Introduction
Colorectal cancer (CRC) is one of the common
malignancies, with an estimation of 693,900 deaths in 2012
(1). Patients with CRC are
frequently diagnosed at an advanced stage due to lack of effective
diagnostic biomarkers and the prognosis is unsatisfactory even with
comprehensive therapies (2–4).
Thus, it is necessary to identify novel biomarkers and therapeutic
targets for CRC patients. Identification of crucial molecules will
help to accelebrate the research on CRC pathogenesis.
With the improvement of high-resolution microarray
and RNA sequencing technology, it has been verified that non-coding
RNAs (ncRNAs) occupies a higher rate of 98% in transcripts of human
genome (5–7). Long non-coding RNAs (lncRNAs), newly
identified counterparts of ncRNAs, have been demonstrated to be
dysregulated and serve as critical regulators in various tumors
(8–10). Current investigations have focused
on the pathogenesis of lncRNAs in multiple cancers (11–14).
Dysregulation of lncRNAs can alter the process of many biological
events, such as cell cycle, apoptosis, invasion, and epigenetic
regulation (15,16). Nie et al (17), found an obvious upregulation of
lncRNA ANRIL in non-small-cell lung cancer (NSCLC) tissue samples.
ANRIL acts as an oncogene in CRC partly via decreasing p21 and
Kruppel like factor 2 (KLF2) expression (17). In contrast, increased lncRNA-LET
inhibits cell proliferation, invasion, and migration in lung cancer
(18). Thus, lncRNAs may be
oncogenes or tumor suppressors to mediate cancer progression. There
is a critical need to investigate correlations between lncRNAs and
carcinogenesis, especially CRC. However, only a small portion of
lncRNAs have been functionally studied, more lncRNAs should be
identified (19).
To detect aberrantly expressed lncRNAs associated
with CRC progression, we analyzed The Cancer Genome Atlas (TCGA)
colon cancer and normal tissue RNA sequencing data (49 normal and
648 cancer samples), and focused on a remarkably overexpressed
lncRNA termed MLK7 antisense RNA 1 (MLK7-AS1). LncRNA MLK7-AS1 is
located on human chromosome 2 with a length of 2551 bp. It was
firstly reported to be significantly upregulated in gastric cancer
(GC) tissues (20). Further,
MLK7-AS1 can serve as an independent prognosis indicator in GC
patients (20). However, the
expression pattern, functional role, and clinical significance of
MLK7-AS1 in CRC still remain uncharacterized. In this study, we
show the first reporting of expression pattern and funtional role
associated with MLK7-AS1 in CRC. MLK7-AS1 is significantly
increased in CRC tissues and cells. Further, MLK7-AS1
overexpression exhibits tight associations with clinicopathologic
factors in CRC patients. In vitro and in vivo
experiments suggested that decreased MLK7-AS1 expression inhibited
cell proliferative capacities, and promoted cell cycle arrest and
apoptosis in CRC. Consistently, MLK7-AS1 overexpression showed
opposite effects.
Cyclin-dependent kinase inhibitors (CKIs) are known
to modulate cell cycle progression and function as tumor
suppressors (21,22). p21 is an essential member of CKIs
family, which includes p15, p16, p27 and p57 (21). Mounting studies reveal that lncRNAs
can alter cancer cell phenotypes through silencing tumor
suppressors and CKIs are involved in biological functions induced
by lncRNAs (10,23,24).
Thus, we further investigated the alteration of CKIs family
expression levels in DLD-1 and LOVO cells with MLK7-AS1 knockdown.
Our findings revealed that decreased MLK7-AS1 expression levels
remarkably activated p21 expression at both mRNA and protein
levels. Therefore, p21 is partly involved in MLK7-AS1-induced
proliferation in CRC. Overall, MLK7-AS1 has potential as a
biomarker for CRC patients and promotes CRC cells proliferation
partly through suppressing p21 expression.
Materials and methods
Expression profiling data retrieval
and analysis of lncRNAs in colorectal cancer
Expression profiling data of CRC and normal tissue
samples were downloaded from the TCGA dataset. The expression data
are collected from cancer patients before therapeutic intervention
(25). The BAM files and
normalized probe-level intensity files can be achieved from the
Atlas of Non-coding RNAs in Cancer (TANRIC, http://bioinformatics.mdanderson.org/main/TANRIC:Overview)
database (25). Gene annotations
accords to GENCODE Release 19 annotation for lncRNAs.Reads per
kilobase per million mapped reads (RPKM) values were calculated
using TCGA RNA-sequencing data in the BAM files.
Tumor tissue samples and matched
non-tumor tissue samples
The CRC tissues (n=50) and matched non-tumor tissue
samples (n=50) were obtained from CRC patients with surgical
resection in Cancer Hospital of China Medical University. The
patients who participated in this study do so in the context of
informed consents. Median value was selected as cut-off point to
better compare correlations between clinical pathological factors
and the expression level of certain gene in patients with cancer
(10,26,27).
In the present study, we divided 50 PC patients into two groups:
the high MLK7-AS1 group (n=25, fold change above the median value);
and the low MLK7-AS1 group (n=25, fold change below the median
value) according to the median value of MLK7-AS1 levels. The study
was approved by the Cancer Hospital of China Medical University
Ethics Committee. The collected tissue samples were instantly
stored in a liquid nitrogen, and then transferred to be kept at
−80°C.
Cell culture
Human colonic epithelial cells (HCoEpiC) and CRC
cells (SW480, HCT116, LOVO and DLD-1) are all cultured in DMEM
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 mg/ml streptomycin, and 100 U/ml penicillin (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C with 5% O2.
Reverse transcription-quantitative
polymerase chain reaction PCR (RT-qPCR) analyses
A Reverse Transcription Kit (Thermo Fisher
Scientific Inc.) was used to reverse total RNA into cDNA. Then,
cDNA and primers were used to perform qRT-PCR assays on 7500
Real-Time PCR System (Thermo Fisher Scientific Inc.). The specific
primers are listed in Table II.
The results downloaded from this instrument were then normalized to
GAPDH expression. The collected data were analyzed, expressed
relative to threshold cycle values, and then switched to fold
changes. Each sample was analyzed in triplicate.
 | Table II.Sequences of specific primers for
reverse transcription-quantitative polymerase chain reaction. |
Table II.
Sequences of specific primers for
reverse transcription-quantitative polymerase chain reaction.
| Primer sequences
(5′-3′) |
|---|
|
|---|
| Gene | Forward | Reverse |
|---|
| MLK7-AS1 |
CAGCCTCCCGAGTTGAGTAA |
CAAATGACACGAGCCTTCCT |
| GAPDH |
GAAGAGAGAGACCCTCACGCTG |
ACTGTGAGGAGGGGAGATTCAGT |
| p15 |
ACGGAGTCAACCGTTTCGGGAG |
GGTCGGGTGAGAGTGGCAGG |
| p16 |
ATGGAGCCTTCGGCTGACT |
GGCCTCCGACCGTAACTATT |
| p21 |
CAGCAGAGGAAGACCATGTG |
GGCGTTTGGAGTGGTAGAAA |
| p27 |
TGCAACCGACGATTCTTCTACTCAA |
CAAGCAGTGATGTATCTGATAAACAAGG |
| p57 |
CACGATGGAGCGTCTTGTC |
CCTGCTGGAAGTCGTAATCC |
Cell transfection
CRC cell lines were transfected with three
individual MLK7-AS1 (MLK7-AS1 no. 1, no. 2 and no. 3), scrambled
negative control (NC) small interfering RNAs (siRNAs), as well as
vectors such as pcDNA-MLK7-AS1, empty vector, and
sh-MLK7-AS1-vector. SiRNAs were transfected into cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Plasmid vectors were extracted by DNA Midiprep kit (Qiagen GmbH,
Hilden, Germany) and transfected into cells using Fugene (Roche
Diagnostics, Basel, Switzerland). The full-length complementary DNA
of MLK7-AS1 was synthesized by Realgene (China), and subcloned into
the pcDNA3.1(+) vector (Invitrogen; Thermo Fisher Scientific,
Inc.). Up to transfection after 48 h, collected cells were used to
conduct qRT-PCR and western blot experiments. The sequences for
siRNAs and shRNAs are listed in Table III.
 | Table III.Sequences of siRNAs and shRNAs. |
Table III.
