Introduction
Hepatic fibrosis (HF) is a chronic injury to the
liver characterized by excess production of extracellular matrix
(ECM) proteins. This chronic process undermines the architecture
and normal function of the liver, and leads to fibrosis, cirrhosis
and eventually hepatocellular carcinoma. Chronic fibrosis, however,
is pathological change that may induce organ failure, formation of
scar tissue and even result in mortality (1). Previous data has indicated that
fibrosis accounts for almost 50% of all-cause mortality in
industrialized countries, making this an urgent problem to be
solved by clinicians (2).
The pathogenesis of fibrosis is broadly similar, but
the regeneration capacity of the liver is remarkable. Therefore,
early HF is not readily detected. Angiogenesis is the formation of
new blood vessels from pre-existing ones, and it is the stress
response of an organism to injury. Hepatic angiogenesis has been
observed in different inflammatory, fibrotic and ischemic
conditions. Hepatic angiogenesis and overexpression of moieties in
hepatic stellate cells (HSCs) are key factors in HF pathogenesis
(3). Experimental studies have
demonstrated that appropriate anti-angiogenic therapy may lead to
significant inhibition of HF progression, decreases in inflammatory
infiltrates and α-smooth muscle actin (SMA)-positive
myofibroblasts, and a decrease in portal pressure (4,5).
Hepatic angiogenesis is regulated by growth factors
expressed by hepatocytes. These growth factors include transforming
growth factor (TGF)-β, vascular endothelial growth factor (VEGF),
epidermal growth factor, insulin-like growth factor-1, fibroblast
growth factor (FGF) and platelet-derived growth factor (PDGF).
Levels of these growth factors have been identified to be increased
significantly in cases of fibrosis and cirrhosis of the liver.
Among these growth factors, VEGF is the best
characterized, due to its mitogenic properties for endothelial
cells. Also, its association with angiogenesis and HF has been
confirmed. VEGF may induce growth of new blood vessels as a
response to hepatic injury, which is essential for HF (6). In addition, the Ras/Rapidly
accelerated fibrosarcoma/mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase (ERK) signaling
pathway, also known as the ERK pathway, is the most representative
of mitogen-activated protein kinase (MAPK) pathways (7), and serves an important part in
angiogenesis (8).
HF is the early and only reversible stage of
cirrhosis and cancer of the liver (9). Delaying or reversing HF may prevent
the development of these pathologies. Therefore, identifying novel
drugs that may prevent HF is important.
Rhizoma Paridis grows primarily in Southwest
China and, as a traditional Chinese medicine, has been used widely
in the treatment of chronic liver disease (10). In certain ethnic groups in China,
Rhizoma Paridis is used for the treatment of fractures,
snake bites and abscesses, due to its heat-clearing and detoxifying
properties (11). Rhizoma
Paridis also exhibits marked anti-tumor activity (12).
Previously, we demonstrated that Rhizoma
Paridis protects the liver and exhibits anti-HF effects
(13). We suggested that the
primary active components of Rhizoma Paridis that protect
against liver injury are Rhizoma Paridis saponins (RPS). One
of the mechanisms by which RPS is active against HF is the
regulation of expression of the RasGAP-activating-like protein
1/ERK1/2 signaling pathway. RPS may inhibit the proliferation and
activation of HSCs by inhibiting the ERK pathway and, ultimately,
inhibiting or reversing HF (14).
However, the association between VEGF, PDGF and
ERKI/2 in HF has not been conclusively demonstrated. The present
study aimed to ascertain the effect of RPS on
angiogenesis-associated factors including VEGF, PDGF and ERK1/2,
and whether RPS exerts anti-HF effects through affecting the
VEGF/ERK1/2 pathway, by creating a HF model in rats using carbon
tetrachloride (CCl4).
Materials and methods
RPS preparation
The dried rhizomes of Rhizoma Paridis were
purchased from the Chinese Herbal Medicine Pharmacy of The First
Affiliated Hospital, Anhui University of Chinese Medicine (Hefei,
China) following identification by Professor Hua-sheng Pen (Anhui
University of Chinese Medicine).
RPS was prepared as described previously (14) and its yield was 1.15%. The content
of steroidal saponins in RPS was high when absorbance was measured
at 408 nm (53.22 g steroidal saponins/100 g RPS). Ultra-performance
liquid chromatography-evaporative light-scattering detection
(UPLC-ELSD) was used to determine the contents of the saponins
polyphyllin-VII, -VI, -II and -I in RPS in comparison with
reference substances as described below, and the contents were
identified to be 2.41, 3.15, 2.49 and 9.92%, respectively.
Analyses of RPS by UPLC-ELSD
UPLC-ELSD was performed using an Acquity UPLC
H-Class system (Waters Corporation, Milford, MA, USA) consisting of
an autosampler and a quaternary pump coupled with an Acquity ELSD
detector. An Acquity UPLC BEH C18 column (100 mm × 2.1
mm, 1.7 µm, Waters China, Ltd., Shantin, Hong Kong, China) was used
for all separations, column temperature was 30°C and the analysis
time was 8 min. UPLC conditions were: Solvent A, acetonitrile;
solvent B, water; gradient, 0–2.5 min (40–45% A), 2.5–3.5 min
(45–55% A), 3.5–4.5 min (55–60% A), 4.5–6.5 min (65–40% A). The
flow rate was 0.45 ml/min, and the injection volume was 2 µl. ELSD
conditions were: Gain, 500; nebulizer model, heated; drift tube
temperature was 70°C; gas pressure was 275.8 kPa. The internal
standards were saponins polyphyllin-VII (133.6 µg/ml), -VI (84.0
µg/ml), -II (1,1804 µg/ml) and -I (99.6 µg/ml). The UPLC
chromatograph of mixed standard compounds and PRS were processed by
the Similarity Evaluation Software of Traditional Chinese Medicine
Injection (National Pharmacopoeia Commission of China. Edition A,
2004).
Chemicals
CCl4 was purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). TRIzol™ was obtained from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). PCR
MasterMix™ was from Thermo Fisher Scientific, Inc. A chemistry
analyzer (7600) was purchased from Hitachi, Ltd., (Tokyo,
Japan).
