Introduction
OS is the most common pediatric primary malignant
bone tumor. The 5-year survival rates for children (0–14 years old)
and adolescents (15–19 years old) are 69.5 and 63.4%, respectively
(1), whereas for metastatic
patients, it is only 20–30% (2).
At present, the pathogenesis and etiology of OS remain to be fully
elucidated. A strategy that was implemented to determine treatment
based on the histological response did not lead to any improvement
in the survival rate (3). Early
diagnosis and the improvement of therapeutic strategies are
therefore urgently required.
MicroRNAs (miRNAs) are single-stranded RNA species
that are highly conserved, between 20 and 24 nucleotides in length,
and mature miRNAs are able to inhibit the translation and
degradation of mRNAs by binding to their 3′-untranslated regions
(UTRs) (4). Researchers have
identified >2,000 miRNAs in Homo sapiens, and one-third
of mRNAs are hypothesized to be co-regulated in the genome
(5). At present, miRNAs have been
demonstrated to be widely involved in physiological and
pathological processes associated with cancer, including the
differentiation, proliferation and apoptosis of cancer cells
(6). Recently, increasing evidence
has indicated that miRNAs are closely associated with the
development and metastasis of OS; for example, miRNA (miR)-34a in
OS targets the Notch pathway and causes Notch1 downregulation,
resulting in cell cycle arrest and apoptosis (7,8). The
study of target genes and gene pathways of miRNAs is conducive to
revealing the molecular mechanisms underpinning the development and
metastasis of OS. Researchers have demonstrated that miR-542-3p is
implicated in the progression of various types of tumors, including
neuroblastoma, gastric cancer, bladder cancer and astrocytoma, via
targeted inhibition of angiopoietin-2 (9–12). A
recent study verified that miR-542-3p is an oncogene of OS, which
is able to enhance cell proliferation and migration at an
overexpressed level in OS, and one of its target genes is VANGL
planar cell polarity protein 2 (VANGL2) (13). However, the target gene(s) of
miR-542-3p, and its underlying mechanism(s) in OS, remain
unclear.
In the present study, the high expression level of
miR-542-3p in OS, based on the continuous variables of the Gene
Expression Omnibus (GEO) database and PubMed, was first confirmed.
Subsequently, the potential target genes of miR-542-3p were
identified using bioinformatics software and gene expression
profiles. Using the Database for Annotation, Visualization and
Integrated Discovery (DAVID), version 6.8, the biological value of
miR-542-3p was identified using gene ontology (GO) enrichment and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses.
The top 10 results of the GO enrichment analysis were selected, and
the KEGG results revealed the highlighted pathways of miR-542-3p.
Taken together, the identification of the potential target genes
and biological functions of miR-542-3p has provided novel insights
into the role of differentially expressed genes (DEGs) in OS.
miR-542-3p may therefore be a novel marker useful for diagnosis and
treatment of patients with OS.
Materials and methods
Study of a comparison of the
expression levels of miR-542-3p between patients with OS and normal
controls (NC)
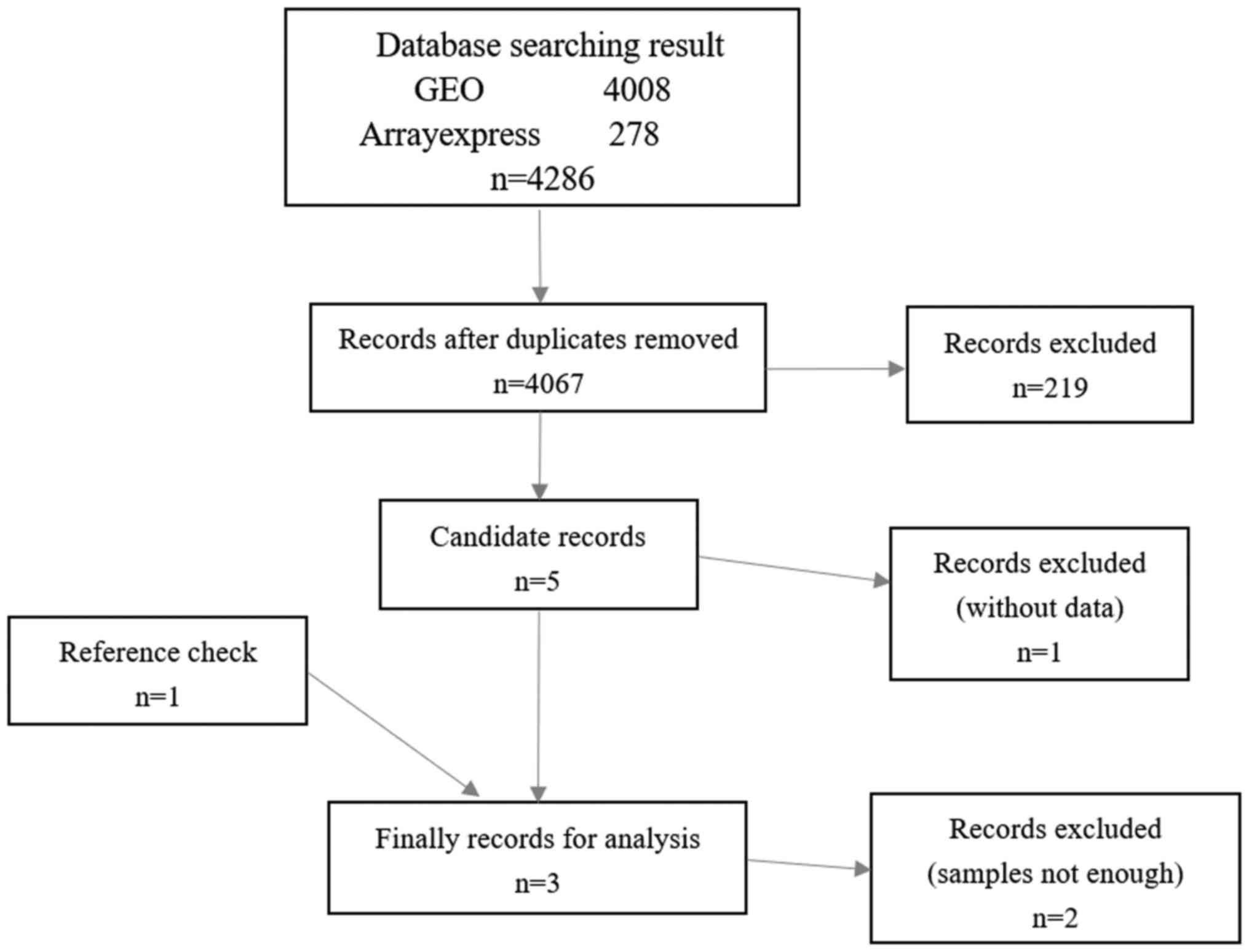
As presented in Fig.
