Introduction
Activating transcription factor 3 (ATF3) is a member
of the ATF/cyclic adenosine 5′-monophosphate response element
binding protein (CREB) subfamily. A role for the ATF/CREB family in
controlling the progression of hepatocellular carcinoma (HCC) has
been suggested (1). ATF3 is an
early stress response gene that is rapidly induced in cells
following exposure to a wide range of stress stimuli (2). ATF3 may be rapidly induced in cells
by a wide range of stress signals and may be activated by several
important signal transduction pathways, including the mitogen
activated protein kinase (MAPK) (3), c-myc avian myelocytomatosis viral
oncogene (4), tumour protein p53
(p53) (5), and transforming growth
factor-β TGF-β/Mothers against decapentaplegic (6) pathways, which are involved in cell
proliferation, differentiation, transformation and apoptosis
(7). The activation of ATF3 has
been demonstrated to be a negative or positive regulator of these
processes. Its precise effects remain controversial due to
differences in the tumour types and cell lines examined. ATF3 has
been indicated to promote (8) and
inhibit (9) cellular proliferation
and metastasis. In addition, it also has been suggested to exhibit
pro- and anti-apoptotic effects, and to regulate cell cycle
progression. To improve the understanding of how ATF3 acts in human
HCC, the expression of ATF3 was evaluated in clinical samples of
human HCC using quantitative polymerase chain reaction (qPCR),
immunohistochemical (IHC) staining and a western blot analysis, and
its expression was associated with the clinicopathological
parameters of human HCC tissues in our previous study (10). It was identified that the
expression of ATF3 was low in HCC tissues, and the protein level
was decreased in patients with capsule invasion compared with those
without. Therefore, it was inferred, based on the low expression of
ATF3, that it may function as a tumour suppressor during human
hepatocellular oncogenesis.
To improve the understanding of the cytological
mechanisms by which ATF3 functions during the process of liver
cancer formation, HepG2 cells were infected by an LV-ATF3-enhanced
green fluorescent protein (EGFP) overexpression vector. Then, the
biological behaviour of the cells with and without ATF3
overexpression was compared. The behaviours examined included cell
proliferation, migration, apoptosis rate and cell cycle
progression. The data from the present study may assist to clarify
the association between ATF3 and human liver cancer.
Materials and methods
Cell culture
HepG2 cells were purchased from the Cellbank of
Shanghai Institutes for Life Science, Chinese Academy of Sciences
(Shanghai, China) and were maintained at 37°C in an atmosphere of
5% CO2. Cell culture reagents were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA) unless otherwise
indicated. The cells were grown in complete culture medium
(high-glucose) consisting of Dulbecco's modified Eagle's medium
(DMEM), supplemented with 10% heat-inactivated foetal bovine serum
(FBS) and 1% penicillin/streptomycin.
Construction of lentiviral ATF3
overexpression vectors and cell transfection
Based on the ATF3 gene sequence in GenBank
(NM_001674; http://www.ncbi.nlm.nih.gov/nuccore), primers that
specifically matched ATF3 mRNA were designed (forward,
GAGGATCCCCGGGTACCGGTC GCCACCATGATGCTTCAACACCCAGG; reverse, TCCTT
GTAGTCCATACCGCTCTGCAATGTTCCTTCT; 587 bp). The lentiviral vector
GV287-EGFP (Shanghai GeneChem Co., Ltd., Shanghai, China) was used
to construct the ATF3 mRNA-expressing vector. The non-transfected
group and mock vehicle group transfected with an empty vector
without ATF3 mRNA were used as the negative control (NC). The
GV287-EGFP vector was linearized by digestion with the restriction
endonucleases AgeI and BamHI, and the inserted
sequences were ligated with an In-Fusion PCR Cloning kit (Clontech
Laboratories, Inc., Mountainview, CA, USA) to produce the human
ATF3 mRNA vectors (LV-ATF3-EGFP and LV-NC-EGFP). DNA sequencing was
used to verify all inserted sequences. Using
Lipofectamine® 2000 (Invitrogen: Thermo Fisher
Scientific, Inc.), 293T cells (Cellbank of Shanghai Institutes for
Life Science, Chinese Academy of Sciences) were co-transfected with
20 µg ATF3 mRNA vectors and the 15 µg pHelper1.0 and 10 µg
pHelper2.0 plasmids (Addgene, Inc., Cambridge, MA, USA) for 48 h to
generate lentiviruses. The viral titres were determined, and the
lentiviral particles 2×109 TU/ml were used to infect
HepG2 2×105 cells at 37°C for 72 h. Successfully
transfected cells, which expressed EGFP, were selected and grown in
cell culture for subsequent investigation.
Western blotting
HepG2 cells were lysed in ice-cold lysis buffer [150
mM NaCl, 100 mM Tris (pH 8.0), 1% Tween-20, 1 mM EDTA, 1 mM
phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, 10 µg/ml
trypsin, and 10 µg/ml leupeptin]. The lysate was left on ice for 20
min and then centrifuged at 4°C, 1,200 × g for 10 min. The
clarified supernatant was collected, and the protein concentration
was measured by using a Biotek protein assay kit (Elx800; BioTek
Instruments, Winooski, VT, USA). Protein lysate (60 µg per well),
was separated by 12% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. Membranes were incubated with 5% non-fat milk
for 1 h at room temperature and then with mouse monoclonal
anti-human ATF3 antibody (1:100 dilution, ab58668; Abcam,
Cambridge, MA, USA) at 4°C overnight. The membranes were washed and
stained with a horseradish-peroxidase-conjugated secondary antibody
(at 1:1,000 dilution, A0216, Beyotime Institute of Biotechnology,
Shanghai, China). Proteins were visualized using Enhanced
Chemiluminescence Plus system (Beyotime Institute of Biotechnology)
and exposed to autoradiography film (Kodak, Rochester, NY, USA).
