Introduction
Brucellosis is an important neglected zoonotic
disease (1). The causative
pathogen of this disease is Brucella (a facultative
intracellular Gram-negative bacterium). In order to control this
disease in domestic animals, few attenuated vaccines, including
B. melitensis Rev1, B. abortus S19 and RB51 have been
introduced (2). Brucellosis
has been reported to exist in wildlife populations since the early
part of the 20th Century. At the beginning of this century in the
USA, Brucella abortus was a problem in elk and bison in the
Greater Yellowstone Area (3).
B. suis is prevalent in millions of feral swine in the
majority of the southern states, and caribou and reindeer in Alaska
are infected with B. suis biovar 4 (3). However, the existing vaccines were
considered too virulent or unsafe for humans (4).
To develop safe and efficacious vaccines, a number
of different strategies, including the development of subunit
vaccines (5), the utilization of
bacterial vectors (6) and the
overexpression of protective homologous antigens (7,8),
have been applied. In addition, another strategy was developed
involving immunization with DNA vaccines, which encode a protective
antigen (9,10). It was noted that DNA vaccines may
be effective vaccines due to their strong cell-mediated immune
(CMI) responses, which serve an important role in protection
against intracellular pathogens (11). Various animal models demonstrated
the protective roles of DNA vaccination against different viral,
fungal and parasitic diseases (12–14).
With regard to brucellosis, a number of previous studies
demonstrated that specific DNA vaccines [for example, the SEN1002
and SEN1395 genes (15), Cu-Zn
superoxide dismutase (16) and
lumazine synthase (17)] were able
to induce a significant level of protection in mice.
As a member of the two-component BvrR/BvrS system,
BvrR is necessary for Brucella virulence (18,19).
Previous studies demonstrated that dysfunction of BvrR may alter
the expression of the type IV secretion system and specific
principal outer membrane proteins, in addition to the pattern of
lipid A acylation (20–22). At present, studies on BvrR have
primarily focused on its functions. In the present study, the
immunogenicity and protective ability of the BvrR gene were
demonstrated to function as a DNA vaccine.
Materials and methods
Bacterial strains and vector
B. abortus S19 and B. suis S2 were
purchased from Tanon Science and Technology, Co., Ltd. (Shanghai,
China). These bacterial stains were qualified by standard
biochemical tests prior to experimentation. The bacterial cells
were cultured in tryptose-soy broth (Qingdao Hope Bio-Technology
Co., Ltd, Qingdao, China) for 72 h at 37°C under aerobic
conditions. For the inoculation experiments, the bacterial
suspension was adjusted spectrophotometrically to 2×108
colony forming units (CFU). All experiments with live
Brucella were conducted in biosafety level 2
laboratories.
E. coli strains DH5α and BL21 (DE3; Takara
Biotechnology Co., Ltd., Dalian, China) were used for cloning of
the various plasmid constructs and recombinant protein expression,
respectively. The E. coli were cultured at 37°C in lysogeny
broth (Sangon Biotech Co., Ltd., Shanghai, China) with 100 µg
ampicillin/ml. The eukaryotic vector pCDNA3.0 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and prokaryotic vector
pET28a (Merck KGaA, Darmstadt, Germany) were used to construct
plasmids for the DNA vaccine and recombinant protein expression,
respectively.
Animals and grouping
A total of 75 pathogen-free female BALB/c mice (20±2
g, 6-weeks old) were purchased from the Animal Center at the
Academy of Military Medical Sciences (Changchun, China), which were
fed with commercial mouse chow and water ad libitum in clean
conditions (18–22°C, 40–70% relative humidity and 10–14 h
light/dark cycle) at the laboratory animal center of Shenyang
Agricultural University (Shenyang, China). The mice were randomly
divided into three groups (n=25): The pcDNA-BvrR immunization
group; pcDNA control group; and the PBS control group. The
pcDNA-BvrR immunization group and the pcDNA control group were
injected with pcDNA-BvrR plasmid and pcDNA plasmid at a
concentration of 1 µg/µl in the hindlimb tibialis anterior muscle,
and 100 µl of each was injected into the mice. The PBS control
group was injected with 100 µl PBS. The first immunization was
performed at day 0, the second immunization was at day 14 and the
third immunization was at day 28. Samples were collected 1 week
following each immunization. On the 7th day following each
immunization, the blood of five mice was taken for serum testing.
At the same time, spleens were taken for relevant experiments.
Finally, the 10 remaining mice were used for the challenge
experiments. All the animal experiments were approved by the
Laboratory Animal Welfare and Ethical review committee of Shenyang
Agricultural University.
BvrR DNA vaccine construction
The primers for BvrR were designed according to the
corresponding genome sequence (GenBank accession no. AF005157.1;
http://www.ncbi.nlm.nih.gov/genbank/): BvrR forward,
5′-AAAAGGATCCGCCACCATGAAGGAAGCTTCGGCAACG-3′ and BvrR reverse,
5′-AAAACTCGAGTACGCTTCCCGGAAACGATAAC-3′. Kozak sequences and
restriction sites for EcoRI and XhoI were transferred
into the oligonucleotides to aid expression and cloning,
respectively.
