Introduction
Dihydromyricetin, also known as ampelopsin, is a
dihydroflavonol flavonoid that is found in ampelopsis, and its
content in vine tea can reach up to 30% (1). It is also distributed in Myricaceae,
Guttiferae, Euphorbiaceae, Burseraceae, Leguminosae, Sapotaceae and
Clethraceae plants (2). Previous
studies have verified that dihydromyricetin possesses multiple
pharmacological effects, including antitumor, anti-inflammation,
anti-oxidation, anti-alcohol, liver protection, anti-pathogen and
blood lipid regulation activities (3). In addition, dihydromyricetin
possesses bioactivities, such as an anti-hypertension effect,
thrombosis suppression in vivo and hypoglycemic action
(2).
The toxic effect of alcohol affects the vital organs
of the body, particularly the liver. It has been reported that the
overall and per capita alcohol consumption have been increasing
(4). Therefore, the harmful
effects of alcohol on the human body, and more specifically on the
liver, have attracted increasing attention (5). Alcoholic liver disease (ALD) is a
liver disease induced by long-term heavy drinking (5). Generally, fatty liver is the initial
manifestation of ALD (6), which
can then progress into alcoholic hepatitis and alcoholic fibrosis
as alcohol consumption continues (6), finally leading to alcoholic cirrhosis
and hepatocellular carcinoma. In the presence of alcohol
stimulation, excessive lipid input results in liver lipoprotein
synthesis disorder and insufficient fatty acid oxidation. As a
result, the fat is deposited in the liver, thus giving rise to
fatty liver (6).
Nuclear factor (NF)-κB is a common nuclear
transcription factor in cells, with p50/p65 as the heterodimer
form. NF-κB at an inactivated state can bind with the IkB inhibitor
(7). It is involved in immune and
inflammatory reactions, in the pathological and acute stage
response of certain diseases, and in responses to cell
proliferation and viral infection (7). In addition, NF-κB is widely involved
in gene transcription and apoptosis (7); therefore, it has a relatively high
expression in numerous diseases. Studies have suggested that NF-κB
is a promising novel target of anti-inflammatory treatment.
Therefore, the present study aimed to determine the effect of
dihydromyricetin on fatty liver and its possible mechanism.
Materials and methods
Experimental animals
The study was conducted in accordance with the
Guidelines for Animal Experimentation and approval obtained by the
ethics committee of the Sixth People's Hospital of Qingdao
(Qingdao, China). Male Sprague-Dawley rats (weight, 170±20 g; age,
4–5 weeks) were obtained from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The rats were maintained at
22–23°C, 55–60% humidity and a 12/12-h light/dark cycle, and
provided with certified standard chow and tap water ad
libitum. Rats were randomly assigned to three groups, including
the normal (n=6), model (n=7) and dihydromyricetin groups (n=7). In
the model and dihydromyricetin groups, rats were fed with a
high-fat diet (36.5% fat, 44.6% carbohydrate and 18.9% protein) for
12 weeks. In the dihydromyricetin group, rats were intravenously
given 2 mg/kg dihydromyricetin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) every 3 days for 12 weeks as previously
described (8).
Histological assessment
Following treatment with dihydromyricetin for 12
weeks, rats were sacrificed using decollation under 35 mg/kg
pentobarbital, and liver tissue samples were collected. The tissues
were fixed with 4% paraformaldehyde for 24 h, embedded in paraffin
wax and then cut into 3-µM sections. Subsequently, the sections
were stained with Oil Red O for 5 min and examined under a BX51
microscope (Olympus Corporation, Tokyo, Japan).
Enzyme-linked immunosorbent assay
(ELISA)
Following treatment with dihydromyricetin, liver
tissue samples were collected and homogenized by centrifugation at
18,000 × g for 10 min at 4°C. The supernatant was used to measure
the triglyceride (F001), albumin (ALB; A028-1), collagen I (H142),
alanine aminotransferase (ALT; C009-2), aspartate aminotransferase
(AST; C010-2), malondialdehyde (MDA; A003-1), superoxide dismutase
(SOD; A001-3), glutathione (GSH; A006-2), glutathione peroxidase
(GSH-Px; A005), tumor necrosis factor-α (TNF-α; H052), interleukin
(IL)-1β (H002), IL-6 (H007), IL-18 (H015), caspase-3 (G015),
caspase-8 (G017) and caspase-9 (G018), and inducible nitric oxide
synthase (iNOS, A014-1-1) levels with the corresponding ELISA kits
(Nanjing Jiancheng Biology Engineering Institute, Nanjing,
China).
Western blotting
Subsequent to treatment of rats with
dihydromyricetin, total protein was extracted from frozen liver
tissue using a radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China), and the protein
concentration was determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China).
Next, 50-µg protein samples were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred
onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica,
MA, USA). The membrane was blocked with 5% non-fat milk in TBST for
1 h at 37°C, followed by overnight incubation at 4°C with the
following primary antibodies: ALB (sc-271604; 1:2,000), collagen I
(sc-8786; 1:1,000), peroxisome proliferator-activated receptor α
(PPARα; sc-1982; 1:1,000), NF-κB (sc-71675; 1:1,000), p53
(sc-47698; 1:1,000), B-cell lymphoma 2-associated X protein (Bax;
sc-6236; 1:1,000) and GAPDH (sc-51631; 1:5,000; all purchased from
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Membranes were
then incubated with horseradish peroxidase-conjugated goat
anti-rabbit and anti-rat IgG secondary antibodies (sc-2004 and
sc-2005; 1:5,000; Santa Cruz Biotechnology, Inc.) at 37°C for 1
h.