1, miR-542-3p expression profiles of OS were searched from the
GEO (https://www.ncbi.nlm.nih.gov/gds/?term=) and
ArrayExpress (https://www.ebi.ac.uk/arrayexpress/) databases to
identify the expression level of miR-542-3p in OS. The search
strategy employed was as follows: [(‘bone’ OR ‘bones’) AND
(‘sarcoma’ OR ‘sarcomas’)] OR (‘osteosarcoma’ OR ‘osteosarcomas’)
AND “Homo sapiens”] [porgn_txid9606]. In addition, the PubMed
database (https://www.ncbi.nlm.nih.gov/pubmed/?term=) was also
searched using the following retrieval strategy: (‘microRNAs’
[medical subject heading (MeSH) terms] OR ‘microRNAs’ (All fields)
OR ‘osteosarcoma’ (All fields)], retrieving results from all
studies published up to March 2018 to ensure that any relevant
publications were not overlooked. Exclusion criteria were as
follows: Only studies of miR-542-3p expression that involved a
comparison being made between OS and NC were analyzed, and studies
that were duplicates or lacked a sufficient sample size (i.e., the
samples of each group were <3) were also excluded.
Qualifying OS gene expression profiles
and identification of DEGs
To understand the biological role of target genes of
miR-542-3p in OS, qualifying gene expression profiles from the GEO
database were selected for further study. In order to obtain DEGs
between OS and NC (normal bone or osteoblasts or mesenchymal stem
cells), 14 expression profiles were selected for analysis, and the
characteristics of the individual studies are presented in Table I. The raw data file was uploaded to
the online resource GEO2R (https://www.ncbi.nlm.nih.gov/geo/info/geo2r.html).
The truncated standard, logFC<0, was used to determine the DEGs
with statistical significance. Qualifying genes were classified as
such if they appeared in no fewer than six expression profiles, and
these were used for further study. A search was also performed for
gene expression chips that were designed for the transfection of
miRNA or miR-542-3p mimics. The expression profile GSE47363
(14), based on GPL10558 (Illumina
HumanHT-12 v4.0 Expression BeadChip Support; Illumina, Inc., San
Diego, CA, USA), was the only one that qualified. Subsequently, the
OS gene expression profiles and the OS expression profile with the
DEGs transfected with miR-542-3p were overlaid.
 | Table I.Characteristics of individual
studies. |
Table I.
Characteristics of individual
studies.
| GEO ID | Sample count
(osteosarcoma/normal control) | Platform | Sample sources | Tissue |
|---|
| GSE14359 | 18/2 | GPL96 [HG-U133A]
Affymetrix Human Genome U133A Array | in vivo | Bone, lung |
| GSE12865 | 12/2 | GPL6244
[HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array [transcript
(gene) version] | in vivo | Bone |
| GSE11414 | 4/2 | GPL6244
[HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array [transcript
(gene) version] | in
vitro | Bone |
| GSE42352 | 103/15 | GPL10295 Illumina
human-6 v2.0 expression beadchip (using nuIDs as identifier) | in vivo/in
vitro | Bone |
| GSE36001 | 19/6 | GPL6102 Illumina
human-6 v2.0 expression beadchip | in
vitro | Bone |
| GSE32964 | 35/1 | GPL6947 Illumina
HumanHT-12 V3.0 expression beadchip | in vivo | Bone |
| GSE68591 | 10/2 | GPL11028
[HuEx-1_0-st] Affymetrix Human Exon 1.0 ST Array [HuEx-1_0-st-v2,
coreR3, A20071112, EP.cdf] | in
vitro | Bone |
| GSE70414 | 5/1 | GPL570
[HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | in
vitro | Bone |
| GSE56001 | 3/5 | GPL10558 Illumina
HumanHT-12 V4.0 expression beadchip | in vivo | Bone |
| GSE39262 | 10/1 | GPL96 [HG-U133A]
Affymetrix Human Genome U133A Array | in
vitro | Bone |
| GSE28424 | 19/4 | GPL13376 Illumina
HumanWG-6 v2.0 expression beadchip | in
vitro | Bone |
| GSE14789 | 3/1 | GPL571 [HG-U133A_2]
Affymetrix Human Genome U133A 2.0 Array | in
vitro | Bone |
| GSE5045 | 5/2 | GPL1120 SuperArray
GEArray Q Series Human Angiogenesis Gene Array | in vivo | Bone |
| GSE5045 | 5/3 | GPL1133 SuperArray
GEArray Q Series Human Tumor Metastasis Gene Array | in vivo | Bone |
Prediction of target genes of
miR-542-3p in OS based on MirWalk2.0
The miR-542-3p target genes were predicted based on
miRWalk2.0, the comprehensive atlas of predicted and validated
miRNA-target interactions (http://zmf.umm.uni-heidelberg.de/mirwalk2), using 12
predictive software packages, including DIANA-microT v4.0
(http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microtv4/index),
DIANA-microT-CDS (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index),
miRanda release 2010 (http://www.microrna.org/microrna/getDownloads.do),
mirBridge [(http://mirsystem.cgm.ntu.edu.tw/)), miRDB4.0
(http://mirdb.org/miRDB/download.html), miRmap
(https://mirmap.ezlab.org/), miRNAMap
(ftp://mirnamap.mbc.nctu.edu.tw/), PicTar2 (https://dorina.mdc-berlin.de/rbp_browser/download_hg19.html),
PITA (https://genie.weizmann.ac.il/pubs/mir07/mir07_dyn_data.html)],
RNA22v2 (https://cm.jefferson.edu/rna22/), RNAhybrid2.1
(https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/dl_pre-page.html)
and Targetscan version 6.2 (http://www.targetscan.org/cgi-bin/targetscan/data_download.cgi?db=vert_61).
The complete data were downloaded from the platform, and genes that
featured in more than four of the predictive software packages were
subsequently selected as putative target genes of miR-542-3p in
OS.
Potential target genes of miR-542-3p
in OS
As previously mentioned, DEGs in OS gene expression
microarrays and predicted target genes of miR-542-3p based on
miRWalk2.0 were collected. These genes were subsequently overlaid,
and 1,035 potential target genes of miR-542-3p in OS were finally
obtained. In addition, target genes of miR-542-3p in OS reported in
the literature were collected. The inclusion criteria were as
follows: i) miR-542-3p target genes that were identified in OS; and
ii) a dual luciferase reporter assay or RNA binding protein
immunoprecipitation assay had been used for verification of the
target genes.
Bioinformatics analyses of the
potential target genes
DAVID version 6.8 was used to perform GO enrichment
and KEGG pathway analyses of the potential target genes of
miR-542-3p in OS, and the threshold value was set at P<0.05. In
addition, in order to examine the interrelationships between these
genes, protein-protein interaction (PPI) network analysis was
performed using the STRING database (https://string-db.org/). Genes that were revealed to
be hub genes were designated as the key target genes of miR-542-3p
in OS. The correlation between proteins was evaluated using a
reliability scoring threshold >0.9.