Blots with mouse monoclonal β-Actin antibody (at 1:1,000 dilution,
AA128; Beyotime Institute of Biotechnology) were similarly
generated to ensure that equal amounts of protein were loaded in
the wells.
Cell proliferation
HepG2 cells were divided into three groups: An
overexpression group (OE); a mock vehicle-transfected negative
control group (NC); and a non-transfection group (control).
Cell proliferation was measured using an MTT
colorimetric assay from Beijing Dingguo Changsheng Biotechnology
Co. Ltd. (Beijing, China). All the cells were seeded in 96-well
plates and cultured with complete culture medium. Cell
proliferation was measured on days 1–5 at 490 nm in an ELIASA plate
reader (Elx800; BioTek Instruments) following the addition of
dimethyl sulfoxide to each well. The cell proliferation rate was
calculated relative to the optical density at 490 nm of day 1.
Cell migration
Transwell migration assays were performed in 24-well
dishes with cell culture plate inserts (Corning Incorporated,
Corning, NY, USA). The upper chamber had a 3 µm pore size and was
coated with a polycarbonate membrane, with 100 µl serum-free
medium/well, and the plates were incubated at 37°C for 1–2 h. The
lower chamber consisted of 600 µl DMEM medium supplemented with 10%
FBS. Actively growing cells (1×105 cells) were diluted
in 100 µl serum-free medium supplemented with 0.1% bovine serum
albumin (Absin Biochemical Company, Shanghai, China), added to the
upper chamber and incubated at 37°C for 16 h. Following incubation,
the medium from the upper chamber was removed, and the cells on the
upper surface of the membrane were rinsed and removed with a cotton
swab. The upper chambers were stained at 37°C for 20 min with
Giesma solution (Sigma-Aldrich; MercK KGaA, Darmstadt, Germany).
Images of the migrated cells were captured by an inverted
fluorescence microscope (magnification, ×100, Olympus Corporation,
Tokyo, Japan), and their optical densities at 570 nm were measured
by ELIASA microplate reader (Elx800; Bio Tek Instruments).
Cell apoptosis
HepG2 cells were collected following infection with
the lentiviruses for 5 days; then, the cells were washed twice with
cold PBS and binding buffer. The collected cells were resuspended
in staining buffer and then added into Annexin V-allophycocyanin
(APC) staining solution and incubated for 10–15 min at room
temperature in the dark. Flow cytometric analysis was performed
using a FACSCalibur instrument (BD Biosciences, Franklin Lakes, NY,
USA). The results were analysed by FlowJo7.6 software (FlowJo LLC,
Ashland, OR, USA).
Cell cycle progress
HepG2 cells (third to fourth generation) were
collected following infection with the lentivirus; then, the
collected cells were washed and resuspended with cold PBS.
Subsequent to fixation with 70% ethyl alcohol at 4°C overnight, the
cell suspension was added into 50 µg/ml propidium iodide staining
solution and incubated for 30 min at 37°C in the dark. Flow
cytometric analysis was performed using a FACSCalibur instrument,
and the results were analysed using FlowJo7.6 software (FlowJo
LLC).
Statistical analysis
The statistical package SPSS 19.0 was used to
perform the statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
The optical density (OD) 490 value, OD570/OD490
value, apoptotic rate and cell percentages were representative of
the MTT, Transwell, apoptosis and cell cycle results, respectively.
All results presented as mean ± standard deviation.
Tumour-associated variables in the Transwell and apoptosis results
were examined using a one-way analysis of variance (ANOVA) to
compare the differences among groups, and followed by a Least
Significant Difference test between groups. For the variables
associated with the MTT and cell cycle assays, statistical
significance was determined according to an ANOVA with a randomized
block design, followed by a Least Significant Difference test.
Results
To investigate the functional significance of ATF3
expression in HepG2 cells, HepG2 cells were transfected with
lentiviral vectors expressing ATF3 mRNA. The mRNA expression was
associated with increased ATF3 protein expression, as demonstrated
in Fig. 1. The MTT assay indicated
that the overexpression of ATF3 resulted in HepG2 growth inhibition
(Fig. 2). However, there were no
marked differences in the cell migration detected by the Transwell
assay, as presented in Fig. 3.
Figs. 4 and 5 indicated that the rate of HepG2
apoptosis was accelerated and that the cells underwent cycle arrest
following ATF3 overexpression, respectively, as determined by flow
cytometric analyses.