Sequences of siRNAs and shRNAs.
| siRNAs |
|
|
si-MLK7-AS1 no. 1 |
GAGAGUACUUUGGUCACCACGGGAA |
|
si-MLK7-AS1 no. 2 |
CCAAGGGCUCUGUUCAUAAACUGUU |
|
si-MLK7-AS1 no. 3 |
CCAAGCUACUUGUAAUCCUUCCAAA |
| shRNAs |
|
|
sh-MLK7-AS1 no. 1 |
CACCGTTCCCGTGGTGACCAAAGTACTCTCCGAAGAGAGTACTTTGGTCACCACGGGAA |
|
sh-MLK7-AS1 no. 2 |
CACCGAACAGTTTATGAACAGAGCCCTTGGCGAACCAAGGGCTCTGTTCATAAACTG |
|
sh-MLK7-AS1 no. 3 |
CACCGTTTGGAAGGATTACAAGTAGCTTGGCGAACCAAGCTACTTGTAATCCTTCCAAA |
Assays of MTT and colony
formation
The cells are cultured in 96-well plates. MTT
experiments were conducted to test cell viability at
490-nm-wavelength by Microplate Reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). For assays of colony formation, cells with
transfection after 48 h are placed in six-well plates. Up to 14
days, methanol-fixed colonies were stained with crystal violet of
0.1% and then colony numbers in each group can be counted.
EdU assay
5-ethynyl2-deoxyuridine (EdU) labeling/detection kit
(Guangzhou RiboBio Co., Ltd., Guangzhou, China) was used to assess
proliferating cells. SW480 and LOVO cells were cultivated in
96-well plates and transfected with siRNAs for 48 h. Then, the
cells were cultured in EdU labeling medium and incubated for 2 h at
37°C under 5% CO2. After treatment with 4%
paraformaldehyde and 0.5% Triton X-100, anti-EdU working solution
was ued to stain cells. And DAPI was used to label cell nuclei. The
percentage of EdU-positive cells was calculated from five random
fields in three wells.
Flow cytometry
DLD1 and LOVO cells with 48 h transfection of
si-MLK7-AS1 no. 1, no. 2 and si-NC were stained with FITC-Annexin-V
and propidium iodide (PI). Then, flow cytometry
(FACScan®; BD Biosciences, Franklin Lakes, NJ, USA) was
used to detect alterations of cell cycle. Furthermore, cells were
classified into viable cells, dead cells, early apoptotic cells,
and apoptotic cells through analysis of flow cytometry.
Western blot analysis and
antibodies
RIPA buffer with proteinase inhibitor cocktail
(Medchem Express, NJ, USA) was used to lyse cells. The membranes
were incubated with antibodies against CDK2, CDK4 and P21. GAPDH
was used as a reference control. All the antibodies are obtained
from Cell Signaling Technology, Inc., (Danvers, MA, USA).
Xenograft model in nude mice
Male athymic BALB/c nude mice of 4 weeks old were
purchased from Institute of laboratory animal medicine, Chinese
Academy of Medical Sciences. The study accords with rules of Cancer
Hospital of China Medical University Ethics Committee. A total of
100 µl empty-vector-transfected or sh-MLK7-AS1-transfected DLD-1
cells was respectively injected into a single side of each mouse.
Measurement of tumor volume was conducted every four days. Up to
twenty-four days after injection, the mice were killed and tumors
removed from the mice were kept in 4% paraformaldehyde for further
research.
Immunohistochemical (IHC)
analysis
The tumor tissues derived from control group and
sh-MLK7-AS1 group were immunostained for H&E and Ki67.
Anti-Ki67 from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA)
was used to present the percentage of positive cells, further
revealing the proliferative activities in tumor tissues.
Statistical analysis
All assays were repeated for three times. The data
were analyzed with SPSS v17.0 software program (SPSS, Inc.,
Chicago, IL, USA) and presented as mean ± SD (standard deviation).
Student's t test (two tailed) was used to compare data derived from
different groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
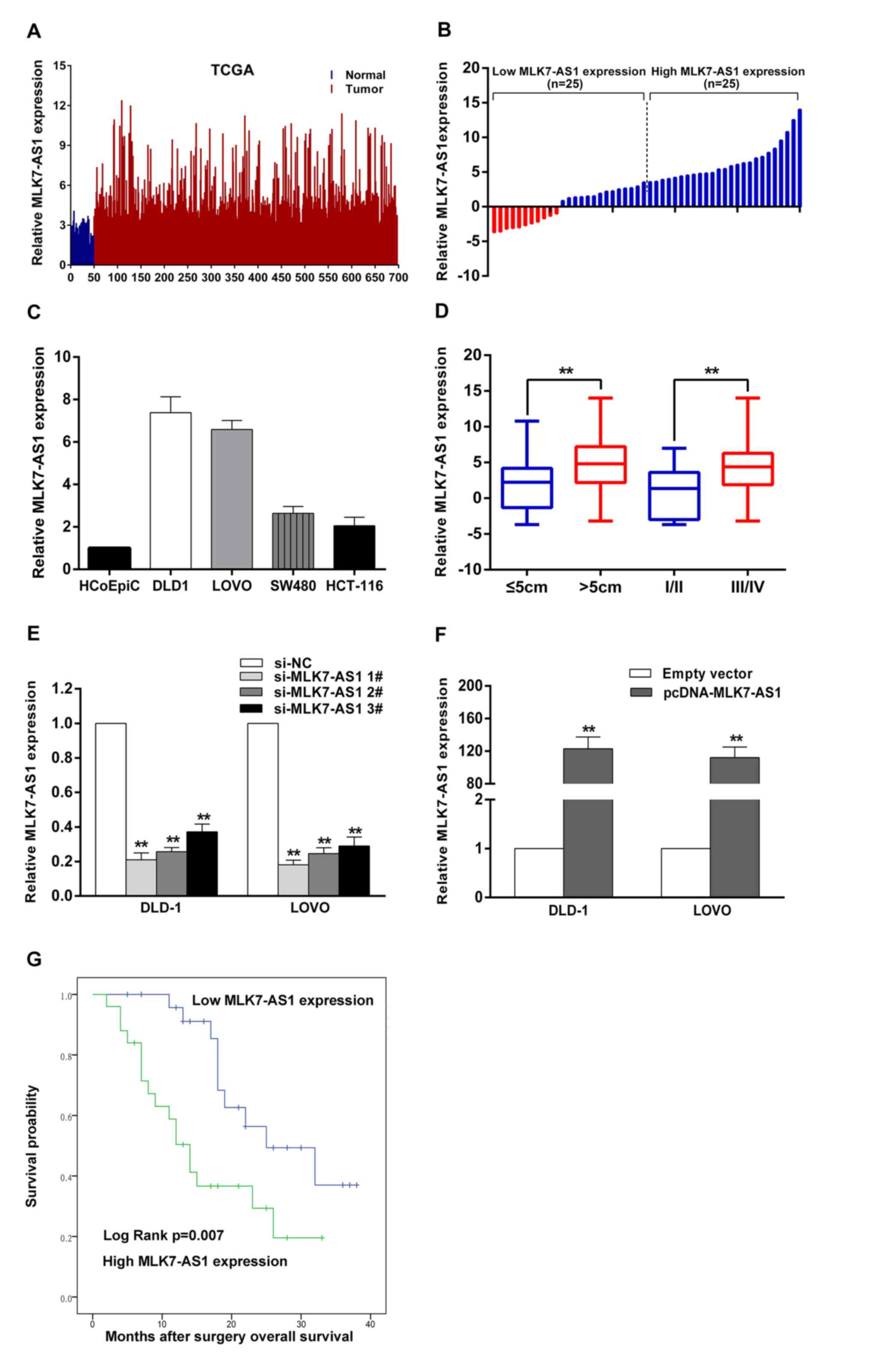
MLK7-AS1 is obviously upregulated in
CRC tissue samples, and increased MLK7-AS1 expression is
significantly correlated with several clinicopathological factors
and poor prognosis in patients with CRC
To investigate aberrantly expressed lncRNAs involved
in CRC, we analyzed the data downloaded from the TCGA dataset, and
found that lncRNA MLK7-AS1 exhibited obvious upregulation in CRC
tissue samples relative to normal tissues (Fig. 1A). Then, qRT-PCR experiments were
used to determine the expression levels of MLK7-AS1 in 50 pairs CRC
tissue samples and matched non-tumor tissue samples. MLK7-AS1
exhibits significant upregulation in CRC tissues. Fold change
>1.5 was recognized to be significant. Then, we divided CRC
patients into high (above the median value, n=25) and low (below
the median value, n=25) MLK7-AS1 expression groups to better study
correlations between MLK7-AS1 expression levels and
clinicopathological features (Fig.