Statistical analysis
The expression data of miR-542-3p, comparing between
OS and NC, was analyzed using the software package Stata (version
12.0; StataCorp LP, College Station, TX, USA). Continuous variable
analysis was performed on three studies. The expression level of
miR-542-3p in OS was evaluated using a fixed-effects model if there
was significant heterogeneity. Otherwise, a random effects model
was used. A Q test based on the χ2 test was used to
measure the heterogeneity of study effects distributions. No
significant heterogeneity among the studies was considered to exist
where the measure of heterogeneity, I2, was demonstrated
to be ≤50%. A funnel plot was made to evaluate the risk of bias in
the included studies. Two-tailed P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-542-3p is upregulated in OS
In order to detect the function of miR-542-3p in OS,
two miRNA expression profiles (GSE28423 and GSE69524) and two
papers that contained expression data of miR-542-3p in OS were
collected (PMID29103020 and PMID25352048, although it was not
possible to obtain the expression values of the continuous variable
with PMID25352048). The original data were downloaded, from which
the expression data of miR-542-3p were extracted, and a continuous
variable analysis was subsequently performed using Stata software
(Table II). The diagnostic
sensitivity and specificity of miR-542-3p in patients with OS are
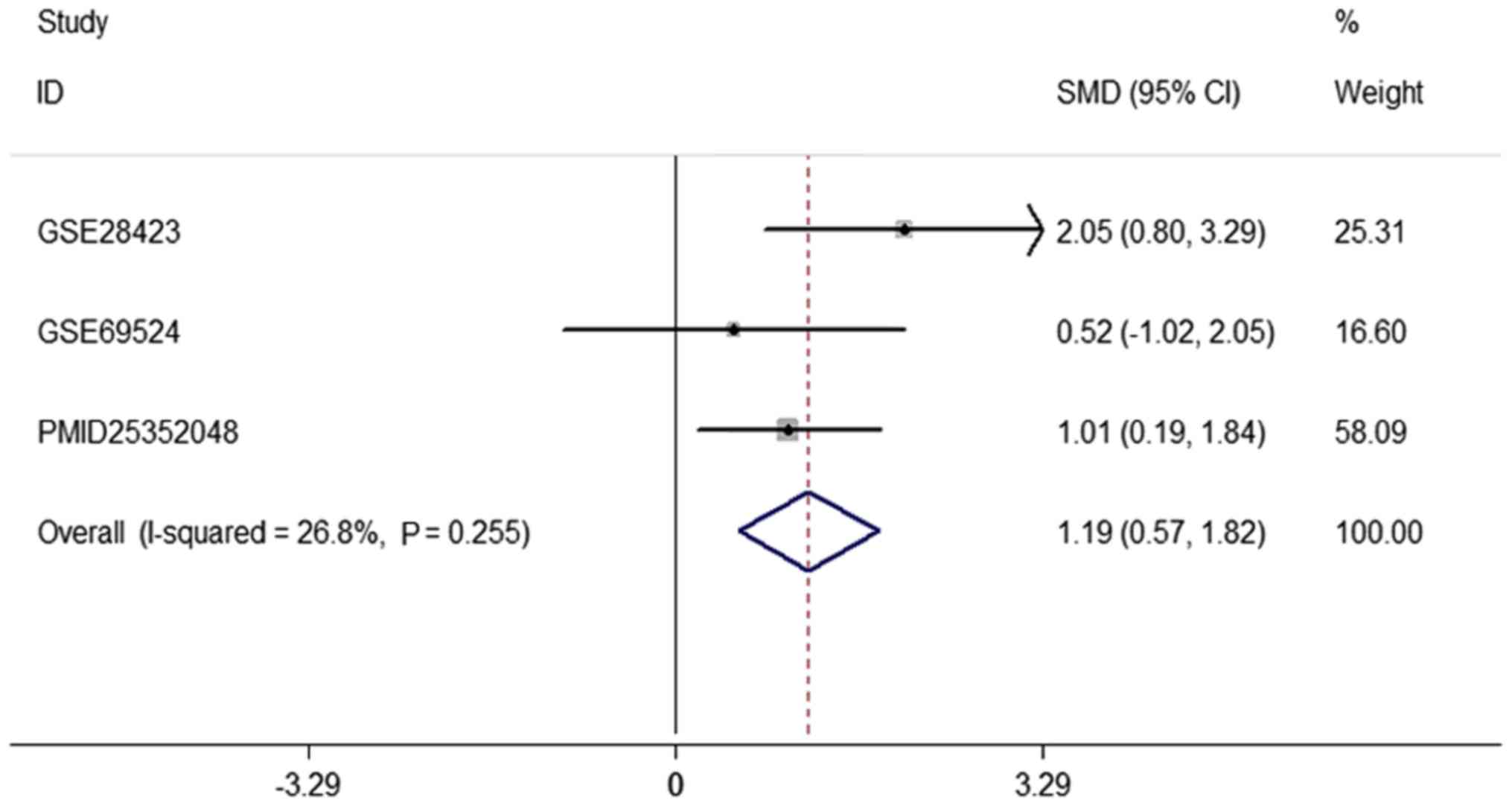
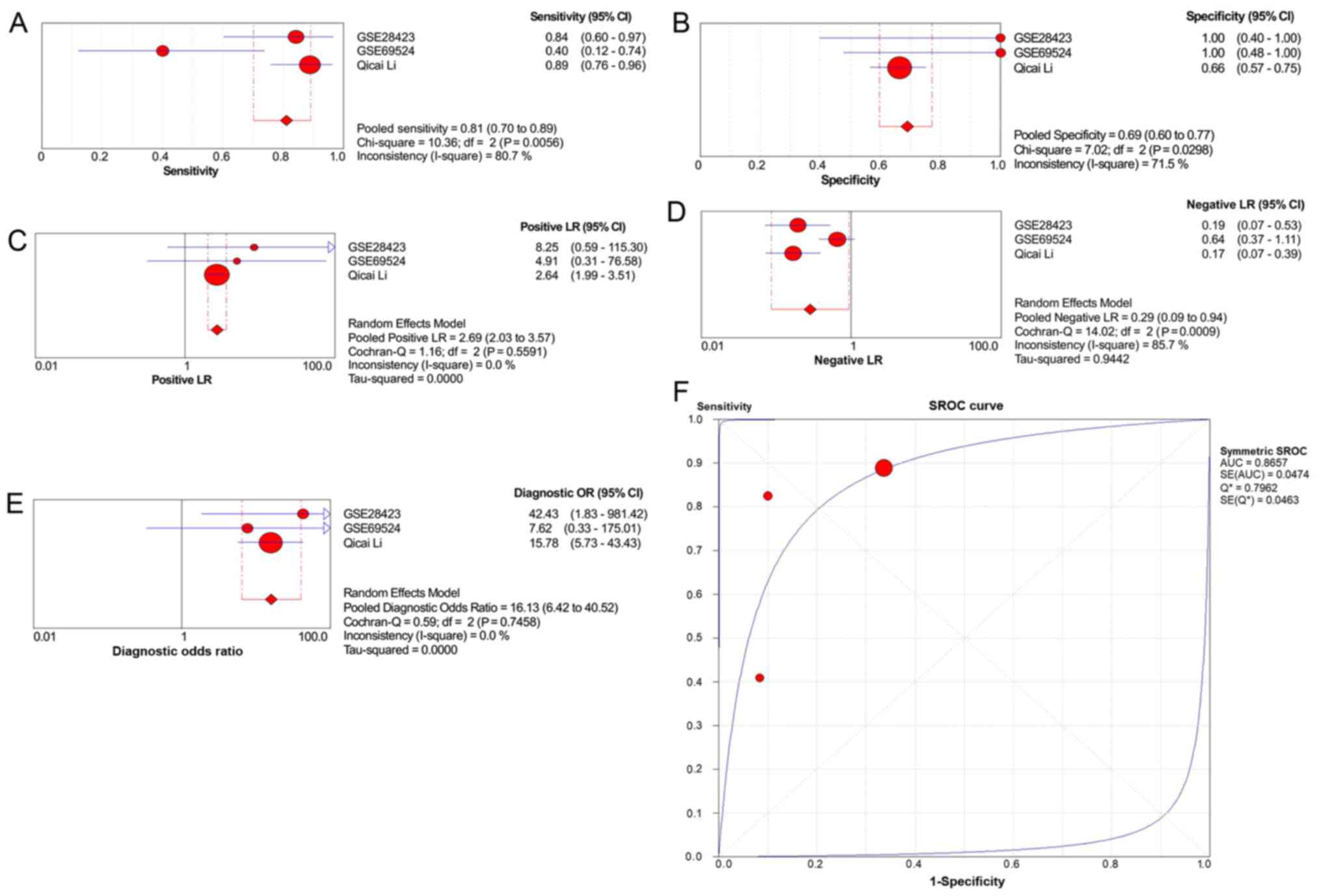
presented in the Forest map (Fig.
