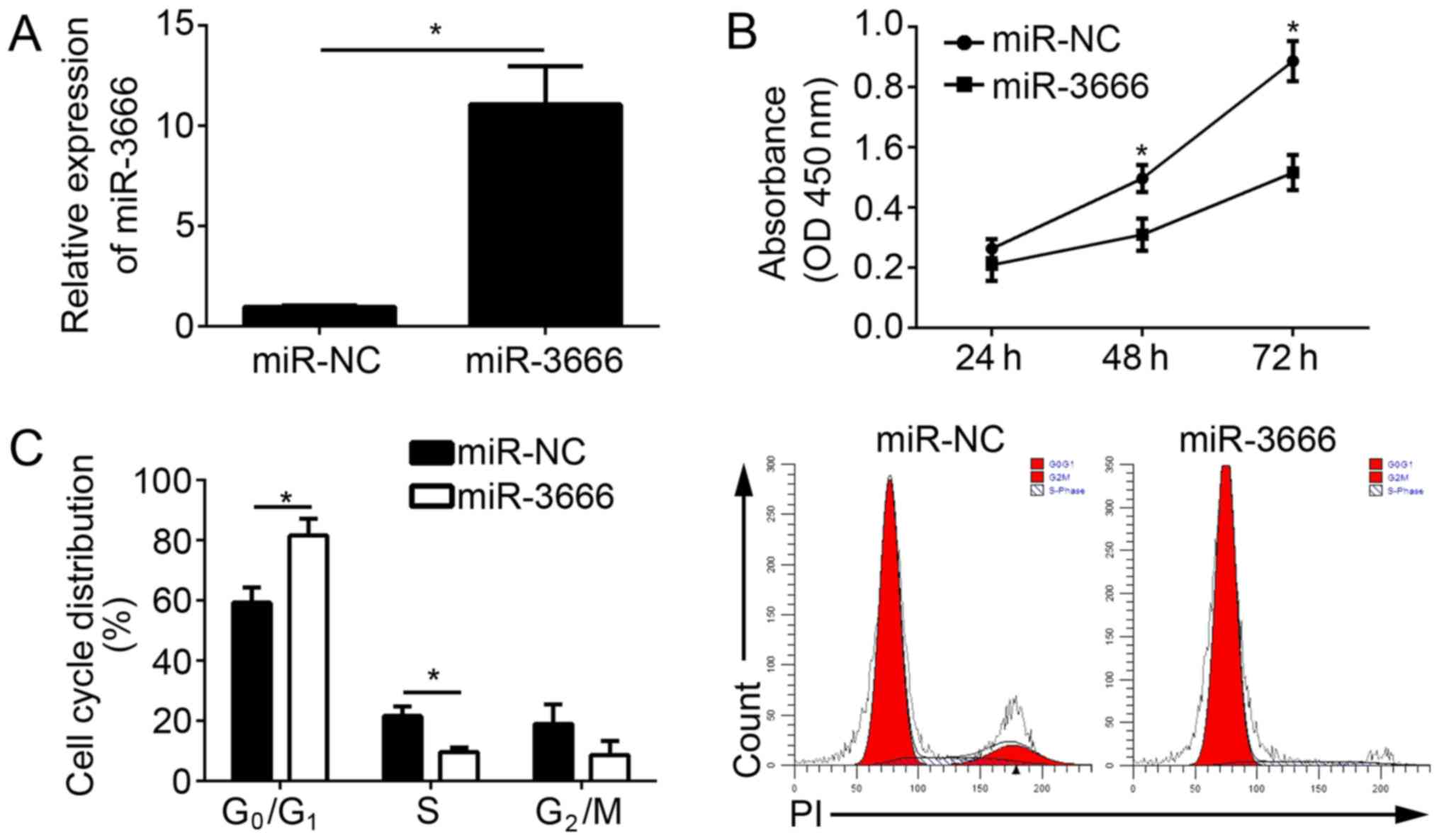
miR-3666 mimics. The RT-qPCR results indicated that miR-3666 was
significantly upregulated in U251 cells transfected with miR-3666
mimics compared with the miR-NC group (Fig. 2A; P<0.05). CCK-8 assays were
conducted to analyze the effect of miR-3666 on cell proliferation.
