Introduction
Radiation is an important environmental factor that
affects human health. Previous studies have suggested that ionizing
radiation causes direct and indirect cell death via DNA strand
breaks and free radical formation (1). Free radicals destroy cellular
membranes, which are made of polyunsaturated fatty acids and are
highly susceptible to oxidative damage, eventually resulting in
programmed cell death (2,3). In addition, oxidative damage leading
to lipid peroxidation activates cellular components that may have
serious effects on cells, and results in various of diseases
(4–6). Due to developments in and application
of nuclear technology in industry, agriculture and medicine,
exposure to ionizing radiation may occur in nuclear accidents and
cancer treatment, thus, the likelihood of people suffering from
radiation damage has increased. This harmful environment can
potentially cause genetic mutations and damage the human
hematopoietic, immune, reproductive, digestive and nervous systems
(7,8).
In an attempt to find effective, reliable and
inexpensive anti-radiation drugs, research has been conducted into
identifying plant-derived radio-protectors. At present, numerous
natural plant-based compounds have displayed a diverse array of
biological activities that may be relevant to the mitigation of
ionizing radiation-induced damage in mammalian systems. These
include puerarin, tea polyphenols, emodin and quercetin (9–13),
which could potentially protect against γ-radiation-induced
toxicity by inhibiting DNA damage and oxidative stress.
Salvia militarize, also known as Danshen, is
one of the most widely used herbs in traditional Chinese medicine.
It has been demonstrated to have positive effects on stasis, blood
flow activation and menstruation, thus has been used to treat a
variety of diseases (14). Modern
pharmacological studies have demonstrated that Salvia
protects vascular endothelial cells, acts as an anti-arrhythmic,
works to combat atherosclerosis, improves microcirculation,
protects the heart, increases coronary blood flow, improves
myocardial ischemia, has an anti-inflammatory act against lipid
peroxidation and pulmonary fibrosis, scavenges radicals and
protects liver cells (15–20). It has also been used in the
treatment of coronary artery disease and other cardiovascular
disorders (21).
The primary ingredients of Salvia militarize
include fat-soluble components, such as tanshinone,
cryptotanshinone and water-soluble components, including danshensu,
salvianolic acid A, B, and C. The most abundant constituent among
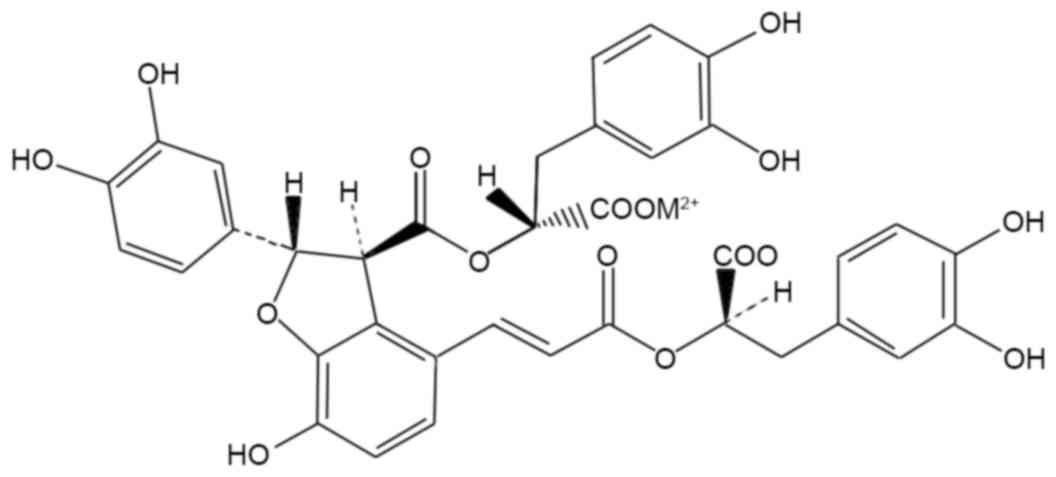
the water-soluble compounds is, Salvianolic acid B (SB; Fig. 1) which is a potent antioxidant and
acts as a scavenger of oxygen free radicals to protect against
ischemia-reperfusion injuries in heart (22), spinal cord I/R injury (23) and 6-hydroxydopamine induced
apoptosis in SH-SY5Y cells (24).
However, no study investigating the protective effects of SB on
radiation-induced oxidative damage has been reported. Therefore,
the aim of the current study was to evaluate if SB treatment had a
protective effect on mice injured by ionizing irradiation and to
elucidate its potential pharmacological mechanism.
Materials and methods
Reagents
Salvianolic acid B (purity >98%) was purchased
from the National Institute for the Control of Pharmaceutical and
Biological Products (Beijing, China) and dissolved in saline.
Ethinylestradiol (purity >98%), used as a positive control drug
for treating radiation induced damage, was purchased from Adamas
Reagent Ltd. (Shanghai, China) and dissolved in oil for injection.
The superoxide dismutase (SOD) and malondialdehyde (MDA) kits were
purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). Cell lysis buffer was obtained from Cell Signaling
Technology, Inc. (Shanghai, China), the bicinchoninic acid (BCA)
protein quantification kit (cat. no. W041), erythrocyte diluents,
platelet diluents and leukocytes diluents were all purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The
other chemicals used were of reagent grade from Sinopharm Chemical
Reagent Co., Ltd (Shanghai, China).
Animals
A total of 30 male and 30 female Kuming (KM) mice of
SPF grade, weighing 18–20 g were obtained from the Laboratory
Animal Services Center, Guangzhou University of Chinese Medicine
(Guangzhou, China). They were acclimatized in an air-conditioned
room maintained at a temperature between 20–25°C, humidity of
55±5%, 12-h light/dark cycle and with free access to food and
water. The animal studies were approved by the Animal Ethics
Committee of Guangzhou University of Chinese Medicine.
γ-irradiation and drug
administration
Following a week of acclimation, the KM mice were
randomly divided into six groups with ten mice in each group:
Normal group, model group (radiation injury only),
ethinylestradiol-positive control group, and three SB dosage groups
(low, SB-L; medium, SB-M; and high, SB-H). The mice in the positive
control group were injected intraperitoneally with 5 mg/kg of
ethynylestradiol, the mice in the SB groups were treated with 5,
12.5, 20 mg/kg of SB, respectively, while mice in normal and model
groups were injected with 0.1 ml/10 g of body weight/day of saline.
To achieve bone marrow suppression, all mice, except those in the
normal group, were exposed to 60Co-γ ray radiation
(Foshan Plastics Group Co., Ltd., Foshan, China) at a total dose of
6 Gy (0.1 Gy/min, for 185 sec). Drug administration started from
day 1 post-irradiation, and lasted for a total of 14 days, with one
dose each day.