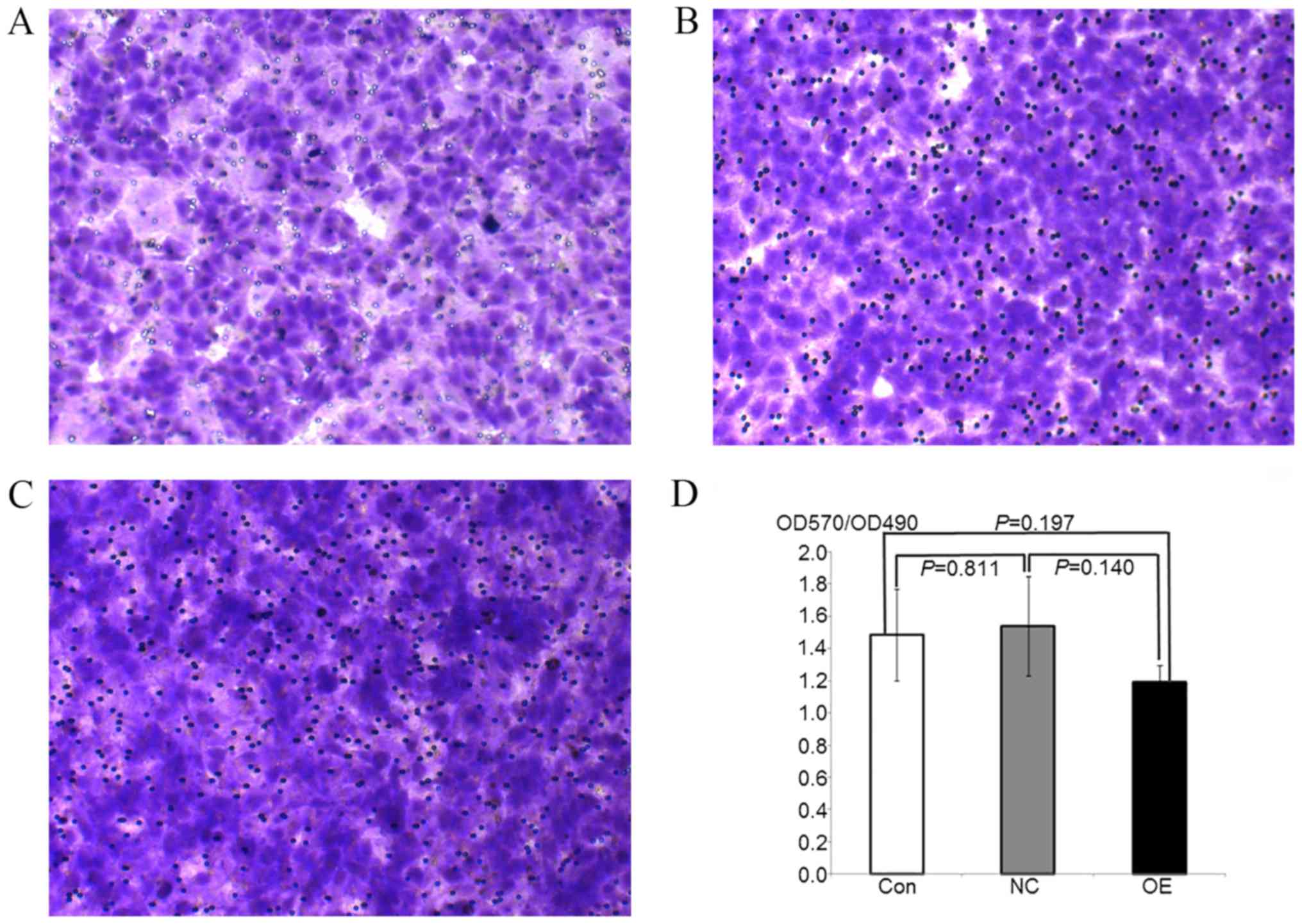 | Figure 3.HepG2 transfer ability following
overexpression of ATF3 is detected by Transwell assay. (A-C)
Microscope images of Giesma-stained HepG2 transfer cells in (A) OE,
(B) NC and (C) control groups. Magnification, ×100. (D) Histograms
of OD570/OD490 values in OE, NC and control groups. Data are
presented as the mean ± standard deviation. The transfer difference
at the OE, NC and control groups was not marked, meanwhile no
significance between groups was observed (P>0.05). ATF3,
activating transcription factor 3; OE, overexpression; NC, negative
control; Con, control; OD, optical density. |
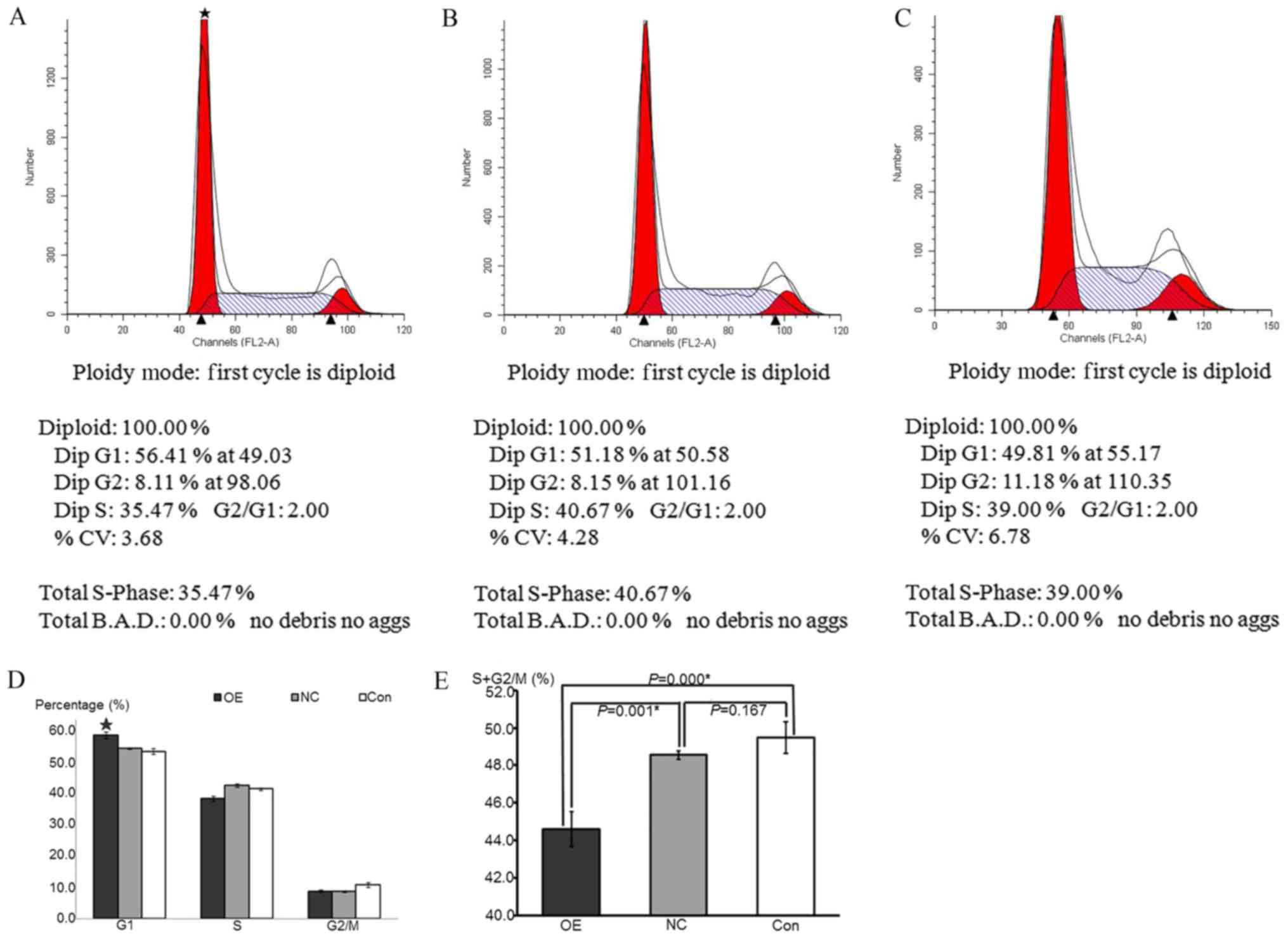 | Figure 5.HepG2 cell cycle progression following
overexpression of ATF3 was detected by propidium iodide staining.