The results demonstrated that the overexpression of miR-3666
suppressed the proliferation of U251 cells (Fig. 2B) and decreased the number of
colonies (data not shown). To determine whether impaired
proliferation by miR-3666 was induced by an aberrant cell cycle,
the cell cycle distribution in U251 cells was measured by
fluorescence-activated cell sorting (FACS). The results
demonstrated that the overexpression of miR-3666 significantly
increased the cells in G0/G1 phase and
significantly decreased the number of cells in the S phase
(Fig. 2C; P<0.05). In
conclusion, the data suggested that miR-3666 suppressed the
proliferation and cell cycle progression of GBM cells.
miR-3666 inhibits the migration and
invasion of GBM cells
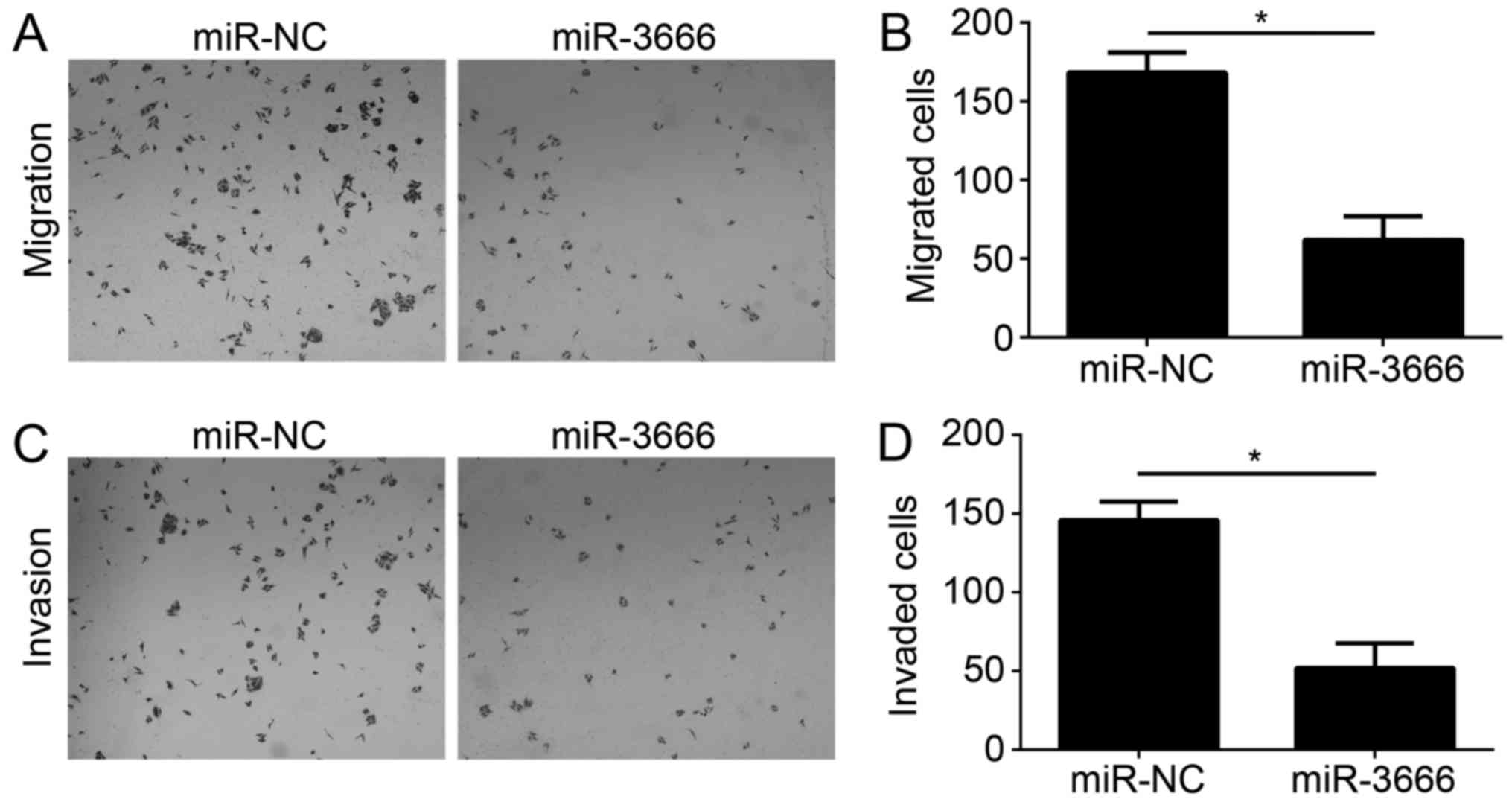
Tumor metastasis is a principal cause of malignancy.