Statistical analysis
The data were expressed as the mean ± standard
deviation using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). All data
were analyzed by one-way analysis of variance, followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Dihydromyricetin treatment prevents
the development of fatty liver in a rat model
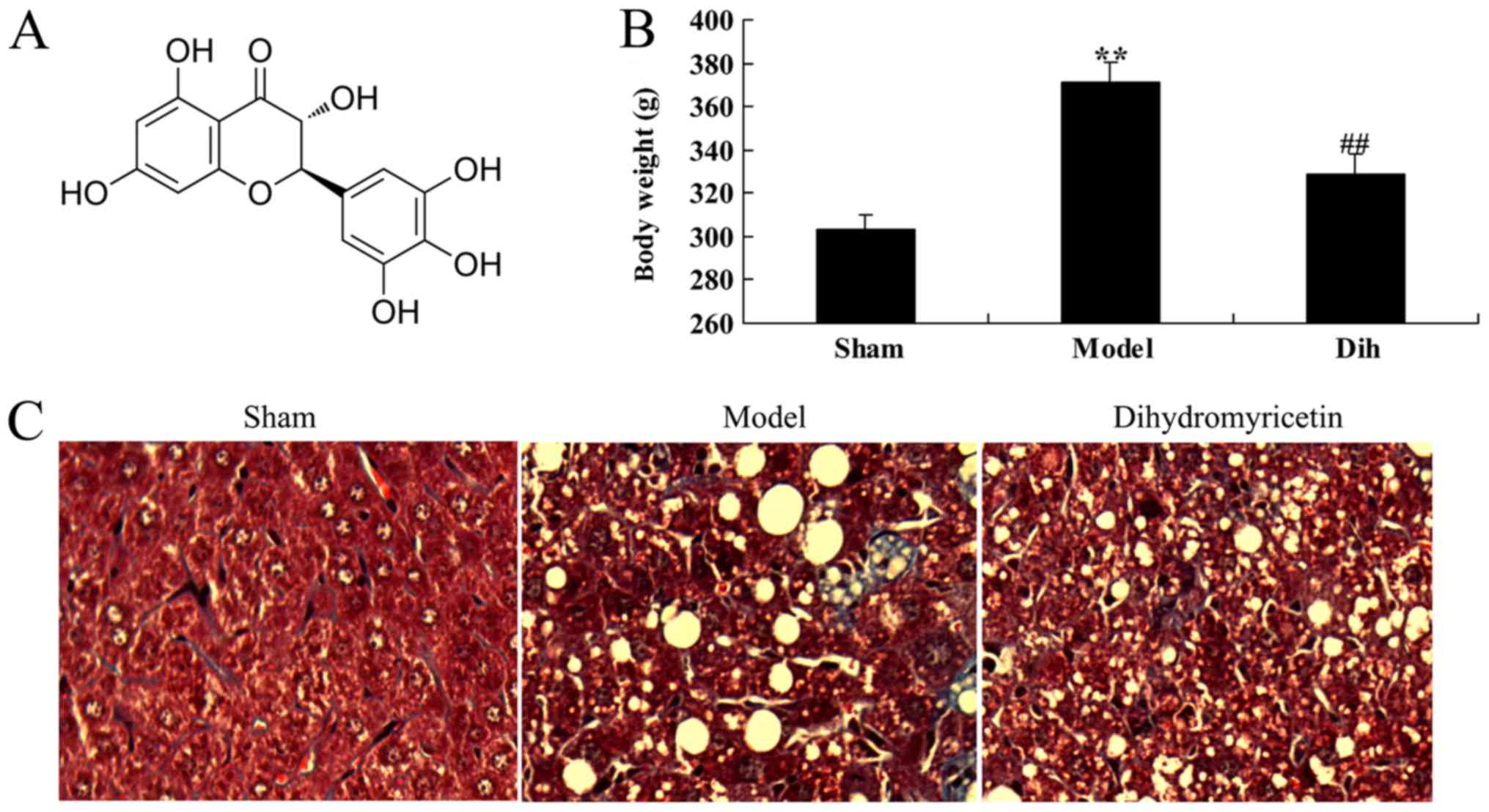
The chemical structure of dihydromyricetin is shown
in Fig. 1A. First, the effects of
dihydromyricetin on preventing fatty liver development were
assessed in a rat model. As a result of a high-fat diet, the body
weight was increased in the rat model of fatty liver as compared
with that in the normal control group (Fig. 1B). Oil Red O staining of liver
sections revealed steatosis in the liver tissues of rat in the
fatty liver model group, which was absent in the normal control
group tissues (Fig. 1C). However,
treatment with dihydromyricetin markedly reduced the body weight
and liver steatosis determined by Oil Red O staining in the fatty
liver rat model, as compared with the untreated model group
(Fig. 1B and C).