(A-C) Flow cytometry cycle diagram of (A) OE, (B) NC and (C) Con
groups. DipG1 was representative of the proportion of cells in the
G0/G1 phase; DipG2 was representative of the proportion of cells in
the G2/M phase; and DipS was representative of the proportion of
cells in the S phase. (D) Composite histograms of the cell
proportion in G0/G1, S and G2/M phases among the OE, NC and Control
groups. Data are presented as the mean ± standard deviation. The
G0/G1 proportion in OE group was significantly increased compared
with the NC and control groups, while the S and G2/M proportion
were not significance compared with other groups. *P<0.05 vs. NC
and control groups. (E) Histograms of the S+G2/M phase cell
proportions in the OE, NC and control groups. Data are presented as
the mean ± standard deviation. The proportion sum in OE group was
significantly decreased compared with the NC and control groups,
while no significance between the NC and control groups was
observed. *P<0.05 vs. NC and control groups. ATF3, activating
transcription factor 3; OE, overexpression; NC, negative control;
Con, control. |
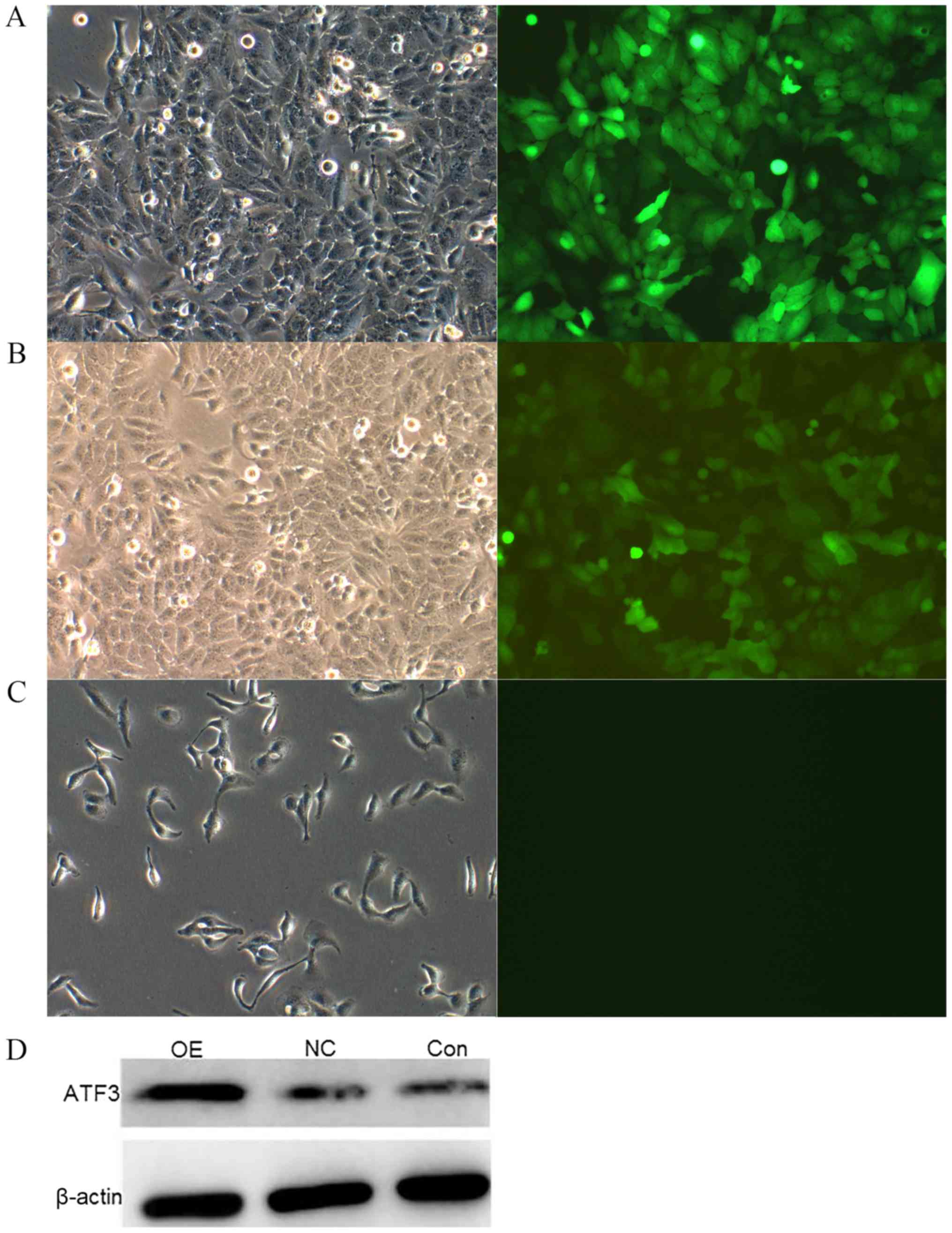
ATF3 is successfully overexpressed in
HepG2 cells
To examine the biological activity of ATF3, ATF3 was
cloned into the lentiviral vector GV287-EGFP. Stable pools of HepG2
cells containing full-length ATF3 were generated. The
overexpression of ATF3 was confirmed in these cells by fluorescence
microscope and western blot analysis. Relative to the NC and
control groups, the OE group exhibited a marked increase in ATF3
protein expression (Fig. 1).