1B). As shown in Fig. 1D and
Table I, increased MLK7-AS1
expression was obviously linked with tumor size (P=0.011), TNM
stage (P=0.031), and lymph node metastasis (P=0.031) in CRC
patients.
 | Table I.Correlation between MLK7-AS1
expression and clinicopathological factors of colorectal cancer
patients. |
Table I.
Correlation between MLK7-AS1
expression and clinicopathological factors of colorectal cancer
patients.
|
| MLK7-AS1
expression |
|
|---|
|
|
|
|
|---|
| Variables | High | Low | P-value |
|---|
| Age (years) |
|
|
|
|
>60 | 11 | 16 | 0.156 |
|
≤60 | 14 | 9 |
|
| Gender |
|
|
|
|
Male | 17 | 17 | 1.000 |
|
Female | 8 | 8 |
|
| Tumor size |
|
|
|
| ≤5
cm | 9 | 18 | 0.011a |
| >5
cm | 16 | 7 |
|
| TNM stage |
|
|
|
|
I/II | 4 | 11 | 0.031a |
|
III/IV | 21 | 14 |
|
| Lymph node
metastasis |
|
|
|
|
Positive | 21 | 14 | 0.031a |
|
Negative | 4 | 11 |
|
To detect the correlation between MLK7-AS1
expression and the prognosis of CRC patients, Kaplan-Meier analysis
and log-rank test were used to explore the effects of MLK7-AS1 on
overall survival of CRC patients (Fig.
1G). The median survival time for cases with high MLK7-AS1
expression was 14 months, whereas it was 25 months for low MLK7-AS1
expression. Furthermore, the overall survival rate over 2 years for
the low MLK7-AS1 expression group was 32%, while it was 16% for the
high MLK7-AS1 expression group. Our findings show that MLK7-AS1 is
an unfavourable prognostic factor for CRC patients.
Regulation of MLK7-AS1 expression in
CRC cell lines
We detected MLK7-AS1 expression levels in CRC cell
lines, found that MLK7-AS1 exhibited higher levels in DLD-1 and
LOVO cells (Fig. 1C). In attempt
to evaluate the role of MLK7-AS1 in CRC cells, MLK7-AS1 expression
was decreased by transfection with siRNAs or shRNA vector. And qPCR
assays were used to test the interference efficiencies of three
siRNAs transfected in CRC cells. Si-MLK7-AS1 no. 1 and si-MLK7-AS1
no. 2 exhibited more efficient silencing abilities than si-MLK7-AS1
no. 3 (Fig. 1E). Thus, we selected
si-MLK7-AS1 no. 1 and si-MLK7-AS1 no. 2 for all subsequent assays.
Moreover, we also examine the expression levels of MLK7-AS1 in
DLD-1 and LOVO cells transfected with pcDNA-MLK7-AS1. Compared with
the NC, MLK7-AS1 expression significantly increased in
pcDNA-MLK7-AS1-transfected CRC cells (Fig. 1F).
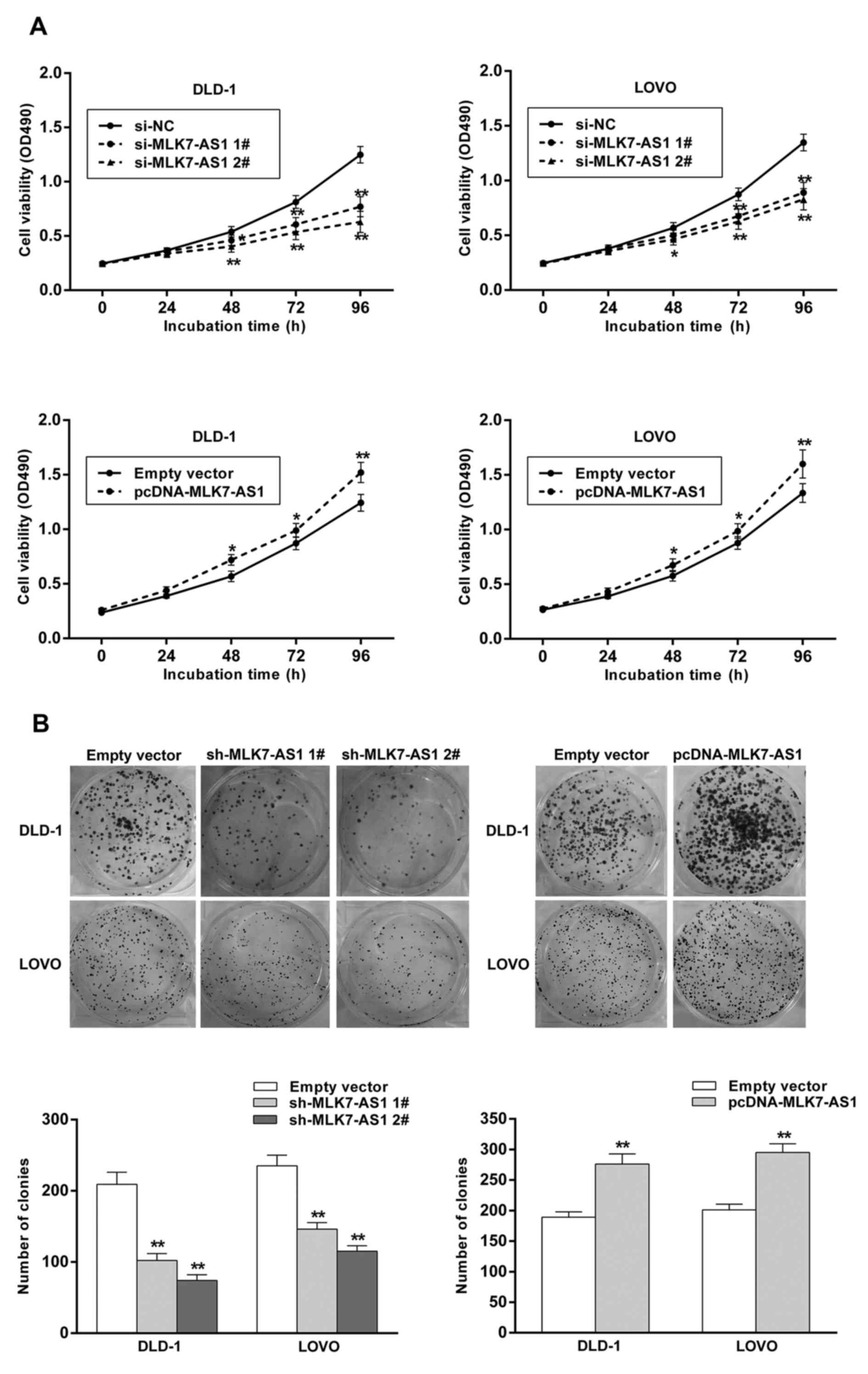
The effects of MLK7-AS1 dysregulation
on cell viability and colony-formation ability in CRC cells
MTT experiments were performed to test CRC cells
viability, and the results demonstrated that cell viabilities of
DLD1 and LOVO cells following transfection with si-MLK7-AS1 no. 1
or si-MLK7-AS1 no. 2 were obviously suppressed compared with
control cells (Fig. 2A).