2). The pooled sensitivity, specificity, positive likelihood
ratio, negative likelihood ratio and diagnostic odds ratio were
0.81 [95% confidence interval (CI), 0.70–0.89], 0.69 (95% CI,
0.60–0.77), 2.69 (95% CI, 2.03–3.57), 0.29 (95% CI, 0.09–0.94) and
16.13 (95% CI, 6.42–40.52), respectively (Fig. 3A-E). The area under the summary
receiver operating characteristic (SROC) curve was 0.8657 (Fig. 3F). In addition, the statistical
analysis yielded the following values for the listed parameters:
P=0.255; standard mean deviation=1.19; 95% CI, 0.57–1.8;
heterogeneity according to the χ2 test,
I2=26.8% (Fig. 2).
miR-542-3p was identified to be overexpressed in OS tissues and in
OS cell lines. As there were fewer than 10 studies, tests for
funnel plot asymmetry were not used in the meta-analysis (15).
 | Table II.Results of continuous variable
analysis for identifying miR-542-3p expression level in
osteosarcoma. |
Table II.
Results of continuous variable
analysis for identifying miR-542-3p expression level in
osteosarcoma.
|
|
| n | Mean ± standard
deviation |
|---|
|
|
|
|
|
|---|
| Study | Year | Case | Control | Case | Control |
|---|
| GSE28423 | 2012 | 19 | 4 | 4.9500±1.4447 | 1.8996±1.7431 |
| GSE69524 | 2015 | 10 | 2 | 4.6094±0.7349 | 4.2477±0.0892 |
| PMID25352048 | 2015 | 13 | 13 | 9.5084±9.1297 | 2.7254±2.4619 |
Prediction of target genes of
miR-542-3p
Based on the GEO dataset and the miRWalk2.0 atlas of
predicted and experimentally verified miRNA target binding sites,
the analysis data of the potential target genes of miR-542-3p were
extracted from the gene expression profile of OS. The 12
bioinformatics software packages mentioned above were used to
predict the target genes of miR-542-3p, and only those genes that
appeared in more than four of the prediction software packages were
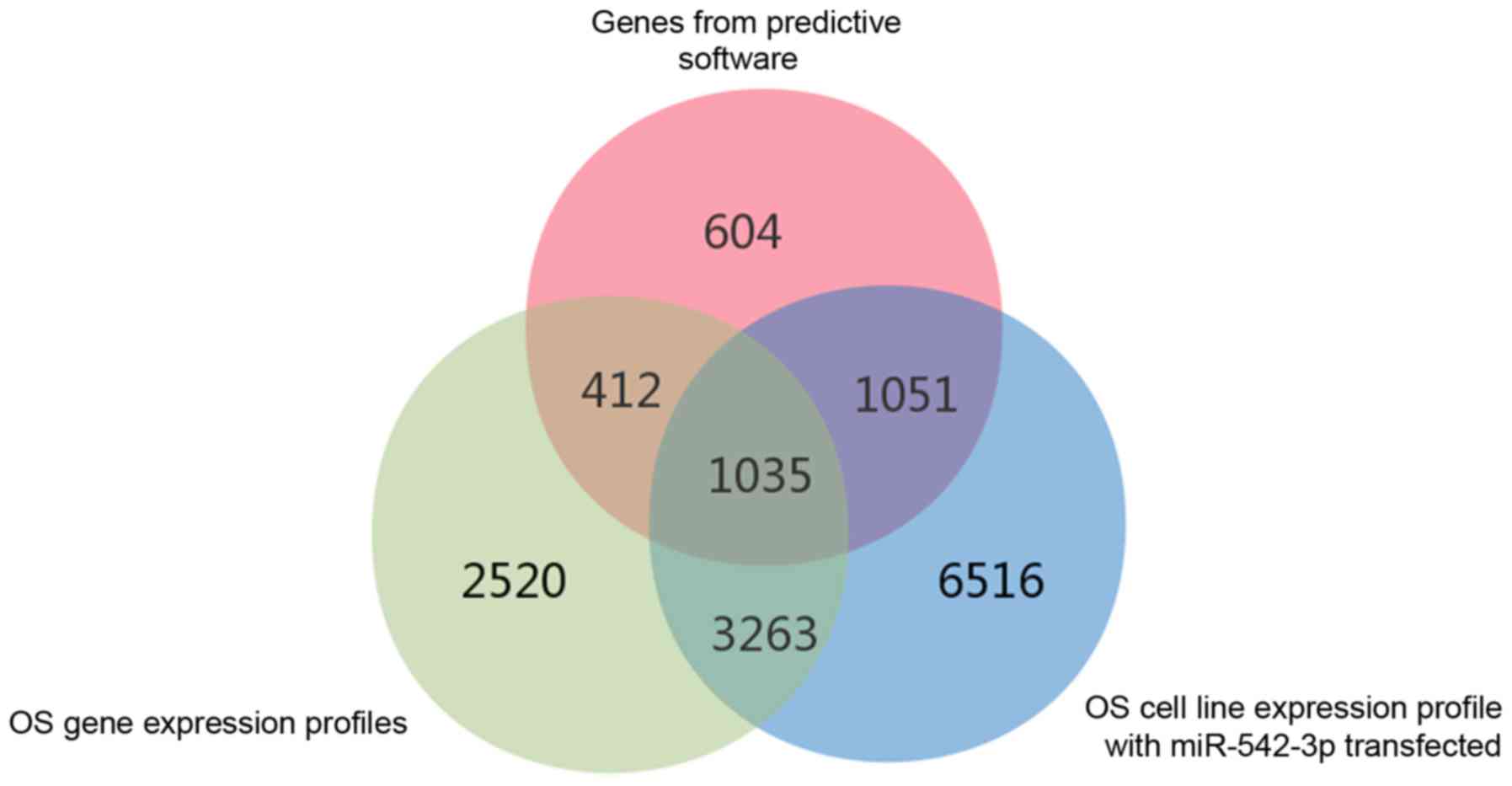
retained. The overlapping potential target genes are presented in
Fig. 4; VANGL2 was an experimental
verification gene identified in the literature, and this was also
involved in the above analysis.