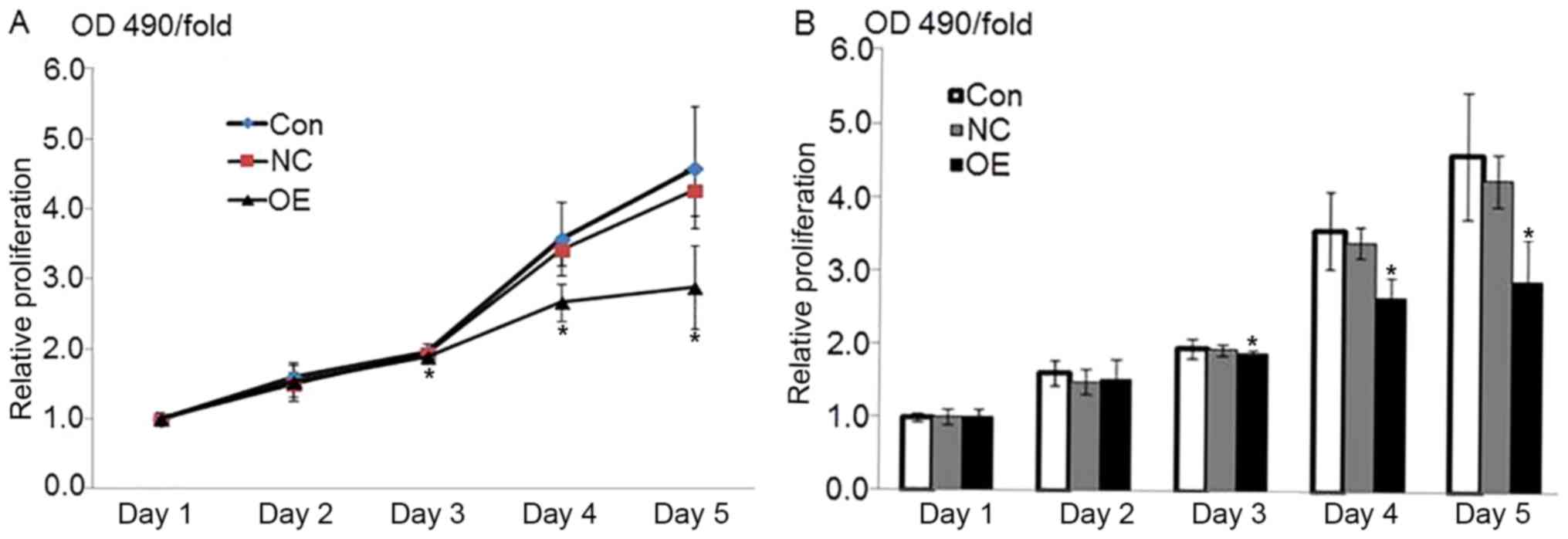
Overexpression of ATF3 inhibits the
growth of HepG2 cells
The optical density at 490 nm on the first day was
considered the reference coefficient. The percent viability was
calculated relative to this value as the fold-change (OD490/fold)
from the second day to the fifth day. The MTT-OD490 values were
representative of cell growth activity. The results indicated that
the value in the OE group was significantly decreased compared with
that in the NC and control groups beginning from the third day
following lentiviral vector infection (P<0.05), whereas no
significant differences between the NC and control groups were
observed (P=0.637; Fig. 2). These
data indicated that the overexpression of ATF3 may inhibit the
growth of HepG2 cells.
Overexpression of ATF3 does not affect
the migration of HepG2 cells
To examine the effect of cell proliferation on the
number of cells that migrated across the Transwell membrane, the
optical density at 570 nm was calculated relative to the
corresponding MTT-OD490 values. The OD570/OD490 values were
representative of the numbers of cells that migrated through the
chamber membrane. The results indicated no significant differences
in the number of cells that migrated in the OE, NC and control
groups, and there were no significant differences between the
various groups (P>0.05; Fig.
3). These data indicated that the overexpression of ATF3 had no
significant effects on the migration of HepG2 cells.