Furthermore, MLK7-AS1 knockdown impaired CRC cells clonogenic
survival in DLD-1 and LOVO cells (Fig.
2B). Consistently, the results of MLK7-AS1 overexpression
showed opposite effects (Fig. 2A and
B). These findings indicated the effects of MLK7-AS1 on CRC
proliferation.
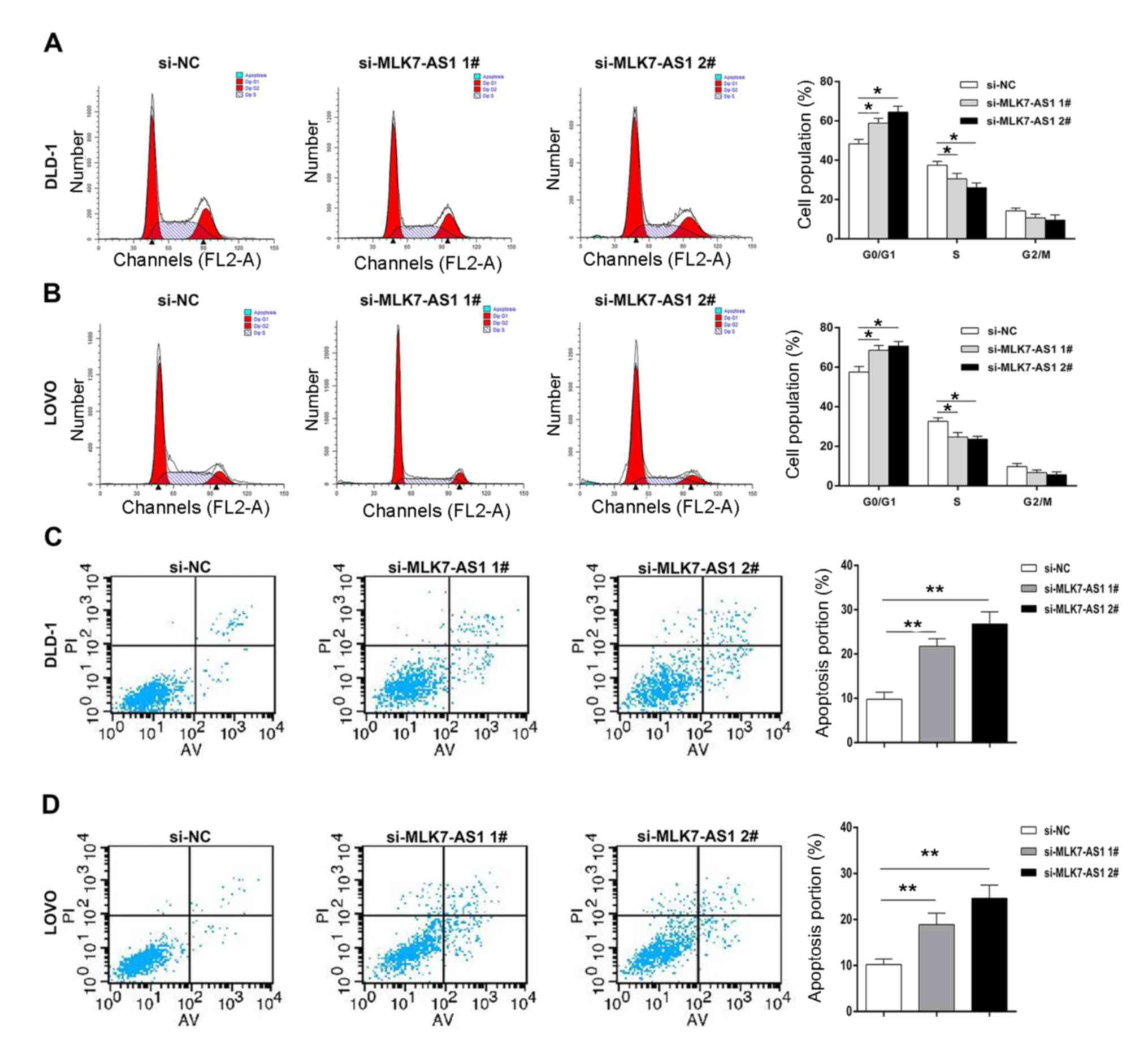
The function of MLK7-AS1 in cell cycle
progression and apoptosis of CRC cell lines
To explore whether MLK7-AS1 is involved in cell
cycle regulations, flow cytometry assays were performed in DLD-1
and LOVO cell lines. Compared with control cells, DLD1 and LOVO
cells with MLK7-AS1 knockdown showed an obvious G1/G0 phase arrest
(Fig. 3A and B). Importantly, flow
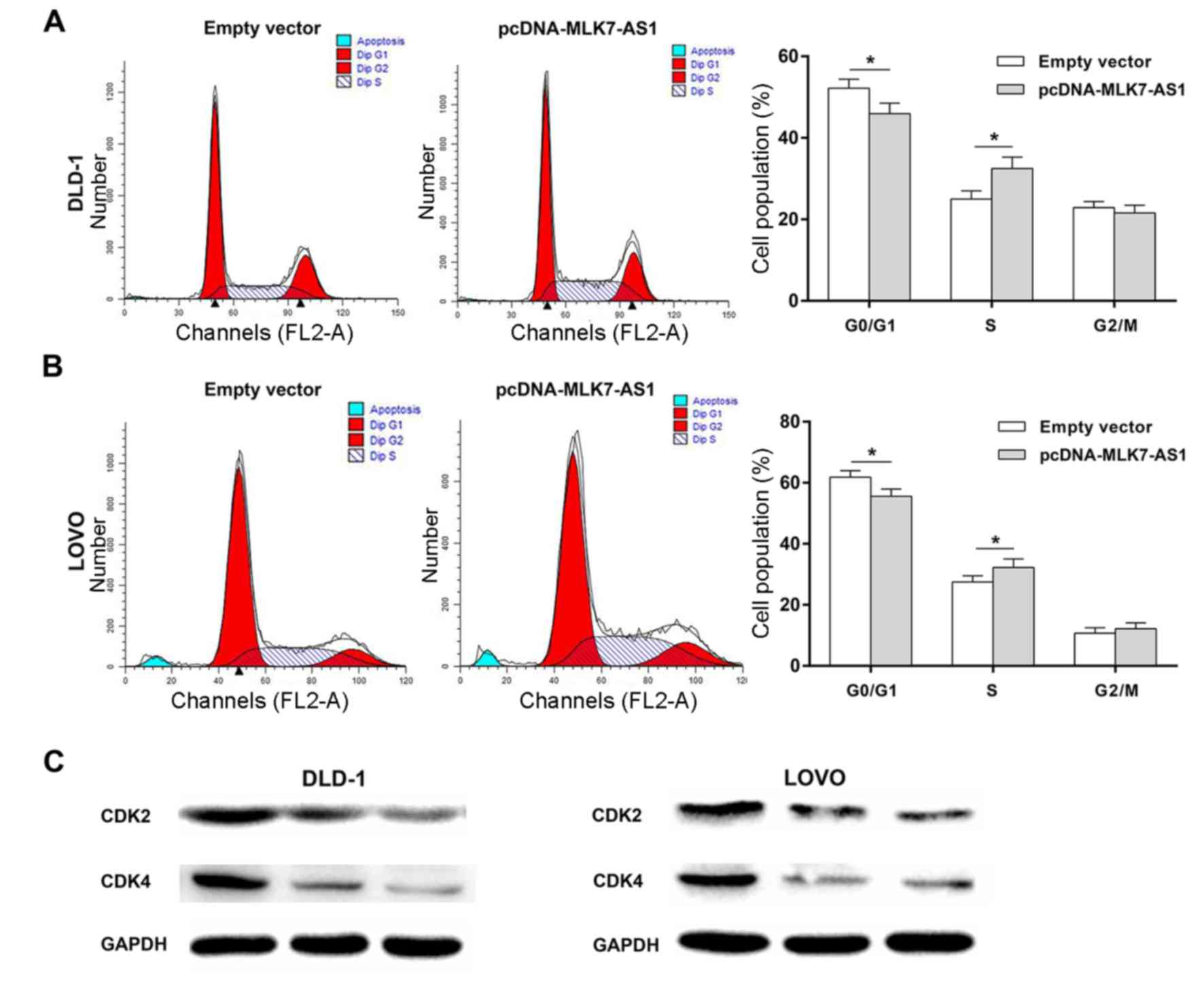
cytometry experiments were further used to study the effects of
MLK7-AS1 overexpression on cell cycle regulation in DLD-1 and LOVO
cell lines. Consistently, the results showed that DLD-1 and LOVO
cells with MLK7-AS1 overexpression had a significant decrease in