Rabbit anti-phosphorylated (p)-ERK1/2 polyclonal
antibody was obtained from Cell Signaling Technology, Inc. (cat.
no. 4370S, Danvers, MA, USA). VEGF (ab19645), PDGF (aab55160) and
α-SMA (ab20979) polyclonal antibodies were purchased from Abcam
(Cambridge, UK). ERK1/2 rat anti-human monoclonal antibody (cat.
no. 1544S) was purchased from Beijing Biosynthesis Biotechnology
Co., Ltd. (Beijing, China). Alanine aminotransferase (ALT; cat. no.
c009-2), aspartate aminotransferase (AST; cat. no. c010-2),
glutathione (GSH; cat. no. a006-2), malondialdehyde (MDA; cat. no.
a003-4) and superoxide dismutase (SOD; cat. no. a001-1-1) kits were
obtained from the Nanjing Jiancheng Institute of Biotechnology
(Nanjing, China).
Animal experimental model
All experiments were performed in accordance with
national legislations and local guidelines. The study protocol was
approved by the Committee on the Ethics of Animal Experiments of
Anhui University of Chinese Medicine (approval no.
2012AH-036-03).
A total of 40 male Sprague-Dawley rats (180–200 g)
were purchased from the Laboratory Animal Center, Medical
University of Anhui Province (Hefei, China). Rats were housed in a
room with a controlled environment (25°C and 12 h light-dark cycle)
and specific pathogen-free conditions.
After 1 week of acclimation, rats were divided
randomly and equally into four groups: Control; model; RPS high
dose (RPS-H); and RPS low dose (RPS-L). With the exception of the
rats in the control group, rats in each group were injected with
50% CCl4 dissolved in olive oil (2.0 ml/kg body
weight/rat) twice a week for 16 consecutive weeks to induce HF
(15,16).
A total of 12 weeks after modeling, the control
group was administered the same amount of physiologic (0.9%) saline
as gavage; no additional processing was performed in the model
group, whereas the RPS-H and RPS-L groups were treated with RPS
(300 and 150 mg/10 ml/kg body weight/rat, respectively) once a day
as gavage. The optimal dose of PRS was determined in our previous
studies, and it was confirmed that the toxicity would not cause
harm to the experimental animals at this dose (13,17).
Rats were sacrificed via exsanguination and cervical
dislocation at the end of treatment. For 12 h prior to sacrifice,
rats were not fed and anesthetized [2% pentobarbital sodium (40
mg/kg body weight/rat), i.v.], A total of ~10 ml blood was
extracted from each rat from the abdominal aorta for measurement of
serum biochemical parameters, and liver biopsies from each animal
were collected for histology and immunohistochemical (IHC)
analyses. The remaining liver samples were snap-frozen to extract
total RNA and proteins for molecular analyses.
Liver pathology
Pathological changes in liver tissues were observed
by hematoxylin and eosin (H&E; 1%) staining of paraffin
sections for 30–60 sec at 25°C. Tissues were fixed in 4%
paraformaldehyde at 55°C for 1 h. HF extent was determined by
Masson trichrome (1%) staining for 5–10 min at 55°C. Analysis was
conducted with an optical light microscope (magnification,
×400).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from liver samples using
TRIzol® (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol, and stored at −80°C until use. Then, 3
µg RNA was placed in a 0.2 ml Eppendorf tube for PCR analysis
according to the manufacturer's instructions. For RT, an RNA
Extraction kit was used, and qPCR was conducted using a
Fluorescence Qauntitative PCR kit (both from Sangon Biotech Co.,
Ltd., Shanghai, China). The thermocycler conditions for RT-qPCR
were: Initial denaturation: 95°C for 5 min, followed by 30 cycles
of 95°C for 10 sec and 60°C for 30 sec, and final extension was
72°C for 10 min. The primer for each indicator is summarized in
Table I (18).
 | Table I.Primer sequences and the length of
VEGF, PDGF, ERK1/2 and α-SMA genes. |
Table I.
Primer sequences and the length of
VEGF, PDGF, ERK1/2 and α-SMA genes.
| Gene | Primer sequences
(5′-3′) | Fragment length,
bp | Tm, °C |
|---|
| ERK1 | F:
5′-CCATCCCAAGAGGACCTAAA-3′ | 273 | 55 |
|
| R:
5′-ATCATCCAGCTCCATGTCAA-3′ |
|
|
| ERK2 | F:
5′-CGCGCTACACTAATCTCTCG-3′ | 470 | 58 |
|
| R:
5′-ATCATGGTCTGGATCTGCAA-3′ |
|
|
| VEGF | F:
5′-GTCTACCAGCGCAGCTATTG-3′ | 491 | 97 |
|
| R:
5′-AGTCGAGTCGGAAAGCTCAT-3′ |
|
|
| PDGF | F:
5′-CACAGGACGGCTTGAAGATA-3′ | 355 | 101 |
|
| R:
5′-ACACCTCTGTACGCGTCTTG-3′ |
|
|
| GAPDH | F:
5′-CAAGGTCATCCATGACAACTTTG-3′ | 496 | 55 |
|
| R:
5′-GTCCACCACCCTGTTGCTGTAG-3′ |
|
|
Western blot analysis
Liver tissues (100 mg) were lysed with 1 ml cell
extraction buffer, which was prepared according to methods
described previously (14).
Lysates were centrifuged at 12,000 × g for 10 min at 4°C, and the
supernatant was collected. Protein samples were separated by
SDS-PAGE on 8% gels, transferred to polyvinylidene fluoride (PVDF)
membranes and blocked in TBST (TBS buffer with 0.24% Tween-20, pH
7.4, plus 5% skimmed milk powder) for 14–16 h at 4°C. PVDF
membranes were incubated overnight at 4°C with primary antibodies
against VEGF (1:1,000), PDGF (1:1,000), phosphorylated (p)-ERK1/2
(1:2,000), α-SMA (1:1,000) and β-actin (1:800). Following washing 5
times for 10 min each in TBST, bound proteins were detected with a
secondary antibody (1:10,000; cat. no. 140829, Tiangen Biochemical
Technology (Beijing) Co., Ltd., (Beijing, China) according to
manufacturer's instructions. β-actin values were used to normalize
expression of VEGF, p-PDGF, p-ERK1/2 and α-SMA. Protein expression
was analyzed using ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Measurement of serum biochemical
parameters
Blood was collected and centrifuged at 3,000 × g for
10 min at room temperature. The serum was separated and stored at
−70°C. A chemistry analyzer (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) was used to determine serum levels of ALT and AST. The
thiobarbituric acid reactive substances method (19) was used to measure MDA formation, to
determine levels of lipid peroxidation in the liver. Commercial
kits were utilized to analyze the activities of SOD and
GSH-peroxidase (Px).