PPI network analysis
The potential target genes were integrated and
analyzed using PPI network analysis. A total of eight hub genes
[ubiquitin-60S ribosomal protein L40 (UBA52), Ras-related C3
botulinum toxin substrate (RAC1), mitogen-activated protein kinase
1 (MAPK1), epidermal growth factor receptor (EGFR), cystic fibrosis
transmembrane conductance regulator (CFTR), phosphoinositide
3-kinase regulatory subunit 1 (PI3KR1), AKT serine/threonine kinase
1 (AKT1), and actin-related protein 2/3 complex subunit 1A
(ARPC1A)] were identified in the PPI network, which were associated
with >20 other types of genes. These hub genes were therefore
designated as the key target genes of miR-542-3p in OS (Table III).
 | Table III.Top ten protein-protein interaction
nodes according to STRING. |
Table III.
Top ten protein-protein interaction
nodes according to STRING.
| Node1 | Node2 | Experimentally
determined interaction | Database
annotated | Automated text
mining | Combined score |
|---|
| ACLY | OGDH | 0 | 0.536 | 0.795 | 0.999 |
| ATP6V1A | ATP6V1E1 | 0.932 | 0.9 | 0.826 | 0.999 |
| ATP6V1C1 | ATP6V1E1 | 0.921 | 0.9 | 0.829 | 0.999 |
| UPF2 | UPF1 | 0.965 | 0.9 | 0.98 | 0.999 |
| SEC61A1 | SEC61B | 0.895 | 0.9 | 0.87 | 0.999 |
| AP4E1 | AP4S1 | 0.922 | 0.9 | 0.87 | 0.999 |
| CALM1 | NOS3 | 0.98 | 0.8 | 0.919 | 0.999 |
| RPS27L | RPS23 | 0.973 | 0.9 | 0.144 | 0.999 |
| RANGAP1 | UBE2I | 0.973 | 0.9 | 0.921 | 0.999 |
| FOXO3 | AKT1 | 0.854 | 0.9 | 0.955 | 0.999 |
GO enrichment and KEGG pathway
analysis
GO enrichment was used to detect the role of the
1,036 target genes involved the biological process of OS. Three
categories were included in GO enrichment analysis: The biological
process (BP), cellular component (CC), and molecular function (MF)
categories. For BPs, the target genes were predominantly associated
with 10 GO terms, including ‘positive regulation of transcription,
DNA-templated’, ‘intracellular signal transduction’ and ‘positive
regulation of apoptotic process’. Regarding MFs, the target genes
were mainly enriched with 10 GO terms, including ‘protein binding’,
‘actin binding’ and ‘cadherin binding involved in cell-cell
adhesion’. For CCs, the target genes were also associated with 10
GO terms, including ‘cytoplasm’, ‘extracellular exosome’ and
‘cytosol’. The top ten most significant GO enrichment terms are
described in Table IV. The KEGG
results revealed the signaling pathways of the target genes of
miR-542-3p of particular interest, including the insulin
(P=1.04×10−5), PI3K-AKT (P=3.17×10−5), and
sphingolipid (P=3.91×10−5) signaling pathways.
Statistically significant pathways are described in Table V.
 | Table IV.GO functional annotation of
miR-542-3p target genes. |
Table IV.
GO functional annotation of
miR-542-3p target genes.
| A, Biological
process |
|---|
|
|---|
| GO ID | GO term | Count | % | P-value |
|---|
| GO:0006605 | Protein
targeting | 11 |
6.57×10−3 |
4.86×10−5 |
| GO:0035556 | Intracellular
signal transduction | 44 |
2.63×10−2 |
5.76×10−5 |
| GO:0043065 | Positive regulation
of apoptotic process | 35 |
2.09×10−2 |
1.05×10−4 |
| GO:0051056 | Regulation of small
GTPase mediated signal transduction | 20 |
1.20×10−2 |
2.15×10−4 |
| GO:0043401 | Steroid hormone
mediated signaling pathway | 12 |
7.17×10−3 |
3.23×10−4 |
| GO:0007050 | Cell cycle
arrest | 20 |
1.20×10−2 |
4.17×10−4 |
| GO:0008286 | Insulin receptor
signaling pathway | 14 |
8.37×10−3 |
4.35×10−4 |
| GO:0060348 | Bone
development | 10 |
5.98×10−3 |
4.99×10−4 |
| GO:0045893 | Positive regulation
of transcription, DNA-templated | 48 |
2.87×10−2 |
1.00×10−3 |
| GO:0060020 | Bergmann glial cell
differentiation | 5 |
2.99×10−3 |
1.05×10−3 |
|
| B, Molecular
function |
|
| GO ID | GO term | Count | % | P-value |
|
| GO:0003779 | Actin binding | 37 |
2.21×10−2 |
2.75×10−6 |
| GO:0051117 | ATPase binding | 15 |
8.96×10−3 |
5.72×10−5 |
| GO:0003707 | Steroid hormone
receptor activity | 12 |
7.17×10−3 |
2.49×10−4 |
| GO:0005516 | Calmodulin
binding | 23 |
1.37×10−2 |
1.01×10−3 |
| GO:0005158 | Insulin receptor
binding | 8 |
4.78×10−3 |
1.42×10−3 |
| GO:0051015 | Actin filament
binding | 17 |
1.02×10−2 |
3.11×10−3 |
| GO:0098641 | Cadherin binding
involved in cell-cell adhesion | 29 |
1.73×10−2 |
3.91×10−3 |
| GO:0008092 | Cytoskeletal
protein binding | 9 |
5.38×10−3 |
5.02×10−3 |
| GO:0004707 | MAP kinase
activity | 5 |
2.99×10−3 |
6.38×10−3 |
|
| C, Cellular
component |
|
| GO ID | GO term | Count | % | P-value |
|
| GO:0005829 | Cytosol | 255 |
1.52×10−1 |
1.01×10−9 |
| GO:0005925 | Focal adhesion | 52 |
3.11×10−2 |
5.35×10−9 |
| GO:0005737 | Cytoplasm | 362 |
2.16×10−1 |
2.13×10−8 |
| GO:0005789 | Endoplasmic
reticulum membrane | 83 |
4.96×10−2 |
4.33×10−7 |
| GO:0000139 | Golgi membrane | 61 |
3.65×10−2 |
2.11×10−6 |
| GO:0070062 | Extracellular
exosome | 206 |
1.23×10−1 |
2.66×10−6 |
| GO:0005856 | Cytoskeleton | 43 |
2.57×10−2 |
5.23×10−6 |
| GO:0043234 | Protein
complex | 44 |
2.63×10−2 |
3.04×10−5 |
| GO:0005794 | Golgi
apparatus | 76 |
4.54×10−2 |
3.40×10−5 |
 | Table V.Top 35 enriched KEGG pathway of
target genes. |
Table V.