Determination of peripheral blood cell
counts
Blood was drawn from the tail vein of all the groups
of mice pre-irradiation and on days 3, 7 and 14 post-irradiation
and then mixed with erythrocyte diluents, leukocyte diluents and
platelet diluents, respectively. The peripheral white blood cell
(WBC), red blood cell (RBC) and platelet (PLT) counts were then
determined.
Bone marrow nucleated cell counts
A total of 1 h after the final drug administration
on day 14, all mice were sacrificed by cervical dislocation and the
bone marrows were flushed from the whole femoral bone with 1 ml
phosphate-buffered saline (PBS) and mixed homogenously to make a
cell suspension. The cell suspension was added to a solution of 60%
lymphocyte separation medium (density 1.077 g/ml, Cat. no. 17-829E,
Lonza Group, Ltd., Basel, Switzerland), and centrifuged using the
Heraeus Laboratory Centrifuge (Model Biofuge Pico, Heraeus,
Germany) at 18°C at 300 × g for 30 min. The white ring layer was
separated by a sterile plastic pipette after the plasma fluid was
deprived. The nucleated cells were diluted with PBS and centrifuged
under the same conditions. The cell pellets were then suspended
homogenously, and were used to count nucleated cells under the
optical microscope.
Determination of protein content of
bone marrow cells
Lysis buffer was added to the bone marrow cell
suspension, incubated on ice for 30 min, and centrifuged using the
Heraeus Laboratory Centrifuge at 4°C at 13,000 × g for 10 min. The
supernatant was separated, dispensed, stored at −80°C. The protein
concentration was determined by the BCA method.
Thymus and spleen indices
On day 14 post-radiation, all animals were
sacrificed and the spleen and thymus were removed and weighed. The
thymus and spleen indices were calculated by dividing organ weight
by body weight according to the following formulae. Thymus
index=thymus weight (mg)/body weight (g)*10; spleen index=spleen
weight (mg)/body weight (g)*10.
Measures of antioxidant capacity
On day 14 post-radiation, blood samples were
obtained from the post-ocular venous plex from mice that were
fasted overnight. They were anesthetized with diethyl ether. The
blood was allowed to clot at room temperature for 1 h prior to
centrifugation using the Heraeus Laboratory Centrifuge at 4°C at
1,200 × g for 15 min to separate the serum. Serum SOD and MDA
levels were detected by ELISA test kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression levels of BTB and CNC homology 1
(Bach1) and nuclear factor (erythroid-derived 2)-like 2 protein
(Nrf2) were determined using RT-qPCR. RNA extraction was prepared
using TRIzol reagent (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's instructions. A Total RNA (1
mg) was used to synthesize the first strand of cDNA using Bestar
qPCR RT kit (DBI Bioscience, Heidelberg, Germany). The mRNA
expression was evaluated by qPCR on Stratagene Mx3000P Real time
PCR platform (Agilent Technologies, Inc., Santa Clara, CA, USA)
with SYBR Green PCR core reagents. The PCR reaction system (20 µl)
contained qPCR master mix 10 µl, plus forward and reverse primers
(10 µM) 1.0 µl, cDNA template 1 µl and ddH2O 8 µl.
β-actin was applied as the internal reference. The following
primers were synthesized and applied: Nrf2, forward
5′-GGTTGCCCACATTCCCAAAT-3′ and reverse 5′-AGCAATGAAGACTGGGCTCT3′;
Bach1, forward 5′-TAGTGTGGAGCGAGAAGTGG-3′ and reverse
5′-ACCTAACCACGGACACTCAG-3′; β-actin, forward
5′-CATTGCTGACAGGATGCAGA-3′ and reverse 5′-CTGCTGGAAGGTGGACAGTGA-3′.
The reaction procedure was initiated with denaturation at 94°C for
2 min and followed by 40 repeated cycles (denaturation at 94°C for
20 sec, annealing at 58°C for 20 sec and extension at 72°C for 20
sec). The Ct-value for each sample was calculated with the
ΔΔCq-method (25), and the results
were expressed as 2−ΔΔCq to analyze the fold change.
Western blotting
Western blot analysis was performed as described
previously (26). Briefly,
portions of liver were homogenized for each group and nucleoprotein
was extracted from samples by using Nuclear Protein Extraction kit
(Beyotime Institute of Biotechnology, Wuhan, China) according to
the manufacturer's instructions. Proteins (20–40 µg) were loaded in
each lane. Membranes were incubated overnight at 4°C with anti-Nrf2
(cat. no. ab89443; 1:1,000; Abcam, Cambridge, UK), anti-Bach1 (cat.
no. NBP1-88722; 1:500; Novus Biologicals Ltd., Cambridge, UK) and
anti-Bax (cat. no. 2772; 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) antibodies. Blots were subsequently incubated
with horseradish peroxidase-conjugated secondary antibodies (cat
nos. A4416 and A6154; 1:10,000; Sigma-Aldrich; Merck KGaA) for 1 h
at room temperature. Histone 3 (Sigma-Aldrich; Merck KGaA) served
as a control.
Cellular ROS detection assay
ROS were detected with 2,7-dichlorofluorescein
diacetate (DCFH-DA; Beyotime Institute of Biotechnology) according
to the manufacturers' instructions. Livers were obtained from the
mice of each group. Collagenase and trypsin were added to
enzymolyze liver tissues into single-cell suspension. Cells were
incubated with DCFH-DA at a final concentration of 10 mM for 30 min
and washed 3 times with HEPES buffer in the dark. Subsequently
cells were washed with PBS three times. Following centrifugation,
cell pellets were suspended in PBS for immediate analysis by flow
cytometry.