Therefore, the effect of miR-3666 on GBM cell migration and
invasion was analyzed. Transwell migration and Matrigel invasion
assays were conducted with U251 cells transfected with miR-NC or
miR-3666. The results demonstrated that ectopic expression of
miR-3666 significantly decreased the number of migrated and invaded
cells (Fig. 3A; P<0.05).
KDM2A is a target of miR-3666
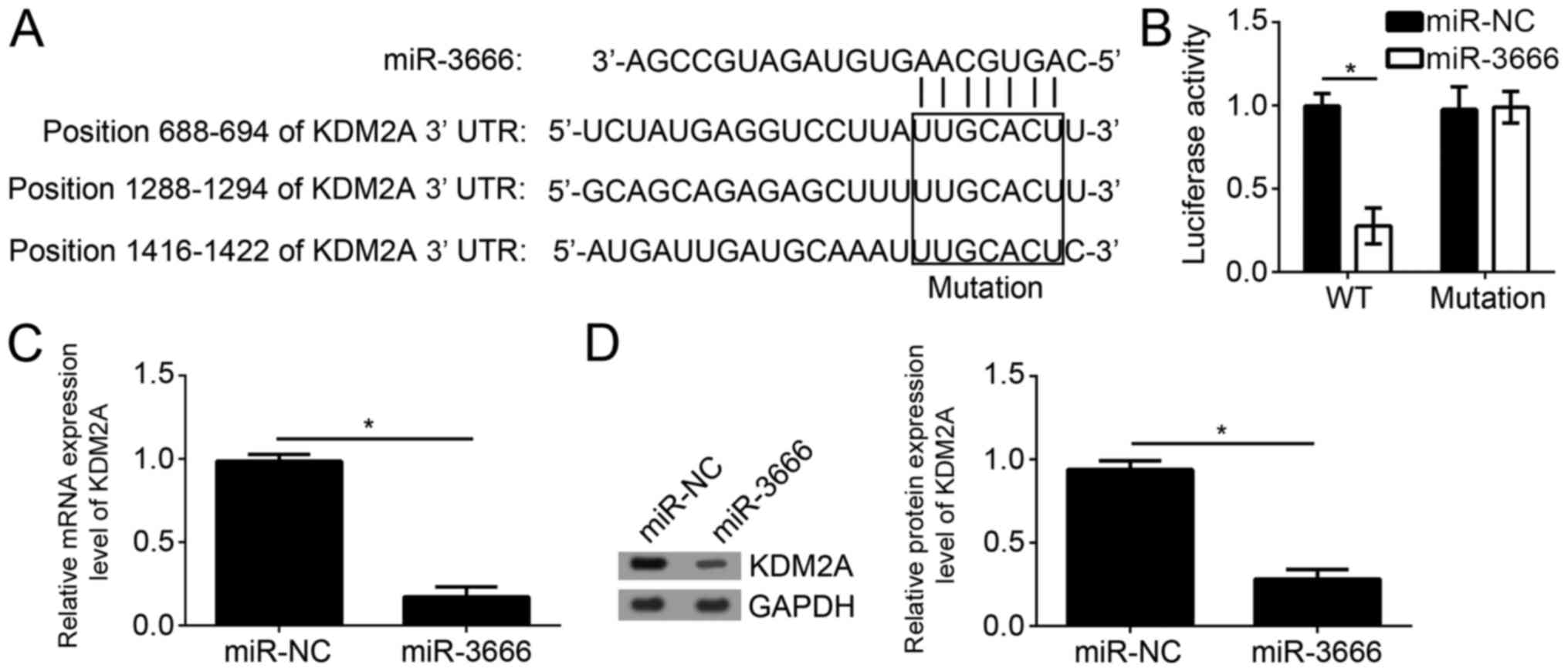
To further examine the mechanism of miR-3666, the
target genes of miR-3666 were searched for in GBM cells. By
bioinformatics analysis using TargetScan7 (http://www.targetscan.org/vert_71/), it was identified
that KDM2A was one of the most potential targets. There are three
potential binding sites of miR-3666 in the 3′-UTR region of KDM2A
(Fig. 4A). To verify the direct
interaction between miR-3666 and KDM2A mRNA, luciferase reporter
assays were performed with the reporter plasmid containing the WT
or mutant 3′-UTR region of KDM2A mRNA. The results suggested that
overexpression of miR-3666 significantly decreased the luciferase
activity in U251 cells transfected with WT reporter plasmid;
however, not the mutant plasmid (Fig.