Statistical analyses
Data are the mean ± standard deviation. Statistical
significance was determined by one-way analysis of variance
followed by the Tukey multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS v21.0 (IBM Corp.,
Armonk, NY, USA).
Results
UPLC-ELSD
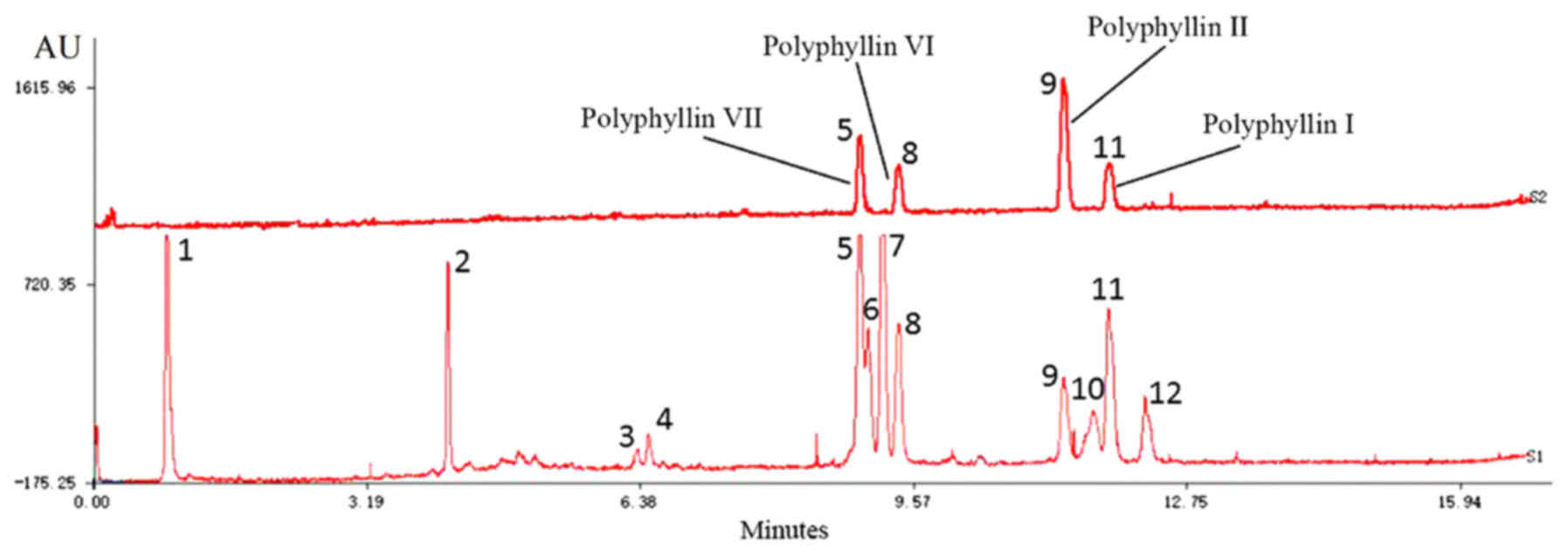
Fig. 1 demonstrates
that UPLC-ELSD detected 12 peaks within 15 min. Compared with
authentic reference substances at individual peak retention times,
4 compounds [polyphyllin-VII (5),
polyphyllin-VI (8), polyphyllin-II
(9) and polyphyllin-I (11)] were verified in mixed standard
compounds; the others were not identified.
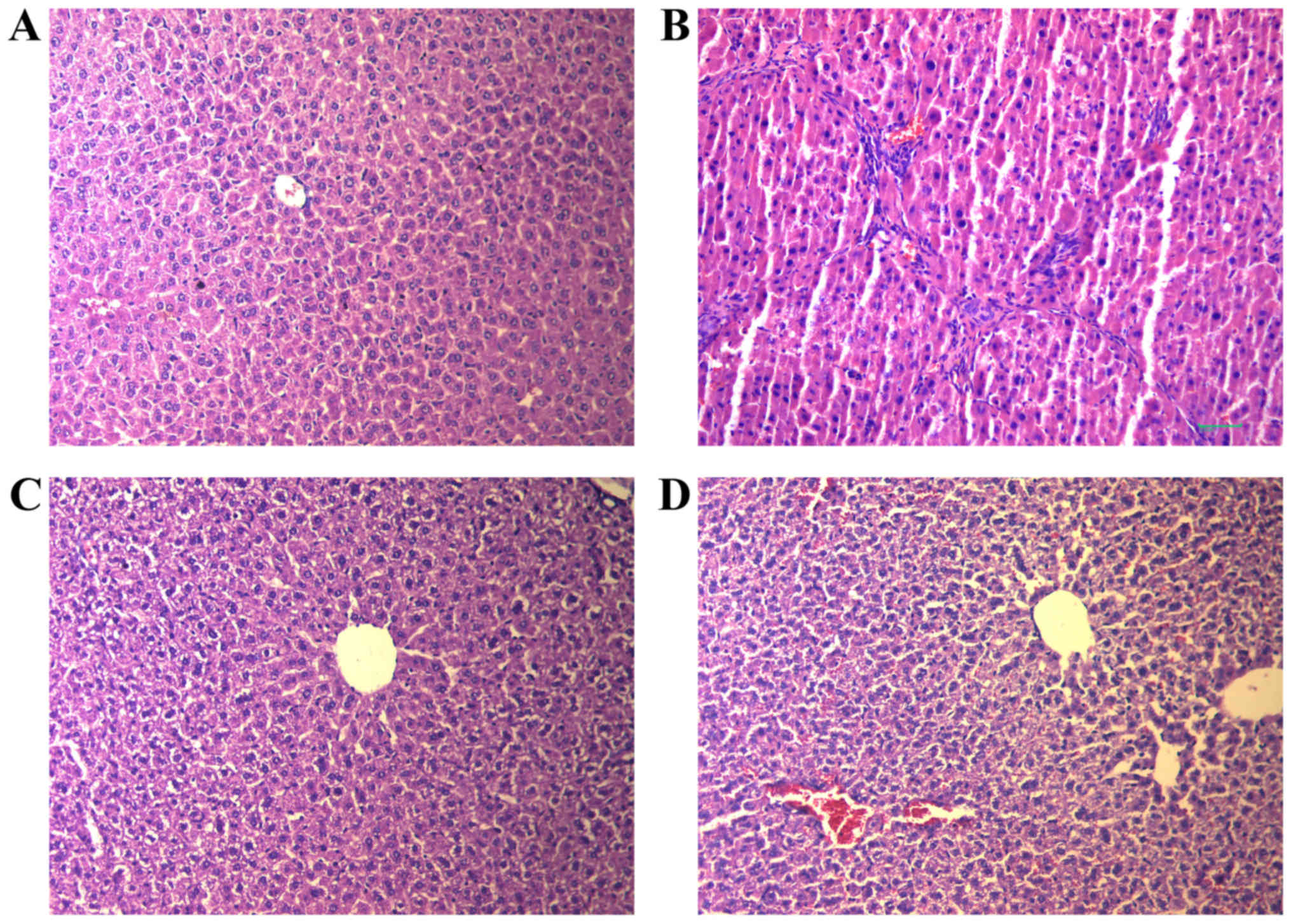
Histopathology
The effects of RPS on CCl4-induced liver
injury as evaluated by H&E and Masson's trichrome staining are
demonstrated in Fig. 2. There were
no significant alterations in the liver tissue of rats in the
control group. The hepatic lobular architecture was normal, and
little proliferation of connective tissue was noted (Fig. 2A). However, there was massive
hepatocellular necrosis in rats of the model group, with