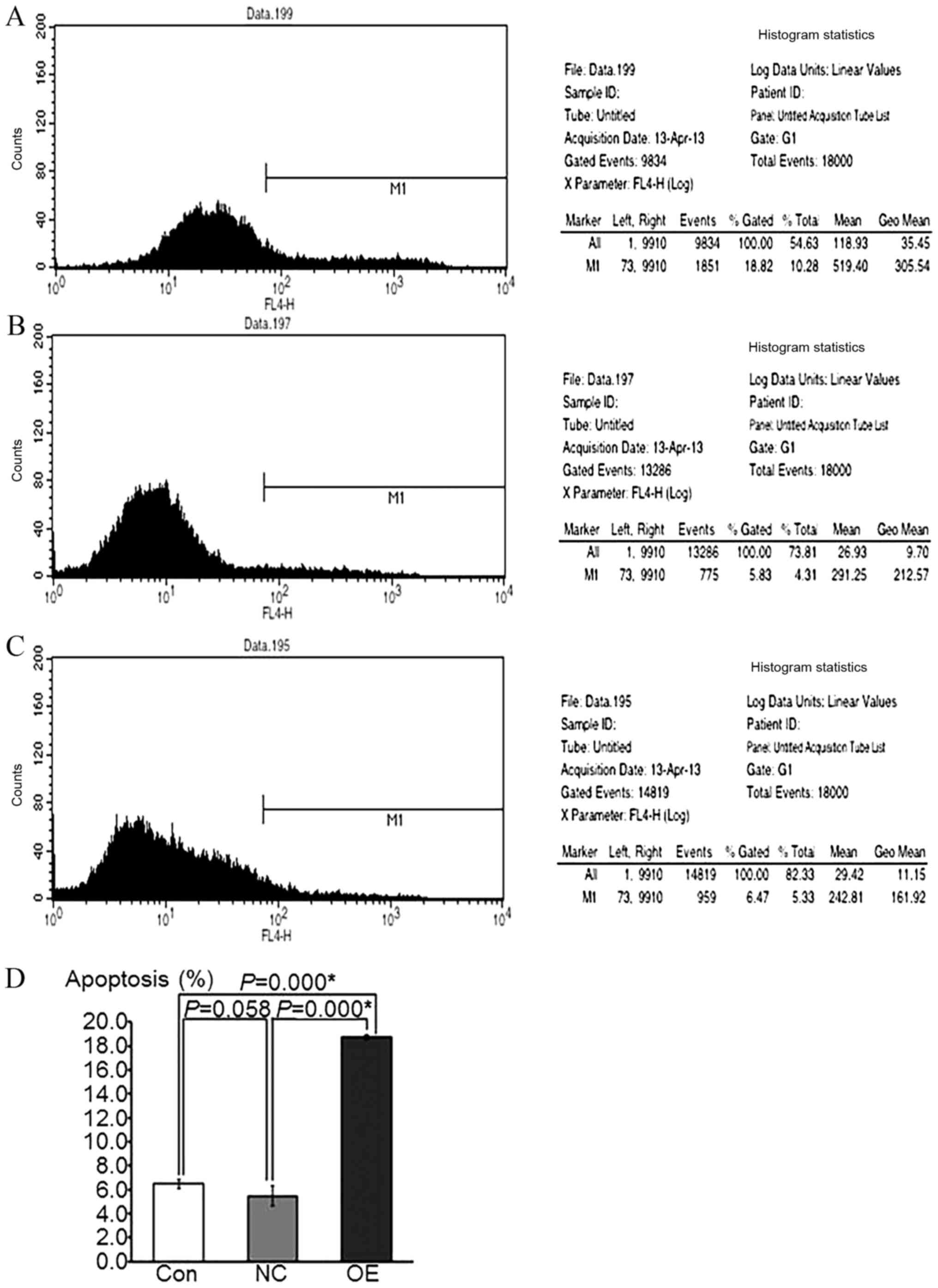
Overexpression of ATF3 increases the
apoptotic activity in HepG2 cells
The APC fluorescence intensity was examined by flow
cytometry in the FL4 channel, and the fluorescence intensity of
EGFP, which was used as a reporter gene, was measured in the FL1
channel. The percentage of apoptotic cells, which was
representative of the cell apoptotic rate, was calculated by
comparing these 2 fluorescence intensities. The results
demonstrated that the apoptotic rate in the OE group was
significantly increased compared with that in the NC and control
groups (P<0.05), whereas there was no significant difference
between the NC and control groups (P=0.058; Fig. 4). These data suggest that the
overexpression of ATF3 may increase the apoptosis of HepG2
cells.
Overexpression of ATF3 decreases cell
cycle progression in HepG2 cells
The numbers of cells in each phase of the cell cycle
were detected by flow cytometry using a FACSCalibur instrument. The
cell proportions in various phases of the cell cycle were
calculated to demonstrate the specific cell growth states. The
results indicated that the proportion of cells in the G0/G1 phase
in the OE group was significantly increased compared with that in
the NC and control groups (P<0.05), whereas the proportions of
cells in the S and G2/M phases were not significantly different
compared with the other groups (P>0.05). There was no
significant difference between the NC and control groups in any of
the phases of the cell cycle (P=0.183). The sum of the S and G2/M
cell proportions was representative of cell proliferation. The
results were the same as those of the MTT assay, which suggested
that the sum of the S and G2/M cell proportions in the OE group was
significantly decreased compared with that in the NC and control
groups (P<0.05), whereas there was no significant difference
between the NC and control groups (P=0.167; Fig. 5). These data indicated that the
overexpression of ATF3 inhibited cell proliferation by inducing
cell cycle arrest at the G0/G1 phase.
Discussion
Lentiviral vectors are now the most commonly used
tool in genetic intervention experiments. GV287, pHelper1.0 and
pHelper2.0 plasmids were used as an integrated overexpression
system for the target gene ATF3 in the present study. HepG2 cells
were successfully infected with the recombinant plasmid generated
in the Institute of Oncology, with a marked green fluorescent
signal and a high level of ATF3 protein expression.
Following the overexpression of ATF3 in HepG2 cells,
proliferation was markedly inhibited from the third day, as
indicated by the MTT assay. From the third day until the fifth day
following infection, the proliferation of the HepG2 cells in the OE
group was significantly decreased compared with that in the NC and
control groups. Similar to the results of the MTT assay, the sum of
the cells in the S and G2/M phases of the cell cycle in the OE
group was also markedly decreased compared with that in the other
groups, as detected by flow cytometry. In addition, the proportion
of cells in the G0/G1 phase in the OE group was significantly
increased compared with that in the NC and control groups. These
consistent results demonstrated that cell cycle progression in
HepG2 cells may be blocked in the mitosis stage by the
overexpression of the ATF3 protein. Ultimately, cell viability was
decreased in the tumour cells with high ATF3 levels.