4B; P<0.05). Furthermore, it was observed that
overexpression of miR-3666 significantly downregulated the mRNA
expression level of KDM2A in U251 cells (Fig. 4C; P<0.05). Consistently, western
blot analysis additionally suggested that miR-3666 overexpression
significantly inhibited the protein expression level of KDM2A in
U251 cells (Fig. 4D;
P<0.05).
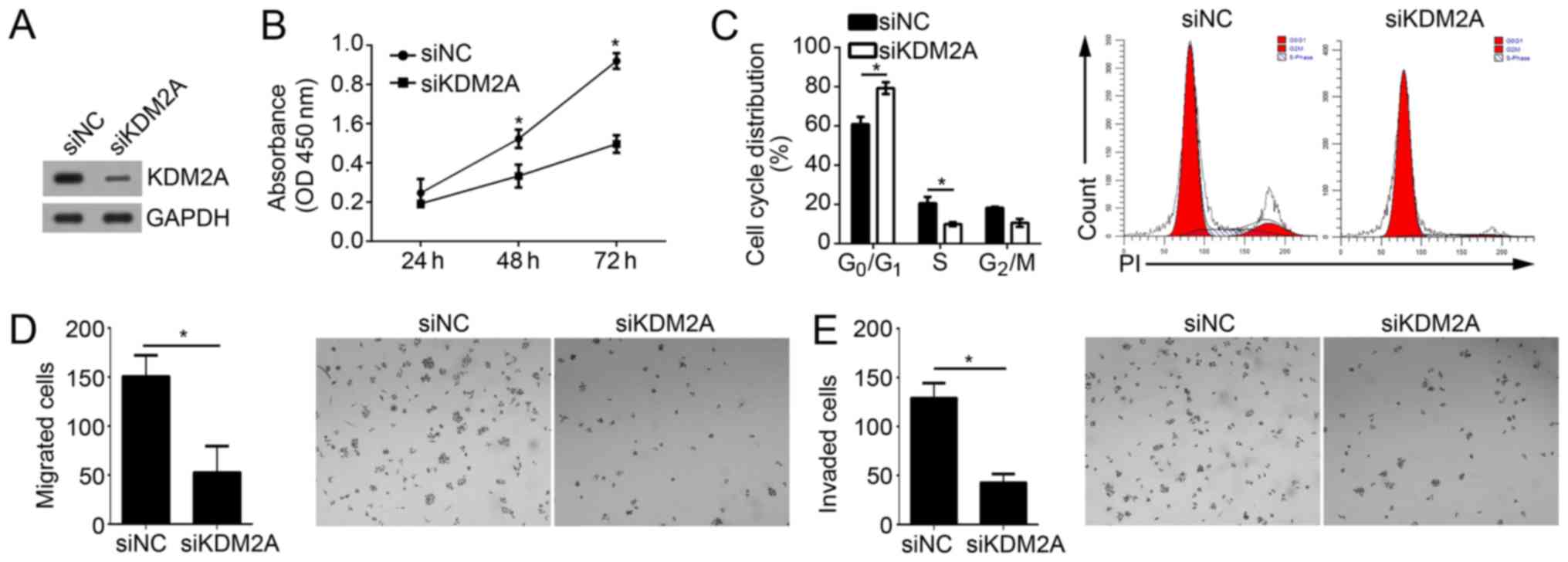
Knockdown of KDM2A suppresses the
proliferation, migration and invasion of GBM cells
To determine whether KDM2A is important in
miR-3666-mediated inhibition of GBM cell proliferation, migration
and invasion, the effect of KDM2A on these activities in U251 cell
was investigated. Upon transfection with specific siRNA against
KDM2A, the KDM2A expression level was effectively knocked down in
U251 cells (Fig. 5A). CCK-8 assays
demonstrated that knockdown of KDM2A suppressed the proliferative
ability of U251 cells (Fig. 5B).