Top 35 enriched KEGG pathway of
target genes.
| KEGG ID | KEGG term | Count | P-value |
|---|
| hsa04910 | Insulin signaling
pathway | 24 |
1.04×10−5 |
| hsa04151 | PI3K-Akt signaling
pathway | 42 |
3.17×10−5 |
| hsa04071 | Sphingolipid
signaling pathway | 21 |
3.91×10−5 |
| hsa04152 | AMPK signaling
pathway | 21 |
5.00×10−5 |
| hsa04510 | Focal adhesion | 29 |
5.95×10−5 |
| hsa04722 | Neurotrophin
signaling pathway | 20 |
1.24×10−4 |
| hsa05231 | Choline metabolism
in cancer | 18 |
1.31×10−4 |
| hsa05200 | Pathways in
cancer | 44 |
1.43×10−4 |
| hsa04150 | mTOR signaling
pathway | 13 |
1.75×10−4 |
| hsa04919 | Thyroid hormone
signaling pathway | 19 |
1.94×10−4 |
| hsa04022 | cGMP-PKG signaling
pathway | 23 |
5.23×10−4 |
| hsa05212 | Pancreatic
cancer | 13 |
5.34×10−4 |
| hsa04068 | FoxO signaling
pathway | 20 |
5.35×10−4 |
| hsa05205 | Proteoglycans in
cancer | 26 |
5.46×10−4 |
| hsa04931 | Insulin
resistance | 17 |
8.94×10−4 |
| hsa04611 | Platelet
activation | 19 |
9.89×10−4 |
| hsa05213 | Endometrial
cancer | 11 |
1.11×10−3 |
| hsa05160 | Hepatitis C | 19 |
1.29×10−3 |
| hsa04141 | Protein processing
in endoplasmic reticulum | 22 |
1.61×10−3 |
| hsa05131 | Shigellosis | 12 |
1.67×10−3 |
| hsa05221 | Acute myeloid
leukemia | 11 |
1.99×10−3 |
| hsa00512 | Mucin type O-Glycan
biosynthesis | 8 |
2.35×10−3 |
| hsa05120 | Epithelial cell
signaling in Helicobacter pylori infection | 12 |
2.44×10−3 |
| hsa05215 | Prostate
cancer | 14 |
2.72×10−3 |
| hsa04664 | Fc epsilon RI
signaling pathway | 12 |
2.75×10−3 |
| hsa04261 | Adrenergic
signaling in cardiomyocytes | 19 |
3.69×10−3 |
| hsa05218 | Melanoma | 12 |
3.89×10−3 |
| hsa05210 | Colorectal
cancer | 11 |
4.33×10−3 |
| hsa05211 | Renal cell
carcinoma | 11 |
6.11×10−3 |
| hsa04014 | Ras signaling
pathway | 25 |
6.27×10−3 |
| hsa04380 | Osteoclast
differentiation | 17 |
6.59×10−3 |
| hsa05223 | Non-small cell lung
cancer | 10 |
6.82×10−3 |
| hsa04012 | ErbB signaling
pathway | 13 |
6.86×10−3 |
| hsa04066 | HIF-1 signaling
pathway | 14 |
6.96×10−3 |
| hsa04915 | Estrogen signaling
pathway | 14 |
7.58×10−3 |
Discussion
Although miR-542-3p was demonstrated to be
overexpressed in OS, promoting the proliferation and migration of
OS (13), the number of samples
used to evaluate miR-542-3p in OS and noncancerous tissues was
relatively small, and the molecular mechanisms underlying the role
of miR-542-3p in OS remain to be fully elucidated. In the present
study, the upregulation of miR-542-3p was verified through
continuous variables, and the heterogeneity test revealed slight
heterogeneity (I2<50%). The diagnostic ability of
miR-542-3p in OS was investigated by constructing the SROC curve.
The area under the curve value of the SROC curve indicated that
miR-542-3p may serve as a putative diagnostic target for OS.
However, the number of qualifying studies and the sample size of
the present study remain a limiting factor, and further studies
involving larger sample sizes are required to better evaluate the
diagnostic capacity of miR-542-3p in OS.
The present study revealed that miR-542-3p was
overexpressed in OS. Li et al (13) also demonstrated that miR-542-3p is
highly expressed in OS, and carcinogenicity was increased via
targeting of VANGL2. However, lower expression levels of miR-542-3p
were identified in numerous other tumor types in previous studies
(15–17), and miR-542-3p was reported to exert
a tumor-suppressing effect, contrary to what was identified in the
present study. Liu et al (16) reported that miR-542-3p is
downregulated in non-small cell lung cancer cells, and functions as
a tumor suppressor via upregulation of the mitochondrial protein,
mitochondrial rRNA methyltransferase 2. Tao et al (17) demonstrated that miR-542-3p is
downregulated in hepatocellular carcinoma tissues, and is a tumor
suppressor gene that regulates hepatoma metastasis and
epithelialization by targeting ubiquitin protein ligase E3C. Rang
et al (18) demonstrated
that miR-542-3p is underexpressed in melanoma, and inhibits
cellular invasion and metastasis through its action on PIM1
proto-oncogene, serine/threonine kinase. Considered altogether, the
previous reports support the notion that overexpression of
miR-542-3p may be used as a target for the diagnosis and therapy of
OS, although the contrasting results obtained from the various
studies of the expression of miR-542-3p, and its role in different
types of tumors, merit further study.
Subsequently, 1,035 downregulated target genes were
screened from the gene chip of OS and bioinformatics prediction
software, also including OS cell line expression profiles with
transfected miR-542-3p.
Due to the mutation and methylation of genes,
differential expression of genes may arise. High-throughput
technology has emerged as an effective method of detecting the
expression levels of genes across the entire genome, and this
process serves a vital role in identifying abnormal genomic
alterations. As a consequence, an increasing number of DEGs
associated with OS have been identified through the application of
this technology (19,20). Compared with the simple prediction
of target genes of miR-542-3p in OS via predictive software, to
have combined these data with DEGs from the OS expression chips has
undoubtedly resulted in increased specificity. OS cell line
expression profiles with transfected miR-542-3p are typically used
to simulate the expression of miR-542-3p in OS, in order to
identify upregulated or downregulated expression of target genes,
with the purpose of investigating the potential role of miR-542-3p,
and taking this approach has provided more convincing results in
the present study.
Using the STRING database to identify hub genes,
eight loci were eventually screened out: UBA52, RAC1, MAPK1, EGFR,
CFTR, PIK3R1, AKT1 and ARPC1A. These genes are likely to be key
target genes of miR-542-3p in OS. More detailed information on
these hub genes in OS was subsequently sought in the present
study.
RAC1 is a small GTPase, which acts as a molecular
switch controlling multiple signaling pathways (21). Geng et al (22) identified RAC1 as a direct target
gene of miR-224 in OS, and the authors of that study considered
that RAC1 to be an oncogene of OS that is associated with the
sensitivity of OS to cisplatin. Tan et al (23) demonstrated that chitosan promoted
the apoptosis of OS cells by downregulating RAC1.
In a bioinformatics study, Li et al (24) predicted that the MAPK1 gene is the
most important gene associated with OS. Their findings were
consistent with the results of the present study; however, the role
of this gene in OS is yet to be verified experimentally.
EGFR is a receptor tyrosine kinase that is
associated with the pathogenesis of numerous types of cancer
(25), modulating the growth,
signaling, differentiation, adhesion, migration and survival of
cancer cells (26). Hou et
al (27) considered that the
interaction between transforming growth factor-α and EGFR triggers
the activation of PI3K and AKT, which in turn activates nuclear
factor-κB, leading to the expression of intercellular adhesion
molecule 1 and the promotion of the migration of human OS cells.
Zhang et al (28)
demonstrated that toosendanin (a triterpenoid extracted from
Chinese traditional medicine) inhibited OS by blocking signal
transducer and activator of transcription 3 (STAT3) dimerization
and impairing the formation of the complex between STAT3 and
EGFR.