Statistical analysis
All data were expressed as the mean ± standard
deviation. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. One-way analysis of variance was
followed by Tukey's post-hoc test. Student's t-test analysis was
used for assessing significant differences between the normal and
model groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of SB on peripheral blood count
of irradiated mice
Bone marrow is highly sensitive to radiation and
exposure often damages the hematopoietic cells. Thus, it was
investigated whether treatment with SB had beneficial effects on
hematopoietic function of irradiated mice. To this end, the cell
counts of peripheral WBC, RBC and PLT were measured with and
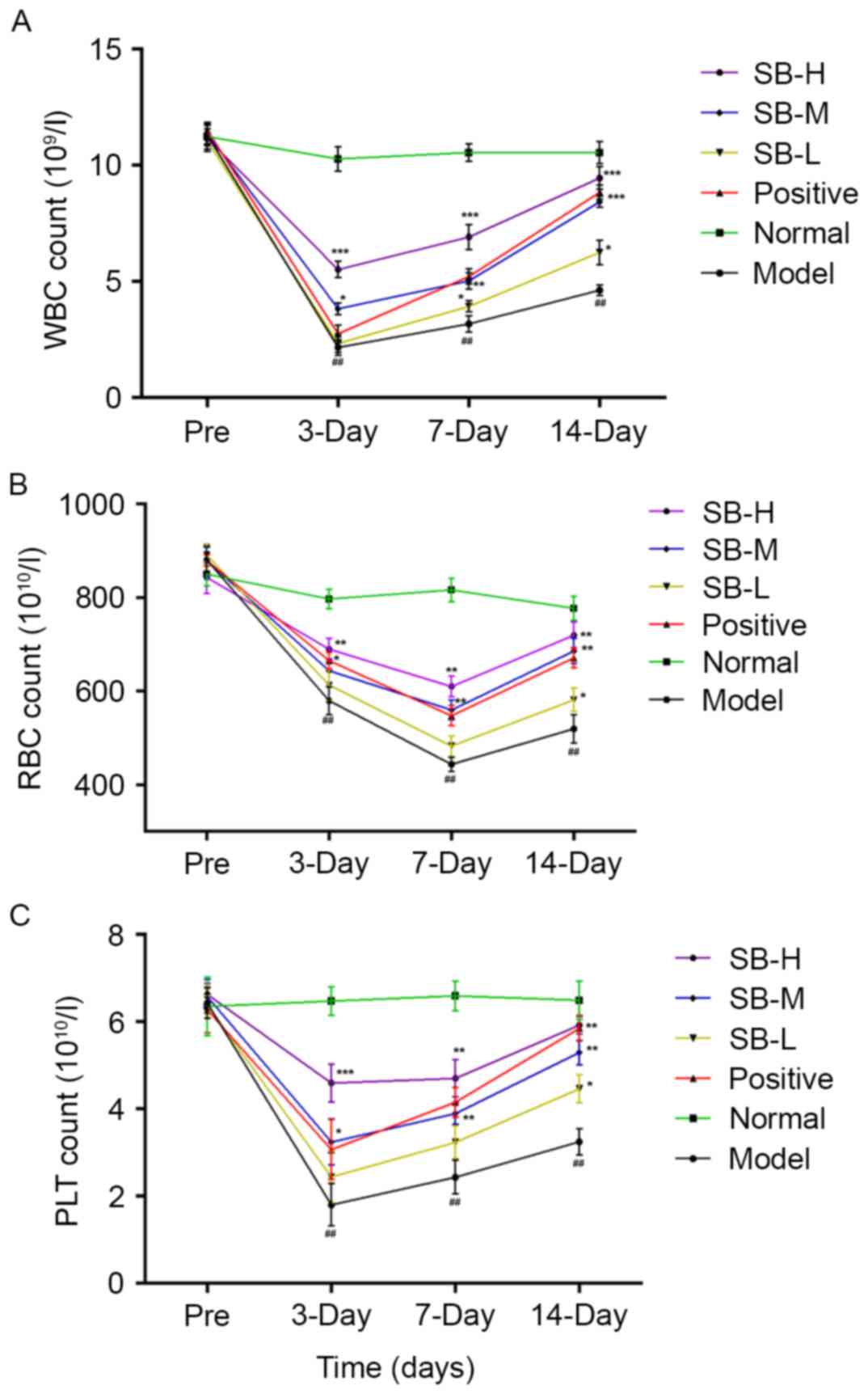
without treatment of SB from radiated mice. As presented in
Fig. 2, the trend of peripheral
WBC, RBC and PLT in mice were in the normal range and no
significant differences were observed between the groups prior to
radiation. However, following exposure to 60Co-γ
radiation at a dose of 60 Gy, the peripheral WBC, RBC and PLT
counts decreased significantly after γ radiation comparing with the
normal group (P<0.01). Although cell counts in the model group
exhibited a marginal auto-recovery on days 7 and 14, SB treatment
significantly raised the count of WBC, RBC and PLT on days 3, 7 and
14, compared with the model group. These results suggest that SB
alleviates the reduction in the number of peripheral WBC, RBC and
PLT induced by radiation and resulted in less damage to the
mice.
Effect of SB on nucleated cells in
bone marrow of radiated mice
Radiation destroys the microenvironment of the bone
marrow, thereby affecting hematopoietic function. The decrease in
nucleated cells of the bone marrow indicates disorders of
hematopoietic system. The number of nucleated cells in the bone
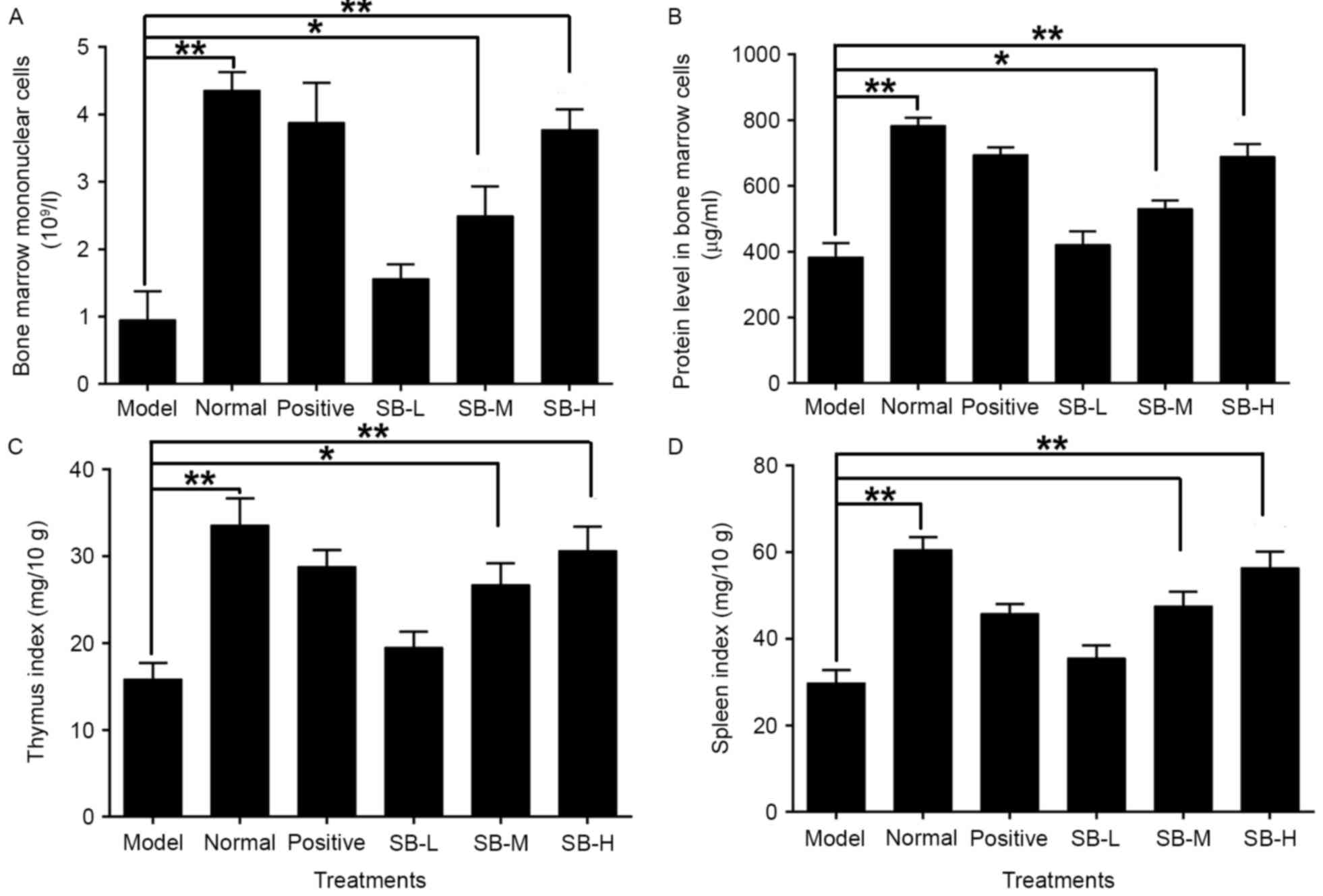
marrow decreased significantly (P<0.01) in the model group as
compared with the normal group (Fig.