B. suis S2 chromosomal DNA was used as the
template for amplifying the coding region of the BvrR gene. The DNA
(Takara Biotechnology Co., Ltd.) parameters of the polymerase chain
reaction (PCR) were as follows: 30 cycles at 94°C for 30 sec, 50°C
for 30 sec and 72°C for 45 sec. A 1.5% agarose gel was used to
purify the PCR amplified product, which was digested by
EcoRI and XhoI restriction enzymes (Takara
Biotechnology Co., Ltd.) and ligated using T4 DNA ligase (Takara
Biotechnology Co., Ltd.) into the pCDNA3.0 vector. The pCDNA-BvrR
plasmid was verified by DNA sequencing following purification using
the UNIQ-500 Column Endotoxin-Free Plasmid Maxi-Preps kit (Sangon
Biotech Co., Ltd.; data not shown).
Protein expression and
purification
The BvrR gene was inserted into a pET28a vector
between the restriction sites of EcoRI and XhoI, and
DNA sequencing was verified (data not shown). E. coli BL21
(DE3) cells harboring pET-BvrR were induced in the auto-induction
medium ZYP-5052 (22). The
resulting protein contained a His6-tag in its
N-terminus.
The His6-tagged BvrR was purified by
His-trap FF crude (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA)
affinity chromatography (data not shown) and verified with an
anti-histidine monoclonal antibody (cat. no. D199987; Sangon
Biotech Co., Ltd.) and Brucella polyclonal antibody (cat. no. Z244;
China Veterinary Culture Collection Center, Beijing, China) by
western blot analysis. Determination of protein concentration was
performed using a Bradford assay. Protein samples (20 µg) were
loaded onto a 12% SDS-PAGE gel for separation. Following this,
proteins were transferred to nitrocellulose membranes and then
blocked with 5% bovine serum albumin (BSA; cat. no. B600036; Sangon
Biotech Co., Ltd.) at room temperature for 2 h. Membranes were
subsequently incubated with anti-His mouse monoclonal antibodies
(1:500; cat. no. D199987; Sangon Biotech Co., Ltd.) with 3% BSA
overnight at 4°C. Following this, membranes were incubated with
horseradish peroxidase-conjugated rabbit anti-mouse IgG secondary
antibodies (1:1,000 dilution with 1% BSA; cat. no. D110098; Sangon
Biotech Co., Ltd.) at room temperature for 2 h. A horseradish
catalase 3,3′-diaminobenzidine color kit (cat. no. C520017; Sangon
Biotech Co., Ltd.) was used for the visualization of proteins.
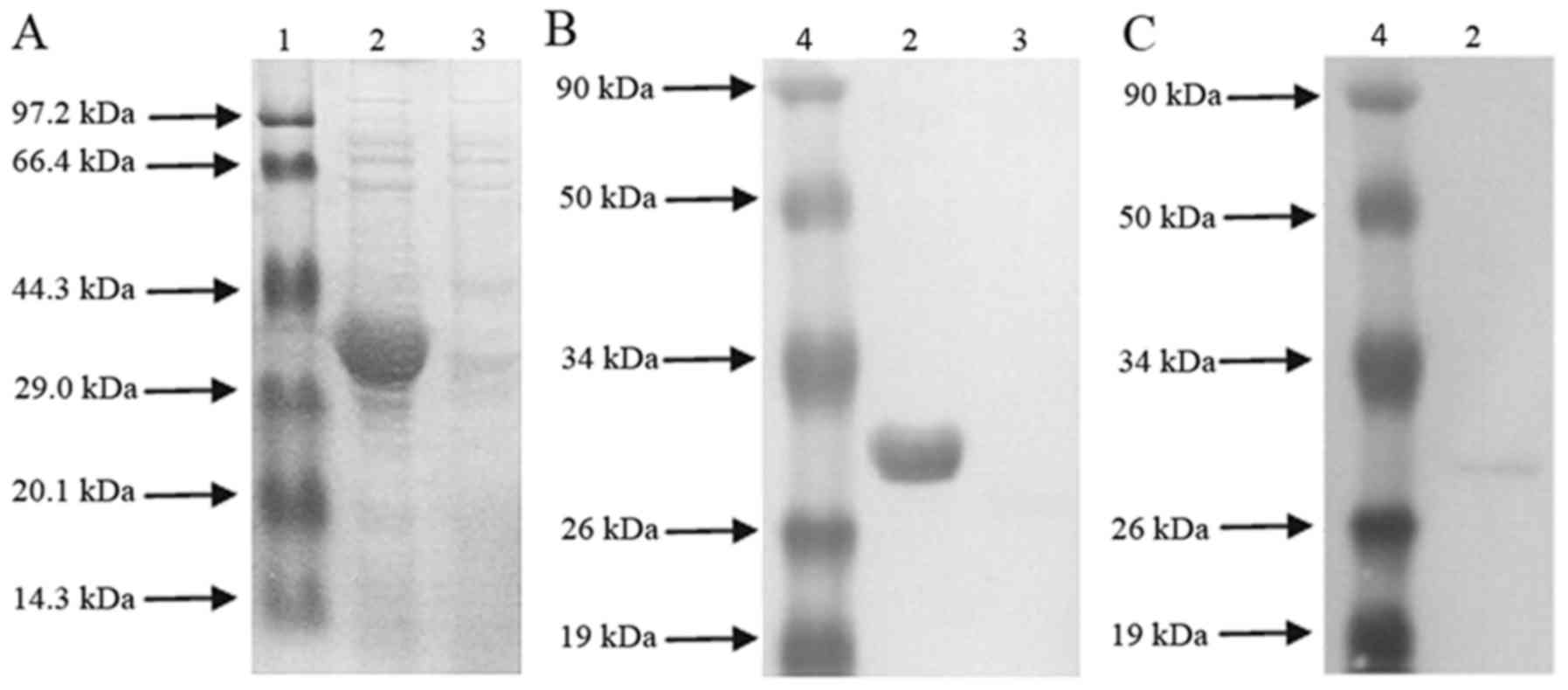
SDS-PAGE was used to separate recombinant His6-BvrR
protein. Lane 1 was a low molecular weight protein marker (Takara
Biotechnology Co., Ltd., Dalian, China and MBI Fermentas, Vilnius,
Lithuania), Lane 2 was the recombinant His6-BvrR
protein, and Lane 3 was the negative control. Determination of
His6-tagged BvrR protein concentration was performed using a
Bradford assay. Finally, Bradford assay was used for analysis of
the recombinant BvrR (rBvrR) protein or in vitro stimulation
of lymphocytes.
Immunization
The immunological studies were performed in three
groups. Following anesthetization with inhaled halothane, different
