Introduction
As the most common type of human arthritis and
musculoskeletal disease, osteoarthritis (OA) is a degenerative and
chronic joint disorder caused by the deterioration of hyaline
cartilage, accompanied by chondrocyte hypertrophy, angiogenesis,
chondrogenesis, and variable degrees of inflammation without
systemic effects (1,2). Several important inflammatory
factors, including interleukin (IL)-1β, tumor necrosis factor α,
nitric oxide, matrix metalloproteinases (MMPs) and eicosanoids, are
actively synthesized during dysfunctional cartilage homeostasis,
which result in increased nuclear factor (NF)-κB and catabolic
activity (3–5). Although numerous studies have
revealed the contribution of genetic factors to OA, inflammatory
factors and altered chondrocyte responses also contribute to OA
progression (6–8). The etiology of OA is thought to be
influenced by aging, genetics, trauma and obesity. Furthermore, a
molecular target for treating OA has yet to be identified (9,10).
As a result, the current treatment options predominantly consist of
pain management, and no disease-modifying agent to effectively
treat OA is currently available.
High-mobility group box chromosomal protein (HMGB-1)
is a ubiquitous nuclear DNA-binding protein with a mass of ~27 kDa,
which contains an amino acid sequence that is highly conserved
between rodents and humans (11).
Activation of HMGB-1 is typically triggered by necrotic cells,
macrophages or other myeloid cells in response to an inflammatory
stimulus (12). Previous studies
have detected high levels of HMGB-1 in the synovial fluid of
patients with rheumatoid arthritis and collagen-induced arthritis
animal models (11,13). These high HMGB-1 levels were shown
to induce MMP and cytokine production, as well as angiogenesis, by
enhancing oxidative stress in vitro (14). In addition, in vitro studies
suggest that the chondrocyte hypertrophy and increased synthesis of
type X collagen caused by OA may be driven by the HMGB-1 receptor,
the receptor for advanced glycated end-products (RAGE) (15). As an important pro-inflammatory
mediator, HMGB1, along with its receptor, have been associated with
the onset and progression of cancers and arthritis (16,17);
however, a limited number of studies have investigated HMGB1 and
its various downstream genes as possible therapeutic targets in OA
(18,19).
Chrysin (CH; 5,7-dihydroxyflavone), an important
natural plant flavonoid, has been demonstrated to exert
antioxidative, anti-allergic, anti-inflammatory, antifibrotic and
anti-apoptotic effects in the central nervous and immune systems
(20,21). However, few studies have
investigated the potential use of CH for treating OA, despite the
recently demonstrated ability of CH to inhibit inflammatory factor
stimulation, and to produce therapeutic effects in human OA
chondrocytes in vitro (22).
Results of previous studies have suggested an
upregulation of HMGB-1 and inflammatory cytokine expression,
including IL-6 or IL-8, in OA cartilage (23,24).
Accordingly, the present study was designed to determine whether
treatment with CH improved the characteristics of human OA
chondrocytes by activating HMGB1, and thereby altering the
production of inflammatory factors. The alterations in cellular
function and inflammatory factors which occurred following HMGB-1
silencing were also examined. To the best our knowledge, this is
the first study to evaluate the protective effects of CH, and to
investigate the involvement of HMGB-1 in OA in vitro. The
results of the present study may assist in the discovery of novel
treatments for OA.
Materials and methods
Cell culture and treatment
Human chondrocytes (HC-a) were obtained from
Shanghai CAFA Biological Technology (Shanghai, China). Cells were
cultured for 24 h in high glucose-Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (both
from HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and 1%
penicillin/streptomycin (Corning Incorporation, Corning, NY, USA)
in a humidified atmosphere with 5% CO2 at 37°C.
Following culture, the cells were diluted to single
cell suspensions and seeded into 6-well plates (1×104
cells/well). Next, an OA model was induced by incubating the cells
with IL-1β (200 µM) for 24 h at 37°C. For CH treatment, the cells
were incubated with 0, 0.4, and 4 µM CH (cat. no. C80105,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 24 h at 37°C,
respectively. For transfection, the 50 nM HMGB1 siRNA (siRNA) and
50 nM negative control (NC) oligonucleotides were synthesized from
Shanghai GenePharma, Co., Ltd. (Shanghai, China). The sequences
were si-HMGB1, 5′-CCCGUUAUGAAAGAGAAAUUU-3′ (sense),
5′-AUUUCUCUUUCAUAAUGGGUU-3′ (antisense); si-NC,
5′-UUCGUCUGUACUCCACAUATT-3′ (sense), 5′-GAUGUCUUCUACAGUCCGATT-3′
(antisense). The cells were transfected with si-NC or si-HMGB1 by
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 48 h at 37°C following the
manufacturer's protocols. Non-treated cells were used as a blank
group.
Thus, the initial experimental groups were as
follows: i) Blank (non-treated cells); ii) OA model (treated with
200 µM IL-1β); and iii) CH (treated with 200 µM IL-1β and the
indicated concentration of CH). The transfection experimental
groups were: i) Blank (non-treated cells); ii) OA model (treated
with 200 µM IL-1β); iii) NC (treated with 200 µM IL-1β, si-NC); iv)
siRNA (treated with 200 µM IL-1β and si-HMGB1); and v) CH + siRNA
(treated with 200 µM IL-1β, si-HMGB1 and CH).
Apoptosis assay
Apoptotic cells were quantified using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (Merck KGaA). Cells were collected by 0.25% trypsin
digestion, washed with PBS and re-suspended in 200 µl binding
buffer containing 5 µl Annexin V (10 µg/ml) in DMEM with FBS at
37°C for 10 min in the dark. The cells were subsequently incubated
with 10 µl PI (20 µg/ml) for 15 min at room temperature and
analyzed with the EPICS® XL™ flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA). Data acquisition and
analyses were performed using CellQuest™ software
version 5.1 (BD Biosciences, Franklin Lakes, NJ, USA). Early and
late apoptotic cells were detected in the lower and upper right
quadrants of the flow cytometry plots presented late apoptosis, and
lower right represented early apoptosis. The percentage of
apoptotic cells was presented for both early and late apoptotic
cells.