Dihydromyricetin treatment prevents
liver function deterioration in a fatty liver rat model
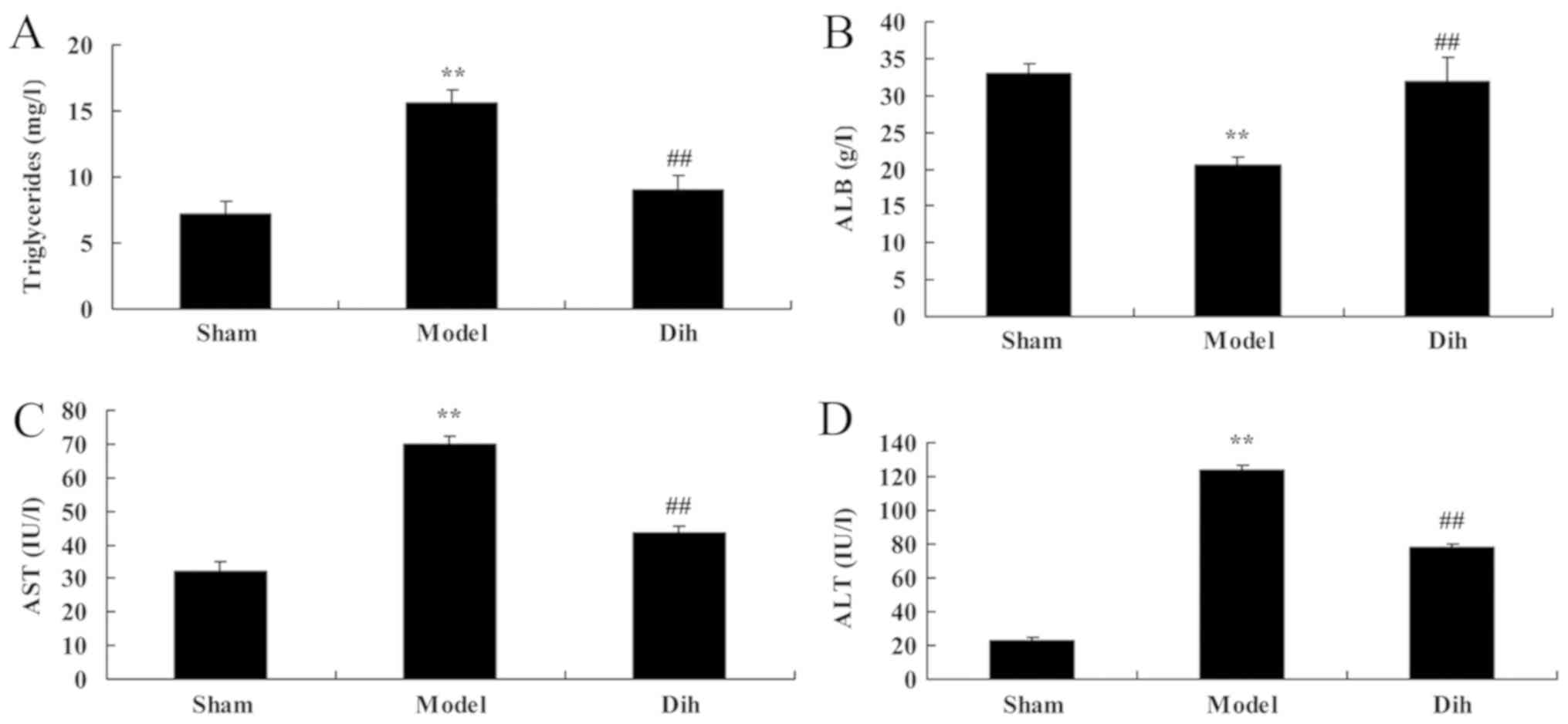
The levels of triglycerides, ALB, ALT and AST were
decreased in the rat model of fatty liver, as compared with those
in the normal control group (Fig.
2). By contrast, dihydromyricetin administration in the fatty
liver rat model significantly increased the levels of
triglycerides, ALB, ALT and AST as compared with those in the
untreated model group, and indicating the inhibition of liver
steatosis in rats (Fig. 2).
Dihydromyricetin treatment inhibits
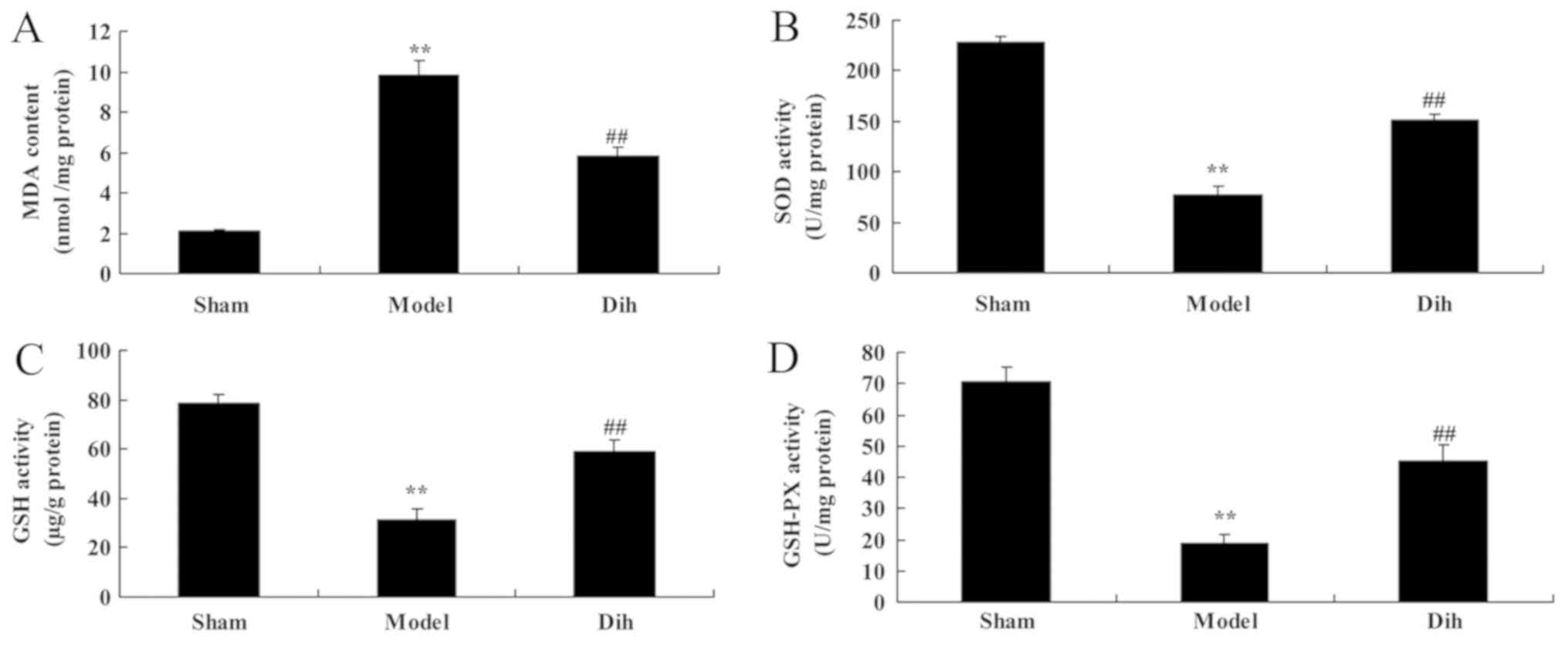
oxidative stress in a fatty liver rat model
In rat of the fatty liver model group, the MDA level
was increased, while the levels of SOD, GSH and GSH-Px were
decreased, as compared with those in the normal control group
(Fig. 3). However, treatment with
dihydromyricetin significantly reduced the MDA level, and
significantly increased SOD, GSH and GSH-Px levels, as compared
with those of rat in the fatty liver model group without
dihydromyricetin administration (Fig.