groups of experimental mice were inoculated separately in the
tibialis anterior muscle with 100 µg pCDNA-BvrR, pCDNA3.0 vector or
PBS at 0, 14 and 28 days.
On the 7th day following each vaccination, blood was
collected from five mice and the serum samples from each group were
kept in sterile microfuge tubes. The final serum was kept at −70°C
until further use.
Measurement of specific immunoglobulin
antibodies and their isotypes
Pooled serum collected from five mice of the
different groups at 7, 21 and 35 days was used for detecting
specific antibodies with the purified rBvrR proteins by indirect
ELISA. Serum at 35 days was used for the determination of the
antibody subtypes. A total of 3 µg/ml purified rBvrR proteins
diluted with carbonate buffer (0.05 M; pH 9.6) were applied for
coating the wells of polystyrene plates at 4°C overnight. The
plates were washed with PBS with 0.05% Tween-20 (PBS-T) buffer
three times, and skimmed milk powder (3%) in PBS-T was used to
block for 1 h at 37°C. The plates were subsequently incubated with
serial dilutions of serum or the negative control starting from a
1:200 dilution for 3 h at room temperature, followed by washing
four times. Horseradish peroxidase-conjugated anti-mouse
immunoglobulin G (IgG; D720358), IgG1 (D720359) and IgG2a (D720360,
all Sangon Biotech Co., Ltd. at 100 µl/well) was added into the
wells and incubated at 37°C for 1 h. Following washing four times
at room temperature for 30 min, 100 µl substrate solution was
added, and incubated in the dark at room temperature for 20 min.
Finally, 100 µl 0.5 M sulfuric acid per well was added to stop the
enzymatic reaction, and the absorbance was measured at 450 nm. The
titer was expressed as the optical density (OD).
Splenocyte cultures and lymphocyte
proliferation
Under aseptic conditions, mice were sacrificed to
obtain their spleens at 7, 21 and 35 days following the first
vaccination. The spleen was mixed intensively with chilled PBS to
collect the splenocytes. The flushed PBS, including splenocytes and
red blood cells was layered slowly onto an equal volume of
lymphocyte separation medium and centrifuged at 4°C, 1,000 × g for
40 min. The interface, including splenocytes, was collected and
washed with chilled PBS and finally washed with RPMI-1640 (10%
newborn calf serum; 2 mM L-glutamine; 100 µg/ml streptomycin; and
100 IU/ml penicillin; Gibco; Thermo Fisher Scientific, Inc.). In
the presence of rBvrR (1 µg/ml), splenocytes at a density of
4×105 viable cells were cultured at 37°C for 72 h with
5% CO2 in 96-well plates. Subsequently, 10 µl MTT (5
mg/ml thiazolyl blue in RPMI-1640) was added, and was incubated at
37°C for 4 h. Later, the frozen crystals were obtained by
centrifugation at 4°C, 1,000 × g for 10 min. A total of 150 µl
dimethyl sulfoxide per well was used to dissolve the crystals
following pipetting of the supernatant. Finally, the absorbance was
measured at 570 nm. The stimulation indices were calculated as the
ratio between absorbance values of stimulated cells and
unstimulated cells.