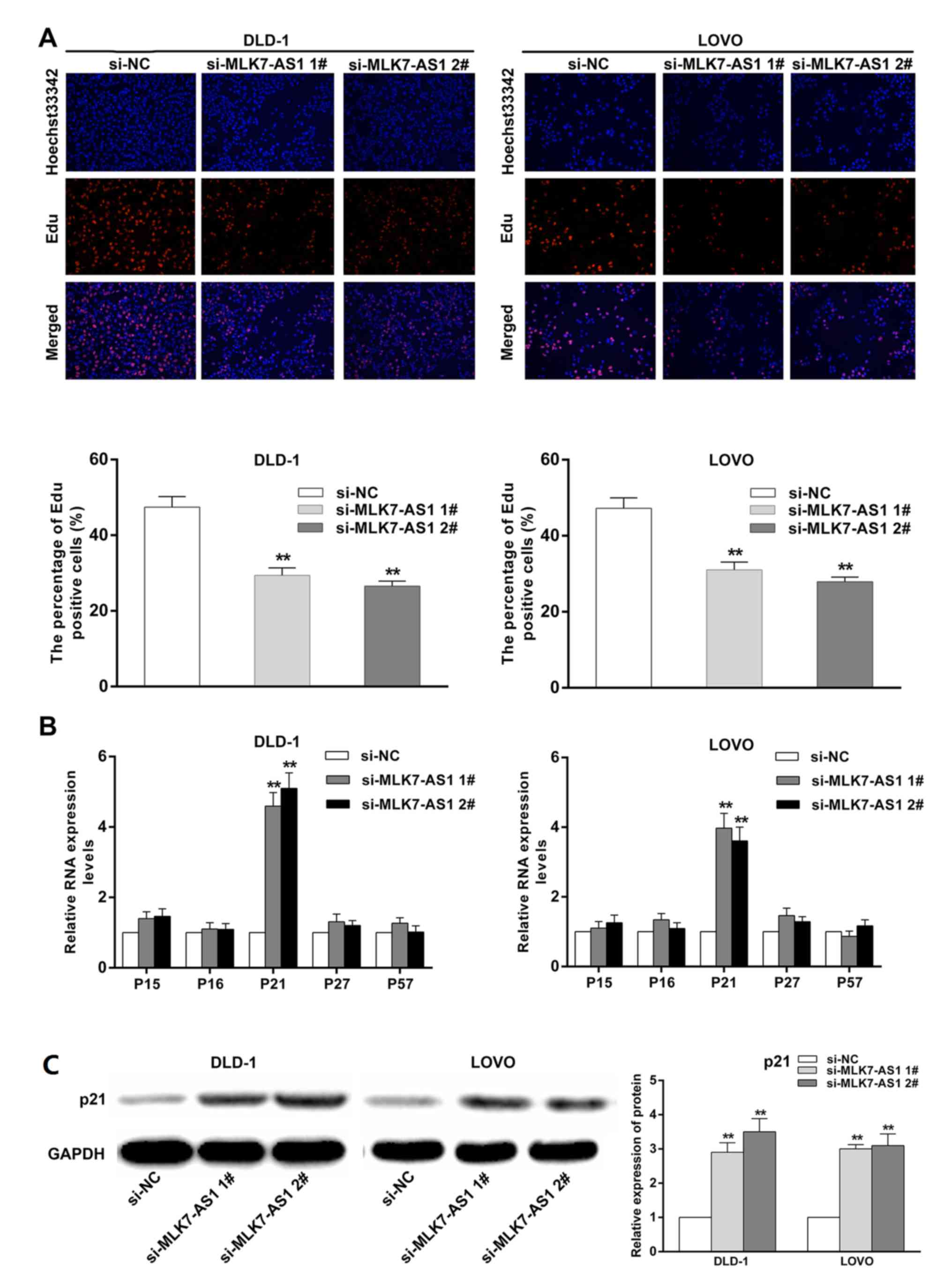
G1/G0 phase and an obvious increase in G2/S phase (Fig. 4A and B). Edu staining assays also
revealed the proliferation promotion mediated by MLK7-AS1 in CRC
(Fig. 5A). As known to all, CKIs
exert indispensable roles in cell cycle progression and serve as
tumor suppressors in many cancers, including CRC (28) Moreover, western blot assays
revealed the significant alteration of cyclin dependent kinase 2
(CDK2) and cyclin dependent kinase 4 (CDK4) in DLD-1 and LOVO cells
with MLK7-AS1 knockdown (Fig.
4C).
To comfirm the findings that MLK7-AS1 exerts
regulatory roles in cell cycle regulation, we investigated the
alteration of CKIs family in DLD-1 and LOVO cells following
transfection with si-MLK7-AS1 no. 1 or si-MLK7-AS1 no. 2.
Interestingly, qPCR and western blot experiments both demonstrated
that p21 was dramatically increased in DLD-1 and LOVO cells with
MLK7-AS1 knockdown (Fig. 5B).