alterations in adipose tissue and massive infiltration of
lymphocytes causing a severe inflammatory reaction in the portal
tract (Fig. 2B). However, the
pathological changes induced by CCl4 treatment were
attenuated considerably in RPS-H and RPS-L groups, with decreases
in HF, severe steatosis, necrosis and collagen deposition (Fig. 2C and D).
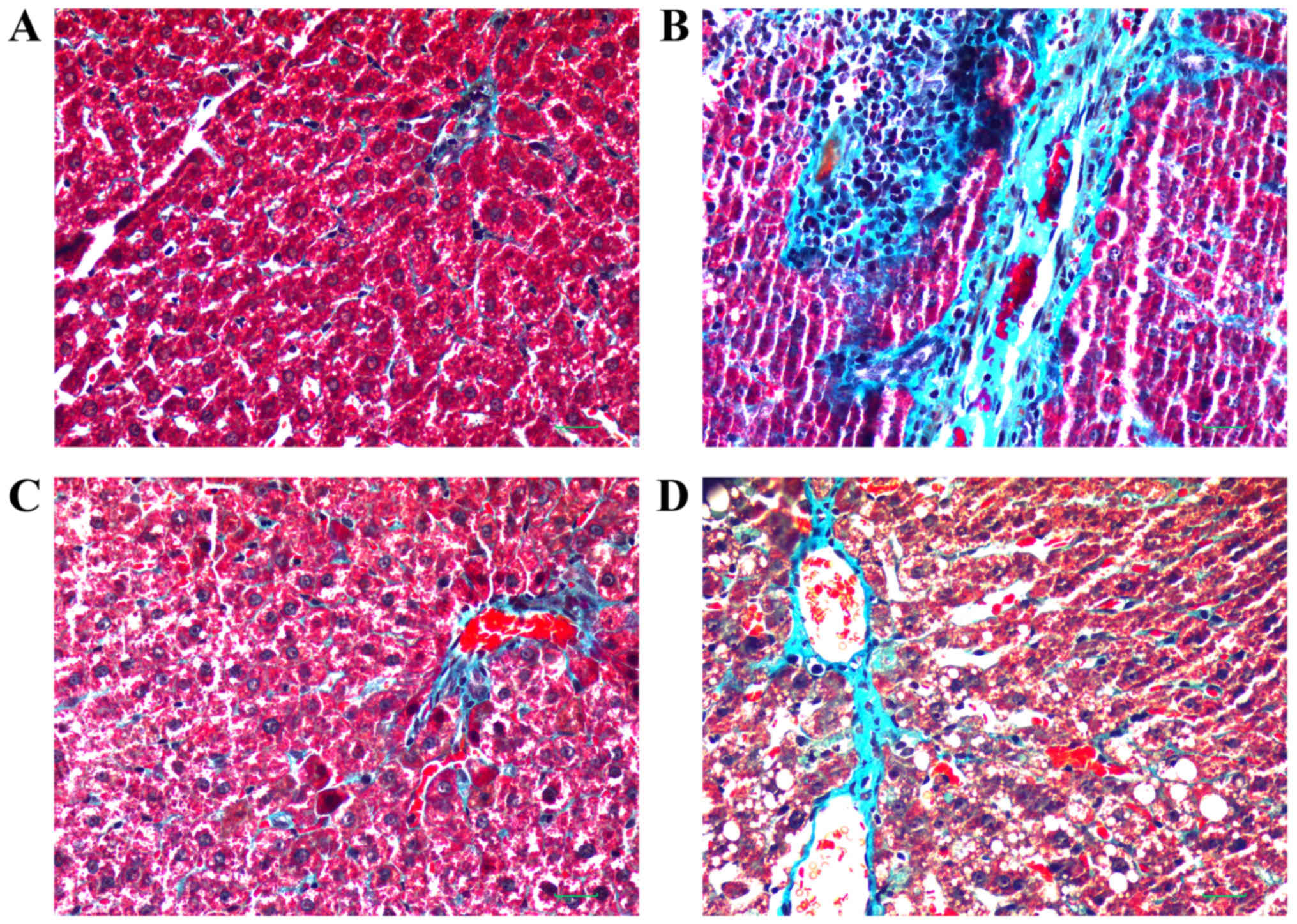
Masson's trichrome staining of liver sections is
presented in Fig. 3. The central
veins and radiating hepatic cords exhibited an intact lobular
architecture in the control group (Fig. 3A). In the model group, hepatic
lobules were observed but hepatic cords were irregular. Ballooning
degeneration was present, and inflammation was observed in portal
areas with proliferation of the small bile ducts. Hyperplasia of
collagen fibers was marked and intersected at multiple portal
areas. Bridging of collagen fibers connected portal areas with
central veins and, as a result, pseudolobuli were formed (Fig. 3B). Compared with the model group,
RPS-H and RPS-L groups also exhibited ballooning degeneration, and
proliferation of fibrous connective tissue in the portal area.