Additionally, following the overexpression of ATF3
in HepG2 cells, the apoptotic rate in the OE group was markedly
increased compared with that in the NC and control groups, as
detected by flow cytometry. The rates of tumour cell apoptosis were
accelerated by the high level of ATF3 protein. However, HepG2
migration was not markedly different among the three groups as a
whole, as indicated by the Transwell assay. Nevertheless, the
OD570/OD490 value, which represents cell mobility, was decreased in
the OE group compared with the NC and control groups. Therefore,
there was a tendency for the HepG2 cells with ATF3 overexpression
to exhibit decreased cell migration. These results were consistent
with our previous study (10),
which demonstrated that capsule invasion was weaker in liver cancer
tissues with a high level of ATF3 protein.
Combined with the data from our previous study
(10), our results indicate that
the ATF3 level is low in human HCC tissues, and there was a
decreased protein level in patients with capsule invasion. All
these results indicate that ATF3 may serve a role as a tumour
suppressor during human hepatocellular oncogenesis.
The present study provides mechanistic insights into
this phenomenon of ATF3 as a tumour suppressor. The changes in the
biological behaviour of HepG2 cells with and without ATF3
overexpression were explored, and it was observed that the cells
overexpressing ATF3 exhibited slower viability, accelerated
apoptosis and cell cycle arrest. These data provide insight into
how ATF3 affects liver cancer cells.
According to previous studies, ATF3 is primarily
activated by 2 important signal transduction pathways, c-Jun
N-terminal kinase/stress-activated protein kinase (JNK/SAPK) and
P38/MAPK (11). Certain studies
have identified that persistent activation of the JNK/SAPK
signalling pathway is closely associated with cell apoptosis
(12), and the P38/MAPK pathway is
crucial for promoting apoptosis (13). ATF3 suppresses oncogenic networks,
induces cell death and inhibits malignant dissemination in a number
of cancer types (14), including
glioblastoma and colon, prostate, bladder and lung cancer (9,15–18).
In a study by Wang et al (19), the expression of the NOXA gene,
which is a key mediator of apoptosis, may be enhanced by ATF3. All
these data indicate that ATF3 expression and tumour cell death are
closely associated. Certain studies hypothesize that this
association is likely to be context-dependent (20,21).
In addition, several studies have revealed that ATF3
may induce cell cycle arrest to decrease tumour formation. A study
by Feng et al (22)
explored whether ATF3 may regulate lung small cancer cells
proliferation through regulation of the cyclin D1-associated
pathway. In an additional study, the ATF3 protein was demonstrated
to slow cell progression from the G1 to S phase, and to moderately
suppress cell growth (23). A
study by James et al (24)
demonstrated decreased cyclin-dependent kinase activity and
hypophosphorylation of pocket proteins in response to ATF3
upregulation in mature chondrocytes, which would have resulted in
cell cycle exit and increased activity of runt-related
transcription factor 2, thereby terminating chondrocyte
differentiation. Additionally, there is certain evidence indicating
that ATF3 may suppress cell migration and invasion (9,25).
These results agree with the data from the present study. At
present, our studies on HepG2 cells have verified certain basic
results regarding the association between ATF3 and tumour formation
and have illustrated that a high level of ATF3 may inhibit liver
cancer development.
However, ATF3 is unlikely to be solely responsible
for the development of liver cancer. The context-dependent role
that ATF3 serves in tumours is due to the intricate networks formed
with other genes and proteins. Future studies will focus on the
protein-protein interactions of ATF3 to investigate how ATF3 and
its critical partners function in liver cancer oncogenesis. Whether
an intervention targeting ATF3 may be used as a novel strategy for
liver cancer prevention and treatment is an interesting question
and remains to be resolved.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Fujian Provincial Education Office (grant no. 2013B009) and The
Special Fund of Fujian Provincial Finance Department of China
(grant no. 2014–1262).
Availability of data and materials
The data and materials are available from the first
author and corresponding author on reasonable request.
Authors' contributions
XL made substantial contributions to analyzing data
and the writing of the manuscript. SZ performed the construction of
lentiviral ATF3 overexpression vectors and cell transfection. HC
and JL performed the examination of biological behaviour of HepG2
cells. AH made substantial contributions to the design of the
present study and quality control. All the authors have approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATF3
|
activating transcription factor 3
|
|
HCC
|
hepatocellular carcinoma
|
|
LV
|
lentivirus
|
References
|
1
|
Brunacci C, Piobbico D, Bartoli D,
Castelli M, Pieroni S, Bellet MM, Viola-Magni M, Della Fazia MA and
Servillo G: Identification and characterization of a novel peptide
interacting with cAMP-responsive elements binding and
cAMP-responsive elements modulator in mouse liver. Liver Int.
30:388–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilchrist M, Henderson WR Jr, Morotti A,
Johnson CD, Nachman A, Schmitz F, Smith KD and Aderem A: A key role
for ATF3 in regulating mast cell survival and mediator release.
Blood. 115:4734–4741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Du Z and Jiang B: The research progress of
ATF3 and tumor. J Gannan Univ Med. 30:163–166. 2010.