Cytokine ELISA
Cytokines in the culture supernatants of spleen
cells were determined by mouse interferon (IFN)-γ and interleukin
(IL)-4 ELISA kits (cat. no. 558258; BD Biosciences, Franklin Lakes,
NJ, USA; and cat. no. D720336; Sangon Biotech Co., Ltd.). All
assays were performed in triplicate. The absorbance values of
standards were used to obtain a linear regression equation, and
concentrations of IFN-γ and IL-4 were calculated.
Protection experiments
The protection experiments were performed by
vaccinating mice intramuscularly with B. abortus S19.
Simultaneously, mice were vaccinated with PBS and 108
CFU of B. suis S2 as a negative and positive control,
respectively. A total of 42 days following the first vaccination,
mice were challenged with 108 CFU of S19 by
intramuscular injection. A total of 2 weeks later, infected mice
were sacrificed to obtain their spleens, which were removed
aseptically and triturated. A 10 µl dilution of spleen lysate
diluted in triplicate was used to measure the CFU of
Brucella. Colonies were counted subsequent to all the plates
being incubated at 37°C with 5% CO2 for 3 days. Finally,
the protection was obtained by subtracting the mean of
log10 CFU of the experimental groups from that of the
corresponding PBS group.
Statistical analysis
Data are presented as the mean ± standard deviation
and evaluated using the SPSS 15.0 program for Windows (SPSS, Inc.,
Chicago, IL, USA). The data for the antibodies, lymphocyte
proliferation and cytokines were analyzed with paired-samples
t-test. Multiple groups were compared using one-way analysis of
variance, and Newman-Keuls method was subsequently used for
pairwise comparison. Tukey's honest significant difference
procedure was used for the data for the protection experiments.
P<0.05 was considered indicate a statistically significant
difference.
Results
Expression and purification of the
recombinant His6-BvrR protein
To obtain the rBvrR, E. coli harboring the
plasmid pET28a-BvrR was induced for expression. The molecular
weight (MW) of the expressed protein detected by SDS-PAGE was 31
kDa, which was consistent with the theoretical MW of
His6-BvrR (Fig. 1A).
Subsequently, rBvrR protein was confirmed by an anti-histidine
monoclonal antibody in the western blot analysis (Fig. 1B). The appearance of a specific
band at ~31 kDa coincided with the expected size, demonstrating
that the purified BvrR protein exhibited immunoreactivity with the
polyclonal antibodies of Brucella (Fig. 1C).
BvrR is involved in humoral
immunity
ELISA was used to measure the titers of anti-BvrR
antibodies in serum from mice immunized separately with pCDNA-BvrR,
pCDNA3.0 vector or PBS as a control. The serum from mice vaccinated
with pCDNA-BvrR was reactive to the antibody of BvrR between the
first and fifth week post-vaccination and the value of
OD450 ranged between 0.8 and 1.4 (Table I). The OD450 value of
IgG in pCDNA-BvrR group was significantly higher compared with the
pCDNA3.0 vector or PBS control groups (P<0.05). However, the
OD450 value was not different between the pCDNA3.0
vector and PBS control groups (P>0.05; Table I).
