Introduction
Arginase is the enzyme that functions in the last
step of the urea cycle to hydrolyzes L-arginine (L-Arg) to
L-ornithine and urea. The arginase isoforms, arginase I and II, are
encoded by distinct genes, show 60% sequence homology, and exhibit
different tissue distribution and subcellular localization
patterns. Arginase I is a cytosolic enzyme that is abundantly
expressed in the liver, whereas arginase II is a mitochondrial
protein that is predominantly expressed in the kidney (1,2). In
addition to its primary function in the urea cycle, arginase
exhibits various biological activities in vascular smooth muscle
cells (VSMCs). In VSMCs of the rat carotid artery, inhibition or
knockdown of arginase I led to attenuated medial and neointimal DNA
synthesis and neointima formation (3). In mouse VSMCs, arginase II is
involved in cell senescence and apoptosis (4). In our previous studies, we
demonstrated that the proliferation of rat VSMCs, induced by the
inflammatory cytokine, interleukin-1β, was suppressed by inhibition
of arginase in a cGMP-dependent manner (5). Inhibition of arginase II also
inhibited Ca2+ uptake into the mitochondria of native
low-density lipoprotein-stimulated human aortic smooth muscle
cells, thus arginase II activity may regulate Ca2+
levels in the cytosol and the mitochondria (6). Furthermore, we recently reported that
inhibition of arginase II activated endothelial nitric oxide
synthase by increasing the cytosolic Ca2+ level
[(Ca2+)] in a p32-dependent manner (7).
The modulation of smooth muscle tone by various
factors in the vasculature plays a role in the control of lumen
diameter, which associated with blood pressure and organ blood flow
(8–10). All smooth muscle cells produce
force and contraction through cross-bridge cycling between actin
and myosin filaments. The contraction response is the result of
myosin-light chain kinase (MLCK) and myosin-light chain phosphatase
(MLCP) activation. In VSMCs, this process is dependent on the
Ca2+-mediated change in the myosin filaments (9). Increased intracellular
Ca2+ is a primary target of the EF-hand
Ca2+-binding protein calmodulin, and then the
Ca2+/calmodulin complex activates MLCK via
Ca2+/calmodulin-dependent protein kinase II (CaMKII)
phosphorylation. Contraction can also occur independent of
Ca2+ through the activation of the RhoA/Rho kinase
pathway, which leads to MLCP inactivation and maintenance of the
contraction (11).
Because the contractile activity of VSMCs plays an
important role in the regulation of vascular tone in the vessels,
its dysregulation can result in endothelial dysfunction leading to
cardiovascular diseases. Therefore, identifying natural compounds
that can effectively inhibit arginase to control this contractile
activity would offer a new strategy for the treatment and
prevention of vascular conditions. Resveratrol (RSV) is a small
polyphenol found in grapes, berries, and peanuts, and is considered
to protect against cardiovascular diseases by reducing platelet
aggregation, low-density lipoprotein oxidation, prostagladin
synthesis, and endothelial cells activation by tumor necrosis
factor-α, as well as by activating and inducing endothelial nitric
oxide synthase (12–14). RSV has been known to activate to
SirT proteins that post-translationally regulate protein activity
by deacetylation, and RSV-dependent activation of SirT has showed a
marked reduction in the signs of aging. On the other hand,
inhibitors of SirT, such as sirtinol and splitomicin, were proposed
to treat cancer and human immunodeficiency virus infection
(15). Moreover, RSV was found to
prevent angiotensin II-stimulated hypertrophy (16) and NADPH oxidase-dependent
proliferation (17), as well as to
induce differentiation to a contractile phenotype in VSMCs
(18). However, the effect of RSV
on the regulation of VSMCs contractility remains unknown. We
hypothesized that RSV regulates VSMCs contractions by inhibiting
arginase. To test this hypothesis, we treated rat VSMCs with RSV
and determined the effects on arginase activity and
[Ca2+]c, as well as the effecs on contraction by
evaluating the change in CaMKII-dependent MLC20 phosphorylation. We
further determined the effects of RSV on vascular contractility
using an ex vivo vessel tension assay with thoracic aortas
of mice. Our results should highlighted the fundamental mechanisms
of VSMCs contractility and the potential role of arginase
inhibition as a clinical treatment.
Materials and methods
Materials
RSV (trans−1,2-(3,4′, 5-Trihydroxydiphenyl)
ethylene; Fig. 1) was purchased
from Tokyo Chemical Industry inc. (Tokyo, Japan) and the purity was
shown to be ≥99% by gas chromatographic analysis.
2(S)-amino-6-boronohexanoic acid (ABH) was purchased from
Calbiochem. Co. (La Jolla, CA, USA). All other reagents were
purchased from Sigma unless otherwise stated.
VSMCs isolation and maintenance
Vascular smooth muscle cells (VSMCs) were isolated
from the thoracic aortas and the upper parts of the abdominal aorta
of 10–12-week old Sprague Dawley male rats (n=4, DBL Co., Chungbuk
Korea) as previously described (19) with minor modifications. Briefly,
the rat were anesthetized with isoflurane, aortas were stripped and
cut into 2 mm pieces that were treated with 1 mg/ml of type II
collagenase (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) for 1 h to remove the endothelial cells, and then the
cells were washed with the culture medum. The de-endothelialized
pieces of aorta were incubated with culture medium in a gelatin
(0.1%)-coated culture dish for approximately 10 d. The VSMCs
cultures were confirmed to be >95% pure by immunocytochemical
staining for α-smooth muscle actin. VSMCs that were passaged 2–5
times were used in the subsequent experiments. The culture medium
consisted of Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS), 0.5X smooth muscle growth
supplement (Cascade Biologics, Portland, OR, USA), 100 U/ml
penicillin, 100 µg/ml streptomycin, 8 mM HEPES, and 2 mM glutamine.
The VSMCs were cultured at 37°C in a humidified 5% CO2
incubator.