|
|
4
|
Tamura K, Hua B, Adachi S, Guney I,
Kawauchi J, Morioka M, Tamamori-Adachi M, Tanaka Y, Nakabeppu Y,
Sunamori M, et al: Stress response gene ATF3 is a target of c-myc
in serum-induced cell proliferation. EMBO J. 24:2590–2601. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taketani K, Kawauchi J, Tanaka-Okamoto M,
Ishizaki H, Tanaka Y, Sakai T, Miyoshi J, Maehara Y and Kitajima S:
Key role of ATF3 in p53-dependent DR5 induction upon DNA damage of
human colon cancer cells. Oncogene. 31:2210–2221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin X, Wolford CC, Chang YS, McConoughey
SJ, Ramsey SA, Aderem A and Hai T: ATF3, an adaptive-response gene,
enhances TGF-{beta} signaling and cancer-initiating cell features
in breast cancer cells. J Cell Sci. 123:3558–3565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma S, Pang C, Song L, Guo F and Sun H:
Activating transcription factor 3 is overexpressed in human glioma
and its knockdown in glioblastoma cells causes growth inhibition
both in vitro and in vivo. Int J Mol Med. 35:1561–1573. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka Y, Nakamura A, Morioka MS, Inoue S,
Tamamori-Adachi M, Yamada K, Taketani K, Kawauchi J, Tanaka-Okamoto
M, Miyoshi J, et al: Systems analysis of ATF3 in stress response
and cancer reveals opposing effects on pro-apoptotic genes in p53
pathway. PLoS One. 6:e268482011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hackl C, Lang SA, Moser C, Mori A,
Fichtner-Feigl S, Hellerbrand C, Dietmeier W, Schlitt HJ, Geissler
EK and Stoeltzing O: Activating transcription factor-3 (ATF3)
functions as a tumor suppressor in colon cancer and is up-regulated
upon heat-shock protein 90 (Hsp90) inhibition. BMC Cancer.
10:6682010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiaoyan L, Shengbing Z, Yu Z, Lin Z,
Chengjie L, Jingfeng L and Aimin H: Low expression of activating
transcription factor 3 in human hepatocellular carcinoma and its
clinicopathological significance. Int J Mol Med. 210:477–481.
2014.
|
|
11
|
Maciag AE, Nandurdikar RS, Hong SY,
Chakrapani H, Diwan B, Morris NL, Shami PJ, Shiao YH, Anderson LM,
Keefer LK and Saavedra JE: Activation of the c-Jun N-terminal
kinase/activating transcription factor 3 (ATF3) pathway
characterizes effective arylated diazeniumdiolate-based nitric
oxide-releasing anticancer prodrugs. J Med Chem. 54:7751–7758.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Sun F, Sun W, Shi H, Yong Y, Liu S
and Liu L: Busuishengxue ranules mediate their effects upon
non-severe aplastic anemia via mitogen-activated protein
kinase/extracellular signal-regulated kinase pathway. J Tradit Chin
Med. 34:23–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slattery ML, Lundgreen A and Wolff RK:
Dietary influence on MAPK-signaling pathways and risk of colon and
rectal cancer. Nutr Cancer. 65:729–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei S, Wang H, Lu C, Malmut S, Zhang J,
Ren S, Yu G, Wang W, Tang DD and Yan C: The activating
transcription factor 3 protein suppresses the oncogenic function of
mutant p53 proteins. J Biol Chem. 289:8947–8959. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gargiulo G, Cesaroni M, Serresi M, de
Vries N, Hulsman D, Bruggeman SW, Lancini C and van Lohuizen M: In
vivo RNAi screen for BMI1 targets identifies TGF-β/BMP-ER stress
pathways as key regulators of neural- and malignant glioma-stem
cell homeostasis. Cancer Cell. 23:660–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang X, Li X and Guo B: KLF6 induces
apoptosis in prostate cancer cells through up-regulation of ATF3. J
Biol Chem. 283:29795–29801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan X, Yu L, Li J, Xie G, Rong T, Zhang
L, Chen J, Meng Q, Irving AT, Wang D, et al: ATF3 suppresses
metastasis of bladder cancer by regulating gelsolin-mediated
remodeling of the actin cytoskeleton. Cancer Res. 73:3625–3637.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jan YH, Tsai HY, Yang CJ, Huang MS, Yang
YF, Lai TC, Lee CH, Jeng YM, Huang CY, Su JL, et al: Adenylate
kinase-4 is a marker of poor clinical outcomes that promotes
metastasis of lung cancer by downregulating the transcription
factor ATF3. Cancer Res. 72:5119–5129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Mora-Jensen H, Weniger MA,
Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner
A and Ye Y: ERAD inhibitors integrate ER stress with an epigenetic
mechanism to activate BH3-only protein NOXA in cancer cells. Proc
Natl Acad Sci USA. 106:2200–2205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu D, Wolfgang CD and Hai T: Activating
transcription factor ATF3, a stress inducible gene, suppresses Ras
stimulated tumorigenesis. J Biol Chem. 281:10473–10481. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang G, Lunardi A, Zhang J, Chen Z, Ala U,
Webster KA, Tay Y, Gonzalez-Billalabeitia E, Egia A, Shaffer DR, et
al: Zbtb7a suppresses prostate cancer through repression of a
Sox9-dependent pathway for cellular senescence bypass and tumor
invasion. Nat Genet. 45:739–746. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng J, Sun Q, Wu T, Lu J, Qu L, Sun Y,
Tian L, Zhang B, Li D and Liu M: Upregulation of ATF-3 is
correlated with prognosis and proliferation of laryngeal cancer by
regulating Cyclin D1 expression. Int J Clin Exp Pathol.
6:2064–2070. 2013.PubMed/NCBI
|
|
23
|
Fan F, Jin S, Amundson SA, Tong T, Fan W,
Zhao H, Zhu X, Mazzacurati L, Li X, Petrik KL, et al: ATF3
induction following DNA damages regulated by distinct signaling
pathways and over expression of ATF3 protein suppresses cells
growth. Oncogene. 21:7488–7496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
James CG, Woods A, Underhill TM and Beier
F: The transcription factor ATF3 is up-regulated during chondrocyte
differentiation and represses cyclin D1 and A gene transcription.
BMC Mol Biol. 7:302006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zigler M, Villares GJ, Dobroff AS, Wang H,
Huang L, Braeuer RR, Kamiya T, Melnikova VO, Song R, Friedman R, et
al: Expression of Id-1 is regulated by MCAM/MUC18: A missing link
in melanoma progression. Cancer Res. 71:3494–3504. 2011. View Article : Google Scholar : PubMed/NCBI
|