 | Table I.Optical density 450 values of
immunoglobulin G among the three groups on different days. |
Table I.
Optical density 450 values of
immunoglobulin G among the three groups on different days.
| Groups | 7 days | 21 days | 35 days |
|---|
| pcDNA-BvrR | 0.81±0.05 | 1.05±0.11 | 1.40±0.57 |
| pcDNA vector |
0.50±0.01a |
0.52±0.08a |
0.52±0.04a |
| PBS control |
0.49±0.04a |
0.51±0.03a |
0.50±0.21a |
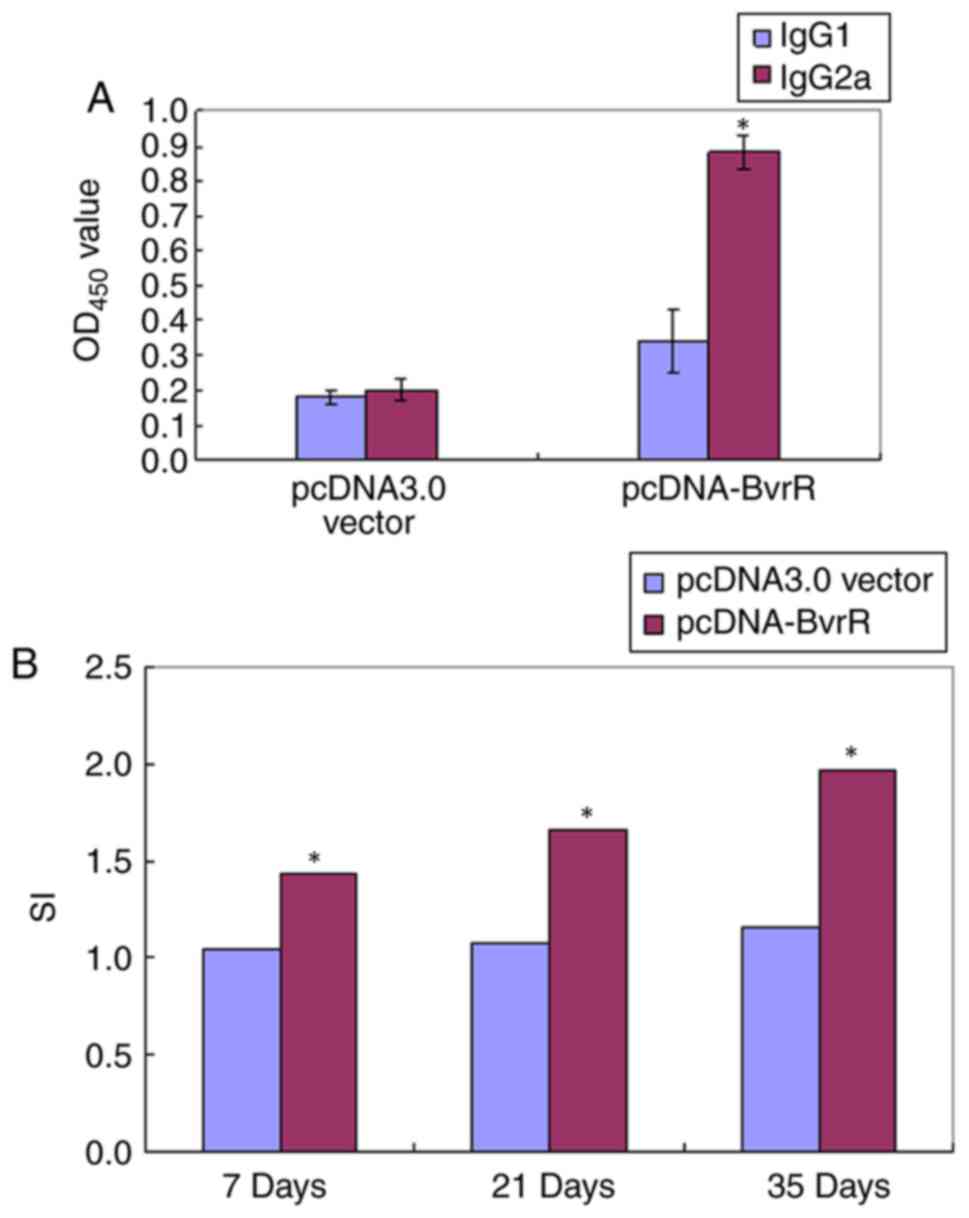
Subtype analysis suggested that the anti-BvrR
antibody in pCDNA-BvrR-immunized mice was primarily the IgG2a
subtype at 35 days of post-vaccination. The OD450 value
of the specific IgG2a subtype was increased compared with the
specific IgG1 subtype in the pCDNA-BvrR group (P<0.05); however,
not significantly increased in the PBS control group (Fig. 2A).
Role of BvrR in lymphocyte
proliferation
To test the CMI response to the Brucella
rBvrR protein, the proliferation rate of spleen cells from
immunized mice was determined. As demonstrated in Fig. 2B, at 1-week post-booster, the
splenocytes from mice of the pCDNA-BvrR group demonstrated
significant proliferative activity compared with the pCDNA3.0
vector group (P<0.05). This phenomenon also existed at 35 days
post-vaccination.
Determining the expression levels of
IFN-γ and IL-4
The cultured splenocyte supernatant of the mice was
assessed to determine the expression levels of IFN-γ and IL-4
following stimulation with rBvrR. An increased expression level of
IFN-γ was identified in supernatants of cell cultures from
pCDNA-BvrR-immunized animals, which reached a peak (95 pg/ml) at 35
days post-vaccination, compared with the pCDNA3.0 vector and PBS
groups (P<0.05; Table II).
Notably, the levels of IL-4 were not significantly different among
the three groups (Table II).
 | Table II.Determining expression levels of
IFN-γ and IL-4 in immunized mice. |
Table II.
Determining expression levels of
IFN-γ and IL-4 in immunized mice.
| Factor | Group | 7 days | 21 days | 35 days |
|---|
| IFN-γ | pcDNA-BvrR | 31.500±1.800 | 42.000±11.200 |
95.000±23.000a |
|
| pcDNA vector | 19.800±1.600 | 20.000±3.100 |
25.000±2.000b |
|
| PBS control | 20.500±3.200 | 19.600±2.100 |
22.500±1.500b |
| IL-4 | pcDNA-BvrR | 5.747±0.046 | 5.701±0.200 | 5.697±0.015 |
|
| pcDNA vector | 5.625±0.109 | 5.725±0.050 | 5.700±0.050 |
|
| PBS control | 5.708±0.095 | 5.733±0.038 | 5.750±0.075 |
Protection of B. abortus S19
challenge
To test the protective efficacy of BvrR, mice were
sacrificed on the 14th day post-challenge. The protection efficacy
was calculated as the reduction of bacteria number in the spleens
from immunized mice compared with control mice receiving PBS. When
the log10 CFU of B. abortus S19 was measured at
2-weeks post-challenge, it was indicated that the maximum clearance
was observed in the positive control group (B. suis S2;
1.415) or the pCDNA-BvrR group (0.814), which were significantly
different in the pCDNA3.0 vector and PBS groups (P<0.05;
Table III).
 | Table III.Protection against challenge with
B. abortus S19 in mice following immunization with the DNA
vaccine pCDNA-BvrR. |
Table III.