3A). Administering the mice with three different doses of SB
noticeably increased the number of nucleated cells in the bone
marrow suggesting that treatment with SB may have a protective
effect on bone marrow hematopoietic cells.
Impact of SB on protein synthesis in
bone marrow cells of radiated mice
A significant proportion of damage resulting from
ionizing radiation is associated with the production of reactive
oxygen species (ROS), which oxidize DNA, proteins, lipids and
cofactors. Ionizing radiation breaks DNA strands and indirectly
impact protein synthesis of bone marrow cells. In addition,
radiation directly damages protein, including ROS-induced protein
chemical alterations via carbonization and nitrosylation (27). Thus, protein synthesis was assessed
in mice exposed to radiation. Ionizing radiation markedly decreased
the protein content in bone marrow cells as compared with the
normal group (P<0.01; Fig. 3B),
whereas the treatment of SB significantly increased the protein
levels in bone marrow cells. This suggests that SB may protect bone
marrow cells from oxidative damage of protein.
Effect of SB on the spleen and thymus
of radiated mice
Radiation damage to the thymus and spleen destroys
the immune system, increasing the probability of complications. To
investigate the effect of SB on the thymus and spleen of irradiated
mice, the thymus and spleen indices were evaluated. As presented in
Fig. 3C and D, the thymus and
spleen indices were both reduced significantly as compared with the
normal group (P<0.01), whereas treatment with three different
doses of SB significantly increased the thymus and spleen indices
of irradiated mice in a dose-dependent manner. This suggests that
SB treatment can significantly neutralize the damage caused by
radiation in the spleen and thymus of radiated mice.
Effect of SB on oxidation resistance
in radiated mice
Ionizing radiation affects the formation of free
oxygen radicals. These act on the cellular membrane and cause lipid
peroxidation, resulting in functional and metabolic changes of the
membrane. To evaluate the effect of SB on oxidative stress, the
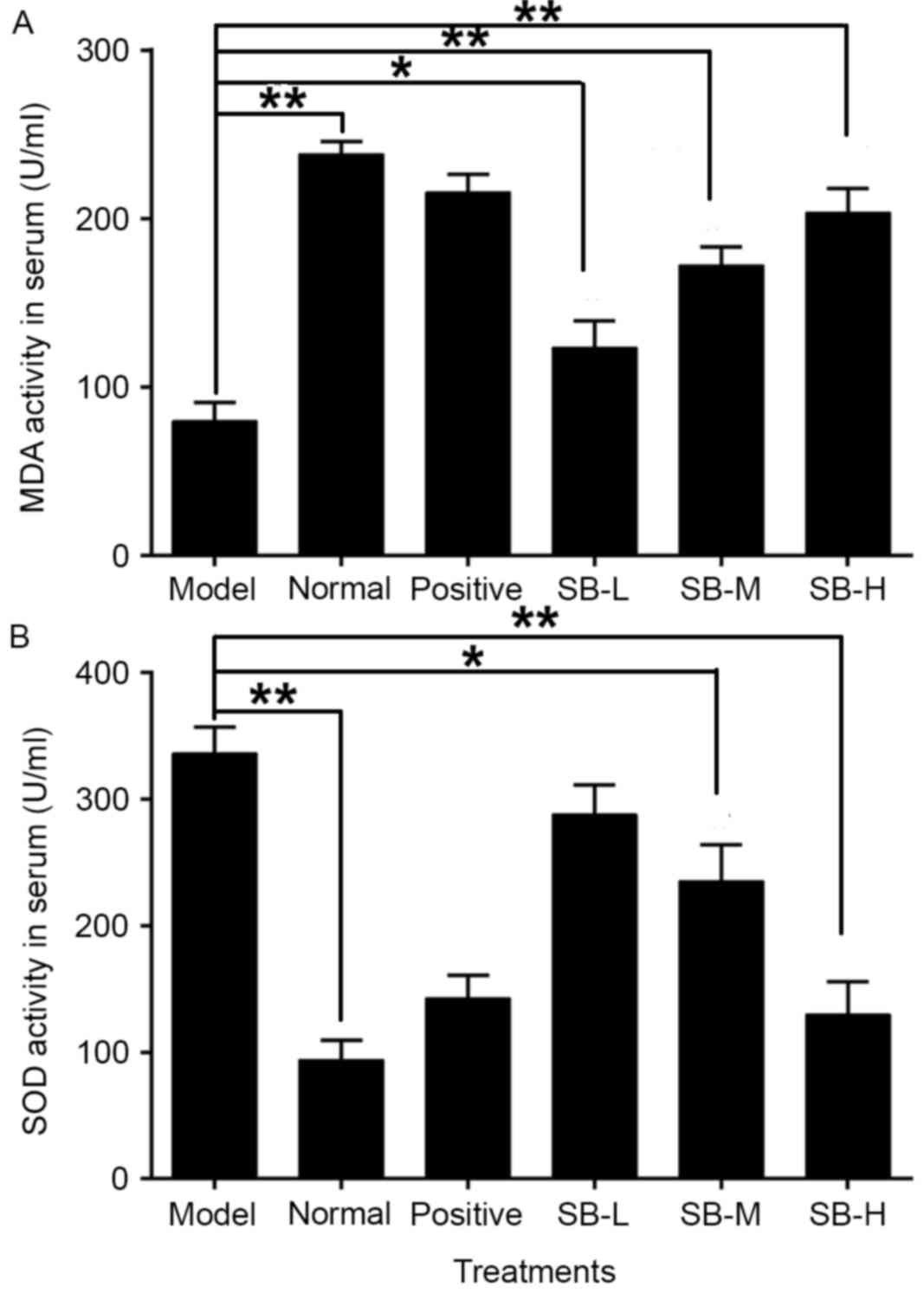
levels of serum SOD and MDA were assessed. SOD activity of radiated
mice in serum was significantly increased (P<0.01), whereas MDA
levels were significantly reduced comparing with the normal group
(P<0.01; Fig. 4). SB treatment
markedly increased MDA levels and down-regulated SOD levels, in a
dose-dependent manner comparing with the model group. Meanwhile,
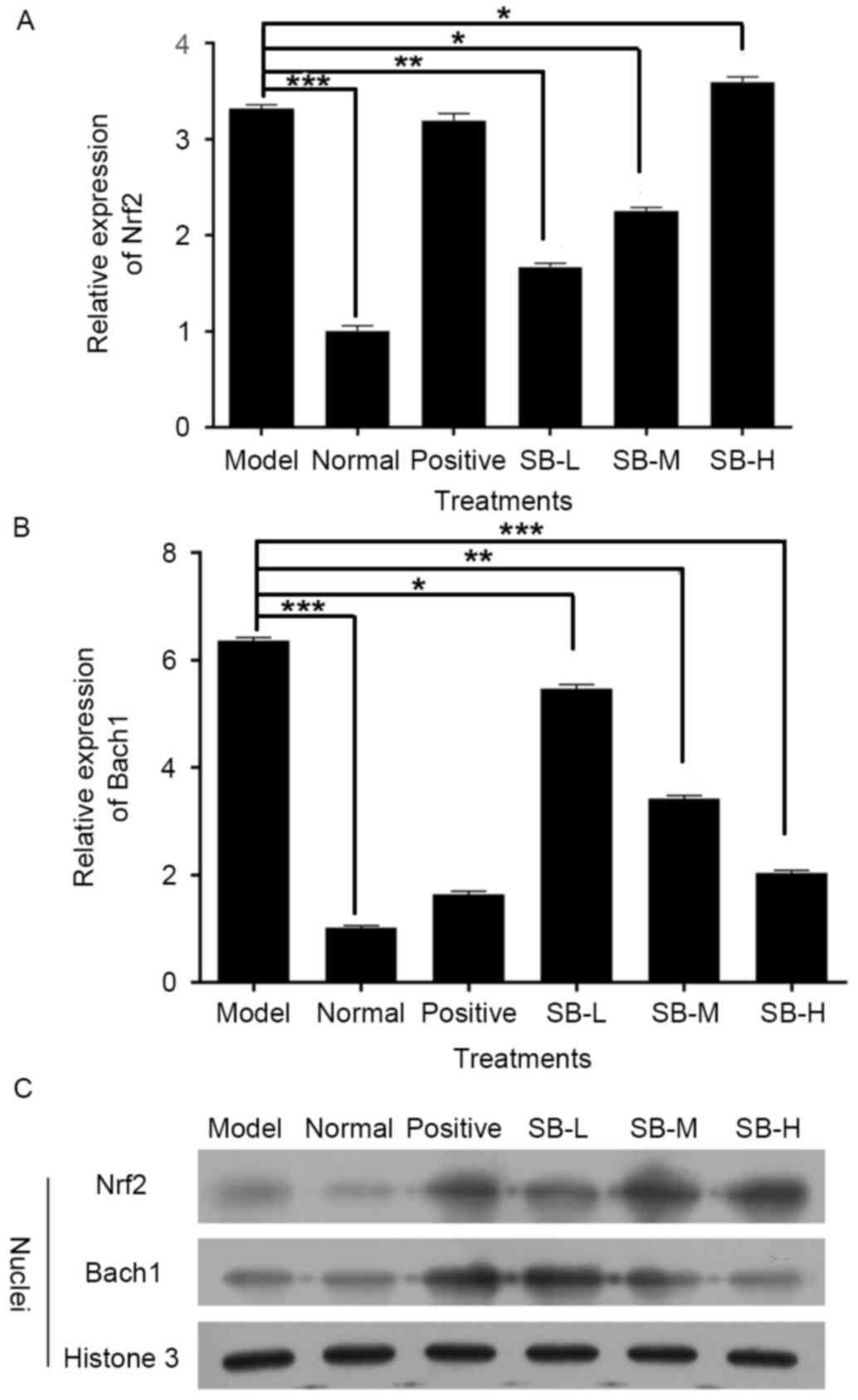
the expression level of Nrf2 in radiated mice decreased and Bach1,
which competed with Nrf2 for binding to antioxidant response
element, was markedly increased (P<0.001, Fig. 5A and B). Notably, this changing
tendency was completely reversed following treatment with SB
compared with the model group (Fig. 5A