Immunofluorescence staining
Cells were plated onto coverslips and incubated in
RPMI-1640 medium (cat. no. 11875-093; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS for 24 h at 37°C. Following
treatment, cells were fixed with 4% paraformaldehyde for 20 min at
4°C, incubated in 0.3% Triton X-100-PBS for 10 min at room
temperature and subsequently blocked with 5% goat serum at 37°C for
30 min. The cells were incubated with anti-human HMGB-1 (1:2,000;
cat no. M-1702-100; Biosensis Pty Ltd., Thebarton, Australia) at
4°C overnight, followed by incubation with goat anti-human
immunoglobulin G conjugated to Cy3 (1:400; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) at 37°C for 1 h. Nuclei
were counter stained with DAPI (1:1,000; Sigma-Aldrich; Merck KGaA)
for 5 min at room temperature. Images were obtained using an
inverted fluorescence microscope (Olympus Corporation, Tokyo,
Japan) at ×400 magnification.
Protein isolation and western blot
analysis
The total protein was extracted from cells by
incubation with lysis buffer (12.5 ml Tris HCL, 2 g SDS, 10 ml
glycerol and 67.5 ml distilled water). Nuclear protein was
extracted with NE-PER Nuclear and Cytoplasmic Extraction Reagents
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocols. The concentrations were measured by the
protein assay kit (Qcbio Science Technologies Co., Ltd., Shanghai,
China). The protein (30 µg) were separated by electrophoresis on
Novex® 4–20% Tris-Glycine 12-well polyacrylamide
gradient gels (Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, the separated proteins were transferred onto a
nitrocellulose membrane by using a Protean Mini Cell system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The gel was
blocked with 5% non-fat milk in Tris-buffered saline with 0.1%
Tween-20 (TBST; Merck KGaA) for 2 h at room temperature. The
membrane was incubated with anti-human HMGB-1 (1:10,000, ab77302;
Abcam, Cambridge, UK), anti-GAPDH as a loading control (1:2,000,
sc-47724; Santa Cruz Biotechnology, Inc., Dallas, CA, USA), and
lamin B (1:2,000, ab122919; Abcam) overnight at 4°C, followed by
blotting with horseradish peroxidase-conjugated secondary
antibodies (1:2,000, anti-mouse, cat. no. SC-2005 and anti-rabbit,
cat. no. SC-2004) for 1 h at room temperature; following which, it
was washed again with TBST. Finally, the blots were analyzed by the
enhanced chemiluminescence (ECL) substrate kit an ECL system (both
from GE Healthcare, Chicago, IL, USA).
ELISA
The concentrations of MMP13 (1:5,000, ab9128),
collagenase (1:5,000, ab182881), IL-6 (1:5,000, ab7737) and
collagen α-1 (II) chain (COL2A1, 1:5,000, ab34712) (all from Abcam)
were quantified with commercial human ELISA kits (Elabscience,
Wuhan, China) according to the manufacturer's protocols. All
samples were assayed in duplicate. The mean concentration was
determined for each sample. Stop solution was then added to each
well, and its optical density at 450 nm (OD450) was
immediately measured on an Infinite M200 microtiter plate reader
(Tecan Group, Ltd., Maennedorf, Switzerland).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM Corp., Armonk, NY, USA). Data were presented as the mean
± standard deviation. Student's t-test was used to analyze
differences between two groups, and one-way analysis of variance
followed by Tukey's post-hoc test was used to determine the
significance of differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were independently repeated three times.
Results
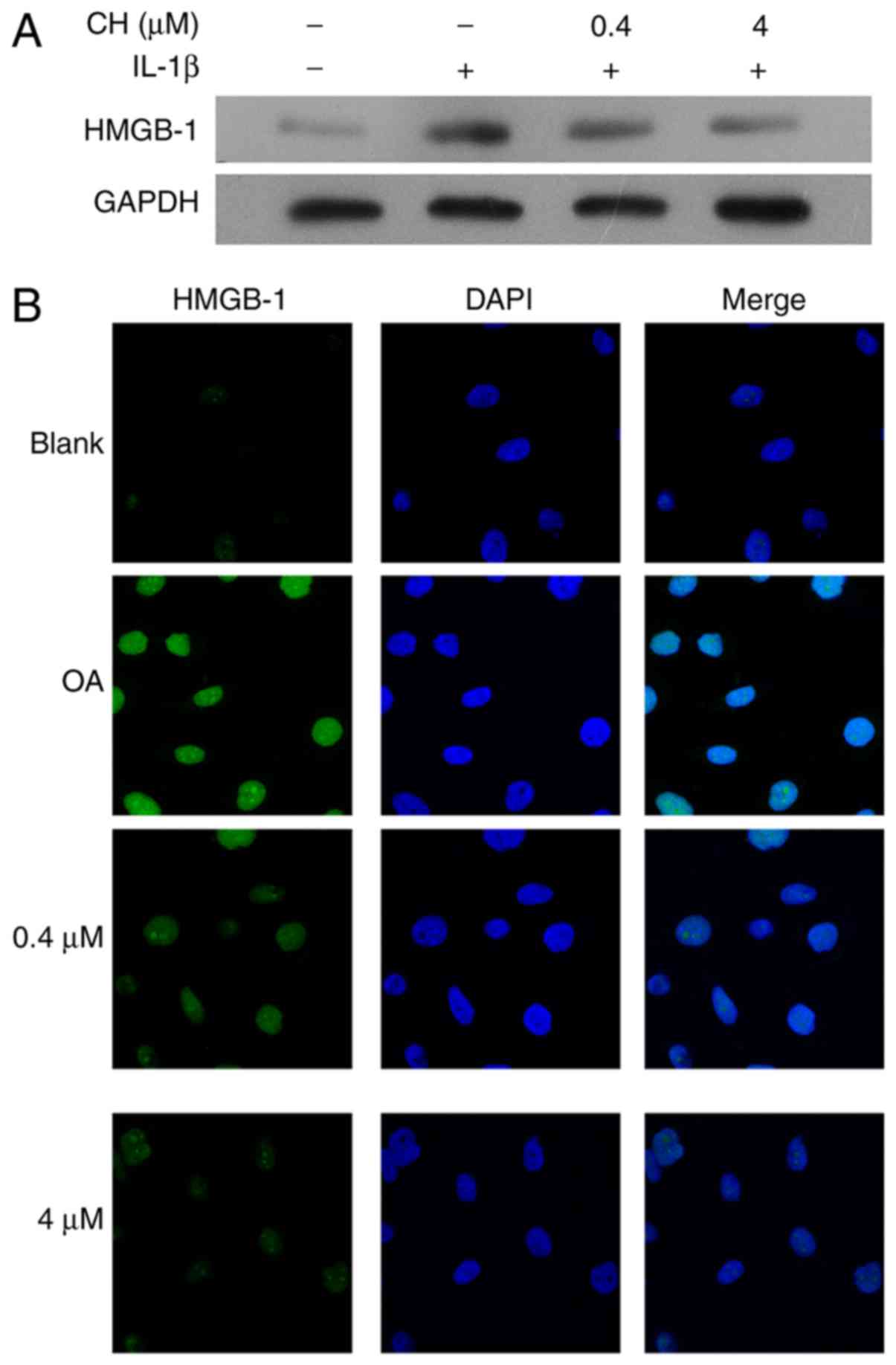
HMGB-1 expression in the human
chondrocyte OA model
To validate the OA cell model used in this study,
HMGB-1 expression was detected in human chondrocytes following
pre-treatment with IL-1β, followed by treatment with CH. The
results demonstrated that the HMGB1 expression levels were
increased in response to IL-1 treatment, but was notably decreased
in the CH treated groups, compared with the OA group (Fig. 1A). The results from
immunofluorescence assays revealed that the increase in HMGB-1
expression in response to IL-1β, followed by a dose-dependent
decrease in HMGB-1 expression in response to CH (Fig. 1B).