Hepatic lobules were divided by collagen fibers. Pseudolobuli
formation was not marked, and hepatic cords were essentially normal
(Fig. 3C and D). The RPS-H group
was improved compared with the RPS-L group in terms of treatment
effect, but the levels of blue staining of the thick fibers in
these two groups were markedly decreased compared with the model
group following Masson's trichrome staining.
Biochemical parameters
As indicated in Table
II, compared with the control group, expression of ALT and AST
in the model group was upregulated significantly (4-fold and
2.37-fold higher, respectively, compared with that in the control
group). The MDA content was increased compared with that in the
control group. These data suggested the successful establishment of
a HF model in rats using CCl4.
 | Table II.Alterations in serum biochemical
parameters and activity of antioxidant enzymes. |
Table II.
Alterations in serum biochemical
parameters and activity of antioxidant enzymes.
| Groups | n | ALT (U/l) | AST (U/l) | MDA (nmol/mg
protein) | SOD (U/mg
protein) | GSH (U/mg
protein) |
|---|
| Control | 10 | 20.64±4.38 | 47.30±6.41 | 0.82±0.09 | 98.45±17.50 | 273.26±44.37 |
| Model | 10 |
80.91±18.93a |
112.50±19.23a |
1.20±0.18a |
70.34±14.74a |
165.65±49.21a |
| RPS H | 10 |
51.14±14.70b |
80.80±22.60b |
0.90±0.12b |
84.70±15.31c |
244.21±55.62b |
| RPS L | 10 |
60.52±10.29b |
92.50±19.76c |
1.03±0.15c | 79.10±12.90 |
220.91±66.08c |
The content of SOD and GSH-Px in the model group was
decreased compared with that in the control group, with the GSH-Px
content exhibiting a significant decrease. However, a significant
decrease in expression of ALT and AST in the RPS-H and RPS-L groups
was observed compared with the model group. The MDA content also
decreased in RPS-H and RPS-L groups compared with the model group.
Expression of SOD and GSH-Px in the RPS-H and RPS-L groups
exhibited marked upregulation compared with the model group and was
close to that observed in the control group. These results
demonstrated the robust anti-HF effects of RPS: It was able to
increase the activity of SOD and GSH-Px and inhibit lipid
peroxidation in liver tissue simultaneously. Therefore, RPS may
protect the liver from oxygen free radicals and peroxides.
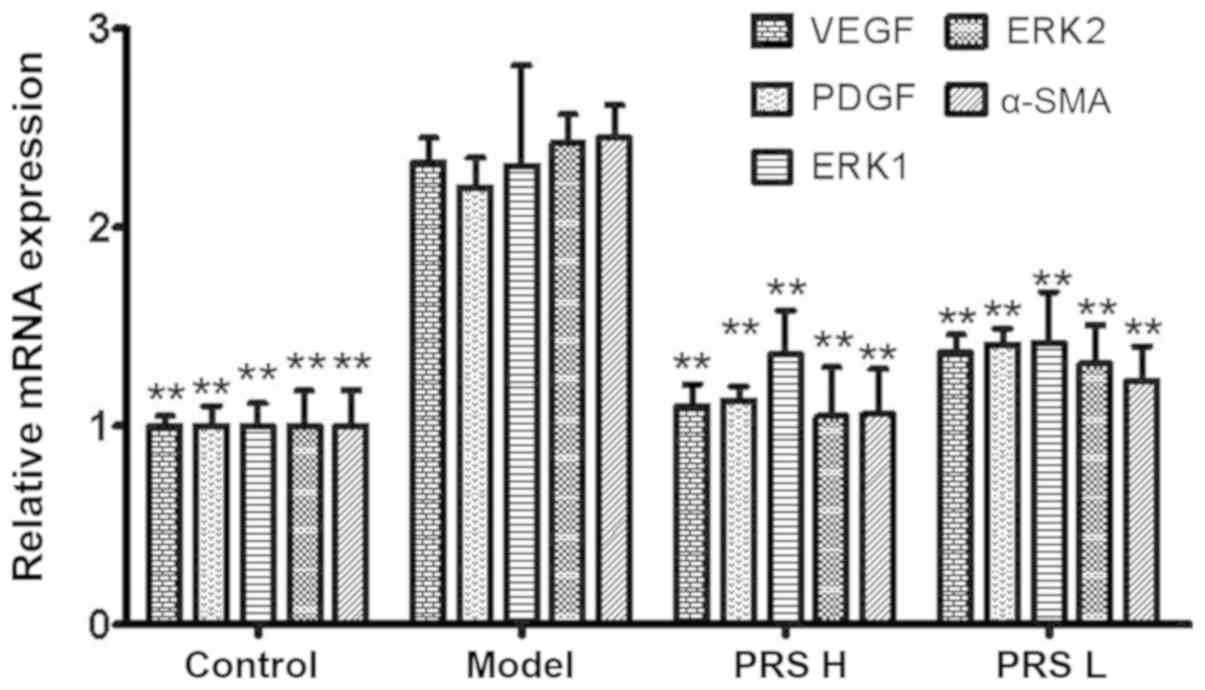
mRNA expression of VEGF, PDGF, ERK1/2
and α-SMA
mRNA expression of VEGF, PDGF, ERK1/2 and α-SMA in
the model group was 2.33-, 2.20-, 2.31-, 2.43- and 2.45-fold
increased, respectively, compared with that in the control group
(Fig. 4). Compared with the model
group, relative mRNA expression of VEGF, PDGF, ERK1/2 and α-SMA in
the RPS groups was decreased significantly (P<0.01). The
decrease in the RPS-H group was even more significant compared with
that observed in the RPS-L group. These results suggested that RPS
treatment of HF in rats may be through downregulation of expression
of angiogenic growth factors in liver tissue, thereby improving
liver microcirculation.