and B), which indicates that SB has a protective effect on
ionizing radiation injury in mice by activating Nrf2-mediated
antioxidant effect. These tendencies were confirmed by western
blotting (Fig. 5C).
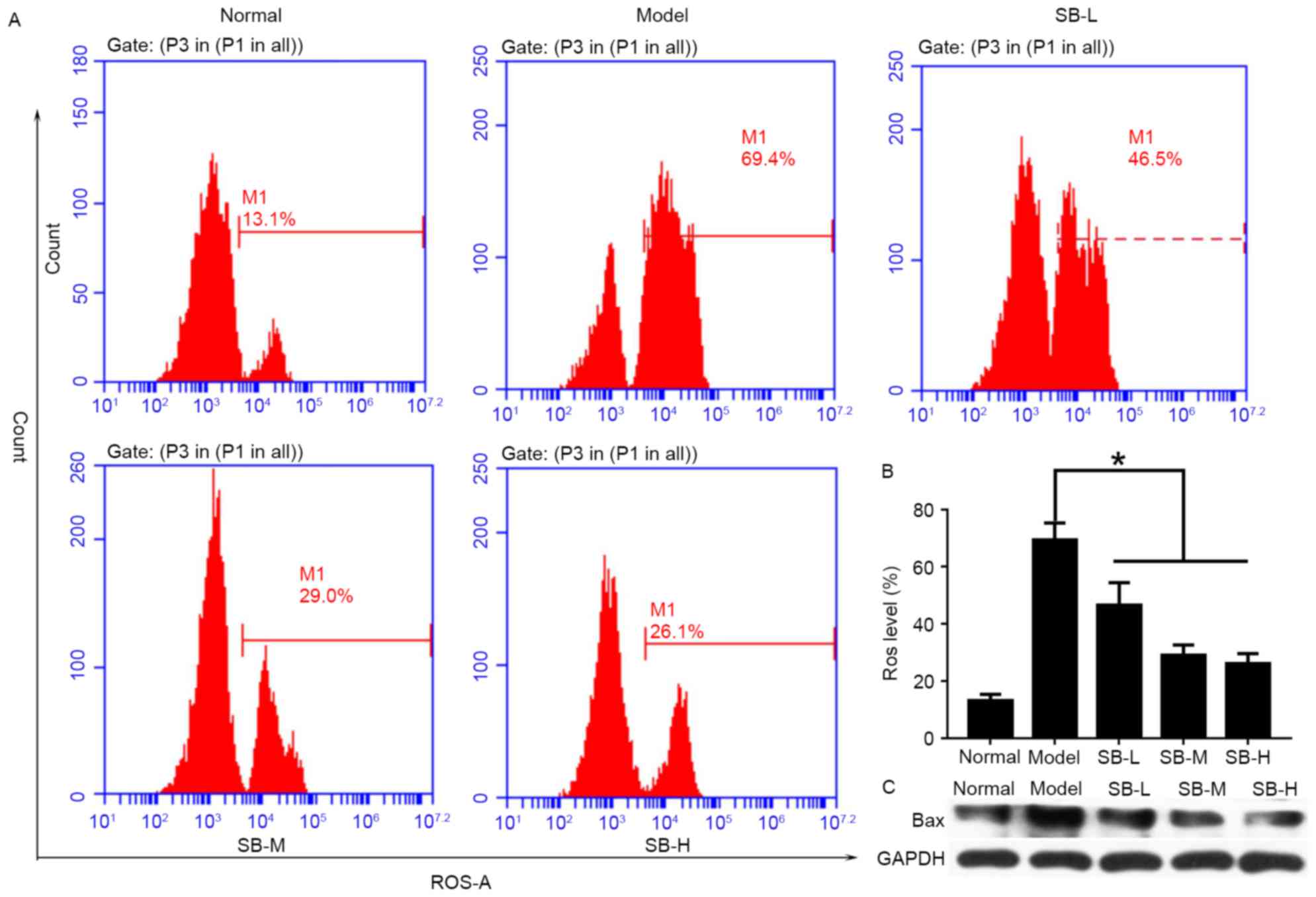
SB reduced ROS level and suppressed
Bax expression
To explore the protective mechanism of SB in
vivo, ROS levels in the liver were detected by flow cytometry
(Fig. 6A). As presented in
Fig. 6, mice in the model group
exhibited the highest levels of ROS, while lower ROS levels was
detected in SB-treated groups. In addition, this inhibitory effect
exhibited a dose-dependent manner (Fig. 6B). Bax protein expression was
suppressed in liver tissue after SB treatment (Fig. 6C). Therefore, it was suggested that
SB could protect mouse livers through reducing ROS levels and
inhibiting the relative apoptotic protein levels.
Discussion
Ionizing radiation exerts adverse biological effects
through direct and indirect processes: Breaks in DNA strands and
production of oxygen free radicals. These oxygen free radicals in
turn damage and mutate cellular DNA, leading to an increased rate
of malignancy with radiation exposure. In addition, free radicals
destroy supporting cellular structures, including organelles and
cell membranes, resulting in programmed cell death (28). Furthermore, generation of free
radicals result in an imbalance of the body's antioxidant system as
they attack polyunsaturated fatty acids of the biofilm, ultimately
leading to a series of damage to the hematopoietic, immune, nervous
and endocrine systems (29). Due
to the limitations and adverse effects of synthetic chemical
anti-radiation drugs, there is a requirement for anti-radiation
ingredients from natural sources, such as plants. Previous studies
have indicated that polysaccharides, alkaloids, coumarins,
flavonoids, saponins and other compounds possess anti-radiation
effects (30–32). The mechanism of this effect may be
attributed to protecting DNA, inhibiting immune injury, protecting
the hematopoietic system and scavenging free radicals.
Radix Salviae miltiorrhizae has been widely
used for thousands of years in traditional Chinese medicine with
little reported toxicity (33).
Salvianolic acid is the main water-soluble component in Radix
Salviae miltiorrhizae. SB is the most abundant component of
Salvianolic acid and is valued for both nutritional and medicinal
purposes (34). SB exhibits higher
scavenging activities than vitamin C against free hydroxyl radicals
[HO(−)], superoxide anion radicals [O2(−)], and 1,
1-diphenyl-2-picryl-hydrazyl radicals and 2-azino-bis
(3-ethylbenzthiazoline-6-sulfonic acid) radicals (35). As presented in Fig. 1, the compound SB contains multiple
phenolic hydroxyl groups, which act as hydrogen donors to provide
protons that combine with the oxygen free radicals generated by