FACS analysis suggested that KDM2A knockdown additionally resulted
in a significant decrease of the cells in S phase and a significant
increase of cells in the G0/G1 phase
(Fig. 5C; P<0.05). Furthermore,
Transwell and Matrigel assays indicated that KDM2A depletion
significantly decreased the abilities of migration and invasion in
U251 cells (Fig. 5D and E;
P<0.05). Collectively, these results suggested that KDM2A
knockdown inhibited the proliferation, migration and invasion of
GBM cells, which suggested that miR-3666 regulates GBM progression
by targeting KDM2A at least partially.
Discussion
The present results demonstrated that miR-3666
serves as a tumor suppressor in GBM cells. Additionally, it was
identified that KDM2A may be a direct target of miR-3666 in U251
cells. Overexpression of miR-3666 or knockdown of KDM2A suppressed
the proliferation, migration and invasion, which suggested that
miR-3666 suppressed the progression of GBM by targeting KDM2A at
least in part.
miRNAs are a group of short regulatory non-coding
RNAs that participate in the regulation of post-transcriptional
gene expression by binding to complementary sequences (16). Accumulating evidence suggested that
miRNAs are involved in various cell processes, including cell
proliferation, apoptosis, migration and invasion (17). Dysregulation of miRNAs is reported
in human cancer, including colorectal cancer (18), glioma (19), cholangiocarcinoma (20), breast cancer (21), non-small cell lung cancer (22), esophageal squamous carcinoma
(23), gastric cancer (24), papillary thyroid cancer (25) and renal cell carcinoma (26). An increasing number of studies
suggest that miRNAs are potential biomarkers for cancer diagnosis
and prognosis, and effective therapeutic targets for tumor
treatments (26–28). Numerous miRNAs have been reported
to regulate the development and progression of GBM (27). For example, Wang et al
(10) reported that miRNA-598
inhibits cell proliferation and invasion of GBM by directly
targeting metastasis associated in colon cancer-1. In addition,
Chen et al (28)
demonstrated that downregulation of miR-205 is associated with GBM
cell migration, invasion and the epithelial-mesenchymal transition,
by targeting zinc finger E-box-binding homeobox 1 via the protein
kinase B/mammalian target of rapamycin signaling pathway. miR-3666
has been demonstrated to inhibit the progression of non-small cell
lung cancer (13) and thyroid
carcinoma (14). However, its
exact role and underlying mechanisms of miR-3666 in GBM require
investigation. In the present study, it was observed that miR-3666
was significantly downregulated in GBM tissues. Furthermore, it was
demonstrated that overexpression of miR-3666 inhibited cell
proliferation, migration and invasion, and arrested cell cycle
progression in U251 cells.
KDM2A is a histone H3 lysine 36 demethylase and
consists of an F-box, a JmjC domain, a CXXC zinc finger, a PHD
domain and three leucine-rich repeat elements (29). A previous study suggested that
KDM2A exerts an oncogenic role in a number of cancer types
(29). Chen et al (30) demonstrated that KDM2A represses
TET2 to increase DNA methylation and dowregulation of tumor
suppressor genes in breast cancer. Huang et al (31) reported that KDM2A enhanced tumor
cell growth and migration in gastric cancer. In addition, Wagner
et al (32) suggested that
KDM2A promotes lung tumorigenesis by epigenetically enhancing
extracellular signal-regulated kinase 1/2 signaling. However, the
function of KDM2A in GBM has not been defined. In the present
study, it was identified that KDM2A is a direct target of miR-3666
in GBM cells. It was demonstrated that overexpression of miR-3666
significantly decreased the mRNA and protein expression levels of
KDM2A in U251 cells. Furthermore, with functional experiments, it
was observed that KDM2A silencing significantly suppressed the
proliferation, migration and invasion of U251 cells, which
suggested that KDM2A is responsible for the function of miR-3336 in
GBM progression at least in part.