Arginase activity assay and
intracellular L-Arg quantification
Arginase activity was determined by quantifying the
urea generated from L-Arg substrate as previously described
(20). Intracellular
concentrations of L-Arg were determined using high-performance
liquid chromatography (HPLC) with pre-column derivatization with
o-phthalaldehyde (OPA) according to a modification of previously
published methods (21). Briefly,
L-Arg (100 µmol/l) and polyamine (30 µmol/l/each) were added to the
cell lysate (0.1 mmol/l) as internal standard. The samples were
extracted on solid-phase extraction cartridges (CBA Bond elute,
Varian), with a recovery rate of 87.5±3.9% to L-Arg. The eluates
were dried over nitrogen and resuspended in double-distilled water
for HPLC analysis. HPLC was performed on a computer-controlled
Waters chromatography system (M600E) consisting of an automatic
injector (M7725i, Waters Co.) and a fluorescence detector (FP-1520,
Jasco Co.). Samples were incubated for 1 min with OPA reagent (5.4
mg/ml OPA in borate buffer, pH 8.4, with 0.4% 2-mercaptoethanol)
before automatic injection into the HPLC system. The OPA
derivatives of L-Arg and polyamine were separated on a 150×4.6 mm-5
µm Zorbax Eclipse XDB-C18 column with the fluorescence detector set
at an excitation of 340 nm and an emission of 450 nm. Samples were
eluted from the column with 0.96% citric acid:methanol (70:30), pH
6.8, at a flow rate of 1.5 ml/min.
Cytosolic Ca2+ measurement
using confocal microscopy and flow cytometry
Direct assessment of the [Ca2+]c was
peformed using an established procedure in which cells were loaded
with the fluorescent agent Fluo-4 AM (ThermoFisher Scientific Co.,
Waltham, MA). Briefly, the cells were incubated with 100 nmol/l
Fluo-4 AM at 37°C for 1 h in a starvation medium. The cells were
then washed to remove excess Fluo-4 AM and incubated in Tyrode's
modified solution (150 mmol/l NaCl, 4 mmol/l KCl, 2 mmol/l
CaCl2, 2 mmol/l MgCl2, 10 mmol/l HEPES, and
10 mmol/l glucose). For detection of Fluo-4 AM fluorescence, 494 nm
excitation, and 506 nm emission filters were used. Intensity values
were normalized according to the initial fluorescence values after
subtraction of the background signal using the Metamorph program
(Molecular Probe). The [Ca2+]c was also determined using
flow cytometry on a FACS Calibur system. The fluorescence intensity
for each sample was determined using CellQuest software, and the
Ca2+ level was calculated by comparing the fluorescence
intensities of treated cells vs. control cells.
MTT assay
The cytotoxic activities of the tested compounds
were measured using MTT 3
(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-based
colorimetric assay. MTT was purchased from Sigma. In brief,
1×104 cells per well were seeded in 96-well plates and
allowed to grow for 24 h. The tested compounds were added to the
wells at the indicated concentrations and the plates were incubated
for an additional 48 h. Then, MTT solution (5 mg/ml) was added and
the plates were incubated for an additional 4 h. The experiment was
performed in triplicate and cell viability was determined by
measuring the optical density at 570 nm.
Western blotting analysis
Cells were lysed in SDS sample buffer (62.5 mmol/l
Tris, pH 6.8, 2% SDS, and 10% glycerol) and sonicated for 5 sec to
reduce sample viscosity. Each sample was resolved by 10% SDS-PAGE,
and transferred to PVDF membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Membranes were incubated with primary
antibodies against phospho-CaMKII, CaMKII, phospho-MLC20, and
MLC-20 (BD Biosciences, San Jose, CA) according to the
manufacturer's protocol and were visualized with peroxidase and
enhanced chemiluminescence (Thermo Fisher Scientific, Inc.). The
phosphorylation levels of the proteins were normalized to the total
protein levels. Densitometry analysis of bands was performed using
ImageJ software (National Institute of Health, Bethesda, MD,
USA).
Vascular tension assay
Thoracic aortas were dissected from C57BL/6 mice
that had been anesthetized with isoflurane, and the fat and
connective tissues were removed from the aortas. The aortas were
cut into 1.5-mm rings, and endothelia were gently removed using a
wooden stick. The aorta sections were then suspended between two
wire stirrups (150 µm) in 10 ml Krebs-ringer solution (95%
O2, 5% CO2, pH 7.4, 37°C) in a myograph
(Multi myograph system DMT-620). One stirrup was connected to a
three-dimensional micromanipulator, and the other was connected to
a force transducer. The rings were passively stretched in 100-mg
increments at 10-min intervals to reach the optimal tone (600 mg).
After the arterial rings had been stretched to their optimal
resting tone, the contractile response to 100 mM KCl was
determined. Responses to the maximal KCl dose were used to
normalize the agonist responses across vessel rings. Dose responses
to the vasoconstrictor phenylephrine (PE;
10−10−10−6 M) were also determined.
Statistical analysis
Data are reported as the mean ± SD. Statistical
significance was determined using the Bonferroni-corrected unpaired
t test for unpaired values. A P-value <0.05 was considered
statistically significance. Dose responses were analyzed using
two-way or one-way analysis of variance (ANOVA) followed by
Bonferroni's post-hoc test, using Graphpad prism 5.0 software.