radiation. This eliminates excessive free radicals in the body and
reduces the production of oxygen free radicals. In addition, it
interrupts the free radical oxidation chain reaction, removes free
radicals, prevents macromolecular damage and serves a role in
radiation protection. Hemograms reflect the functional state of the
body's hematopoietic system, which is particularly sensitive to
radiation injury. Patients exposed to ionizing radiation often
appear to show a sharp decline in the number of peripheral RBC, WBC
and PLT (36). Therefore,
protecting the hematopoietic system or improving the peripheral
blood counts is an important biochemical indicator to evaluate the
protective effect of novel drug candidates against radiation damage
on the body (37). The present
study indicated that radiated mice had a damaged hematopoietic
system and the number of peripheral RBC, WBC and PLT decreased
significantly. SB can significantly improve the peripheral blood
counts of radiated mice, potentially by relieving the injury of
ionizing radiation on hematopoietic system.
Bone marrow tissue is highly sensitive to radiation,
and radiation-induced bone marrow damage leads to changes in bone
marrow cells that are closely associated with the
hematopoietic/stem cell microenvironment damage (38). Mitotically active cells are more
susceptible to the detrimental effects of ionizing radiation,
therefore, anatomic structures with high mitotic rates, such as red
marrow, are more susceptible to the deleterious effects of
radiation exposure (39). Ionizing
radiation breaks DNA strands, and the number of nucleated cells and
protein synthesis in bone marrow cells is affected, however in
addition, radiation causes direct damage to the protein, including
ROS-induced protein chemical alterations such as carbonylation and
nitrosylation (39). The present
study demonstrated that SB protects protein damage caused by
ionizing radiation.
The spleen and the thymus are important immune
organs in mammals, in which immune cells and several immune factors
are produced. They constitute the body's defense system and serve
an important role in providing resistance to a variety of germs or
physical and chemical injuries. Secondly, due to the fact that they
are particularly sensitive to radiation damage, patients often
exhibit a decline in immunity after receiving radiation therapy, a
major side effect of radiation therapy (40). The present study identified that
the thymus and spleen indices of ionizing irradiated mice were
markedly reduced. Administration of SB alleviates the declining
trend caused by ionizing radiation, implying that SB may confer
certain protective effects on radiation-damaged organs.
A significant proportion of the damage from ionizing
radiation primarily results from the production of ROS that oxidize
DNA, proteins, lipids and cofactors (41–43).