In conclusion, the present study, to the best of our
knowledge, for the first time identified the function of miR-3666
in GBM progression, and demonstrated that miR-3666 inhibited the
proliferation, migration and invasion of GBM cell by targeting
KDM2A. The present study suggests that miR-3666 may be a promising
therapeutic target for GBM treatment. However, there were several
limitations in the present study. Whether miR-3666 has an effect on
GBM cell apoptosis was not investigated. Furthermore, the present
study did not use in vivo assays, which are better able to
demonstrate the roles of miR-3666 in GBM. Future studies should
investigate how KDM2A participates in the regulation of GBM
progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS, HY and XS contributed to the conception and
design of the present study, analyzed and interpreted the data, and
wrote the manuscript. JL and DL conducted the experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the protocol for the
present study was approved by the Institutional Ethics Committee of
The Affiliated Huai'an No. 1 Hospital of Nanjing Medical University
and all enrolled patients signed a written informed consent
document.
Patient consent for publication
All patients within the present study provide
consent for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao F, Li M, Kong L, Zhang G and Yu J:
Delineation of radiation therapy target volumes for patients with
postoperative glioblastoma: A review. Onco Targets Ther.
9:3197–3204. 2016.PubMed/NCBI
|
|
2
|
Koekkoek JA, Kerkhof M, Dirven L, Heimans
JJ, Reijneveld JC and Taphoorn MJ: Seizure outcome after
radiotherapy and chemotherapy in low-grade glioma patients: A
systematic review. Neuro Oncol. 17:924–934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bradshaw A, Wickremsekera A, Tan ST, Peng
L, Davis PF and Itinteang T: Cancer stem cell hierarchy in
glioblastoma multiforme. Front Surg. 3:212016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neagu MR and Reardon DA: An update on the
role of immunotherapy and vaccine strategies for primary brain
tumors. Curr Treat Options Oncol. 16:542015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Nie J, Mei Q and Han WD: MicroRNAs:
Novel immunotherapeutic targets in colorectal carcinoma. World J
Gastroenterol. 22:5317–5331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diab M, Muqbil I, Mohammad RM, Azmi AS and
Philip PA: The role of microRNAs in the diagnosis and treatment of
pancreatic adenocarcinoma. J Clin Med. 5(pii): E592016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song H, Rao Y, Zhang G and Kong X:
MicroRNA-384 inhibits the growth and invasion of renal cell
carcinoma cells by targeting astrocyte elevated gene 1. Oncol Res.
Aug 25–2017.(Epub ahead of print).
|
|
8
|
Anninos P, Chatzimichael A, Adamopoulos A,
Kotini A and Tsagas N: A combined study of MEG and pico-Tesla TMS
on children with autism disorder. J Integr Neurosci. 15:497–513.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Qi G, Zhang J, Wu J, Zhou N, Li L
and Ma J: Knockdown of long noncoding RNA small nucleolar RNA host
gene 12 inhibits cell growth and induces apoptosis by upregulating
miR-138 in nonsmall cell lung cancer. DNA Cell Biol. 36:892–900.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang N, Zhang Y and Liang H: microRNA-598
inhibits cell proliferation and invasion of glioblastoma by
directly targeting metastasis associated in colon cancer-1. Oncol
Res. Feb 14–2018.(Epub ahead of print). View Article : Google Scholar
|
|
11
|
Hamam R, Hamam D, Alsaleh KA, Kassem M,
Zaher W, Alfayez M, Aldahmash A and Alajez NM: Circulating
microRNAs in breast cancer: Novel diagnostic and prognostic
biomarkers. Cell Death Dis. 8:e30452017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren J, Yang Y, Xue J, Xi Z, Hu L, Pan SJ
and Sun Q: Long noncoding RNA SNHG7 promotes the progression and
growth of glioblastoma via inhibition of miR-5095. Biochem Biophys
Res Commun. 496:712–718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi H, Ji Y, Zhang D, Liu Y and Fang P:
MicroRNA-3666-induced suppression of SIRT7 inhibits the growth of
non-small cell lung cancer cells. Oncol Rep. 36:3051–3057. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang G, Cai C and Chen L: MicroRNA-3666
regulates thyroid carcinoma cell proliferation via MET. Cell
Physiol Biochem. 38:1030–1039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan J, Zhou K, Tang X, Duan J and Zhao L:
MicroRNA-34a inhibits cell proliferation and induces cell apoptosis
of glioma cells via targeting of Bcl-2. Mol Med Rep. 14:432–438.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hua S, Liu C, Liu L and Wu D: miR-142-3p
inhibits aerobic glycolysis and cell proliferation in
hepatocellular carcinoma via targeting LDHA. Biochem Biophys Res
Commun. 496:947–954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Chen X, Cheng R, Yang F, Yu M, Wang
C, Cui S, Hong Y, Liang H, Liu M, et al: The Jun/miR-22/HuR
regulatory axis contributes to tumourigenesis in colorectal cancer.