Results
RSV shows arginase inhibitory
activity
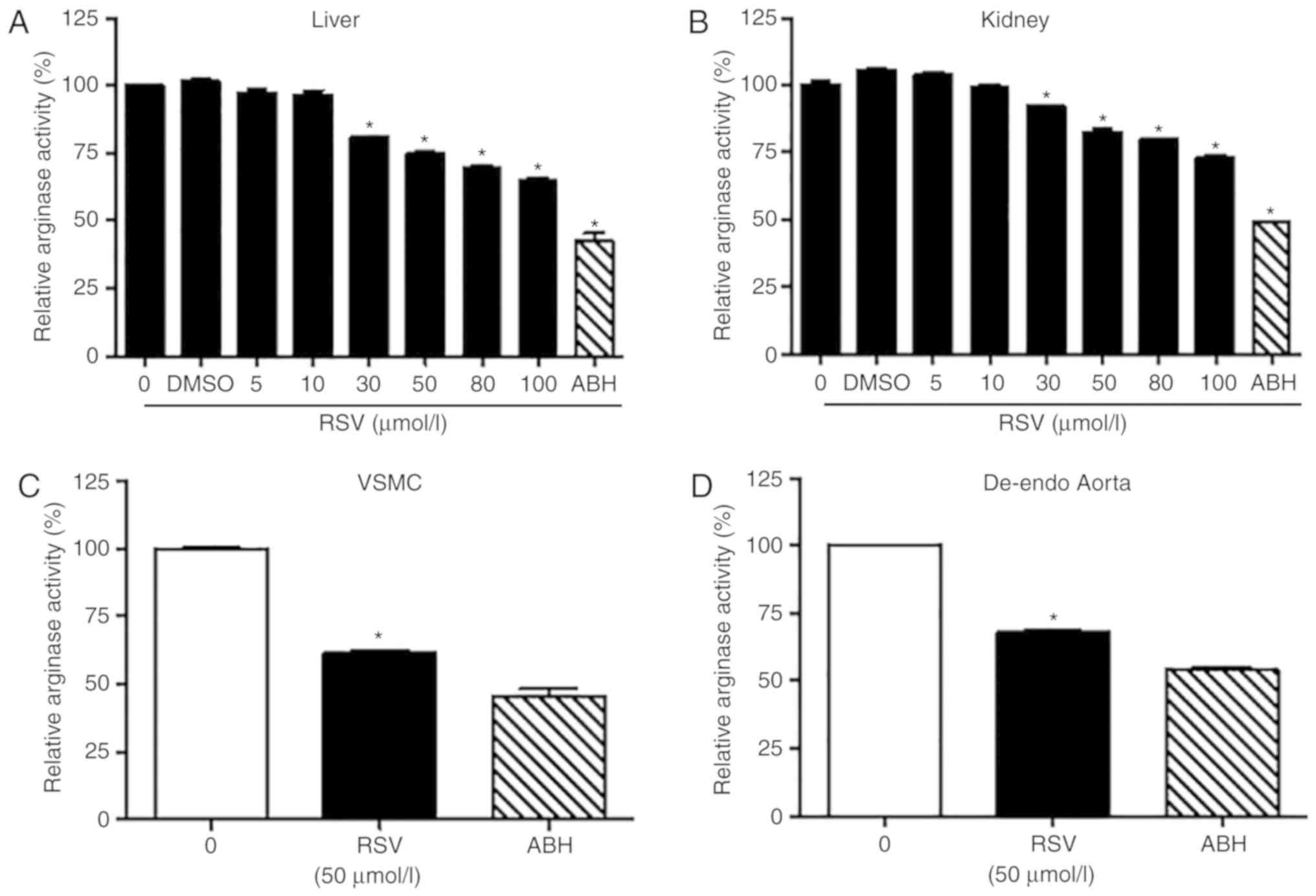
Arginase I and II solutions were prepared from liver
and kidney lysates, respectively, and ability of RSV to inhibit
arginase activity was measured. RSV inhibited arginase I and II
activities in a dose-dependent manner (Fig. 2A and B). At 50 µmol/l of RSV, the
residual activities of arginase I and arginase II were 75.1±1.8 and
82.5±5.8%, respectively. However, ABH, a known arginase inhibitor,
strongly inhibited arginase I and II activity at only 10 µmol/l
(residual arginase I and II activities were 48.9±1.6 and 43.1±2.6%,
respectively). Because effects have preiously been observed with
20% inhibition of arginase activity (20), we used 50 µmol/l RSV for subsequent
experiments. In VSMCs culture and in de-endothelialized aortic
vessels of mice, 50 µmol/l RSV inhibited arginase activity 38.7±3.1
and 32.0±1.3%, respectively (Fig. 2C
and D).
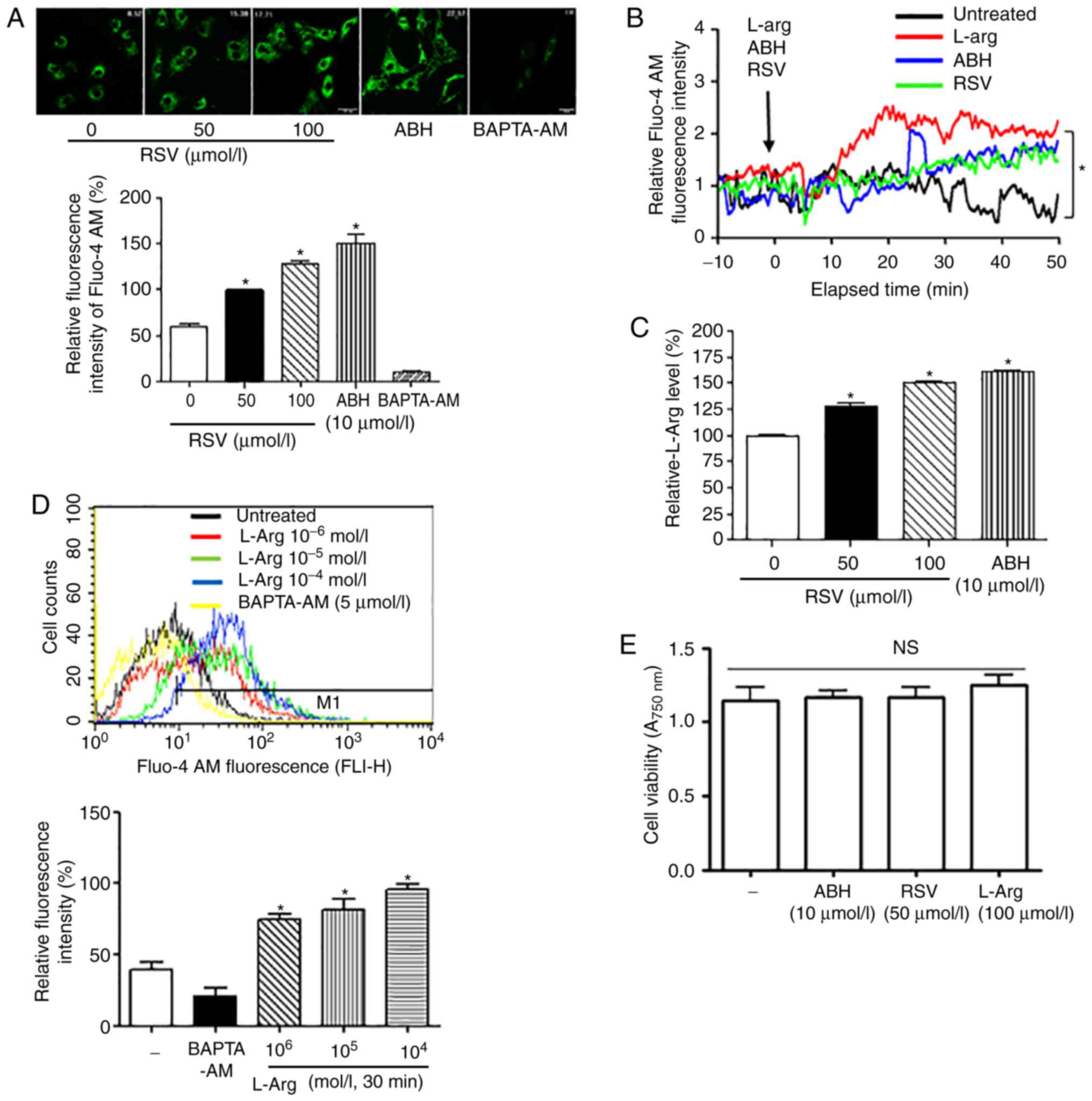
Treatment with RSV increased the
intracellular level of L-Arg, thereby raising the
[Ca2+]c in VSMCs
Because inhibition of arginase induced CaMKII
phosphorylation (22), we
determined whether treatment of RSV would change the
[Ca2+]c level. Both RSV and ABH treatments increased the
[Ca2+]c in VSMCs as observed by microscopy (Fig. 3A; *vs. untreated, P<0.05;
untreated, 60.6±10.6%; RSV 50 µmol/l, 100±2.8%) and real-time
measurement (Fig. 3B; *P<0.05).
Treatment with RSV also increased the intracellular L-Arg
concentration in VSMCs (Fig. 3C;
*vs. untreated, P<0.05; untreated, 100.0±2.8%; RSV 50 µmol/l,
128.5±9.6%). To confirm the effect of arginase inhibition on the
[Ca2+]c, we examined the change in [Ca2+]c
following treatment with L-Arg using flow cytometry. Incubation
with L-Arg significantly increased the [Ca2+]c in a
dose-dependent manner, and BAPTA-AM significantly attenuated the
fluorescent signal of Fluo-4 AM (Fig.
3D; *vs. untreated, P<0.05, untreated vs. L-Arg at
10−6 mol/l, 37.0±5.0 vs. 72.0±3.5%). These results