3).
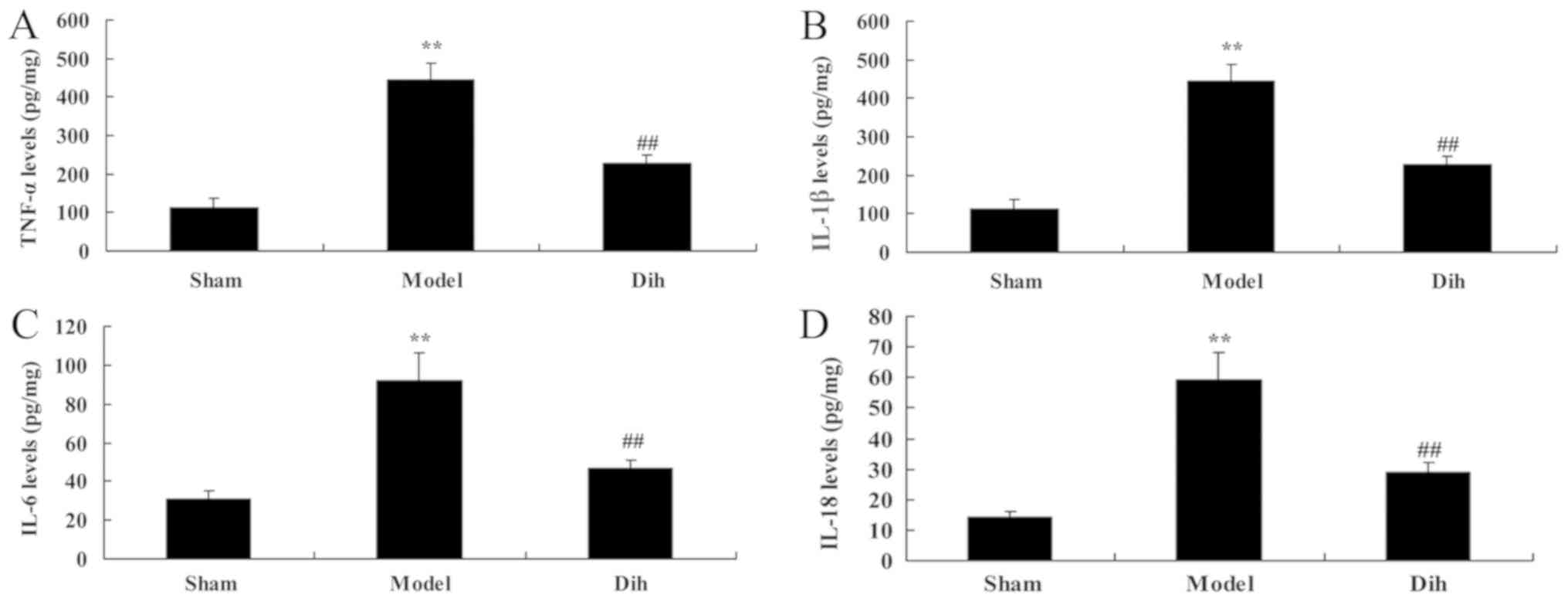
Dihydromyricetin treatment inhibits
inflammation in a fatty liver rat model
Next, the anti-inflammation effects of
dihydromyricetin in rat model of fatty liver were assessed. As
shown in Fig. 4, the levels of
TNF-α, IL-1β, IL-6 and IL-18 were increased in the rat model of
fatty liver, compared with the normal control group (Fig. 4). However, dihydromyricetin
treatment in the rat model of fatty liver reduced the levels of
TNF-α, IL-1β, IL-6 and IL-18 levels, in comparison with those in
model rat without dihydromyricetin administration (Fig. 4).
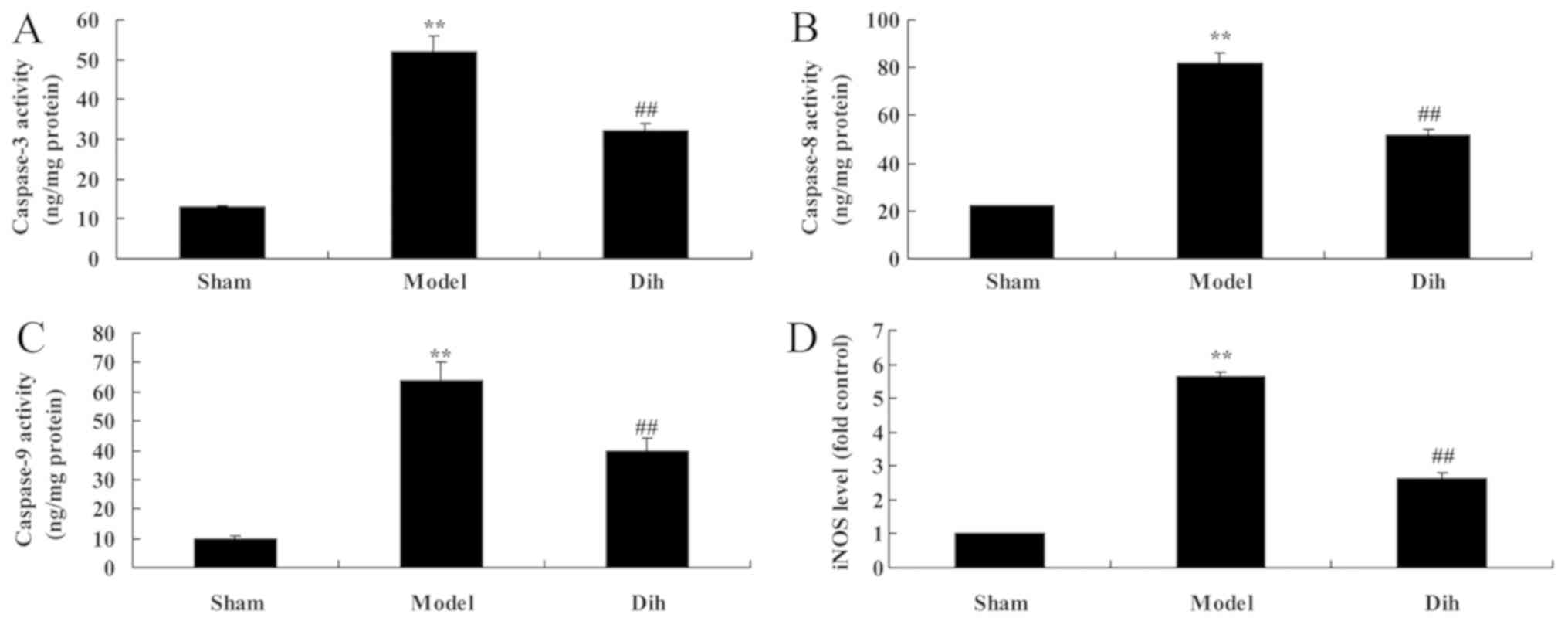
Dihydromyricetin inhibits hepatic cell
apoptosis in a rat model of fatty liver
The anti-apoptotic effects of dihydromyricetin in
the rat model of fatty liver were further evaluated. As shown in
Fig. 5, the activities of
caspase-3, caspase-8 and caspase-9, and inducible nitric oxide
synthase (iNOS) levels were significantly induced in the rat model
of fatty liver, compared with the normal control group. By
contrast, treatment with dihydromyricetin in the rat model of fatty
liver markedly reduced the activities of caspase-3, caspace-8 and
caspase-9, as well as iNOS protein expression, as compared with the
levels in untreated model rat (Fig.