CH treatment alters the expression of
inflammatory mediators and reduces apoptosis
The results of the ELISAs are presented in Fig. 2. Compared with the blank group, the
levels of MMP13, collagenase and IL-6 were significantly increased
in the OA model group; however, that of COL2A1 were significantly
decreased. Compared with the OA model group, the levels of MMP13,
collagenase and IL-6 were significantly decreased in the CH
treatment groups; however that of COL2A1 were significantly
increased in the CH (4 µM) group. The results suggested that CH
treatment inhibited the levels of inflammatory mediators in
IL-1β-induced HC-a cells (P<0.01; Fig. 2).
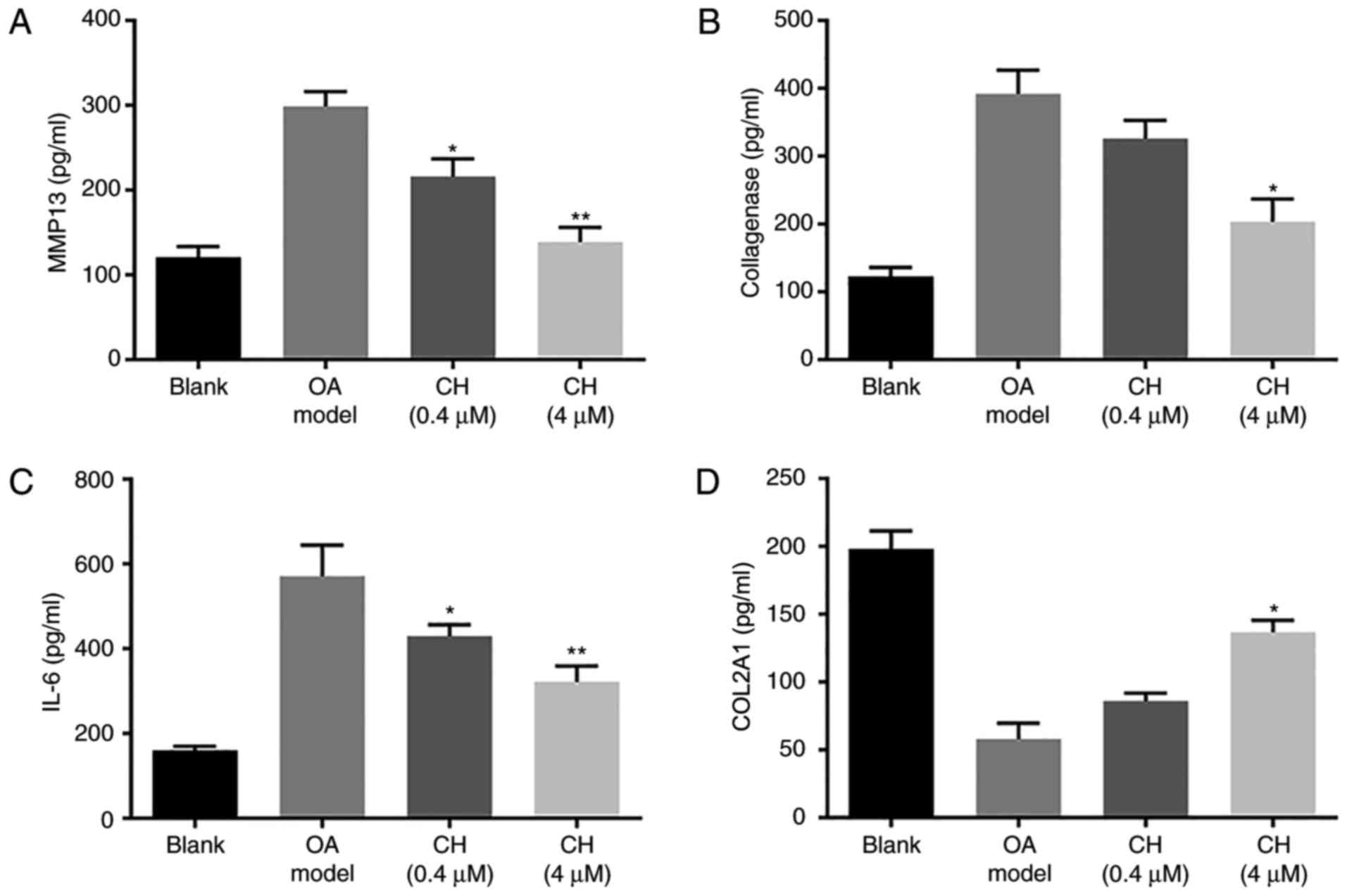 | Figure 2.MMP13, collagenase, IL-6 and COL2A1
expression levels. (A) MMP13, (B) collagenase, (C) IL-6 and (D)
COL2A1 expression in medium 24 h after IL-1β and CH treatments.