indicated that the increased intracellular L-Arg concentration,
which results from arginase inhibition by RSV, plays a crucial role
in the augmentation of [Ca2+]c. In addition, RSV, ABH,
and L-Arg, did not show cytoxic effects on VSMCs (Fig. 3E, ns, not significant).
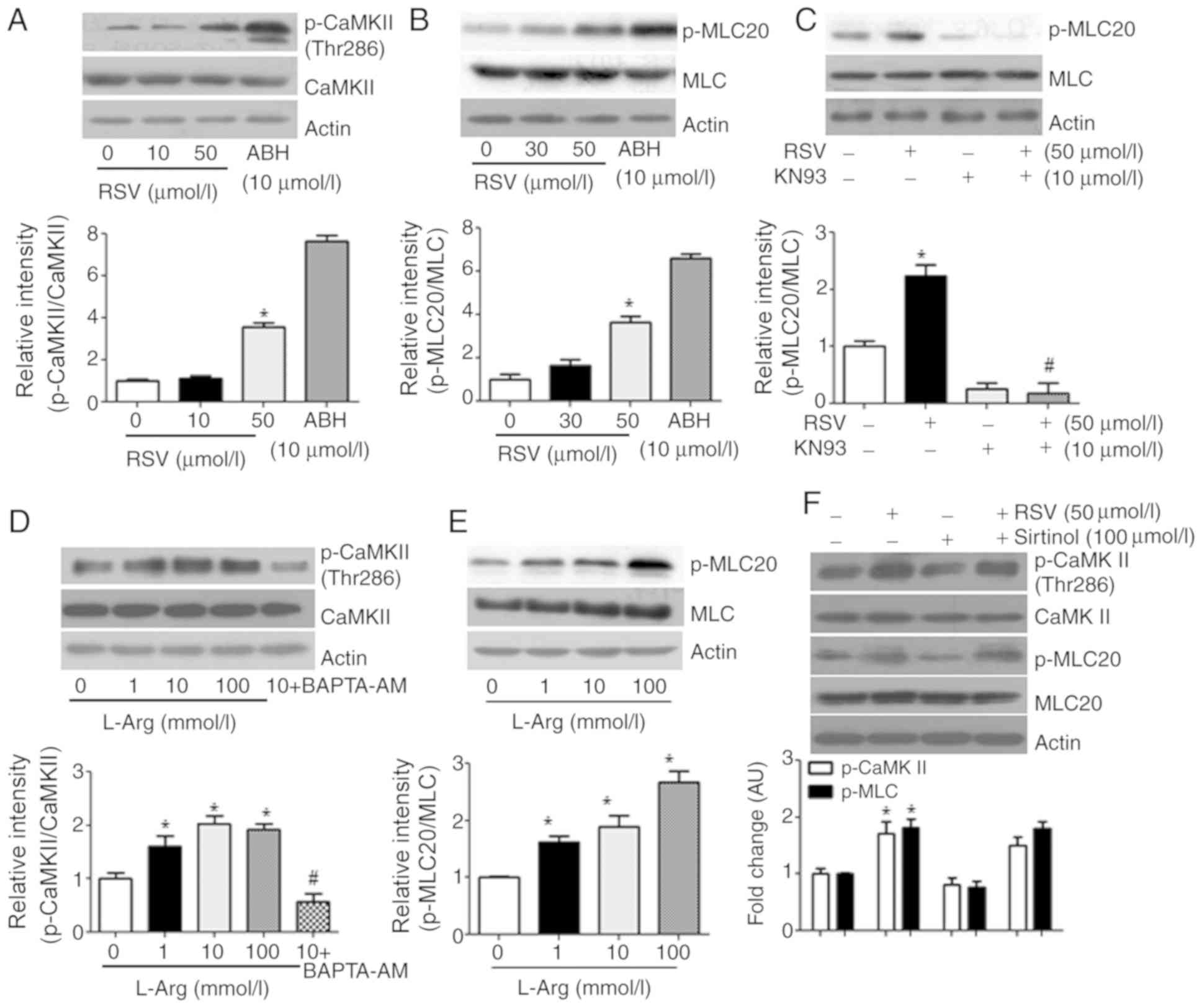
CaMKII activation by RSV associated
with MLC20 phosphorylation
Because RSV increased the [Ca2+]c by an
L-Arg-dependent mechanism, we examined whether RSV may activate
CaMKII. Indeed, 50 µmol/l RSV induced CaMKII phosphorylation at
Thr286 (Fig. 4A; *vs. untreated,
1.0±0.1 vs. 3.5±0.2 AU, P<0.05), which associated with
phosphorylation of MLC20 (Fig. 4B,
*vs. untreated, 1.0±0.2 vs. 3.6±0.3 AU, P<0.05; Fig. 4C, *vs. untreated, 1.0±0.1 vs.
2.2±0.2 AU, P<0.05; #vs. RSV, 1.8±0.1 vs. 2.2±0.2 AU,
P<0.01). Consistently, incubation of VSMCs with L-Arg also
increased the phosphorylation of CaMKII Thr286 (Fig. 4D, *L-Arg (1 mmol/l) vs. untreated,
1.6±0.2 vs. 1.0±0.1 AU, P<0.05; #vs. L-Arg (1
mmol/l), 0.57±0.1 vs. 1.6±0.2 AU, P<0.01) and MLC20 (Fig. 4E; *L-Arg (1 mmol/l) vs. untreated,
1.6±0.2 vs. 1.0±0.01 AU, P<0.05). We also tested whether the
effect of RSV may be derived from activation of Sirt proteins by
treating in VSMCs with RSV and sirtinol, an inhibitor of Sirt
proteins, and we found that the RSV-induced changes in CaMKII and
MLC20 phosphorylation levels were not influenced by sirtinol
(Fig. 4F; phospho-CaMKII, *vs.
untreated control, 1.7±0.2 vs. 1.0±0.1 AU, P<0.01,
#vs. RSV, 1.7±0.2 vs. 1.5±0.15 AU, not significant;
phospho-MLC20, *vs. untreated control, 1.8±0.1 vs. 1.0±0.01 AU,
P<0.01, #vs. RSV, 1.8±0.1 vs. 1.8±0.1 AU, not
significant).
RSV augmented vessel constriction in
de-endothelialized aortas
Because RSV treatment increased MLC20
phosphorylation in VSMCs, we measured the effect of RSV on vascular
tension using de-endothelialized aortas of wild-type (WT) mice. The
de-endothelialized aortic vessels did not respond to the
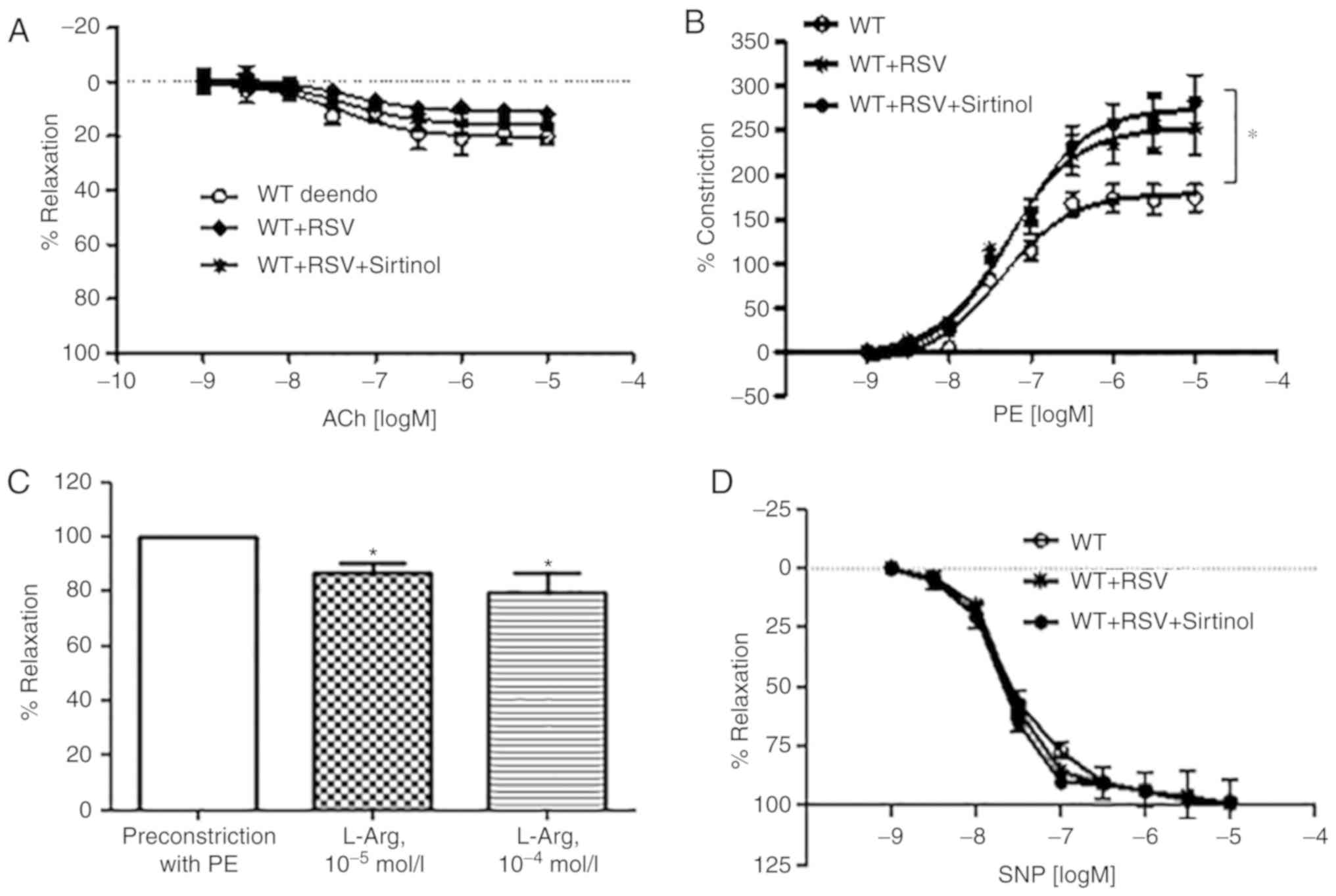
endothelium-dependent vasorelaxant acetylcholine (Ach, Fig. 5A). However, dose responses to PE