5).
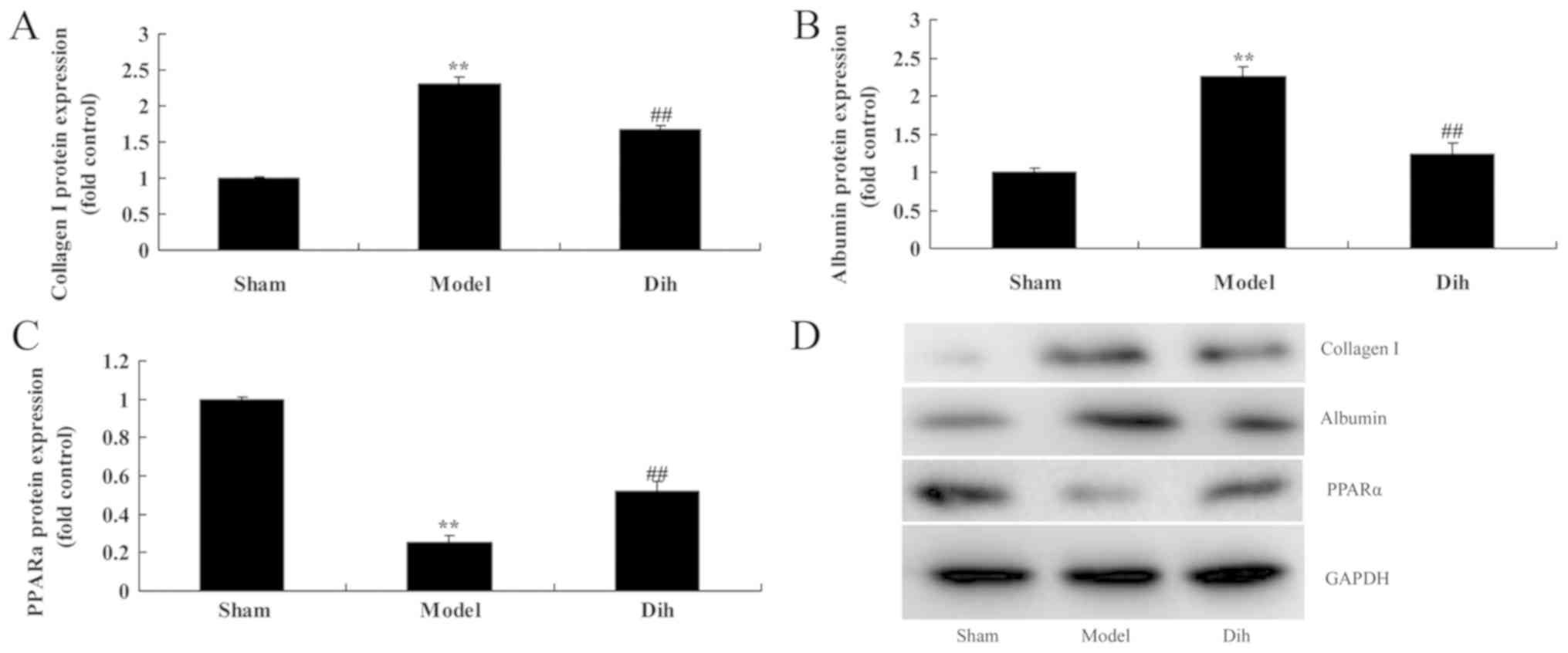
Dihydromyricetin decreases the protein
expression levels of Albumin and collagen I, and induced PPARα
protein expression in the rat model
Subsequently, the current study aimed to investigate
the mechanism of dihydromyricetin in preventing fatty liver
development in a rat model. The expression levels of Albumin and
collagen I were markedly induced, and PPARα protein expression was
suppressed in the rat model of fatty liver, compared with normal
control group (Fig. 6). However,
dihydromyricetin administration in the fatty liver rat model
significantly suppressed the protein levels of ALB and collagen I,
and induced PPARα protein expression in comparison with those in
the untreated model group (Fig.
6).