When normal tissue is exposed to ionizing radiation, the atoms of
water molecules or other oxygen-containing molecules are attacked
by the photon. This results in the excitation and emission of an
electrons from that atom (44). In
addition, the ROS generated attack polyunsaturated fatty acids of
biofilm phospholipids and causes lipid peroxidation (45). MDA is a lipid peroxidation product
of biofilm phospholipid molecules, and its levels reflect the
degree of biofilm damaged by ROS (46). SOD is the key enzyme that removes
ROS intracellularly, and is widely distributed in various tissues
and organs of the body, SOD activity decreases due to aging,
ionizing radiation and environmental pollution, which results in
the destruction of the body's oxygen metabolism homeostasis
(47). Nrf2-mediated antioxidant
induction is a cellular adaptive response to oxidative stress
challenge and it can activate a series of antioxidant enzymes,
including SOD and heme oxygenase and therefore serves a central
role in the protection of cells against oxidative damage (19). The current study demonstrated that
ionizing radiation led to decreased SOD activity and increased MDA
levels in serum. In addition, it suppressed the Nrf2 pathway by
reducing Nrf2 expression levels and elevating Bach1 expression
levels. However, SB supplementation not only restores decreased SOD
activity and increased MDA in radiation injured mice but also
increased Nrf2 expression level and reduced Bach1 expression
levels. In addition, SB reduced ROS level and suppressed Bax
expression in mouse liver. These observations suggest that SB acts
as an anti-oxidant by activating Nrf2 pathway in the serum of
radiation-exposed mice, which may be associated with reducing
oxidative stress and inhibiting apoptosis. The results are in
agreement with a previous study, which observed that SB attenuated
toxin-induced neuronal damage via Nrf2 (48). However, further investigation using
Nrf2 knockout/knockdown mice or specific inhibition of Nrf2
expression is required to confirm the involvement of the Nrf2
pathway in the protective role of SB against radiation damage.
Taken together, it was identified that
supplementation of SB to mice exposed to radiation suppressed the
MDA levels induced by radiation, increased peripheral RBC, WBC and
PLT levels, thymus and spleen indices, and activated the
Nrf2-mediated antioxidant pathway. In conclusion, the results, at
least in part, indicated that SB has a protective effect against
radiation damage and may have valid antioxidant activity in
enhancing immunity and the function of the hematopoietic system.
Therefore, it is inferred that SB could serve as a promising
candidate for adjuvant therapy to alleviate radiation-induced
injuries in humans affected by ionizing radiation. The mechanisms
underlying the anti-radiation effect of SB require further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Guangdong Province Construction of Traditional Chinese Medicine
Strong Province Project (grant no. 20132103), Guangdong Province
Universities and Colleges Pearl River Scholar Funded Scheme (grant
no. 2011), Science & Technology Planning Project of Guangdong
Province Office of Education (grant no. 2014GKXM032) and Science
& Technology Planning Project of Nansha District (grant no.
2016CX003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RZ and GL designed the experiments. RZ, HL and BZ
performed the majority of the experiments. ZL, QZ, TW, YZ, QW and
XL assisted with the experiments. RZ, LZL and HL collected the data
and completed the data analysis. RZ and LZL drafted the manuscript,
and GL and LZL revised the drafts. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The animal studies were approved by the Animal
Ethics Committee of Guangzhou University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pacheco R and Stock H: Effects of
Radiation on Bone. Curr Osteoporos Rep. 11:299–304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giusti AM, Raimondi M, Ravagnan G, Sapora
O and Parasassi T: Human cell membrane oxidative damage induced by
single and fractionated doses of ionizing radiation: A fluorescence
spectroscopic study. Int J Radiat Biol. 74:595–605. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao W, Diz DI and Robbins ME: Oxidative
damage pathways in relation to normal tissue injury. Br J Radiol.
80:S23–S31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rice-Evans C and Burdon R: Free
radical-lipid interactions and their pathological consequences.
Prog Lipid Res. 32:71–110. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esterbauer H: Estimation of peroxidative
damage. A critical review. Pathol Biol (Paris). 44:25–28.
1996.PubMed/NCBI
|
|
6
|
Packer L and Ong ASH: Biological Oxidants
and Antioxidants. Molecular Mechanisms and Health Effects. AOCS
Press; Champaign, IL: 1998,
|
|
7
|
Moulder JE: Radiobiology of nuclear
terrorism: Report on an interagency workshop (Bethesda, MD,
December 17–18, 2001). Int J Radiat Oncol Biol Phys. 54:327–328.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arora R, Gupta D, Chawla R, Sagar R,
Sharma A, Kumar R, Prasad J, Singh S, Samanta N and Sharma RK:
Radioprotection by plant products: Present status and future
prospects. Phytother Res. 19:1–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin LH, Liu CF and Zeng Y: Protective
effects of puerarin on radiation injury of experimental rats. Zhong
Xi Yi Jie He Xue Bao. 3:43–45. 2005.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo S, Hu Y, Liu P, Wang Y, Guo D, Wang D
and Liao H: Protective activity of different concentration of tea
polyphenols and its major compound EGCG against whole body
irradiation-induced injury in mice. Zhongguo Zhong Yao Za Zhi.
35:1328–1332. 2010.(In Chinese). PubMed/NCBI
|
|
11
|
Sharma R and Tiku AB: Emodin, an
anthraquinone derivative, protects against gamma radiation-induced
toxicity by inhibiting DNA damage and oxidative stress. Int J
Radiat Biol. 90:275–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun XC, Li WB, Li QJ, Li SQ, Zhang M and
Xian XH: Spantide inhibits up-regulation of NOS in the pericentral
canal region of the spinal cord in the rat formalin test. Chin J
Pathophysiol. 21:242224262005.
|
|
13
|
Özyurt H, Çevik Ö, Özgen Z, Özden AS,
Çadırcı S, Elmas MA, Ercan F, Gören MZ and Şener G: Quercetin
protects radiation-induced DNA damage and apoptosis in kidney and
bladder tissues of rats. Free Radic Res. 48:1247–1255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su CY, Ming QL, Rahman K, Han T and Qin
LP: Salvia miltiorrhiza: Traditional medicinal uses, chemistry and
pharmacology. Chin J Nat Med. 13:163–182. 2015.PubMed/NCBI
|
|
15
|
Ji XY, Tan BK and Zhu YZ: Salvia
miltiorrhiza and ischemic diseases. Acta Pharmacol Sin.
21:1089–1094. 2000.PubMed/NCBI
|
|
16
|
Chan K, Chui SH, Wong DY, Ha WY, Chan CL
and Wong RN: Protective effects of Danshensu from the aqueous
extract of Salvia miltiorrhiza (Danshen) against
homocysteine-induced endothelial dysfunction. Life Sci.
75:3157–3171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HB, Xu J and Peng Y: Targets of
Danshen's active components for activating blood circulation
activities. Acta Phys-Chim Sin. 26:199–205. 2010.
|
|
18
|
Zhang N, Zou H, Jin L, Wang J, Zhong MF,
Huang P, Gu BQ, Mao SL, Zhang C and Chen H: Biphasic effects of
sodium danshensu on vessel function in isolated rat aorta. Acta
Pharmacol Sin. 31:421–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji W and Gong B: Hypolipidemic activity
and mechanism of purified herbal extract of Salvia miltiorrhiza in
hyperlipidemic rats. J Ethnopharmacol. 119:291–298. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paik YH, Yoon YJ, Lee HC, Jung MK, Kang
SH, Chung SI, Kim JK, Cho JY, Lee KS and Han KH: Antifibrotic
effects of magnesium lithospermate B on hepatic stellate cells and
thioacetamide-induced cirrhotic rats. Exp Mol Med. 43:341–349.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji XY, Tan BK and Zhu YZ: Salvia
miltiorrhiza and ischemic diseases. Acta Pharmacol Sin.