AKT, also referred to as protein kinase B, exerts
numerous roles vital to human physiology and pathology. Counted
among its family members are AKT1/PKBα, AKT2/PKBβ, and AKT3/PKBγ
(29). Zhu et al (30) demonstrated that the Thr308Ala
mutation in AKT1 was able to enhance the cytotoxicity of OS cells
induced by the mechanistic target of rapamycin kinase (mTOR)
inhibitor, XL388. Han et al (31) reported that tissue inhibitors of
metalloproteinase inhibit OS by downregulating AKT1.
Zucchini et al (32) demonstrated that ARPC1A is a
molecule that serves a crucial role in the remodeling of the actin
cytoskeleton, and CD99 wt (the full-length protein of CD99, a
transmembrane protein encoded by the MIC2 gene that is involved in
multiple cellular events, including cell adhesion and migration) is
able to inhibit OS by inhibiting ARPC1A.
Siegel et al (33) identified PIK3R1 as a gene
associated with vascular anomalies, forming a link between
cancer-associated variants and previously described somatic cell
variants of vascular overgrowth syndrome, and the authors of that
study hypothesized that PIK3R may be associated with tumors.
However, the underlying mechanism of PIK3R in OS requires further
investigation, and the other hub genes, including UBA52 and CFTR,
have been rarely reported in OS; therefore, similarly, further
studies are required to elucidate their underlying mechanisms in
OS.
The results of the GO classification enrichment
analysis in the present study led to an identification of the
possible molecular mechanisms associated with target genes of
miR-542-3p in OS, and the GO terms included ‘bone development’,
‘cell cycle arrest’ and ‘intracellular signal transduction’. Jiang
et al (34) confirmed that
transmembrane protein 119 contributes to bone development, in
addition to the proliferation of OS cells, via the induction of
cell cycle arrest at the G0/G1 phase and
apoptosis. Liu et al (35)
confirmed that nuclear receptor binding SET domain protein 3 (NSD3)
inhibited intracellular signal transduction via the downregulation
of a number of genes that had been explored in their previous work,
and therefore NSD3 may serve as a potential molecular target for OS
therapy. The results of the KEGG pathway analysis in the present
study also showed that the target genes of miR-542-3p serve vital
roles in OS. Our hypothesis was that the target genes of miR-542-3p
may be associated with a number of signaling pathways in order to
influence the occurrence and development of OS. Among the 35
significant signaling pathways, the forkhead box O (FOXO),
AMP-activated protein kinase (AMPK) and PI3K-Akt signaling pathways
have been closely associated with human cancer. FOXO proteins are a
subgroup of the FOX superfamily of transcription factors that are
able to antagonistically affect the action of insulin and trigger
tumor inhibition (36). Evidence
has also been published to suggest that inhibiting FOXO1 may
promote cell proliferation, enhance colony formation and lead to
weak osteogenic differentiation in OS cell lines (37). AMPK is an evolutionarily conserved
cellular energy sensor (38).
Activation of AMPK inhibits the growth of liver cancer cells and
SW620 colorectal cancer cells, in addition to OS cells (39–41).
PI3K is an upstream regulator of mTOR, and activation of the AMPK
signaling pathway has been demonstrated to be responsible for the
transformation of OS cells and the poor prognosis of patients with
OS (42). In the present study, a
profound enrichment of pathways regulated by osteoclast
differentiation was also identified, and this phenomenon is one of
the molecular mechanisms that may be involved in the overexpression
of miR-542-3p in OS (43).
An increasing amount of evidence has demonstrated
that the hub genes of miR-542-3p are directly or indirectly able to
regulate the development, occurrence, prognosis, diagnosis and
treatment of OS. However, one limitation of the present study was
that the association between miR-542-3p and its hub genes was not
verified on an experimental level. A large number of these hub
genes have been rarely reported to be associated with miR-542-3p in
OS. The predicted hub genes identified on the basis of the PPI
analysis require further verification via in vivo and in
vitro experiments in the future.
In conclusion, the present study confirmed that
miR-542-3p is highly expressed in OS, and serves as a tumor
promoter. miR-542-3p exerted its role in promoting OS by regulating
target gene networks via specific signaling pathways. The potential
target genes and biological functions of miR-542-3p provide novel
insights into the DEGs of OS, and miR-542-3p may therefore be a
novel target for the early diagnosis and treatment of OS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560371).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL, JNY, WTH, RQH and JM performed the literature
searches, data extraction and statistical analyses, and drafted the
paper. QJW and GC supervised the literature searches, data
extraction and analyses, and reviewed the paper. All authors
confirm that they have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venkatramani R, Murray J, Helman L, Meyer
W, Hicks MJ, Krance R, Lau C, Jo E and Chintagumpala M: Risk-based
therapy for localized osteosarcoma. Pediatr Blood Cancer.
63:412–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Wang Z and Gemeinhart RA:
Progress in microRNA delivery. J Control Release. 172:962–974.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lages E, Ipas H, Guttin A, Nesr H, Berger
F and Issartel JP: MicroRNAs: Molecular features and role in
cancer. Front Biosci (Landmark Ed). 17:2508–2540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Connett P: Water fluoridation: Is fluoride
chemophobia? Br Dent J. 222:323–324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Zhang J, Zhang L, Si M, Yin H and Li
J: Diallyl trisulfide inhibits proliferation, invasion and
angiogenesis of osteosarcoma cells by switching on suppressor
microRNAs and inactivating of Notch-1 signaling. Carcinogenesis.
34:1601–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Althoff K, Lindner S, Odersky A, Mestdagh
P, Beckers A, Karczewski S, Molenaar JJ, Bohrer A, Knauer S,
Speleman F, et al: miR-542-3p exerts tumor suppressive functions in
neuroblastoma by downregulating survivin. Int J Cancer.
136:1308–1320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai J, Zhao J, Zhang N, Xu X, Li R, Yi Y,
Fang L, Zhang L, Li M, Wu J and Zhang H: MicroRNA-542-3p suppresses
tumor cell invasion via targeting AKT pathway in human astrocytoma.
J Biol Chem. 290:24678–24688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Wang S, Han F, Li J, Yu L, Zhou
P, Chen Z, Xue S, Dai C and Li Q: MicroRNA-542-3p suppresses
cellular proliferation of bladder cancer cells through
post-transcriptionally regulating survivin. Gene. 579:146–152.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen X, Si Y, Yang Z, Wang Q, Yuan J and
Zhang X: MicroRNA-542-3p suppresses cell growth of gastric cancer
cells via targeting oncogene astrocyte-elevated gene-1. Med Oncol.
32:3612015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Liu H, Pei J, Wang H and Lv H:
miR-542-3p overexpression is associated with enhanced osteosarcoma
cell proliferation and migration ability by targeting Van Gogh-like
2. Mol Med Rep. 11:851–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Huang JW, Castella M, Huntsman DG
and Taniguchi T: p53 is positively regulated by miR-542-3p. Cancer
Res. 74:3218–3227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sterne JA, Sutton AJ, Ioannidis JP, Terrin
N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH,
et al: Recommendations for examining and interpreting funnel plot
asymmetry in meta-analyses of randomised controlled trials. BMJ.