Expression levels were detected by ELISA. Data are presented as the
mean ± standard deviation. *P<0.05, **P<0.01, vs. OA model
group. CH, chrysin; OA, osteoarthritis model; IL-6, interleukin-6;
MMP13, matrix metalloproteinase 13; COL2A1, collagen α-1 (II)
chain. |
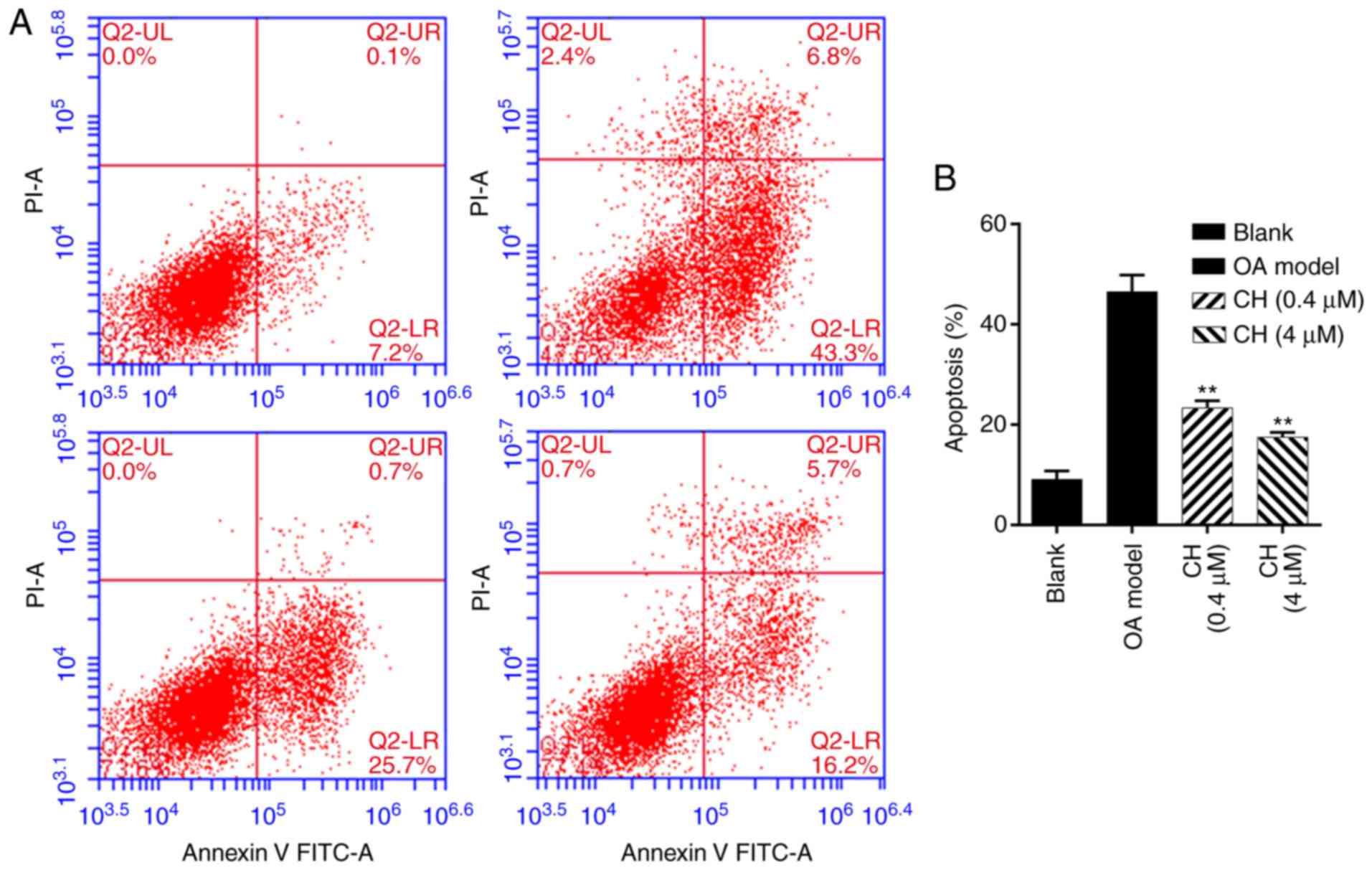
Additionally, cell apoptosis was analyzed by flow
cytometry following treatment with IL-1β and CH. The results
demonstrated that the number of apoptotic cells was significantly
increased in OA model group compared with the blank group, while
the number of apoptotic cells was significantly decreased in the CH
treatment groups compared with the OA model group (P<0.01;
Fig. 3). The results indicated
that CH treatment suppressed the apoptotic ability of IL-1β-induced
HC-a cells.
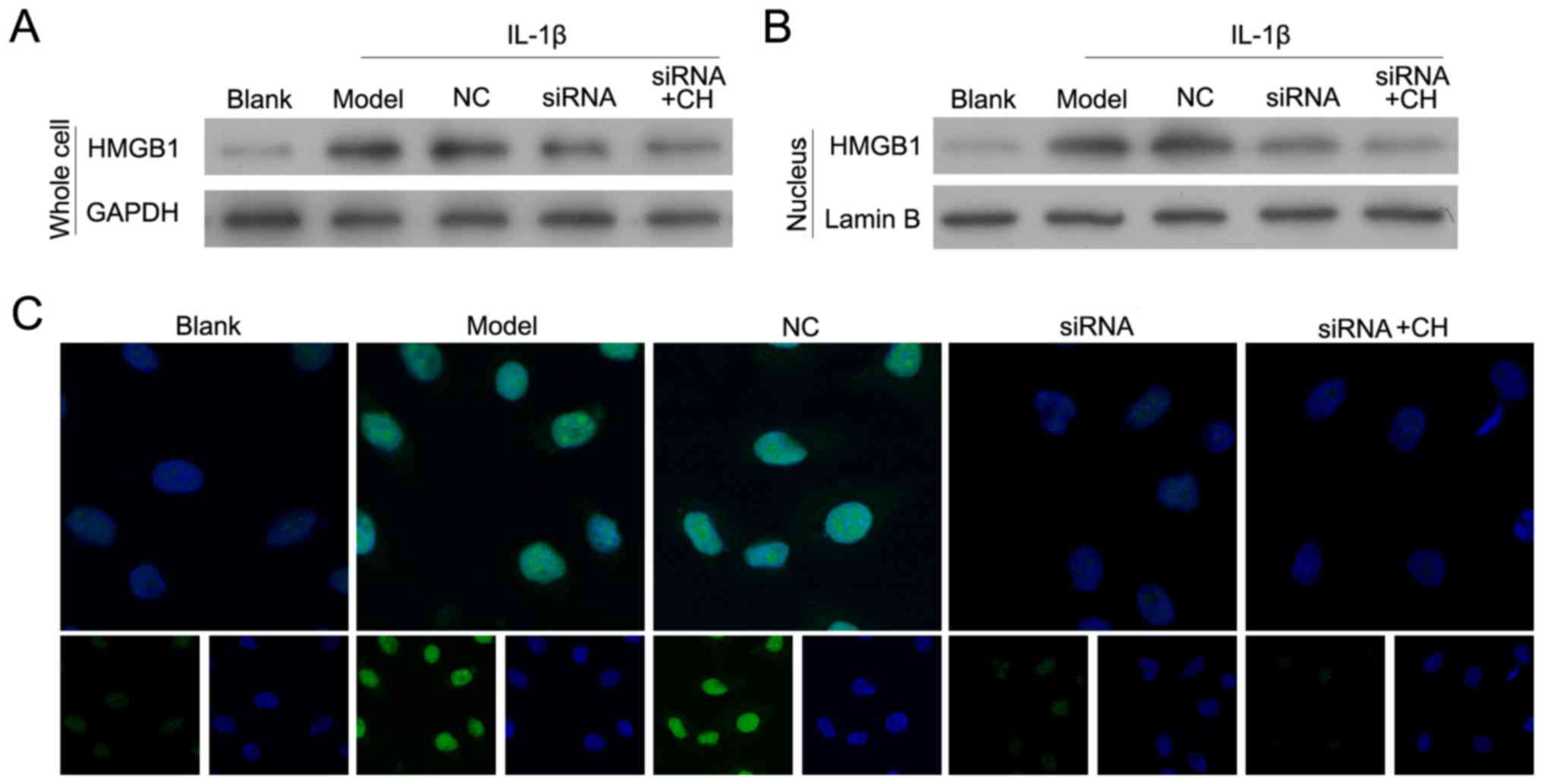
Validation of HMGB-1 knockdown in the
human chondrocyte OA model
Following treatment with IL-1β for 24 h, OA model
chondrocytes were transfected with si-HMGB-1 and/or treated with CH
(4 µM). As presented in Fig. 4A and
B, HMGB-1 expression in the siRNA-transfected cells was
significantly inhibited when compared with HMGB-1 expression in
cells transfected with the si-HMGB-1-NC control (NC group).
Immunofluorescence analyses were performed to demonstrate that in