(Fig. 5B), an
endothelium-independent vasoconstrictor, were enhanced in
RSV-treated vessels (Emax, WT + de-endo vs. WT + RSV + de-endo,
Emax, 179.2±6.6 vs. 253.4±9.8%, logEC50, −7.3±0.1 vs.
−7.2±0.1 mol/l, *P<0.05), and preincubation with 10 µmol/l of
sirtinol did not change the RSV-induced effects (Emax, WT + RSV vs.
WT + RSV + sitinol, Emax, 253.4±9.8 vs. 275.4±9.7%,
logEC50,-7.2±0.1 vs. −7.2±0.1 mol/l, not significant).
Interestingly, treatment of de-endothelialized aortic vessels of WT
mice with L-Arg induced vasoconstriction [Fig. 5C; L-Arg (10−5 mol/l) vs.
PE-preconstriction, 86.6±12.0 vs. 100±0.1%; L-Arg (10−4
mol/l) vs. PE-preconstriction, 79.4±13.2 vs. 100±0.1%, *P<0.05].
However, there was no significant difference in the dose responses
to the nitric oxide (NO) donor sodium nitroprusside (SNP) among the
treatment groups (Fig. 5D).
Additionally, the vasoconstrictive effect of RSV did not differ
upon short-term incubation (4 h) and long-term incubation (16
h).
Discussion
In this study, we demonstrated that RSV at
relatively higher concentrations inhibited arginase I and II
activities and consequently increased the concentration of the
substrate, L-Arg. Interestingly, RSV also increased
[Ca2+]c in VSMCs in a manner dependent on the L-Arg
concentration. The increased [Ca2+]c led to
CaMKII-dependent MLC20 phosphorylation, which was mimicked with
L-Arg stimulation in VSMCs. These RSV-induced effects were
maintained when VSMCs were treated with sirtinol, an inhibitor of
Sirt proteins. Moreover, in a vascular tension assay with
de-endothelialized aortic vessels, vasoconstrictor responses to PE
were significantly enhanced in both RSV- and L-Arg-treated
vessels.
An increase in the level of L-Arg, which results
from arginase inhibition, is beneficial because L-Arg augments
endothelial NO production, which regulates vasoreactivity, platelet
activation, adhesion molecules expression, monocytes infiltration
into the intima, apoptosis of endothelial cells, and proliferation