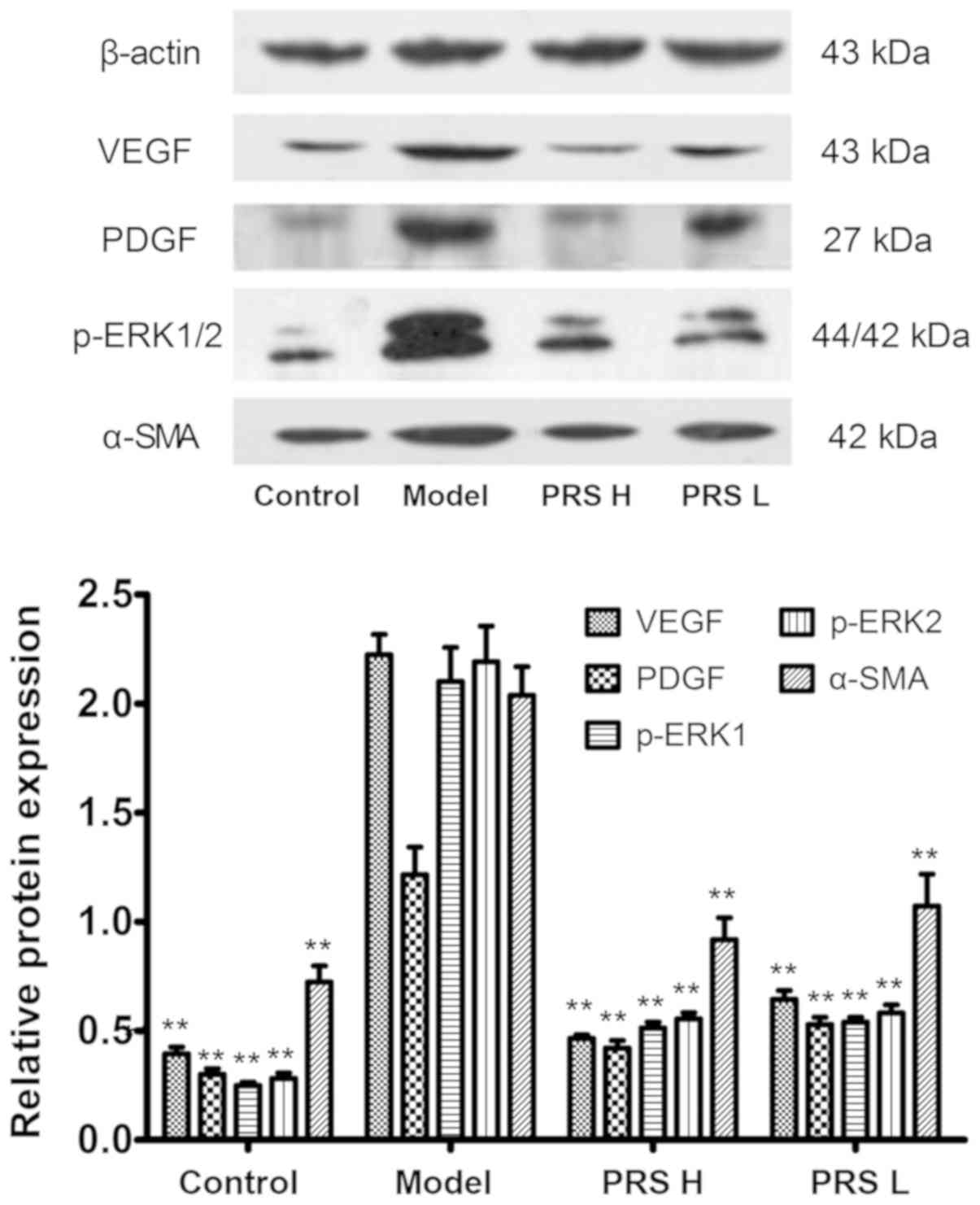
Protein expression of VEGF, PDGF,
p-ERK1/2 and α-SMA
Protein expression of VEGF, PDGF, p-ERK1/2 and α-SMA
in the model group was markedly increased compared with that in the
control group (Fig. 5). Following
RPS treatment, the protein expression levels of VEGF, PDGF,
p-ERK1/2 and α-SMA were decreased significantly (P<0.01)
compared with the model group. The results suggested that the
decrease of VEGF, PDGF, p-ERK1/2 and α-SMA expression may be one of
the mechanisms by which PRS improves liver fibrosis.
Discussion
HF is the result of abnormal proliferation of
connective tissue, and proliferation of connective tissue is a
wound-healing response to various types of chronic injury to the
liver, for example viral infection and exposure to chemicals
(20). At the molecular level, HF
pathogenesis is closely associated with HSCs. The latter may
undergo trans-differentiation into fibrogenic, proliferative and
contractile myofibroblasts (21).
Apoptosis and soluble growth factors may lead to HSC stimulation.
Specific lymphocyte subsets may induce HF. A signaling cascade and
transcription are the basis of fibrogenic effects in HSCs, and each
factor in the cascade may be a target for anti-HF therapy (22). Several vitamins are stored in HSCs.
If the liver is damaged, release of vitamin A is decreased in HSCs,
but increased expression of α-SMA promotes HF (23). VEGF, PDGF, ERK and TGF-β may also
activate HSCs.
Abnormal expression of VEGF occurs under
pathological conditions. In ischemic disease, hypoxia may stimulate
VEGF expression. The resulting marked induction of mitosis of
endothelial cells promotes formation of new blood vessels and
improves the blood supply to tissue. VEGF is a key factor for
ocular neovascularization, and promotes the formation and
development of new blood vessels directly; its expression is
closely associated with disease severity (24).
ERK1/2 belongs to the family of MAPKs which are
involved in the regulation of the proliferation, differentiation,
growth and apoptosis of cells. The term ERK1/2 refers to two
closely associated kinase isoforms (25). ERK enters the nucleus subsequent to
activation, causing changes in the expression of genes for the
substrate, and affecting the growth and proliferation of cells. The
present study identified that ERK was phosphorylated in the MAPK
and VEGF pathways. It has also been identified that upregulation of
p-ERK1/2 was induced by CCl4 in previous studies
(26,27). Injury primarily affects the
post-translational modification of ERK1/2. The effects of injury on
ERK1/2 was demonstrated by drug treatment, indicating the reversal
of liver fibrosis; that is, whether the regulation of p-ERK exists.
Therefore, ERK expression is closely associated with HF. In
addition, a previous study (14),
ERK1/2 protein has been determined by immunohistochemistry.
Therefore, the present study selected to detect p-ERK1/2.