the nucleus, HMGB-1 silenced cells emitted less florescence
compared with cells in the NC group (Fig. 4B and C). The results suggested that
silencing of HMGB1 and CH treatment downregulated the expression
levels of the total and nuclear HMGB-1 protein in OA model
cells.
CH and siRNA cotreatment further
reduces apoptosis
ELISA assays was performed to the detect the
concentrations of MMP13, collagenase, IL-6 and COL2A1. The results
proved that compared with the OA group, the expression of MMP13,
collagenase and IL-6 was decreased following HMGB-1 knockdown,
while COL2A1 expression was increased. Additionally, MMP13,
collagenase and IL-6 expression was further reduced in CH and
si-HMGB-1 treated cells, compared with cells transfected with
si-HMGB-1 alone. Furthermore, COL2A1 expression was significantly
increased in the CH and si-HMGB treatment group, compared with the
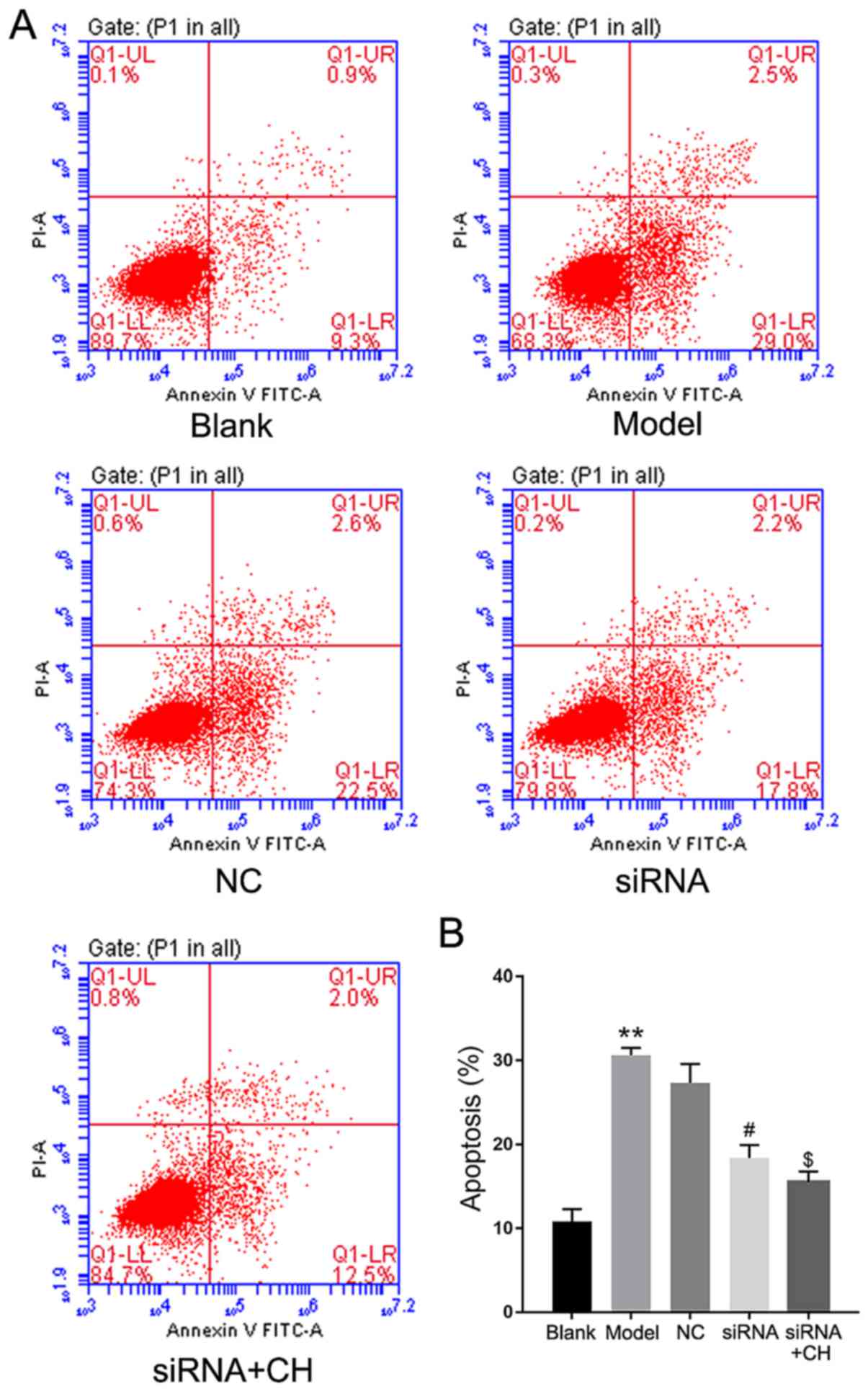
siRNA group (P<0.05, P<0.01; Fig. 5). Flow cytometry revealed that the
number of apoptotic cells was significantly increased in OA model
group compared with the blank group. Silencing of HMGB1
significantly inhibited the apoptotic potential of IL-1β-induced
HC-a cells compared with the NC group; treatment with CH enhanced
the inhibition mediated by HMGB1 siRNA compared with the silencing
group (P<0.05, P<0.01; Fig.