and migration of VSMCs. We previously reported that inhibition of
arginase II suppresses proliferation of VSMCs, which were
stimulated with native low-density lipoprotein, through NADPH
oxidase inactivation (23) and
interleukin-8 production through p38 MAPK inactivation (6). However, L-Arg supplementation
exhibited harmful effects for phathophysiological conditions, such
as diabetic nephropathy (24),
hypertension (25), and peripheral
artery disease (26). Consistent
with these findings, in this study, we found that arginase
inhibition with RSV led to vessels constriction, which may cause
aggravation of a pathological condition in vasculature with
endothelial dysfunction.
RSV, a polyphenol molecule containing two phenyl
rings connected by a methylene bridge (Fig. 1), was the first compound discovered
that could mimic calorie restriction that occurs when sirtuins are
stimulated (27,28). Treatment of RSV was shown to
improves the general health of mice that were fed high-calorie diet
(29); these mice showed a marked
reduction in the signs of aging, including reduced albuminuria and
cataracts formation, decreased inflammation and apoptosis in the
vascular endothelium, increased aortic elasticity, greater motor
coordination, and preserved bone mineral density (30). Furthermore, RSV has been shown to
have several effects on VSMCs (16–18),
however, these studies used various RSV concentrations and found
different concentration-dependent effects. In one study, at a low
dose of RSV, VSMCs differentiation was found to be induced by
SirT1-dependent Akt2 activation, whereas a high dose of RSV
stimulated AMPK, which inhibited the mTORC1 pathway, thereby
relieving the S6K1 inhibition and allowing Akt activation and the
induction of differentiation (18). Although RSV can induce a multiple
signaling cascades, the molecular target of RSV at a high dose has
not been clearly demonstrated to date. Here, we demonstrate that
arginase II, which regulates the mTORC1/S6K1 cascade and AMPK
pathway, may be a target of RSV at a high concentration (31).