The VEGF/ERK1/2 signaling pathway has been
demonstrated to serve a key role in the proliferation of
endothelial cells (28). In bovine
retinal microvascular endothelial cells in vitro, VEGF
stimulated ERK1/2 phosphorylation in a dose-dependent manner to
promote the formation and proliferation of endothelial cells
(29). VEGF and other growth
factors affect cellular functions through the ERK pathway. They
promote the transcription and expression of selected genes, and
thereby initiate the proliferation and differentiation of cells.
This signaling pathway has an important role in the growth,
development and proliferation of cells (30). In the present study, expression
levels of VEGF and p-ERK1/2 in the model group were high; in the
RPS treatment groups, expression of VEGF and p-ERK1/2 was decreased
accordingly. Therefore, we hypothesized that the VEGF/ERK1/2
pathway has a prominent role in HF.
Traditionally, PDGF has been considered to be an
important fibrogenic and proliferative stimulus to HSCs (31). The PDGF family of ligands and
receptors regulate multiple processes. They are also involved in
several pathologic events, including cancer and fibrotic diseases
(32). During the pathogenesis of
fibrotic diseases, PDGF serves a major role in stimulating the
replication, survival and migration of myofibroblasts, and several
pro-inflammatory cytokines mediate their mitogenic effects via
autocrine release of PDGF. PDGF is a potent mitogen that may aid
fibroblast proliferation and secrete fibronectin in liver
fibroblasts (33,34). Large amounts of PDGF stimulate the
ERK1/2 pathway and promote HSC proliferation.
An additional important fibrogenic mediator, TGF-β,
induces secretion of VEGF, FGF and endothelin-1, thereby resulting
in HF. VEGF and FGF-2 induce hepatic vascular proliferation during
HF, and VEGF has an important role in the angiogenesis of HF. VEGF
may also activate HSCs via autocrine or paracrine pathways
(35,36). VEGF induces expression of different
proteases by endothelial cells, and stimulation of endothelial
cells and pro-coagulant activity in monocytes may induce
microvascular remodeling in the liver indirectly; the final stage
involves HF development.
α-SMA expression is upregulated together with an
increased ECM during HSC activation in HF. α-SMA is an important
symbol of HSC activation (37,38).
Therefore, understanding which growth factors are expressed
aberrantly in the signaling cascade and transcription for HSCs is
paramount for developing treatment strategies for HF.
Previously, we demonstrated that RPS exhibits
hepatoprotective and antifibrotic effects in vivo against HF
induced by CCl4 (14).
The anti-HF action of RPS may involve the RAS/ERK1/2 signaling
pathway. In the present study, the expression levels of VEGF, PDGF,
ERK1/2, and α-SMA were measured to explore the mechanism of action
of RPS against HF.
It was identified that CCl4 intoxication
damaged liver function, caused hepatocyte necrosis, and upregulated
the expression levels of the mRNA and phosphorylated proteins of
VEGF, PDGF, ERK1/2 and α-SMA. By contrast, following oral
administration of RPS the severity of HF was relieved
significantly, according to histopathology. Furthermore, the
expression levels of the mRNA and phosphorylated protein of VEGF,
PDGF, ERK1/2 and α-SMA were decreased. The results suggested that
RPS exhibited anti-HF effects in rat livers in vivo.
Therefore, downregulation of the VEGF/ERK pathway and improvement
of angiogenesis may be associated with the anti-HF action of
RPS.
Acknowledgements
The authors appreciate the technical support
provided by Shanghai Aoji Biotechnology Co., Ltd. (Shanghai,
China).
Funding
The present study was supported by a grant from the
Clinical Research Fund of Anhui University of Chinese Medicine
(grant no. 2012zr010).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YHa conceived and designed the study. LP designed
the schema of the VEGF/ERK1/2 signaling and drafted the manuscript.
SR, YS and FFS performed the experiments. YZW and YHo analyzed the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
national legislations and local guidelines. The study protocol was
approved by the Committee on the Ethics of Animal Experiments of
Anhui University of Chinese Medicine (approval no.
2012AH-036-03).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zoheir KMA, Amara AA, Ahmad SF, Mohammad
MA, Ashour AE, Harisa G and Abd-Allah AR: Study of the therapeutic
effects of Lactobacillus and α-lipoic acid against
dimethylnitrosamine-induced liver fibrosis in rats. J Genet Eng
Biotechnol. 12:135–142. 2014. View Article : Google Scholar
|
|
2
|
Friedman SL: Hepatic fibrosis: Emerging
therapies. Dig Dis. 33:504–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elzamly S, Agina HA, Elbalshy AE,
Abuhashim M, Saad E and Abd Elmageed ZY: Integration of VEGF and
α-SMA expression improves the prediction accuracy of fibrosis in
chronic hepatitis C liver biopsy. Appl Immunohistochem Mol Morphol.
25:261–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fernández M, Semela D, Bruix J, Colle I,
Pinzani M and Bosch J: Angiogenesis in liver disease. J Hepatol.
50:604–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valfrè di Bonzo L, Novo E, Cannito S,
Busletta C, Paternostro C, Povero D and Parola M: Angiogenesis and
liver fibrogenesis. Histol Histopathol. 24:1323–1341.
2009.PubMed/NCBI
|
|
6
|
Giatromanolaki A, Kotsiou S, Koukourakis
MI and Sivridis E: Angiogenic factor expression in hepatic
cirrhosis. Mediators Inflamm. 2007:671872007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang F, Li J, Zhu J, Wang D, Chen S and
Bai X: Hydroxysafflor yellow A inhibits angiogenesis of
hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling
pathway in H22 tumor-bearing mice. Eur J Pharmacol. 754:105–114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li DS: 45 cases clinical observation of
matrine in treatment of liver fibrosis of patients with chronic
hepatitis. China Ming Kang Med. 23:2701–2703. 2011.(In
Chinese).