These results highlighted p21 as a novel target gene of MLK7-AS1.
Next, we use flow cytometry to study whether MLK7-AS1 could induce
CRC cells apoptosis. The results showed that downregulation of
MLK7-AS1 by MLK7-AS1 siRNAs significantly increased apoptotic
abilities of CRC cells (Fig. 3C and
D). These findings suggest that MLK7-AS1 promotes CRC cells
proliferative abilities partly via silencing p21 expression.
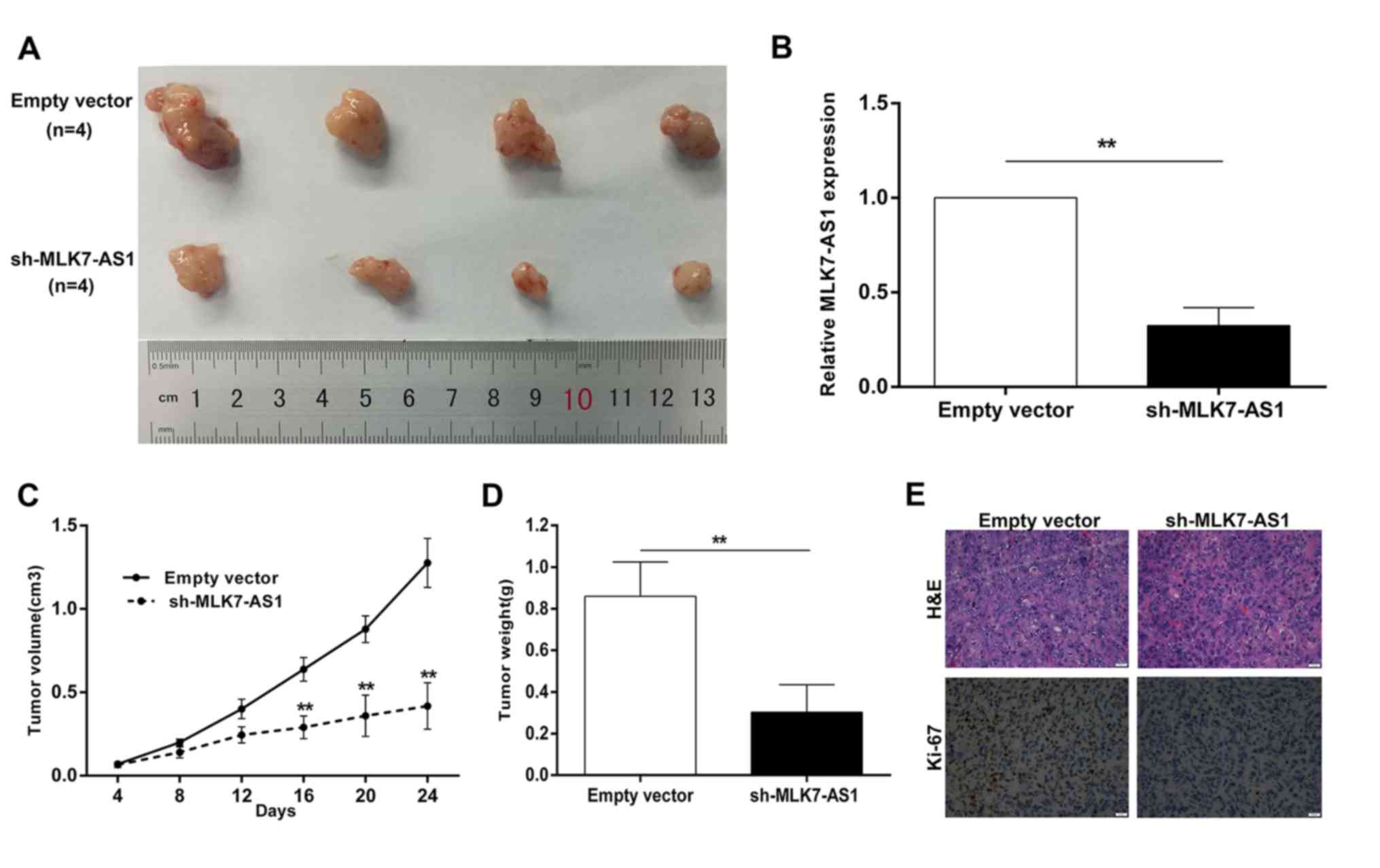
MLK7-AS1 downregulation inhibited
tumor growth in mice
Sh-MLK7-AS1-transfected or empty-vector-transfected
DLD-1 cells are injected into nude mice to establish in vivo
models (10,29). Up to 24 days after injection, the
size of tumor formed from sh-MLK7-AS1-transfected DLD-1 cells was
dramatically smaller than the size of tumor in control group
(Fig. 6A). Additionally, the tumor
volumes and weights were obviously decreased compared with the
controls (Fig. 6C and D). The
expression of MLK7-AS1 in tumors derived from DLD-1 cells with
MLK7-AS1 knockdown exhibited remarkable downregulation, relative to
that of control (Fig. 6B).
Immunohistochemistry (IHC) assays demonstrated that the tumor
tissues formed from DLD1/sh-MLK7-AS1 cells displayed lower Ki-67
expression (Fig. 6E). These
results suggested that MLK7-AS1 knockdown could inhibit CRC cells
growth in vivo.
Discussion
Advances in sequencing technologies facilitate the
completion of new massively human sequencing projects, such as the
Encyclopedia of DNA Elements (ENCODE) and GENCODE (30,31).
LncRNAs are known as newly identified ncRNAs, which occupies a
higher rate of 98% in transcripts of human genome (8). Emerging evidence has highlighted
lncRNAs as critical regulators in multiple biological processes,
and the dysregulation of lncRNAs has been found to exert important
roles in various cancers (8,16,17,23).
For example, lncRNA HOXA11-AS is found to be significantly
upregulated in GC tissues and alters GC cells phenotypes by
modulating cell cycle, apoptosis, and invasion (32). Importantly, lncRNAs exhibit
different cell phenotypes in various cancer, and its expression
patterns are tissue-specific (32). However, only a few of lncRNAs have
been well-studied in cancer progression (33,34).
Thus, more lncRNAs should be investigated in tumors, especially
CRC.
Here, we utilize publicly available data from TCGA
dataset and focus on the overexpressed lncRNAs. LncRNA MLK7-AS1
exhibited obvious upregulation in expression data of CRC and was
screened out as a potential oncogene in CRC progression. Then,
qRT-PCR experiments were performed to validate the expression
levels of MLK7-AS1 in a cohort of 50 paired CRC tissue samples and
matched non-tumor samples. Overexpression of MLK7-AS1 is
significantly correlated with tumor size, TNM stage, and lymph node
metastasis in CRC patients. Functional studies revealed that
MLK7-AS1 knockdown promoted CRC cells apoptotic abilities,
suppressed proliferation in vitro, and contributed to tumor
growth inhibition in vivo. Consistently, upregulation of
MLK7-AS1 showed opposite effects. These findings indicated that
MLK7-AS1 may be an oncogene in CRC progression, suggesting its
utilities as a potential biomarker and a therapeutic target.
Mounting investigations have focused on the
antisense transcripts and its corresponding protein-coding genes.
For example, lncRNA KRT7-AS promotes GC cell progression by
increasing KRT7 expression (35).