In VSMCs, [Ca2+]c is required to maintain
the basal vascular tone, which is modulated by Ca2+
release from the intracellular stores in the sarcoplasmic reticulum
and mitochondria and by Ca2+ entry from the
extracellular space through Ca2+ channels in the plasma
membrane. We found that RSV increased the [Ca2+]c and
the L-Arg level by inhibiting arginase (Fig. 3). Previously, RSV was associated
with intracellular Ca2+ signaling because of the direct
rise in [Ca2+]c (32–34),
which was proposed to originate from the intracellular
Ca2+ store (33).
Interestingly, the endoplasmic reticulum (ER) Ca2+
content in prostate cancer cell lines PC3 and DU145 decreased upon
treatment with RSV (35).
Collectively, these findings suggest that RSV increases in
[Ca2+]c through activation of the IP3
receptor in the ER.
Polyphenols, such as RSV (3,4′,5-trihydroxystilbene)
and piceatannol (3,3′,4′,5-tetrahydroxystilbene, a hydroxylated
analogue of RSV), are secondary metabolites present in many fruits,
vegetables, and medicinal plants. Numerous epidemiological and
experimental studies strongly indicate their potential in the
treatment of chronic diseases, including vascular and cardiac
diseases, obesity, diabetes, and cancer. The positive effects of
polyphenols are partly due to increase in NO production (36). In our previous studies, piceatannol
inhibited the activity of arginase isoforms and reciprocally
regulated the NO production by endothelial NO synthase (37). Because piceatannol showed stronger
arginase inhibitory activity than RSV, it may be useful to compare
two polyphenols for structure-based drug design. RSV is generally
accepted as a beneficial natural product to treat cardiovascular
disorders because increases production of NO, which is a
vasoprotective molecule. The maximal concentration of RSV in
commercial red wine was reported to be 11.2 mg/l (37), which is equivalent to 50 µmol/l.
However, patients with vascular disorders, such as hypertension,
should be careful when taking RSV because RSV may exacerbate blood
pressure as shown in this study. However, in healthy obese man, 150
mg/day of a nutraceutical formulation RSV has been shown to have
beneficial effects, which mimic the effects of calorie restriction
(38).
In conclusion, RSV at a high concentration inhibited
arginase activity and increased the L-Arg level leading to enhanced
Ca2+ signaling by the activation of CaMKII in VSMCs. In
turn, RSV-dependent CaMKII activation elicited MLC20
phosphorylation that augmented vasoconstriction in
de-endothelialized vessels; however, Sirt protein activation by RSV
was not involved in the Ca2+-dependent constriction.
Therefore, although arginase inhibition has shown beneficial
effects for various diseases, care is required when considering the
administration of an arginase inhibitor to patients with vessels
with endothelial dysfunction.
Acknowledgements
The authors would like to thank the Central
Laboratory of Kangwon National University (Chuncheon, Korea) for
technical assistance with the instruments.
Funding
This study was supported by the Basic Science
Research Program of the National Research Foundation of Korea (NRF)
funded by the Ministry of Education, Science and Technology (grant
nos. 2015M3A9B6066968, 2016M3A9B6903185 and 2018R1D1A1B07047959),
and by a 2017 Research Grant from Kangwon National University
(grant no. 2017-S.R).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CIC, BHK, and DH performed the experiments. HJK,
KLH, MHW, and YMK analyzed the data and wrote the manuscript. HKL
and SR designed the study and wrote the manuscript.
Ethics approval and consent to
participate
All experiments were performed with approval of the
Ethics Committee of Kangwon National University (Chuncheon,
Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morris SM Jr: Arginine metabolism:
Boundaries of our knowledge. J Nutr. 137 (6 Suppl 2):1602S–1609S.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pernow J and Jung C: Arginase as a
potential target in the treatment of cardiovascular disease:
Reversal of arginine steal? Cardiovasc Res. 98:334–343. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peyton KJ, Ensenat D, Azam MA, Keswani AN,
Kannan S, Liu XM, Wang H, Tulis DA and Durante W: Arginase promotes
neointima formation in rat injured carotid arteries. Arterioscler
Thromb Vasc Biol. 29:488–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong Y, Yu Y, Montani JP, Yang Z and Ming
XF: Arginase-II induces vascular smooth muscle cell senescence and
apoptosis through p66Shc and p53 independently of its l-arginine
ureahydrolase activity: Implications for atherosclerotic plaque
vulnerability. J Am Heart Assoc. 2:e0000962013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoon J and Ryoo S: Arginase inhibition
reduces interleukin-1β-stimulated vascular smooth muscle cell
proliferation by increasing nitric oxide synthase-dependent nitric
oxide production. Biochem Biophys Res Commun. 435:428–433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koo BH, Yi BG, Jeong MS, Kwon SH, Hoe KL,
Kwon YG, Won MH, Kim YM and Ryoo S: Arginase II inhibition prevents
interleukin-8 production through regulation of p38 MAPK
phosphorylation activated by loss of mitochondrial membrane
potential in nLDL-stimulated hAoSMCs. Exp Mol Med. 50:e4382018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koo BH, Hwang HM, Yi BG, Lim HK, Jeon BH,
Hoe KL, Kwon YG, Won MH, Kim YM, Berkowitz DE and Ryoo S: Arginase
II contributes to the Ca2+/CaMKII/eNOS axis by regulating Ca2+
concentration between the cytosol and mitochondria in a
p32-dependent manner. J Am Heart Assoc. 7:e0095792018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jackson WF: Ion channels and vascular
tone. Hypertension. 35:173–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hilgers RH and Webb RC: Molecular aspects
of arterial smooth muscle contraction: Focus on Rho. Exp Biol Med
(Maywood). 230:829–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Woodrum DA and Brophy CM: The paradox of
smooth muscle physiology. Mol Cell Endocrinol. 177:135–143. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wynne BM, Chiao CW and Webb RC: Vascular
smooth muscle cell signaling mechanisms for contraction to
angiotensin II and endothelin-1. J Am Soc Hypertens. 3:84–95. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitt CA and Dirsch VM: Modulation of
endothelial nitric oxide by plant-derived products. Nitric Oxide.