6).
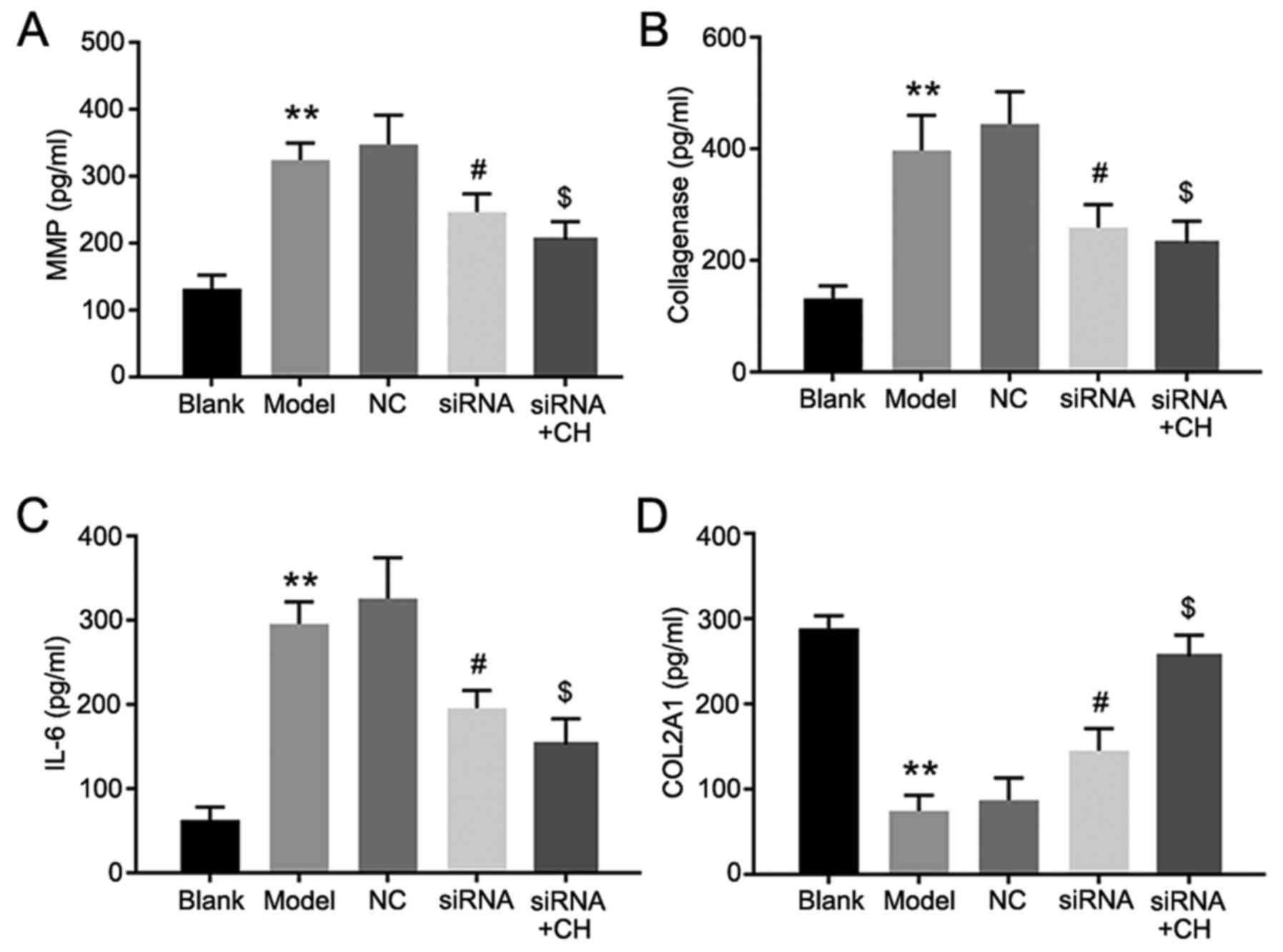 | Figure 5.MMP13, collagenase, IL-6, and COL2A1
expressions levels following the silencing of HMGB-1. (A) MMP13,
(B) collagenase, (C) IL-6 and (D) COL2A1 expression in medium. Data
are presented as the mean ± standard deviation. Experiments were
independently repeated three times. **P<0.01 vs. blank group;
#P<0.05 vs. NC group; $P<0.05 vs. siRNA
group. CH, chrysin; IL-6, interleukin-6; MMP13, matrix
metalloproteinase 13; COL2A1, collagen α-1 (II) chain; siRNA, small
interfering RNA; NC, negative control. |
Discussion
Complementary and alternative medical techniques,
including certain traditional Chinese medicines (TCMs), including
paeonol (25), isofraxidin
(26) and Jingui external lotion
(27), have been widely used for
treating OA for centuries; these are primarily thought to produce
chondroprotective effects and even repair cartilage (28,29).
Consequently, there is growing interest in exploring TCMs as
potential drugs that may aid in reducing inflammation, protecting
cartilage against damage, improving joint function and restoring a
patient's activity level (30).
The present study was designed to evaluate whether CH may be
effective against OA. This was investigated using an in
vitro cartilage cell model to examine if CH exerted a positive
effect on cartilage health through targeting HMGB-1.
As previously reported, HMGB-1 is one of several
nuclear DNA-binding proteins that may be passively released in
response to an inflammatory stimulus resulting from an OA injury
(19). Abundant evidence indicates
that certain complexes (cluster of differentiation 24, siglec-10
and tumor-infiltrating dentric cells) induce innate immune
responses, and the production of inflammatory mediators is induced
by the binding of HMGB1 to bacterial products (31,32).