LncRNA FEZF1-AS1 facilitates cell proliferation and migration in
CRC through mediating FEZF1 expression (36). These studies suggested a novel
mechanism that sense gene regulation can be controlled by antisense
transcripts through forming duplex. LncRNA MLK7-AS1 is located on
the antisense chain of the gene coding MLK7 protein. MLK7 has been
demonstrated to be significantly upregulated in CRC tissues
(37,38). CRC RNA-sequencing data showed that
MLK7 increased by about threefold in CRC tissue samples (8). Both MLK7 and MLK7-AS1 exhibited
obvious upregulation in CRC tissues. However, the regulatory
relationship between MLK7-AS1 and MLK7 remains undefined. The
potential mechanism between MLK7-AS1 and MLK7 deserves to be
focused in the future research.
Recently, it has been revealed that lncRNAs can
increase or inhibit the expression levels of target genes to exert
effects in cancer progression (24). CKIs are known to be critical tumor
suppressors in various cancers and play key roles in cell cycle
regulation (10,22,28)
Importantly, CKIs are involved in the biological role of lncRNAs
(28,39,40).
The abnormal methylation in promoter regions of CKIs leads to gene
expression inhibition, thus contributing to alteration of cell
cycle (41,42). For example, linc00668-mediated cell
proliferation in GC is partly via silencing the expressions of CKIs
at epigenetical levels (24).
Thus, we further examined the expression levels of CKIs in CRC
cells after MLK7-AS1 knockdown. Interestingly, p21 expression was
obviously upregulated at both mRNA and protein levels. Current
studies have highlighted p21 as a critical tumor suppressor in many
cancers, including CRC (21,43).
For example, BRAF activated non-coding RNA (BANCR) could promote
CRC proliferation by silencing p21 (44). The significant increase of p21 can
partly explain the finding that decreased MLK7-AS1 contributes to
significant increase in G1/G0 phase, and activates apoptosis in CRC
cells. Thus, MLK7-AS1 could promote CRC cell proliferation and
induce apoptosis partly via silencing p21 expression. SH2
Domain-Containing Inositol-5′-Phosphatase 1 (SHIP1) has been
reported to be involved in pathways associated with p21 (45). Additionally, dysregulated SHIP1
exerts regulatory effects on cell viability and cell death in many
diseases, including CRC (46–48).
It is also of great significance to investigate the relationship
between MLK7-AS1 and other potential target gene, including
SHIP1.
P21, also known as cyclin dependent kinase inhibitor
1A (CDKN1A), is a crucial component of CKIs family, and has been
elucidated to mediate tumor-suppressive activities in many cancers
(21,49,50).
Current studies have showed the effects of lncRNAs on cell cycle
regulation (51). Identifications
of lncRNAs and associated regulatory target genes in CRC are
important, and will help to explore CRC pathogenesis. Our findings
represent first reporting of remarkable upregulation of MLK7-AS1 in
CRC tissue samples and cell lines. MLK7-AS1 inhibition can decrease
cell proliferative abilities and promote apoptosis in CRC.
Importantly, p21 acts as a novel downstream target of MLK7-AS1. The
oncogenic role of MLK7-AS1 in CRC progression is partly via
silencing p21 expression. Additionally, overexpressed MLK7-AS1
expression may be an unfavourable prognostic factor in CRC
patients. Although the functional role of MLK7-AS1 has been
investigated, mechanistic investigations between MLK7-AS1 and p21
are required to further the understanding of regulatory mechanism.
More numbers of mice can be used to study the effects of MLK7-AS1
dysregulation on tumor growth in vivo in the future. Our
results support the idea that lncRNA MLK7-AS1 promotes the
proliferation in human CRC partly via downregulating p21 expression
and suggest that lncRNA MLK7-AS1 may be a potential therapeutic
target for CRC patients. Theses findings will shed new light on CRC
pathogenesis and promote the development of lncRNAs-directed
diagnosis and treatments.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Fund from the National Natural Science Foundation
of China (grant no. 81672427); the project of ‘Liaoning clinical
research center for colorectal cancer’ (grant no. 2015225005);
‘Liaoning BaiQianWan Talents Program’ [2017] No. B44 and ‘Clinical
capability construction project for Liaoning Provincial Hospitals
(grant no. LNCCC-D42-2015)’.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XZ, RZ, JBL and XFY made substantial contributions
to conception and design. RZ, KJ and WYL were responsible for the
analysis and interpretation of data. RZ, XL, JFZ and SW were
involved the experimental design, drafting the manuscript and
revising it critically for important intellectual content. XZ
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Cancer Hospital of
China Medical University Ethics Committee. Informed consent was
obtained from participants.
Consent for publication
Informed consent was obtained from participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia-Foncillas J and Diaz-Rubio E:
Progress in metastatic colorectal cancer: Growing role of cetuximab
to optimize clinical outcome. Clin Transl Oncol. 12:533–542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dienstmann R, Salazar R and Tabernero J:
Personalizing colon cancer adjuvant therapy: Selecting optimal
treatments for individual patients. J Clin Oncol. 33:1787–1796.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matuchansky C: Colorectal cancer: Some
present aspects of its epidemiology, prevention and screening.
Presse Med. 46:141–144. 2017.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu J, Zhao J, Liu F and Zhang R: Analysis
of mechanism and feature genes of colorectal cancer by
bioinformatic methods. Minerva Med. 108:94–95. 2017.PubMed/NCBI
|
|
7
|
Zhao B, Lu M, Wang D, Li H and He X:
Genome-wide identification of long noncoding RNAs in human
intervertebral disc degeneration by RNA sequencing. Biomed Res Int.
2016:36848752016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su J, Zhang E, Han L, Yin D, Liu Z, He X,
Zhang Y, Lin F, Lin Q, Mao P, et al: Long noncoding RNA BLACAT1
indicates a poor prognosis of colorectal cancer and affects cell
proliferation by epigenetically silencing of p15. Cell Death Dis.
8:e26652017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu X, Yan T, Wang Z, Wu X, Cao G and Zhang
C: LncRNA ZEB2-AS1 promotes bladder cancer cell proliferation and
inhibits apoptosis by regulating miR-27b. Biomed Pharmacother.
96:299–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Fu C, Xu Q and Wei X: Long
non-coding RNA CASC7 inhibits the proliferation and migration of
colon cancer cells via inhibiting microRNA-21. Biomed Pharmacother.
95:1644–1653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kondo Y, Shinjo K and Katsushima K: Long
non-coding RNAs as an epigenetic regulator in human cancers. Cancer
Sci. 108:1927–1933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwok ZH and Tay Y: Long noncoding RNAs:
Lincs between human health and disease. Biochem Soc Trans.
45:805–812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. Rna Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dey BK, Mueller AC and Dutta A: Long
non-coding RNAs as emerging regulators of differentiation,
development, and disease. Transcription. 5:e9440142014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia
R, Liu YW, Liu XH, Zhang EB, Lu KH and Shu YQ: Long noncoding RNA
ANRIL promotes non-small cell lung cancer cell proliferation and
inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer
Ther. 14:268–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu B, Pan CF, He ZC, Wang J, Wang PL, Ma
T, Xia Y and Chen YJ: Long noncoding RNA-LET suppresses tumor
growth and EMT in lung adenocarcinoma. Biomed Res Int.
2016:46934712016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guil S and Esteller M: Cis-acting
noncoding RNAs: Friends and foes. Nat Struct Mol Biol.
19:1068–1075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren W, Zhang J, Li W, Li Z, Hu S, Suo J
and Ying X: A tumor-specific prognostic long non-coding RNA
signature in gastric cancer. Med Sci Monit. 22:3647–3657. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cress WD, Yu P and Wu J: Expression and
alternative splicing of the cyclin-dependent kinase inhibitor-3
gene in human cancer. Int J Biochem Cell Biol. 91:98–101. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun CC, Li SJ, Li G, Hua RX, Zhou XH and
Li DJ: Long intergenic noncoding RNA 00511 acts as an oncogene in
non-small-cell lung cancer by binding to EZH2 and suppressing p57.