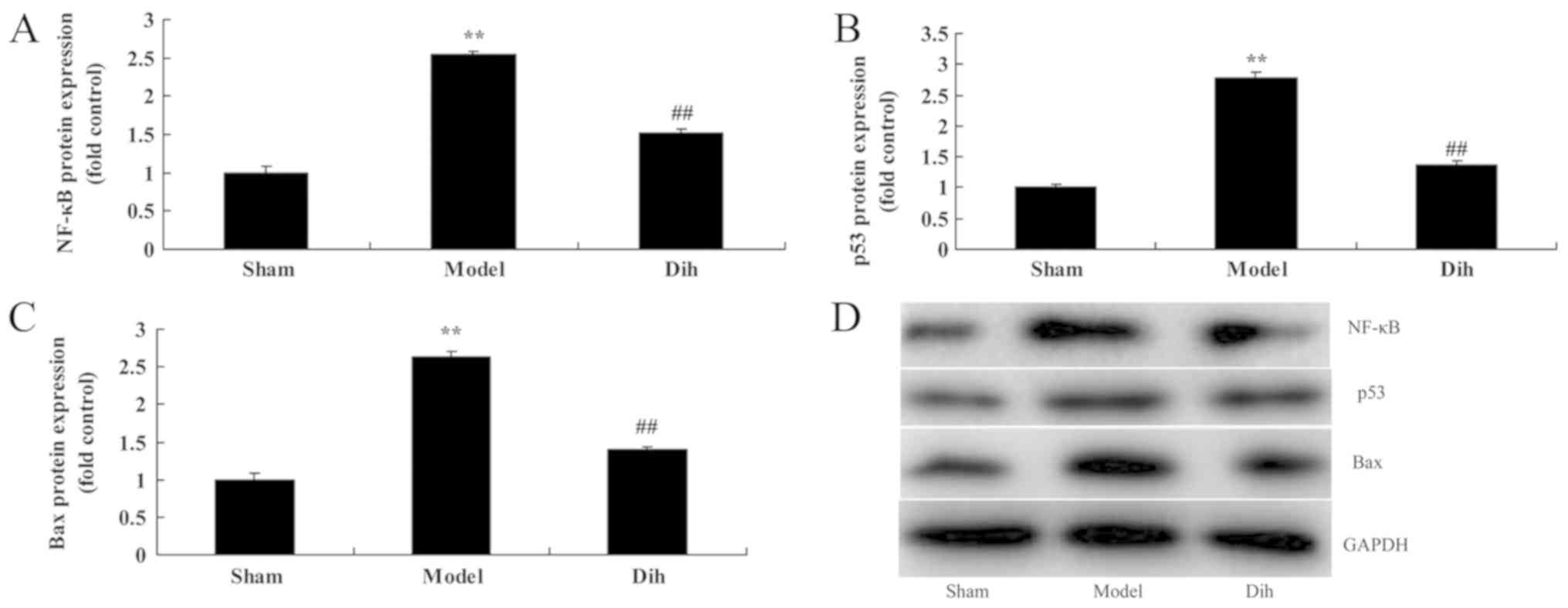
Dihydromyricetin suppresses the
protein levels of NF-κB, p53 and Bax in the rat model
As shown in Fig. 7,
the protein expression levels of NF-κB, p53 and Bax were
significantly enhanced in the rat model of fatty liver, compared
with the normal control group (Fig.
7). However, dihydromyricetin treatment in fatty liver model
rat evidently suppressed the protein expression levels of NF-κB,
p53 and Bax when compared with the model rat without
dihydromyricetin administration (Fig.
7). These results showed that Dihydromyricetin suppresses NF-κB
protein levels to reduce inflammation and decreased p53 and Bax
protein expression to inhibit apoptosis in the rat model.
Discussion
Alcohol consumption may also lead to digestive
disease, chronic gastritis, alcoholic fatty liver, alcoholic
hepatitis and even alcoholic cirrhosis (4). ALD is a disease resulting from
excessive alcohol uptake, which finally leads to hepatic toxic
damage (9). Research indicates
that the major pathogenic factors of ALD are the metabolites of
ethyl alcohol in hepatocytes and the induced metabolic disorder
(9). The disease begins with fatty
degeneration of hepatocytes, and may gradually develop into
alcoholic hepatitis and fibrosis with the increase in alcohol
consumption. Finally, alcoholic cirrhosis and even primary
hepatocellular carcinoma may be developed (10). In the present study, it was
observed that treatment with dihydromyricetin reduced body weight,
triglycerides, ALT and AST levels, and increased ALB levels in a
rat model of fatty liver.
Excessive highly active molecules, such as reactive
oxygen species (ROS) and reactive nitrogen species, are produced in
the presence of harmful stimulations (11). As a result, the oxidative degree
exceeds the scavenging of oxides, and the balance between the
oxidative and anti-oxidative systems is broken. Thus, tissue injury
is induced (12), and such a
process is referred to as oxidative stress. The major alcohol
metabolic pathway exists in the liver (13). The body metabolism produces ROS
(including superoxide anion, hydroxyl radical and hydrogen
peroxide) through different pathways (13), and excessive ROS-induced oxidative
stress response is the important cause of hepatocyte injury.
Alcohol can trigger and aggravate oxidative stress, and reduce the
free radical scavenging and anti-peroxidation capacity (14). As a result, peroxide production
in vivo is increased, thereby promoting the genesis and
development of alcoholic liver injury (12). Oxidative stress and
pro-inflammatory factor production are important factors among
alcohol-induced cell injury mechanisms (13).
The results of the present study suggested that
dihydromyricetin treatment inhibited oxidative stress, inflammation
and liver cell apoptosis in a rat model of fatty liver. Previously,
Chen et al (15) identified
that dihydromyricetin protects against liver
ischemia/reperfusion-induced apoptosis. In addition, Song et
al (1) reported that
dihydromyricetin attenuated angiotensin II-induced cardiac
fibroblast proliferation associated with inhibition of oxidative
stress.
NF-κB, an important transcription factor, is a
nuclear protein binding to κ light chain enhancer of activated B
cells (16). In addition, NF-κB is
the upstream signaling molecule of multiple inflammatory mediators
and can promote their production (17), and is thus key in the inflammatory
reaction. NF-κB also regulates the transcription of various acute
reactive proteins, cytokines and cell adhesion molecules. Thus, it
can directly participate in acute and chronic liver inflammation
(17). Liver injury in alcoholic
fatty liver is closely associated with apoptosis of hepatocytes,
which is in turn associated with the activation of NF-κB (16,18).