21:77–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Opie LH and Lecour S: The red wine
hypothesis: From concepts to protective signalling molecules. Eur
Heart J. 28:1683–1693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leifert WR and Abeywardena MY:
Cardioprotective actions of grape polyphenols. Nutr Res.
28:729–737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Villalba JM and Alcain FJ: Sirtuin
activators and inhibitors. Biofactors. 38:349–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haider UG, Sorescu D, Griendling KK,
Vollmar AM and Dirsch VM: Resveratrol suppresses angiotensin
II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation
and subsequent hypertrophy in rat aortic smooth muscle cells. Mol
Pharmacol. 62:772–777. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ushio-Fukai M, Griendling KK, Becker PL,
Hilenski L, Halleran S and Alexander RW: Epidermal growth factor
receptor transactivation by angiotensin II requires reactive oxygen
species in vascular smooth muscle cells. Arterioscler Thromb Vasc
Biol. 21:489–495. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thompson AM, Martin KA and Rzucidlo EM:
Resveratrol induces vascular smooth muscle cell differentiation
through stimulation of SirT1 and AMPK. PLoS One. 9:e854952014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Lindstedt KA and Kovanen PT: Mast
cell granule remnants carry LDL into smooth muscle cells of the
synthetic phenotype and induce their conversion into foam cells.
Arterioscler Thromb Vasc Biol. 15:801–810. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryoo S, Gupta G, Benjo A, Lim HK, Camara
A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, et al:
Endothelial arginase II: A novel target for the treatment of
atherosclerosis. Circ Res. 102:923–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Böger RH, Bode-Böger SM, Mügge A, Kienke
S, Brandes R, Dwenger A and Frölich JC: Supplementation of
hypercholesterolaemic rabbits with L-arginine reduces the vascular
release of superoxide anions and restores NO production.
Atherosclerosis. 117:273–284. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen MC and Ryoo S: Intravenous
administration of piceatannol, an arginase inhibitor, improves
endothelial dysfunction in aged mice. Korean J Physiol Pharmacol.
21:83–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koo BH, Yi BG, Wang WK, Ko IY, Hoe KL,
Kwon YG, Won MH, Kim YM, Lim HK and Ryoo S: Arginase inhibition
suppresses native low-density lipoprotein-stimulated vascular
smooth muscle cell proliferation by NADPH oxidase inactivation.
Yonsei Med J. 59:366–375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
You H, Gao T, Cooper TK, Morris SM Jr and
Awad AS: Diabetic nephropathy is resistant to oral L-arginine or
L-citrulline supplementation. Am J Physiol Renal Physiol.
307:F1292–F1301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brooks WW, Conrad CH, Robinson KG, Colucci
WS and Bing OH: L-arginine fails to prevent ventricular remodeling
and heart failure in the spontaneously hypertensive rat. Am J
Hypertens. 22:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilson AM, Harada R, Nair N,
Balasubramanian N and Cooke JP: L-arginine supplementation in
peripheral arterial disease: No benefit and possible harm.
Circulation. 116:188–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wood JG, Rogina B, Lavu S, Howitz K,
Helfand SL, Tatar M and Sinclair D: Sirtuin activators mimic
caloric restriction and delay ageing in metazoans. Nature.
430:686–689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pearson KJ, Baur JA, Lewis KN, Peshkin L,
Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et
al: Resveratrol delays age-related deterioration and mimics
transcriptional aspects of dietary restriction without extending
life span. Cell Metab. 8:157–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiong Y, Yepuri G, Forbiteh M, Yu Y,
Montani JP, Yang Z and Ming XF: ARG2 impairs endothelial autophagy
through regulation of MTOR and PRKAA/AMPK signaling in advanced
atherosclerosis. Autophagy. 10:2223–2238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sareen D, Darjatmoko SR, Albert DM and
Polans AS: Mitochondria, calcium, and calpain are key mediators of
resveratrol-induced apoptosis in breast cancer. Mol Pharmacol.
72:1466–1475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garcia-Sanchez L, Santofimia-Castaño P,
Miro-Moran A, Tapia JA, Salido GM and Gonzalez A: Resveratrol
mobilizes Ca2+ from intracellular stores and induces c-Jun
N-terminal kinase activation in tumoral AR42J cells. Mol Cell
Biochem. 362:15–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang HJ, Chou CT, Chang HT, Liang WZ,
Hung TY, Li YD, Fang YC, Kuo CC, Kuo DH, Shieh P and Jan CR:
Mechanisms of resveratrol-induced changes in cytosolic free calcium
ion concentrations and cell viability in OC2 human oral cancer
cells. Hum Exp Toxicol. 34:289–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Selvaraj S, Sun Y, Sukumaran P and Singh
BB: Resveratrol activates autophagic cell death in prostate cancer
cells via downregulation of STIM1 and the mTOR pathway. Mol
Carcinog. 55:818–831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bordage S, Pham TN, Zedet A, Gugglielmetti
AS, Nappey M, Demougeot C and Girard-Thernier C: Investigation of
mammal arginase inhibitory properties of natural ubiquitous
polyphenols by using an optimized colorimetric microplate assay.
Planta Med. 83:647–653. 2017.PubMed/NCBI
|
|
37
|
Woo A, Min B and Ryoo S:
Piceatannol-3′-O-beta-D-glucopyranoside as an active component of
rhubarb activates endothelial nitric oxide synthase through
inhibition of arginase activity. Exp Mol Med. 42:524–532. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Timmers S, Konings E, Bilet L, Houtkooper
RH, van de Weijer T, Goosens GH, Hoeks J, van der Krieken S, Ryu D,
Kersten S, et al: Calorie restriction-like effects of 30 days of
resveratrol supplementation on energy metabolism and metabolic
profile in obese humans. Cell Metab. 14:612–622. 2011. View Article : Google Scholar : PubMed/NCBI
|