|
|
10
|
Man S, Fan W, Liu Z, Gao W, Li Y, Zhang L
and Liu C: Antitumor pathway of Rhizoma Paridis saponins based on
the metabolic regulatory network alterations in H22 hepatocarcinoma
mice. Steroids. 84:17–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Man S, Gao W, Zhang Y, Jin X, Ma C, Huang
X and Li Q: Characterization of steroidal saponins in saponin
extract from Paris polyphylla by liquid chromatography tandem
multi-stage mass spectrometry. Anal Bioanal Chem. 395:495–505.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Man S, Gao W, Zhang Y, Yan L, Ma C, Liu C
and Huang L: Antitumor and antimetastatic activities of Rhizoma
Paridis saponins. Steroids. 74:1051–1056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong Y, Han Y, Luo H, Gui J, Zhang K and
Jiang H: Effect of Chonglou saponin on markers of liver fibrosis of
hepatic fibrosis rats and correlation analysis. J Shanxi Coll Trad
Chin Med. 15:20–22+67. 2014.(In Chinese).
|
|
14
|
Hong Y, Han YQ, Wang YZ, Gao JR, Li YX,
Liu Q and Xia LZ: Paridis Rhizoma sapoinins attenuates liver
fibrosis in rats by regulating the expression of RASAL1/ERK1/2
signal pathway. J Ethnopharmacol. 192:114–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun H, Che QM, Zhao X and Pu XP:
Antifibrotic effects of chronic baicalein administration in a CCl4
liver fibrosis model in rats. Eur J Pharmacol. 631:53–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vatakuti S, Schoonen WG, Elferink ML,
Groothuis GM and Olinga P: Acute toxicity of CCl4 but not of
paracetamol induces a transcriptomic signature of fibrosis in
precision-cut liver slices. Toxicol In Vitro. 29:1012–1020. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Gao W, Man S, Wang J, Li N, Yin S,
Wu S and Liu C: Pharmacological evaluation of sedative-hypnotic
activity and gastro-intestinal toxicity of Rhizoma Paridis
saponins. J Ethnopharmacol. 144:67–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsushita T, Inoue SI and Tanaka R: An
assay method for determining the total lipid content of fish meat
using a 2-thiobarbituric acid reaction. J Am Oil Chem Soc.
87:963–972. 2010. View Article : Google Scholar
|
|
20
|
Choi JH, Sun WJ, Kim HG, Khanal T, Hwang
YP, Lee KJ, Choi CY, Chung YC, Lee YC and Jeong HG: Platycodi Radix
attenuates dimethylnitrosamine-induced liver fibrosis in rats by
inducing Nrf2-mediated antioxidant enzymes. Food Chem Toxicol.
56:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng Y, Cheung KF, Wang N, Liu P,
Nagamatsu T and Yao T: Chinese medicines as a resource for liver
fibrosis treatment. Chin Med. 4:162009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wells RG: The role of matrix stiffness in
hepatic stellate cell activation and liver fibrosis. J Clin
Gastroenterol. 39 (Suppl 2):S158–S161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Argenio G, Mazzone G, Ribecco MT, Lembo
V, Vitaglione P, Guarino M, Morisco F, Napolitano M, Fogliano V and
Caporaso N: Garlic extract attenuating rat liver fibrosis by
inhibiting TGF-β1. Clin Nutr. 32:252–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding Q, Tian XG, Li Y, Wang QZ and Zhang
CQ: Carvedilol may attenuate liver cirrhosis by inhibiting
angiogenesis through the VEGF-Src-ERK signaling pathway. World J
Gastroenterol. 21:9566–9576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keyse SM: Protein phosphatases and the
regulation of mitogen-activated protein kinase signalling. Curr
Opin Cell Biol. 12:186–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sung YC, Liu YC, Chao PH, Chang CC, Jin
PR, Lin TT, Lin JA, Cheng HT, Wang J, Lai CP, et al: Combined
delivery of sorafenib and a MEK inhibitor using CXCR4-targeted
nanoparticles reduces hepatic fibrosis and prevents tumor
development. Theranostics. 8:894–905. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao Q, Gu Y, Jiang Y, Fan L, Wei Z, Jin H,
Yang X, Wang L, Li X, Tai S, et al: Long non-coding RNA Gm2199
rescues liver injury and promotes hepatocyte proliferation through
the upregulation of ERK1/2. Cell Death Dis. 9:6022018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan LH, Chen XL, Di Y and Liu ML:
CCR7/p-ERK1/2/VEGF signaling promotes retinal neovascularization in
a mouse model of oxygen-induced retinopathy. Int J Ophthalmol.
10:862–869. 2017.PubMed/NCBI
|
|
29
|
Bullard LE, Qi X and Penn JS: Role for
extracellular signal-responsive kinase-1 and −2 in retinal
angiogenesis. Invest Ophthalmol Vis Sci. 44:1722–1731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gressner AM: Transdifferentiation of
hepatic stellate cells (Ito cells) to myofibroblasts: A key event
in hepatic fibrogenesis. Kidney Int Suppl. 54:S39–S45.
1996.PubMed/NCBI
|
|
32
|
Lenz B, Klafki HW, Hillemacher T, Frieling
H, Clepce M, Gossler A, Thuerauf N, Winterer G, Kornhuber J and
Bleich S: ERK1/2 protein and mRNA levels in human blood are linked
to smoking behavior. Addict Biol. 17:1026–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonner JC: Regulation of PDGF and its
receptors in fibrotic diseases. Cytokine Growth Factor Rev.
15:255–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gallini R: A PDGFRalpha perspective on
PDGF signalling in developmental and pathological processes.
Geobiology. 7:360–372. 2014.
|
|
35
|
Chaudhary NI, Roth GJ, Hilberg F,
Müller-Quernheim J, Prasse A, Zissel G, Schnapp A and Park JE:
Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis.
Eur Respir J. 29:976–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosmorduc O, Wendum D, Corpechot C, Galy
B, Sebbagh N, Raleigh J, Housset C and Poupon R: Hepatocellular
hypoxia-induced vascular endothelial growth factor expression and
angiogenesis in experimental biliary cirrhosis. Am J Pathol.
155:1065–1073. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Corpechot C, Barbu V, Wendum D, Kinnman N,
Rey C, Poupon R, Housset C and Rosmorduc O: Hypoxia-induced VEGF
and collagen I expressions are associated with angiogenesis and
fibrogenesis in experimental cirrhosis. Hepatology. 35:1010–1021.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nielsen MJ, Nielsen SH, Hansen NUB,
Kristensen JH, Karsdal MA and Leeming DJ: P0525: N-Acetylated alpha
smooth muscle actin levels are increased in hepatic fibrosis but
decreased in hepatocellular carcinoma. J Hepatology. 62:S5122015.
View Article : Google Scholar
|