Protection against challenge with
B. abortus S19 in mice following immunization with the DNA
vaccine pCDNA-BvrR.
| Group | Log CFU | Log units of
protection |
|---|
| PBS | 2.916±0.019 | 0.000a |
| pcDNA | 2.760±0.070 | 0.156a |
| pcDNA-BvrR | 2.102±0.144 | 0.814b |
| B. suis
S2 |
1.557±0.056c | 1.415c |
Discussion
At present, vaccination remains the most successful
method of preventing brucellosis in animals from countries with a
high incidence (23). However,
specific types of live-attenuated vaccines used for controlling
animal brucellosis are disadvantageous to humans (4), leading to the development of novel
vaccines.
It was suggested that tuberculosis may depend on the
T helper-1 (Th1)-type cell-mediated immune response to protect
against infection by an intracellular pathogen, including
Brucella (24,25). A number of studies demonstrated
that DNA vaccines acted on the major histocompatibility complex
class I and II following naked DNA immunization, inducing a wide
range of immune responses, including antibody production, CD8
cytotoxic T cells and CD4 T helper cell activation (10,23,26).
DNA vaccines overcame the disadvantages of acellular vaccines,
including recombinant proteins and synthetic peptides that were not
adequately processed and presented, which resulted in a failure to
induce a strong CMI response as well as to confer a high degree of
protection (6,27). Regarding brucellosis, it was
documented that all the genes or specific epitopes of
Brucella, including Cu/Zn superoxide dismutase (SOD),
ribosomal L7/L12 or lumazine synthase, were able to induce
significant levels of protection in mice (9).
The pathogenesis of Brucella is controlled by
the two-component system BvrR/BvrS (TCS BvrRS) and type IV
secretion machinery VirB (T4SS VirB) (18,28).
Furthermore, the TCS BvrRS and T4SS VirB control the expression of
specific outer membrane proteins through direct and indirect
mechanisms, respectively (21,22).
TCS BvrRS serves an important role in the intracellular replication
of Brucella.
A previous study suggested that a DNA vaccine
encoding Brucella Cu/Zn SOD may be a good candidate for
vaccination against Brucella (29). A wide variety of Brucella
vaccines have been developed for protection against brucellosis;
however, they have had limited acceptance and success. An advantage
of DNA vaccines is that multiple antigens may be expressed;
however, it was essential to fully evaluate the benefits and risks
of these types of Brucella vaccines for the prevention of
brucellosis in animals and particularly humans, including B.
abortus S19, Vaccine strain RB51 and outer membrane vesicles
(30). Plasmid DNA carrying the
BLS gene was additionally a good candidate for vaccination against
Brucella (17). Another
previous study demonstrated a protective immune response induced by
a novel double DNA vaccine encoding the Brucella melitensis
omp31 gene and the E. coli eae gene in a mouse model
(31). All these results suggested
that DNA vaccines demonstrated great immunogenicity and protective
efficacy against infection in a mouse model.
The plasmid DNA containing the BvrR gene was
injected to induce specific humoral and cellular immunities. A
total of 1 week following the first immunization, it was observed
that a weak titer of specific IgG was identified in mice, which was
twice as high at the end of experiment. The induced antibody titers
in pCDNA-BvrR vaccine were lower compared with previous DNA
vaccines against Brucella (29,32).
It was possible that there existed differences in numerous factors,
including the addition of adjuvant, and the method and time of
detection.
Subsequent to in vitro stimulation of splenic
cells, lymphocyte proliferation and cytokine production were
measured to evaluate the T-cell immunity following DNA
immunization. These results demonstrated that rBvrR was able to
elicit increased expression levels of IFN-γ compared with IL-4 and
a strong T cell-proliferative response. Furthermore, the anti-BvrR
antibody was IgG2a-predominant compared with IgG1. Following naked
DNA immunization, IgG2a was the predominant antibody subclass in
responsive mice, indicating that Th1-CD4+ cellular
responses were identified in BALB/c mice (33). Hinkula et al (34) documented that full protection of
mice vaccinated with the specific DNA vaccine and an extra
Th1-specific cellular response were required. Together, it was
concluded that immunization with the plasmid pCDNA-BvrR induced a
Th1 cellular response.
Subsequently, pCDNA-BvrR vaccines from different
strains were investigated in the present study and the protective
efficacy of the pCDNA-BvrR vaccine against more virulent B.
abortus S19 challenge was analyzed. Animals vaccinated with
pCDNA-BvrR demonstrated a log protection of 0.814, which was
markedly increased compared with PBS or the pCDNA3.0 vector, and
lower compared with B. suis S2. Therefore, pCDNA-BvrR
vaccines from different strains were studied in the present study,
and the protective efficacy of the pCDNA-BvrR vaccine against B.
abortus S19 challenge was analyzed. Animals vaccinated with
pCDNA-BvrR demonstrated a log protection of 0.814, which was
markedly increased compared with PBS or the pCDNA3.0 vector, and
lower compared with B. suis S2. All these results
demonstrated that BvrR may be used as a powerful candidate for DNA
vaccination.