Extracellular HMGB1 has been reported to induce cell proliferation,
migration and differentiation by interacting with RAGEs and
toll-like receptors (TLRs), including TLR-2 and TLR-4 (33,34).
Interactions between HMGB1 and phosphatidylserine on the cell
surface inhibit the phagocytosis of apoptotic neutrophils by
macrophages, which may lead to the activation of monocytes,
macrophages and dendritic cells, which prevents the resolution of
inflammation (2,35,36).
Chondrocyte apoptosis is a typical occurrence during
OA progression. In response to structural changes in the cartilage
matrix, chondrocytes serve a critical role in recreating the
anabolic-catabolic balance required for tissue function and matrix
maintenance. Therefore, inhibiting chondrocyte apoptosis while
promoting the maintenance of healthy chondrocytes represents a
potential strategy for preventing cartilage degeneration (37,38).
As TCMs are increasingly being used to treat OA (39), scientists have suggested that
certain TCMs, particularly those used in combination formulas, may
produce therapeutic effects in a synergistic manner. For example,
XuanHuSuo powder (XHSP), a conventional herbal formulation
developed in China, has been extensively used in OA treatment
(40). XHSP has shown reasonable
efficacy as an anti-apoptotic and anti-inflammatory agent when
applied following stimulation with a cytokine (IL-1β) or estrogen
(40). As an active component of
various Chinese herbs, berberine chloride has been demonstrated to
benefit matrix synthesis and cell survival in IL-1β-stimulated
chondrocytes, and displays great therapeutic potential as a
promoter of cartilage repair in rat OA models (41). However, there is no effective way
to target and promote cartilage protection at present. To the best
of our knowledge, the present study was the first to demonstrate
that the herbal extract CH may inhibit chondrocyte apoptosis by
targeting HMGB-1.
Taken together, the data indicated that CH
ameliorated OA in vitro. It does this, at least partially,
by inhibiting various processes mediated by HMGB-1, including
chondrocyte apoptosis, cellular inflammatory responses and
inflammatory cytokine generation. Further studies in animal models
are required to evaluate the safety and efficacy of this herbal
extract.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural
Science Foundation of China (grant no. 81702196) and the Province
Natural Science Fund of Guangdong (grant no. 2017A030313137).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CZ, WZY and ZQH designed the experiments. CZ, CBH
and QHD performed most of the experiments. CZ, CHL, LW and ZYZ
collected and analyzed the data. CZ drafted the manuscript. ZYZ
provided the administrative support.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
References
|
1
|
Erggelet C, Kreuz PC, Mrosek EH,
Schagemann JC, Lahm A, Ducommun PP and Ossendorf C: Autologous
chondrocyte implantation versus ACI using 3D-bioresorbable graft
for the treatment of large full-thickness cartilage lesions of the
knee. Arch Orthop Trauma Surg. 130:957–964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ge X, Shi R and Ma X: The secreted protein
WNT5A regulates condylar chondrocyte proliferation, hypertrophy and
migration. Arch Oral Biol. 82:171–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liao S, Zhou K, Li D, Xie X, Jun F and
Wang J: Schisantherin A suppresses interleukin-1β-induced
inflammation in human chondrocytes via inhibition of NF-κB and
MAPKs activation. Eur J Pharmacol. 780:65–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinez SE, Chen Y, Ho EA, Martinez SA
and Davies NM: Pharmacological effects of a C-phycocyanin-based
multicomponent nutraceutical in an in-vitro canine chondrocyte
model of osteoarthritis. Can J Vet Res. 79:241–249. 2015.PubMed/NCBI
|
|
5
|
Wang HZ, Jin Y, Wang P, Han C, Wang ZP and
Dong MY: Alteration of serum endocan in normal pregnancy and
preeclampsia. Clin Exp Obstet Gynecol. 44:419–422. 2017.PubMed/NCBI
|
|
6
|
Xu L, Peng Q, Xuan W, Feng X, Kong X,
Zhang M, Tan W, Xue M and Wang F: Interleukin-29 enhances synovial
inflammation and cartilage degradation in osteoarthritis. Mediators
Inflamm. 2016:96315102016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu-Bryan R and Terkeltaub R: Emerging
regulators of the inflammatory process in osteoarthritis. Nat Rev
Rheumatol. 11:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruyère O, Cooper C, Arden N, Branco J,
Brandi ML, Herrero-Beaumont G, Berenbaum F, Dennison E, Devogelaer
JP, Hochberg M, et al: Can we identify patients with high risk of
osteoarthritis progression who will respond to treatment? A focus
on epidemiology and phenotype of osteoarthritis. Drugs Aging.
32:179–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kimura T: Progress of research in
osteoarthritis. An overview of the recent knowledge on
osteoarthritis: Pathogenesis, evaluation and therapies. Clin
Calcium. 19:1565–1571. 2009.(In Japanese). PubMed/NCBI
|
|
11
|
Taniguchi N, Kawahara K, Yone K,
Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K,
Matsunaga S, et al: High mobility group box chromosomal protein-1
plays a role in the pathogenesis of rheumatoid arthritis as a novel
cytokine. Arthritis Rheum. 48:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sunahori K, Yamamura M, Yamana J, Takasugi
K, Kawashima M and Makino H: Increased expression of receptor for
advanced glycation end products by synovial tissue macrophages in
rheumatoid arthritis. Arthritis Rheum. 54:97–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kokkola R, Li J, Sundberg E, Aveberger AC,
Palmblad K, Yang H, Tracey KJ, Andersson U and Harris HE:
Successful treatment of collagen-induced arthritis in mice and rats
by targeting extracellular high mobility group box chromosomal
protein-1 activity. Arthritis Rheum. 48:2052–2058. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamada T, Torikai M, Kuwazuru A, Tanaka M,
Horai N, Fukuda T, Yamada S, Nagayama S, Hashiguchi K, Sunahara N,
et al: Extracellular high mobility group box chromosomal protein-1
is a coupling factor for hypoxia and inflammation in arthritis.
Arthritis Rheum. 58:2675–2685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YJ, Sheu ML, Tsai KS, Yang RS and Liu
SH: Advanced glycation end products induce peroxisome
proliferator-activated receptor γ down-regulation-related
inflammatory signals in human chondrocytes via Toll-like receptor-4
and receptor for advanced glycation end products. PLoS One.