343:d40022011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu B, Li J, Zheng M, Ge J, Li J and Yu P:
MiR-542-3p exerts tumor suppressive functions in non-small cell
lung cancer cells by upregulating FTSJ2. Life Sci. 188:87–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao J, Liu Z, Wang Y, Wang L, Yao B, Li Q,
Wang C, Tu K and Liu Q: MiR-542-3p inhibits metastasis and
epithelial-mesenchymal transition of hepatocellular carcinoma by
targeting UBE3C. Biomed Pharmacother. 93:420–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rang Z, Yang G, Wang YW and Cui F:
miR-542-3p suppresses invasion and metastasis by targeting the
proto-oncogene serine/threonine protein kinase, PIM1, in melanoma.
Biochem Biophys Res Commun. 474:315–320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salinas-Souza C, De Andrea C, Bihl M,
Kovac M, Pillay N, Forshew T, Gutteridge A, Ye H, Amary MF,
Tirabosco R, et al: GNAS mutations are not detected in parosteal
and low-grade central osteosarcomas. Mod Pathol. 28:1336–1342.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Z, Chen Y, Fu Y, Yang Y, Zhang Y,
Chen Y and Li D: Meta-analysis of differentially expressed genes in
osteosarcoma based on gene expression data. BMC Med Genet.
15:802014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seong BK, Lau J, Adderley T, Kee L,
Chaukos D, Pienkowska M, Malkin D, Thorner P and Irwin MS: SATB2
enhances migration and invasion in osteosarcoma by regulating genes
involved in cytoskeletal organization. Oncogene. 34:3582–3592.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Geng S, Gu L, Ju F, Zhang H, Wang Y, Tang
H, Bi Z and Yang C: MicroRNA-224 promotes the sensitivity of
osteosarcoma cells to cisplatin by targeting Rac1. J Cell Mol Med.
20:1611–1619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan ML, Shao P, Friedhuber AM, van Moorst
M, Elahy M, Indumathy S, Dunstan DE, Wei Y and Dass CR: The
potential role of free chitosan in bone trauma and bone cancer
management. Biomaterials. 35:7828–7838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, He Y, Hao P and Liu P:
Identification of characteristic gene modules of osteosarcoma using
bioinformatics analysis indicates the possible molecular
pathogenesis. Mol Med Rep. 15:2113–2119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kovacs E, Zorn JA, Huang Y, Barros T and
Kuriyan J: A structural perspective on the regulation of the
epidermal growth factor receptor. Annu Rev Biochem. 84:739–764.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biscaglia F, Rajendran S, Conflitti P,
Benna C, Sommaggio R, Litti L, Mocellin S, Bocchinfuso G, Rosato A,
Palleschi A, et al: Enhanced EGFR targeting activity of plasmonic
nanostructures with engineered GE11 peptide. Adv Healthc Mater.
6:2017.doi: 10.1002/adhm.201700596. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou CH, Lin FL, Tong KB, Hou SM and Liu
JF: Transforming growth factor alpha promotes osteosarcoma
metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochem
Pharmacol. 89:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang T, Li J, Yin F, Lin B, Wang Z, Xu J,
Wang H, Zuo D, Wang G, Hua Y and Cai Z: Toosendanin demonstrates
promising antitumor efficacy in osteosarcoma by targeting STAT3.
Oncogene. 36:6627–6639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang F, Wang Y, Hemmings BA, Rüegg C and
Xue G: PKB/Akt-dependent regulation of inflammation in cancer.
Semin Cancer Biol. 48:62–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu YR, Zhou XZ, Zhu LQ, Yao C, Fang JF,
Zhou F, Deng XW and Zhang YQ: The anti-cancer activity of the
mTORC1/2 dual inhibitor XL388 in preclinical osteosarcoma models.
Oncotarget. 7:49527–49538. 2016.PubMed/NCBI
|
|
31
|
Han XG, Li Y, Mo HM, Li K, Lin D, Zhao CQ,
Zhao J and Tang TT: TIMP3 regulates osteosarcoma cell migration,
invasion, and chemotherapeutic resistances. Tumour Biol.
37:8857–8867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zucchini C, Manara MC, Pinca RS, De
Sanctis P, Guerzoni C, Sciandra M, Lollini PL, Cenacchi G, Picci P,
Valvassori L and Scotlandi K: CD99 suppresses osteosarcoma cell
migration through inhibition of ROCK2 activity. Oncogene.
33:1912–1921. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siegel DH, Cottrell CE, Streicher JL,
Schilter KF, Basel DG, Baselga E, Burrows PE, Ciliberto HM,
Vigh-Conrad KA, Eichenfield LF, et al: Analyzing the genetic
spectrum of vascular anomalies with overgrowth via cancer genomics.
J Invest Dermatol. 138:957–967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang ZH, Peng J, Yang HL, Fu XL, Wang JZ,
Liu L, Jiang JN, Tan YF and Ge ZJ: Upregulation and biological
function of transmembrane protein 119 in osteosarcoma. Exp Mol Med.
49:e3292017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Z, Piao L, Zhuang M, Qiu X, Xu X,
Zhang D, Liu M and Ren D: Silencing of histone methyltransferase
NSD3 reduces cell viability in osteosarcoma with induction of
apoptosis. Oncol Rep. 38:2796–2802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guzman-Perez V, Bumke-Vogt C, Schreiner M,
Mewis I, Borchert A and Pfeiffer AF: Benzylglucosinolate derived
isothiocyanate from tropaeolum majus reduces gluconeogenic gene and
protein expression in human cells. PLoS One. 11:e01623972016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guan H, Tan P, Xie L, Mi B, Fang Z, Li J,
Yue J, Liao H and Li F: FOXO1 inhibits osteosarcoma oncogenesis via
Wnt/β-catenin pathway suppression. Oncogenesis. 4:e1662015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dasgupta B and Chhipa RR: Evolving lessons
on the complex role of AMPK in normal physiology and cancer. Trends
Pharmacol Sci. 37:192–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng L, Yang W, Wu F, Wang C, Yu L, Tang
L, Qiu B, Li Y, Guo L, Wu M, et al: Prognostic significance of AMPK
activation and therapeutic effects of metformin in hepatocellular
carcinoma. Clin Cancer Res. 19:5372–5380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cho SY, Lee HJ, Lee HJ, Jung DB, Kim H,
Sohn EJ, Kim B, Jung JH, Kwon BM and Kim SH: Activation of
AMP-activated protein kinase α and extracelluar signal-regulated
kinase mediates CB-PIC-induced apoptosis in hypoxic SW620
colorectal cancer cells. Evid Based Complement Alternat Med.
2013:9743132013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao Z, Yin JQ, Wu MS, Song G, Xie XB, Zou
C, Tang Q, Wu Y, Lu J, Wang Y, et al: Dihydromyricetin activates
AMP-activated protein kinase and P38(MAPK) exerting antitumor
potential in osteosarcoma. Cancer Prev Res (Phila). 7:927–938.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu K, Dai HB and Qiu ZL: mTOR signaling in
osteosarcoma: Oncogenesis and therapeutic aspects (Review). Oncol
Rep. 36:1219–1225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kureel J, Dixit M, Tyagi AM, Mansoori MN,
Srivastava K, Raghuvanshi A, Maurya R, Trivedi R, Goel A and Singh
D: miR-542-3p suppresses osteoblast cell proliferation and
differentiation, targets BMP-7 signaling and inhibits bone
formation. Cell Death Dis. 5:e10502014. View Article : Google Scholar : PubMed/NCBI
|