Zhou et al (19) indicated
that dihydromyricetin protects against lipopolysaccharide-induced
cardiomyocyte injury via Toll-like receptor 4 and NF-κB
pathways.
NF-κB is activated under the stimulation of
pro-inflammatory factors in ALD, thus upregulating the expression
of pro-inflammatory and chemotactic factors (7). NF-κB activation can enhance the
transcription of pro-inflammatory cytokines (20), which in turn stimulate NF-κB
activation (20). Such positive
feedback reaction can certainly amplify the inflammatory signal and
aggravate tissue injury. The production of abnormal large amounts
of inflammatory factors serves a vital role in the pathogenesis of
ALD (21). Therefore, NF-κB is an
important central link, and control over the production of NF-κB
can control the inflammatory damage of liver, thus serving as an
effective therapeutic target (21). In the present study,
dihydromyricetin suppressed NF-κB, p53 and Bax protein expression
levels in a fatty liver rat model. Zhou et al (19) reported that dihydromyricetin
induced apoptosis and cytoprotective autophagy in human melanoma
cells through ROS-NF-κB signaling. Overall, the NF-κB signaling
pathway regulates a great number of signaling pathways, and further
investigation is required to analyze these signaling pathways.
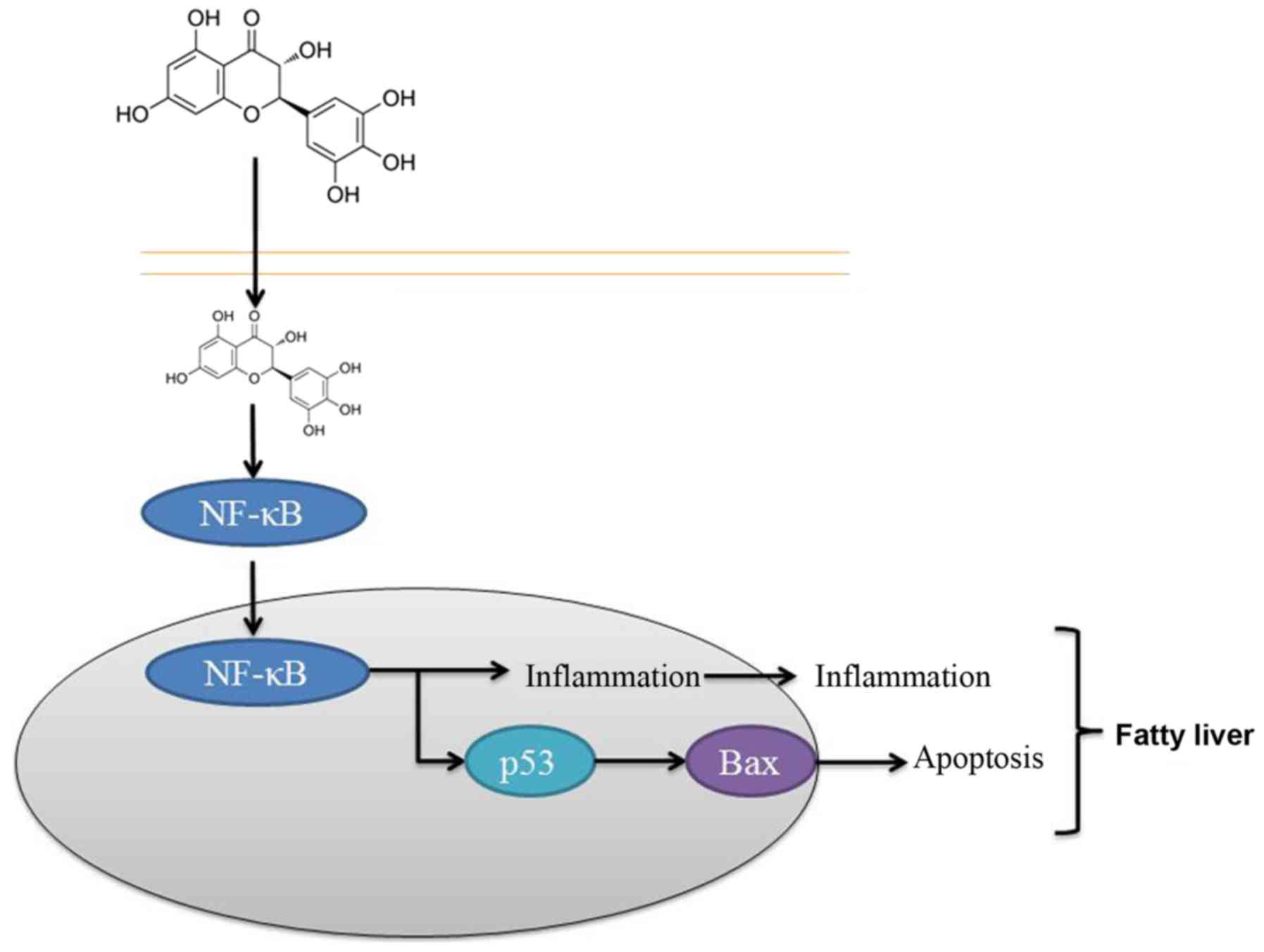
In conclusion, the results of the present study
suggested that dihydromyricetin administration suppressed NF-κB,
p53 and Bax protein expression levels in a fatty liver rat model.
It is, thus, concluded that the protective effect of
dihydromyricetin on the liver was through NF-κB/p53/Bax signaling
pathways in the rat model (Fig.