8:e666112013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sims GP, Rowe DC, Rietdijk ST, Herbst R
and Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev
Immunol. 28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang WJ, Yin SJ and Rong RQ: PKR and HMGB1
expression and function in rheumatoid arthritis. Genet Mol Res.
14:17864–17870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin Y, Chen Y, Wang W, Wang Z, Tang G,
Zhang P, He Z, Liu Y, Dai SM and Shen Q: HMGB1-LPS complex promotes
transformation of osteoarthritis synovial fibroblasts to a
rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death
Dis. 5:e10772014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Guo Y, Wang C and Yu H, Yu X and
Yu H: MicroRNA-142-3p inhibits chondrocyte apoptosis and
inflammation in osteoarthritis by targeting HMGB1. Inflammation.
39:1718–1728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahad A, Ganai AA, Mujeeb M and Siddiqui
WA: Chrysin, an anti-inflammatory molecule, abrogates renal
dysfunction in type 2 diabetic rats. Toxicol Appl Pharmacol.
279:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan R, Khan AQ, Qamar W, Lateef A, Ali F,
Rehman MU, Tahir M, Sharma S and Sultana S: Chrysin abrogates
cisplatin-induced oxidative stress, p53 expression, goblet cell
disintegration and apoptotic responses in the jejunum of Wistar
rats. Br J Nutr. 108:1574–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng W, Tao Z, Cai L, Chen C, Zhang C,
Wang Q, Ying X, Hu W and Chen H: Chrysin attenuates IL-1β-induced
expression of inflammatory mediators by suppressing NF-κB in human
osteoarthritis chondrocytes. Inflammation. 40:1143–1154. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Magna M and Pisetsky DS: The role of HMGB1
in the pathogenesis of inflammatory and autoimmune diseases. Mol
Med. 20:138–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu M, Zhou GM, Wang LH, Zhu L, Liu JM,
Wang XD, Li HT and Chen L: Inhibiting high-mobility group box 1
(HMGB1) attenuates inflammatory cytokine expression and
neurological deficit in ischemic brain injury following cardiac
arrest in rats. Inflammation. 39:1594–1602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu M, Zhong S, Kong R, Shao H, Wang C,
Piao H, Lv W, Chu X and Zhao Y: Paeonol alleviates
interleukin-1β-induced inflammatory responses in chondrocytes
during osteoarthritis. Biomed Pharmacother. 95:914–921. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin J, Li X, Qi W, Yan Y, Chen K, Xue X,
Xu X, Feng Z and Pan X: Isofraxidin inhibits interleukin-1β induced
inflammatory response in human osteoarthritis chondrocytes. Int
Immunopharmacol. 64:238–245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo D, Cao XW, Liu JW, Niu W, Ma ZW, Lin
DK, Chen JY, Lian WD, Ouyang WW and Liu J: Clinical effectiveness
and micro-perfusion alteration of Jingui external lotion in
patients with knee osteoarthritis: Study protocol for a randomized
controlled trial. Trials. 16:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng L, Xiao CZ, Deng ZT and Li RH:
Chondroprotective effects and multitarget mechanisms of fu yuan
capsule in a rat osteoarthritis model. Evid Based Complement
Alternat Med. 2017:89856232017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong P, Xu S, Cao G, Jin W, Guo Y, Cheng
Y, Jin H, Shan L and Xiao L: Chondroprotective activity of a
detoxicated traditional Chinese medicine (Fuzi) of
Aconitum carmichaeli Debx against severe-stage
osteoarthritis model induced by mono-iodoacetate. J Ethnopharmacol.
151:740–744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng BC, Fu XQ, Guo H, Li T, Wu ZZ, Chan
K and Yu ZL: The genus Rosa and arthritis: Overview on
pharmacological perspectives. Pharmacol Res. 114:219–234. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen GY, Tang J, Zheng P and Liu Y: CD24
and Siglec-10 selectively repress tissue damage-induced immune
responses. Science. 323:1722–1725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiba S, Baghdadi M, Akiba H, Yoshiyama H,
Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan
JD, et al: Tumor-infiltrating DCs suppress nucleic acid-mediated
innate immune responses through interactions between the receptor
TIM-3 and the alarmin HMGB1. Nat Immunol. 13:832–842. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian X, Liu C, Shu Z and Chen G: Review:
Therapeutic targeting of HMGB1 in stroke. Curr Drug Deliv.
14:785–790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rosenberg JH, Rai V, Dilisio MF and
Agrawal DK: Damage-associated molecular patterns in the
pathogenesis of osteoarthritis: Potentially novel therapeutic
targets. Mol Cell Biochem. 434:171–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu L, Yang M, Kang R, Dai Y, Yu Y, Gao F,
Wang H, Sun X, Li X, Li J, et al: HMGB1-DNA complex-induced
autophagy limits AIM2 inflammasome activation through RAGE. Biochem
Biophys Res Commun. 450:851–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schaper F, Westra J and Bijl M: Recent
developments in the role of high-mobility group box 1 in systemic
lupus erythematosus. Mol Med. 20:72–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miclea RL, Siebelt M, Finos L, Goeman JJ,
Löwik CW, Oostdijk W, Weinans H, Wit JM, Robanus-Maandag EC and
Karperien M: Inhibition of Gsk3β in cartilage induces
osteoarthritic features through activation of the canonical Wnt
signaling pathway. Osteoarthritis Cartilage. 19:1363–1372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim HA and Blanco FJ: Cell death and
apoptosis in osteoarthritic cartilage. Curr Drug Targets.
8:333–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang M, Jiang L, Wang Q, Chen H and Xu G:
Traditional Chinese medicine for knee osteoarthritis: An overview
of systematic review. PLoS One. 12:e01898842017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang H, He S, Zhang X, Luo S, Zhang B,
Duan X, Zhang Z, Wang W, Wang Y and Sun Y: A network pharmacology
approach to uncover the pharmacological mechanism of xuanhusuo
powder on osteoarthritis. Evid Based Complement Alternat Med.
2016:32469462016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou X, Lin X, Xiong Y, Jiang L, Li W, Li
J and Wu L: Chondroprotective effects of palmatine on
osteoarthritis in vivo and in vitro: A possible mechanism of
inhibiting the Wnt/β-catenin and Hedgehog signaling pathways. Int
Immunopharmacol. 34:129–138. 2016. View Article : Google Scholar : PubMed/NCBI
|