In conclusion, antibody and Th1 cellular responses
were elicited following immunization with the plasmid pCDNA-BvrR,
and protection against B. abortus challenge was obtained.
The present results suggested that BvrR is a promising candidate
for studies of DNA vaccines against brucellosis in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BC acquired data obtained from animal experiments,
performed statistical analyses, designed and modified experimental
protocols, and wrote and revised the manuscript. GW acquired data
obtained from animal experiments and was responsible for the
redrafting of the manuscript. BL performed statistical analyses,
designed and modified experimental protocols, and wrote and revised
the manuscript. ZZ analyzed and interpreted data obtained from
animal experiments and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Laboratory
Animal Welfare and Ethical review committee of Shenyang
Agricultural University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Norman FF, Monge-Maillo B,
Chamorro-Tojeiro S, Pérez-Molina JA and López-Vélez R: Imported
brucellosis: A case series and literature review. Travel Med Infect
Dis. 14:182–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adone R, Muscillo M, La Rosa G, Francia M
and Tarantino M: Antigenic, immunologic and genetic
characterization of rough strains B. abortus RB51, B.
melitensis B115 and B. melitensis B18. PLoS One.
6:e240732011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis DS and Elzer PH: Brucella
vaccines in wildlife. Vet Microbiol. 90:533–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perkins SD, Smither SJ and Atkins HS:
Towards a Brucella vaccine for humans. FEMS Microbiol Rev.
34:379–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bobbala S and Hook S: Is there an optimal
formulation and delivery strategy for subunit vaccines? Pharm Res.
33:2078–2097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baloglu S, Boyle SM, Vemulapalli R,
Sriranganathan N, Schurig GG and Toth TE: Immune responses of mice
to vaccinia virus recombinants expressing either Listeria
monocytogenes partial listeriolysin or Brucella abortus
ribosomal L7/L12 protein. Vet Microbiol. 109:11–17. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vemulapalli R, He Y, Cravero S,
Sriranganathan N, Boyle SM and Schurig GG: Overexpression of
protective antigen as a novel approach to enhance vaccine efficacy
of Brucella abortus strain RB51. Infect Immun. 68:3286–3289.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rajasekaran P, Seleem MN, Contreras A,
Purwantini E, Schurig GG, Sriranganathan N and Boyle SM:
Brucella abortus strain RB51 leucine auxotroph as an
environmentally safe vaccine for plasmid maintenance and antigen
overexpression. Appl Environ Microbiol. 74:7051–7055. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu DH, Hu XD and Cai H: A combined DNA
vaccine encoding BCSP31, SOD, and L7/L12 confers high protection
against Brucella abortus 2308 by inducing specific CTL
responses. DNA Cell Biol. 26:435–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu XD, Yu DH, Chen ST, Li SX and Hong C: A
combined DNA vaccine provides protective immunity against
Mycobacterium bovis Brucella abortus in cattle. DNA Cell
Biol. 28:191–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao HP, Sun JF, Li N, Sun Y, Xia ZH, Wang
Y, Cheng D, Qi QF, Jin ML and Qiu HJ: Assessment of the
cell-mediated immunity induced by alphavirus replicon-vectored DNA
vaccines against classical swine fever in a mouse model. Vet
Immunol Immunopathol. 129:57–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maspi N, Ghaffarifar F, Sharifi Z and
Dalimi A: Codelivery of DNA vaccination encoding LeIF gene and
IL-12 increases protection against Leishmania major infection in
BALB/c mice. Parasite Immunol. 38:228–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oksanen KE, Myllymäki H, Ahava MJ, Mäkinen
L, Parikka M and Rämet M: DNA vaccination boosts Bacillus
Calmette-Guérin protection against mycobacterial infection in
zebrafish. Dev Comp Immunol. 54:89–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Griffin BD, Muthumani K, Warner BM, Majer
A, Hagan M, Audet J, Stein DR, Ranadheera C, Racine T, De La Vega
MA, et al: DNA vaccination protects mice against Zika virus-induced
damage to the testes. Nat Commun. 8:157432017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bello J, Sáez D, Escalona E, Velozo P,
Santiviago CA, Contreras I and Oñate Á: Mucosal immunization of
BALB/c mice with DNA vaccines encoding the SEN1002 and SEN1395 open
reading frames of Salmonella enterica serovar Enteritidis induces
protective immunity. Epidemiol Infect. 144:247–256. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
González-Smith A, Vemulapalli R, Andrews E
and Oñate A: Evaluation of Brucella abortus DNA vaccine by
expression of Cu-Zn superoxide dismutase antigen fused to IL-2.