Mol Cancer. 17:112018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun G, SiMa G, Wu C, Fan Y, Tan Y, Wang Z,
Cheng G and Li J: Decreased MiR-17 in glioma cells increased cell
viability and migration by increasing the expression of Cyclin D1,
p-Akt and Akt. PLoS One. 13:e01905152018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Utaijaratrasmi P, Vaeteewoottacharn K,
Tsunematsu T, Jamjantra P, Wongkham S, Pairojkul C, Khuntikeo N,
Ishimaru N, Sirivatanauksorn Y, Pongpaibul A, et al: The
microRNA-15a-PAI-2 axis in cholangiocarcinoma-associated
fibroblasts promotes migration of cancer cells. Mol Cancer.
17:102018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tahiri A, Aure MR and Kristensen VN:
MicroRNA networks in breast cancer cells. Methods Mol Biol.
1711:55–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu G, Fu D, Jia C, Chai L, Han Y, Liu J,
Wu T, Xie R, Chang Z, Yang H, et al: Reduced miR-105-1 levels are
associated with poor survival of patients with non-small cell lung
cancer. Oncol Lett. 14:7842–7848. 2017.PubMed/NCBI
|
|
23
|
Mei LL, Qiu YT, Wang WJ, Bai J and Shi ZZ:
Overexpression of microRNA-1470 promotes proliferation and
migration, and inhibits senescence of esophageal squamous carcinoma
cells. Oncol. Lett. 14:7753–7758. 2017.
|
|
24
|
Yang H, Wang L, Tang X and Bai W: miR-203a
suppresses cell proliferation by targeting E2F transcription factor
3 in human gastric cancer. Oncol Lett. 14:7687–7690.
2017.PubMed/NCBI
|
|
25
|
Kan Q, Su Y and Yang H: MicroRNA-335 is
downregulated in papillary thyroid cancer and suppresses cancer
cell growth, migration and invasion by directly targeting ZEB2.
Oncol Lett. 14:7622–7628. 2017.PubMed/NCBI
|
|
26
|
Hu B, Wang J and Jin X: MicroRNA-138
suppresses cell proliferation and invasion of renal cell carcinoma
by directly targeting SOX9. Oncol Lett. 14:7583–7588.
2017.PubMed/NCBI
|
|
27
|
Zhang G, Chen L, Khan AA, Li B, Gu B, Lin
F, Su X and Yan J: miRNA-124-3p/neuropilin-1 (NRP-1) axis plays an
important role in mediating glioblastoma growth and angiogenesis.
Int J Cancer. 143:635–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen W, Kong KK, Xu XK, Chen C, Li H, Wang
FY, Peng XF, Zhang Z, Li P, Li JL and Li FC: Downregulation of
miR-205 is associated with glioblastoma cell migration, invasion,
and the epithelial-mesenchymal transition, by targeting ZEB1 via
the Akt/mTOR signaling pathway. Int J Oncol. 52:485–495.
2018.PubMed/NCBI
|
|
29
|
Kong Y, Zou S, Yang F, Xu X, Bu W, Jia J
and Liu Z: RUNX3-mediated up-regulation of miR-29b suppresses the
proliferation and migration of gastric cancer cells by targeting
KDM2A. Cancer Lett. 381:138–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JY, Luo CW, Lai YS, Wu CC and Hung
WC: Lysine demethylase KDM2A inhibits TET2 to promote DNA
methylation and silencing of tumor suppressor genes in breast
cancer. Oncogenesis. 6:e3692017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Y, Liu Y, Yu L, Chen J, Hou J, Cui
L, Ma D and Lu W: Histone demethylase KDM2A promotes tumor cell
growth and migration in gastric cancer. Tumour Biol. 36:271–278.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagner KW, Alam H, Dhar SS, Giri U, Li N,
Wei Y, Giri D, Cascone T, Kim JH, Ye Y, et al: KDM2A promotes lung
tumorigenesis by epigenetically enhancing ERK1/2 signaling. J Clin
Invest. 123:5231–5246. 2013. View
Article : Google Scholar : PubMed/NCBI
|