21:1089–1094. 2000.PubMed/NCBI
|
|
22
|
Zhao GF, Zhang HX and Fan YC: The
protecting effect of Salvianolic acid B on rats with myocardial
ischemia reperfusion injury. J Liaoning Coll Trad Chin Med.
1:55–56. 2004.
|
|
23
|
Fu J, Fan HB, Guo Z, Wang Z, Li XD, Li J
and Pei GX: Salvianolic acid B attenuates spinal cord
ischemia-reperfusion-induced neuronal injury and oxidative stress
by activating the extracellular signal-regulated kinase pathway in
rats. J Surg Res. 188:222–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian LL, Wang XJ, Sun YN, Li CR, Xing YL,
Zhao HB, Duan M, Zhou Z and Wang SQ: Salvianolic acid B, an
antioxidant from Salvia miltiorrhiza, prevents 6-hydroxydopamine
induced apoptosis in SH-SY5Y cells. Int J Biochem Cell Biol.
40:409–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu J, Zhang F, Zhao D, Hong L, Min J,
Zhang L, Li F, Yan Y, Li H, Ma Y and Li Q: ATRA-inhibited
proliferation in glioma cells is associated with subcellular
redistribution of β-catenin via up-regulation of Axin. J
Neurooncol. 87:271–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamori T, Yasui H, Yamazumi M, Wada Y,
Nakamura Y, Nakamura H and Inanami O: Ionizing radiation induces
mitochondrial reactive oxygen species production accompanied by
upregulation of mitochondrial electron transport chain function and
mitochondrial content under control of the cell cycle checkpoint.
Free Radic Biol Med. 53:260–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robbins ME and Zhao W: Chronic oxidative
stress and radiation-induced late normal tissue injury: A review.
Int J Radiat Biol. 80:251–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Epperly M, Bray J, Kraeger S, Zwacka R,
Engelhardt J, Travis E and Greenberger J: Prevention of late
effects of irradiation lung damage by manganese superoxide
dismutase gene therapy. Gene Ther. 5:196–208. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi J, Cheng C, Zhao H, Jing J, Gong N and
Lu W: In vivo anti-radiation activities of the Ulva pertusa
polysaccharides and polysaccharide-iron(III) complex. Int J Biol
Macromol. 60:341–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji R, Zhong K and Li Y and Li Y: Effects
of tea polyphenols on mice survival rate and white blood cell count
after radiation. Wei Sheng Yan Jiu. 31:394–395. 2002.(In Chinese).
PubMed/NCBI
|
|
32
|
Zhang XD, Ma C and Sun X: Advances in
mechanism of natural anti radiation drug. Chin J Radiol Health.
13:228–230. 2004.
|
|
33
|
Ho JH and Hong CY: Salvianolic acids:
Small compounds with multiple mechanisms for cardiovascular
protection. J Biomed Sci. 18:302011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang ZS, Luo P, Dai SH, Liu ZB, Zheng XR
and Chen T: Salvianolic acid B induces apoptosis in human glioma
U87 cells through p38-mediated ROS generation. Cell Mol Neurobiol.
33:921–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao GR, Zhang HM, Ye TX, Xiang ZJ, Yuan
YJ, Guo ZX and Zhao LB: Characterization of the radical scavenging
and antioxidant activities of danshensu and salvianolic acid B.
Food Chem Toxicol. 46:73–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geoffrey P and Jacobs a: A review on the
effects of ionizing radiation on blood and blood components. Radiat
Phys Chem. 53:511–523. 1998. View Article : Google Scholar
|
|
37
|
Edwards JC, Chapman D, Cramp WA and Yatvin
MB: The effects of ionizing radiation on biomembrane structure and
function. Prog Biophys Mol Biol. 43:71–93. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng K, Wu W, Yang S, Huang L, Chen J,
Gong C, Fu Z, Lin R and Tan J: Treatment of radiation-induced acute
intestinal injury with bone marrow-derived mesenchymal stem cells.
Exp Ther Med. 11:2425–2431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kokošová N, Tomášová L, Kisková T and
Šmajda B: Neuronal analysis and behaviour in prenatally
gamma-irradiated rats. Cell Mol Neurobiol. 35:45–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szumiel I: Ionizing radiation-induced cell
death. Int J Radiat Biol. 66:329–341. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kitamura T, Suzuki M, Nishimatsu H,
Kurosaki T, Enomoto Y, Fukuhara H, Kume H, Takeuchi T, Miao L,
Jiangang H and Xiaoqiang L: Final report on low-dose estramustine
phosphate (EMP) monotherapy and very low-dose EMP therapy combined
with LH-RH agonist for previously untreated advanced prostate
cancer. Aktuelle Urol. 41 Suppl 1:S34–S40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wong CM, Marcocci L, Liu L and Suzuki YJ:
Cell signaling by protein carbonylation and decarbonylation.
Antioxid Redox Signal. 12:393–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Breen AP and Murphy JA: Reactions of oxyl
radicals with DNA. Free Radic Biol Med. 18:1033–1077. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ilya Obodovskiy: Effect of ionizing
radiation on biological structures. Fundam Radiat Chem Saf. 87–131.
2015.
|
|
45
|
Pamplona R: Membrane phospholipids,
lipoxidative damage and molecular integrity: A causal role in aging
and longevity. Biochim Biophys Acta. 1777:1249–1262. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kwiecien S, Jasnos K, Magierowski M,
Sliwowski Z, Pajdo R, Brzozowski B, Mach T, Wojcik D and Brzozowski
T: Lipid peroxidation, reactive oxygen species and antioxidative
factors in the pathogenesis of gastric mucosal lesions and
mechanism of protection against oxidative stress-induced gastric
injury. J Physiol Pharmacol. 65:613–622. 2014.PubMed/NCBI
|
|
47
|
Holley AK, Miao L, St Clair DK and St
Clair WH: Redox-modulated phenomena and radiation therapy: The
central role of superoxide dismutases. Antioxid Redox Signal.
20:1567–1589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou J, Qu XD, Li ZY, Wei-Ji, Liu Q, Ma YH
and He JJ: Salvianolic acid B attenuates toxin-induced neuronal
damage via Nrf2-dependent glial cells-mediated protective activity
in Parkinson's disease models. PLoS One. 9:e1016682014. View Article : Google Scholar : PubMed/NCBI
|