Immunobiology. 211:65–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Velikovsky CA, Cassataro J, Giambartolomei
GH, Goldbaum FA, Estein S, Bowden RA, Bruno L, Fossati CA and Spitz
M: A DNA vaccine encoding lumazine synthase from Brucella
abortus induces protective immunity in BALB/c mice. Infect
Immun. 70:2507–2511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
López-Goñi I, Guzmán-Verri C, Manterola L,
Sola-Landa A, Moriyón I and Moreno E: Regulation of Brucella
virulence by the two-component system BvrR/BvrS. Vet Microbiol.
90:329–339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guzmán-Verri C, Manterola L, Sola-Landa A,
Parra A, Cloeckaert A, Garin J, Gorvel JP, Moriyon I, Moreno E and
Lopez-Goni I: The two-component system BvrR/BvrS essential for
Brucella abortus virulence regulates the expression of outer
membrane proteins with counterparts in members of the Rhizobiaceae.
Proc Natl Acad Sci USA. 99:12375–12380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manterola L, Moriyón I, Moreno E,
Sola-Landa A, Weiss DS, Koch MH, Howe J, Brandenburg K and
López-Goñi I: The lipopolysaccharide of Brucella abortus
BvrS/BvrR mutants contains lipid a modifications and has higher
affinity for bactericidal cationic peptides. J Bacteriol.
187:5631–5639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manterola L, Guzmán-Verri C, Chaves-Olarte
E, Barquero-Calvo E, de Miguel MJ, Moriyón I, Grilló MJ, López-Goñi
I and Moreno E: BvrR/BvrS-controlled outer membrane proteins Omp3a
and Omp3b are not essential for Brucella abortus virulence.
Infect Immun. 75:4867–4874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martínez-Núñez C, Altamirano-Silva P,
Alvarado-Guillén F, Moreno E, Guzmán-Verri C and Chaves-Olarte E:
The two-component system BvrR/BvrS regulates the expression of the
type IV secretion system VirB in Brucella abortus. J
Bacteriol. 192:5603–5608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tadesse G: Brucellosis seropositivity in
animals and humans in ethiopia: A meta-analysis. PLoS Negl Trop
Dis. 10:e00050062016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Faurez F, Grasland B, Béven V, Cariolet R,
Keranflec'h A, Henry A, Jestin A and Dory D: The protective immune
response against Pseudorabies virus induced by DNA vaccination is
impaired if the plasmid harbors a functional Porcine circovirus
type 2 rep and origin of replication. Antiviral Res. 96:271–279.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Otani N, Yamanishi K, Sakaguchi Y, Imai Y,
Shima M and Okuno T: Varicella-zoster virus-specific cell-mediated
immunity in subjects with herpes zoster. J Immunol Methods.
377:53–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan Z, Zhou T, Zheng H, Ding Y and Xu Y:
Malaria DNA vaccine gp96NTD-CSP elicits both CSP-specific antibody
and CD8(+) T cell response. Parasitol Res. 114:2333–2339. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Estein SM, Cheves PC, Fiorentino MA,
Cassataro J, Paolicchi FA and Bowden RA: Immunogenicity of
recombinant Omp31 from Brucella melitensis in rams and serum
bactericidal activity against B. ovis. Vet Microbiol. 102:203–213.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lacerda TL, Salcedo SP and Gorvel JP:
Brucella T4SS: The VIP pass inside host cells. Curr Opin
Microbiol. 16:45–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oñate AA, Céspedes S, Cabrera A, Rivers R,
González A, Muñoz C, Folch H and Andrews E: A DNA vaccine encoding
Cu, Zn superoxide dismutase of Brucella abortus induces
protective immunity in BALB/c mice. Infect Immun. 71:4857–4861.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Avila-Calderón ED, Lopez-Merino A,
Sriranganathan N, Boyle SM and Contreras-Rodríguez A: A history of
the development of Brucella vaccines. Biomed Res Int.
2013:7435092013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ranjbar R, Sharifimoghadam S, Saeidi E,
Jonaidi N and Sheikhshahrokh A: Induction of protective immune
responses in mice by double DNA vaccine encoding of Brucella
melitensis Omp31 and Escherichia coli Eae genes. Trop J
Pharm Res. 15:20772016. View Article : Google Scholar
|
|
32
|
Yu DH, Li M, Hu XD and Cai H: A combined
DNA vaccine enhances protective immunity against Mycobacterium
tuberculosis Brucella abortus in the presence of an IL-12
expression vector. Vaccine. 25:6744–6754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lladser A, Párraga M, Quevedo L, Carmen
Molina M, Silva S, Ferreira A, Billetta R and G Quest AF: Naked DNA
immunization as an approach to target the generic tumor antigen
survivin induces humoral and cellular immune responses in mice.
Immunobiology. 211:11–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hinkula J, Devignot S, Åkerström S,
Karlberg H, Wattrang E, Bereczky S, Mousavi-Jazi M, Risinger C,
Lindegren G, Vernersson C, et al: Immunization with DNA plasmids
coding for crimean-congo hemorrhagic fever virus capsid and
envelope proteins and/or virus-like particles induces protection
and survival in challenged mice. J Virol. 91:e02076–e02116. 2017.
View Article : Google Scholar : PubMed/NCBI
|