Mol Ther Nucleic Acids. 5:e3852016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang E, Yin D, Han L, He X, Si X, Chen W,
Xia R, Xu T, Gu D, De W, et al: E2F1-induced upregulation of long
noncoding RNA LINC00668 predicts a poor prognosis of gastric cancer
and promotes cell proliferation through epigenetically silencing of
CKIs. Oncotarget. 7:23212–23226. 2016.PubMed/NCBI
|
|
25
|
Li J, Han L, Roebuck P, Diao L, Liu L,
Yuan Y, Weinstein JN and Liang H: TANRIC: An interactive open
platform to explore the function of lncRNAs in cancer. Cancer Res.
75:3728–3737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao L, Guo H, Zhou B, Feng J, Li Y, Han
T, Liu L, Li L, Zhang S, Liu Y, et al: Long non-coding RNA SNHG5
suppresses gastric cancer progression by trapping MTA2 in the
cytosol. Oncogene. 35:5770–5780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma HW, Xie M, Sun M, Chen TY, Jin RR, Ma
TS, Chen QN, Zhang EB, He XZ, De W and Zhang ZH: The pseudogene
derived long noncoding RNA DUXAP8 promotes gastric cancer cell
proliferation and migration via epigenetically silencing PLEKHO1
expression. Oncotarget. 8:52211–52224. 2016.PubMed/NCBI
|
|
28
|
Kato S, Schwaederle M, Daniels GA,
Piccioni D, Kesari S, Bazhenova L, Shimabukuro K, Parker BA, Fanta
P and Kurzrock R: Cyclin-dependent kinase pathway aberrations in
diverse malignancies: Clinical and molecular characteristics. Cell
Cycle. 14:1252–1259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim T, Jeon YJ, Cui R, Lee JH, Peng Y, Kim
SH, Tili E, Alder H and Croce CM: Role of MYC-regulated long
noncoding RNAs in cell cycle regulation and tumorigenesis. J Natl
Cancer Inst. 107(pii): dju5052015.PubMed/NCBI
|
|
30
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carninci P, Kasukawa T, Katayama S, Gough
J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al:
The transcriptional landscape of the mammalian genome. Science.
309:1559–1563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun M, Nie F, Wang Y, Zhang Z, Hou J, He
D, Xie M, Xu L, De W, Wang Z and Wang J: LncRNA HOXA11-AS promotes
proliferation and invasion of gastric cancer by scaffolding the
chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res.
76:6299–6310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Li B, Zheng Y, Li M, Shi L and Pu X:
Exploring functions of long noncoding RNAs across multiple cancers
through co-expression network. Sci Rep. 7:7542017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Q, Yang H, Wu L, Yao J, Meng X, Jiang
H, Xiao C and Wu F: Identification of specific long non-coding RNA
expression: profile and analysis of association with
clinicopathologic characteristics and BRAF mutation in papillary
thyroid cancer. Thyroid. 26:1719–1732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang B, Song JH, Cheng Y, Abraham JM,
Ibrahim S, Sun Z, Ke X and Meltzer SJ: Long non-coding antisense
RNA KRT7-AS is activated in gastric cancers and supports cancer
cell progression by increasing KRT7 expression. Oncogene.
35:4927–4936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen N, Guo D, Xu Q, Yang M, Wang D, Peng
M, Ding Y, Wang S and Zhou J: Long non-coding RNA FEZF1-AS1
facilitates cell proliferation and migration in colorectal
carcinoma. Oncotarget. 7:11271–11283. 2016.PubMed/NCBI
|
|
37
|
Liu J, McCleland M, Stawiski EW, Gnad F,
Mayba O, Haverty PM, Durinck S, Chen YJ, Klijn C, Jhunjhunwala S,
et al: Integrated exome and transcriptome sequencing reveals ZAK
isoform usage in gastric cancer. Nat Commun. 5:38302014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seshagiri S, Stawiski EW, Durinck S,
Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman
V, Jaiswal BS, et al: Recurrent R-spondin fusions in colon cancer.
Nature. 488:660–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu C, Li S, Dai X, Ma J, Wan J, Jiang H,
Wang P, Liu Z and Zhang H: PRC2 regulates RNA polymerase III
transcribed non-translated RNA gene transcription through EZH2 and
SUZ12 interaction with TFIIIC complex. Nucleic Acids Res.
43:6270–6284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ferrè F, Colantoni A and Helmer-Citterich
M: Revealing protein-lncRNA interaction. Brief Bioinform.
17:106–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu B, Ye X, Du Q, Zhu B, Zhai Q and Li XX:
The long non-coding RNA CRNDE promotes colorectal carcinoma
progression by competitively binding miR-217 with TCF7L2 and
Enhancing the Wnt/β-catenin signaling pathway. Cell Physiol
Biochem. 41:2489–2502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi Y, Liu Y, Wang J, Jie D, Yun T, Li W,
Yan L, Wang K and Feng J: Downregulated long noncoding RNA BANCR
promotes the proliferation of colorectal cancer cells via
downregualtion of p21 expression. PLoS One. 10:e1226792015.
|
|
45
|
An H, Xu H, Zhang M, Zhou J, Feng T, Qian
C, Qi R and Cao X: Src homology 2 domain-containing
inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated
LPS response primarily through a phosphatase activity- and
PI-3K-independent mechanism. Blood. 105:4685–4692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fuhler GM, Brooks R, Toms B, Iyer S, Gengo
EA, Park MY, Gumbleton M, Viernes DR, Chisholm JD and Kerr WG:
Therapeutic potential of SH2 domain-containing
inositol-5′-phosphatase 1 (SHIP1) and SHIP2 inhibition in cancer.
Mol Med. 18:65–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hoekstra E, Das AM, Willemsen M, Swets M,
Kuppen PJ, van der Woude CJ, Bruno MJ, Shah JP, Ten Hagen TL,
Chisholm JD, et al: Lipid phosphatase SHIP2 functions as oncogene
in colorectal cancer by regulating PKB activation. Oncotarget.
7:73525–73540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rouquette-Jazdanian AK, Kortum RL, Li W,
Merrill RK, Nguyen PH, Samelson LE and Sommers CL: miR-155 controls
lymphoproliferation in LAT mutant mice by restraining T-cell
apoptosis via SHIP-1/mTOR and PAK1/FOXO3/BIM pathways. PLoS One.
10:e1318232015. View Article : Google Scholar
|
|
49
|
Won KY, Kim GY, Kim HK, Choi SI, Kim SH,
Bae GE, Lim JU and Lim SJ: Tumoral FOXP3 expression is associated
with favorable clinicopathological variables and good prognosis in
gastric adenocarcinoma: The tumor suppressor function of tumoral
FOXP3 is related with the P21 expression in gastric adenocarcinoma.
Hum Pathol. 68:112–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen Z, Chen X, Chen P, Yu S, Nie F, Lu B,
Zhang T, Zhou Y, Chen Q, Wei C, et al: Long non-coding RNA SNHG20
promotes non-small cell lung cancer cell proliferation and
migration by epigenetically silencing of P21 expression. Cell Death
Dis. 8:e30922017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kitagawa M, Kitagawa K, Kotake Y, Niida H
and Ohhata T: Cell cycle regulation by long non-coding RNAs. Cell
Mol Life Sci. 70:4785–4794. 2013. View Article : Google Scholar : PubMed/NCBI
|