8). These findings suggest that dihydromyricetin is associated
with the pathogenesis of liver fibrosis and that dihydromyricetin
treatment may thus be a strategy for preventing hepatic fibrosis of
patients, although this requires further examination.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XY designed the experiments. LG and HZ performed the
experiments. XY and LG analyzed the data. XY wrote the
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Guidelines for Animal Experimentation and approval obtained by
the ethics committee of the Sixth People's Hospital of Qingdao.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Song Q, Liu L, Yu J, Zhang J, Xu M, Sun L,
Luo H, Feng Z and Meng G: Dihydromyricetin attenuated Ang II
induced cardiac fibroblasts proliferation related to inhibitory of
oxidative stress. Eur J Pharmacol. 807:159–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang H, Hu M, Zhao R, Li P and Li M:
Dihydromyricetin suppresses the proliferation of hepatocellular
carcinoma cells by inducing G2/M arrest through the
Chk1/Chk2/Cdc25C pathway. Oncol Rep. 30:2467–2475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rotman Y: Similarity between studies of
dihydromyricetin and reservatrol for NAFLD. Pharmacol Res.
100:3352015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Houghton D, Thoma C, Hallsworth K, Cassidy
S, Hardy T, Burt AD, Tiniakos D, Hollingsworth KG, Taylor R, Day
CP, et al: Exercise reduces liver lipids and visceral adiposity in
patients with nonalcoholic steatohepatitis in a randomized
controlled trial. Clin Gastroenterol Hepatol. 15:96–102.e3. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alexander KS, Zakai NA, Lidofsky SD,
Callas PW, Judd SE, Tracy RP and Cushman M: Non-alcoholic fatty
liver disease, liver biomarkers and stroke risk: The reasons for
geographic and racial differences in stroke cohort. PLoS One.
13:e01941532018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim W, Kim BG, Lee JS, Lee CK, Yeon JE,
Chang MS, Kim JH, Kim H, Yi S, Lee J, et al: Randomised clinical
trial: The efficacy and safety of oltipraz, a liver X receptor
alpha-inhibitory dithiolethione in patients with non-alcoholic
fatty liver disease. Aliment Pharmacol Ther. 45:1073–1083. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Hai J, Li Z, Zhang Y, Peng H, Li K
and Weng X: Resveratrol modulates autophagy and NF-κB activity in a
murine model for treating non-alcoholic fatty liver disease. Food
Chem Toxicol. 63:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bu W and Luo T: miR-1297 promotes cell
proliferation of non-small cell lung cancer cells: Involving in
PTEN/Akt/Skp2 signaling pathway. DNA Cell Biol. 36:976–982. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan DC, Watts GF, Gan S, Wong AT, Ooi EM
and Barrett PH: Nonalcoholic fatty liver disease as the transducer
of hepatic oversecretion of very-low-density
lipoprotein-apolipoprotein B-100 in obesity. Arterioscler Thromb
Vasc Biol. 30:1043–1050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalinowski P, Paluszkiewicz R,
Ziarkiewicz-Wroblewska B, Wróblewski T, Remiszewski P, Grodzicki M
and Krawczyk M: Liver function in patients with nonalcoholic fatty
liver disease randomized to Roux-en-Y gastric bypass versus sleeve
gastrectomy: A secondary analysis of a randomized clinical trial.
Ann Surg. 266:738–745. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gonzalez-Manan D, D'Espessailles A, Dossi
CG, San Martin M, Mancilla RA and Tapia GS: Rosa mosqueta oil
prevents oxidative stress and inflammation through the upregulation
of PPAR-α and NRF2 in C57BL/6J rat fed a high-fat diet. J Nutr.
147:579–588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukai T, Egawa M, Takeuchi T, Yamashita H
and Kusudo T: Silencing of FABP1 ameliorates hepatic steatosis,
inflammation, and oxidative stress in rat with nonalcoholic fatty
liver disease. FEBS Open Bio. 7:1009–1016. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharifi N, Amani R, Hajiani E and
Cheraghian B: Does vitamin D improve liver enzymes, oxidative
stress, and inflammatory biomarkers in adults with non-alcoholic
fatty liver disease? A randomized clinical trial. Endocrine.
47:70–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Ding YL, Zhang JL, Zhang P, Wang
JQ and Li ZH: Alpinetin improved high fat diet-induced
non-alcoholic fatty liver disease (NAFLD) through improving
oxidative stress, inflammatory response and lipid metabolism.
Biomed Pharmacother. 97:1397–1408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Lv L, Pi H, Qin W, Chen J, Guo D,
Lin J, Chi X, Jiang Z, Yang H and Jiang Y: Dihydromyricetin
protects against liver ischemia/reperfusion induced apoptosis via
activation of FOXO3a-mediated autophagy. Oncotarget. 7:76508–76522.
2016.PubMed/NCBI
|
|
16
|
Willy JA, Young SK, Stevens JL, Masuoka HC
and Wek RC: CHOP links endoplasmic reticulum stress to NF-κB
activation in the pathogenesis of nonalcoholic steatohepatitis. Mol
Biol Cell. 26:2190–2204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shankar E, Zhang A, Franco D and Gupta S:
Betulinic acid-mediated apoptosis in human prostate cancer cells
involves p53 and nuclear Factor-kappa B (NF-κB) Pathways.
Molecules. 22(pii): E2642017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Tan X, Yang D, Lu J, Liu B,
Baiyun R and Zhang Z: Dietary luteolin attenuates chronic liver
injury induced by mercuric chloride via the Nrf2/NF-κB/P53
signaling pathway in rats. Oncotarget. 8:40982–40993.
2017.PubMed/NCBI
|
|
19
|
Zhou DZ, Sun HY, Yue JQ, Peng Y, Chen YM
and Zhong ZJ: Dihydromyricetin induces apoptosis and cytoprotective
autophagy through ROS-NF-κB signalling in human melanoma cells.
Free Radic Res. 51:517–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JY, Song EH, Lee HJ, Oh YK, Choi KH,
Yu DY, Park SI, Seong JK and Kim WH: HBx-induced hepatic steatosis
and apoptosis are regulated by TNFR1- and NF-kappaB-dependent
pathways. J Mol Biol. 397:917–931. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maraslioglu M, Weber R, Korff S, Blattner
C, Nauck C, Henrich D, Jobin C, Marzi I and Lehnert M: Activation
of NF-κB after chronic ethanol intake and haemorrhagic
shock/resuscitation in rat. Br J Pharmacol. 170:506–518. 2013.
View Article : Google Scholar : PubMed/NCBI
|