Introduction
Osteoporosis is a health and socioeconomic problem
characterized by low bone mass and deteriorated bone
microarchitecture, which increases the risk of fragility fractures
(1,2). In the European Union (EU), the
prevalence rate of osteoporosis in individuals aged ≥50 years was
estimated at 6.6 and 22.1% for men and women in 2010, respectively.
Economically, the total cost of osteoporosis to the EU was ~€37.4
billion in 2010 (3). Owing to the
increase in life expectancy and a growing aging population, an
increasing number of people may suffer from osteoporotic fractures
in the future (4). Osteoporosis is
caused by an imbalance between bone formation, mediated by
osteoblasts, and resorption, mediated by osteoclasts (5). Available osteoporosis treatments
include anti-resorption drugs (such as bisphosphonates and
denosumab), calcitonin and estrogen. However, these compounds
exhibit certain limitations; in particular, they decrease the
osteogenic turnover rate, and by decreasing the bone-remodeling
process, they cause a decrease in bone formation (6). Estrogen therapy is not ideal for
long-term osteoporosis therapy, since high levels of estrogen may
induce uterine bleeding, breast cancer and cardiovascular diseases
(7). In addition, anti-resorption
drugs are not able to restore lost bone structure. However,
anabolic agents may stimulate bone formation and increase bone mass
(8). Therefore, it is important to
identify novel safe and effective drugs able to promote bone
formation.
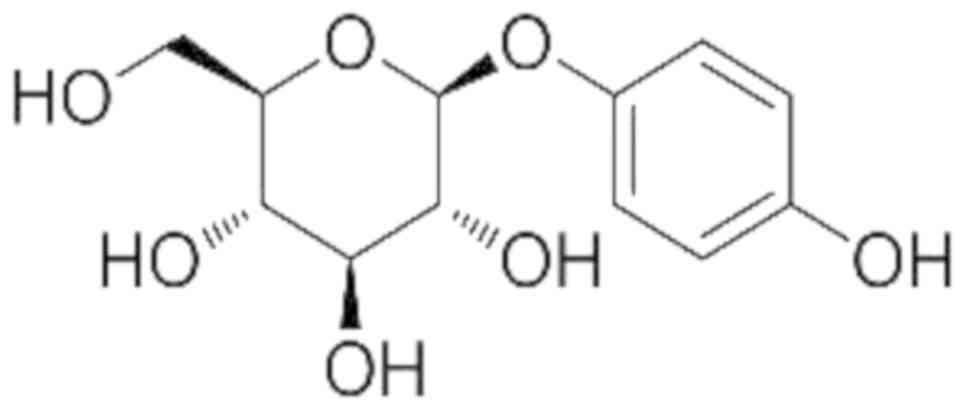
Arbutin (4-hydroxyphenyl-β-D-glucopyranoside;
Fig. 1) is a naturally occurring
hydroquinone derivative (molecular mass 272 Da) present in various
types of plants. High levels of arbutin were identified in plants
including, marjoram (Origanum majorana, Lamiaceae) and pears
(Pyrus communis, Rosaceae) and, in particular, the Ericaceae
family such as bearberry leaves (Arctostaphylos uva-ursi)
(9). Arbutin exhibits various
biological activities. For example, arbutin may be used as a skin
whitening agent; by inhibiting the tyrosinase activity in
melanosomes, arbutin was identified to promote depigmentation
(10,11). A previous study demonstrated that
arbutin may serve a protective role from X-irradiation-induced
apoptosis by decreasing the intracellular levels of hydroxyl
radicals (12). Additionally,
arbutin may inhibit osteoclast differentiation by decreasing the
intracellular levels of superoxide and by downregulating nuclear
factor of activated T cells 1 (13). However, the effects of arbutin on
osteoblast function remain unknown. Therefore, the present study
aimed to investigate the effects and the mechanisms of arbutin on
MC3T3-E1 mouse osteoblast precursor cell proliferation and
differentiation.
The Wnt signaling pathway may affect osteoblast and
osteoclast, directly and indirectly, increasing bone formation and
decreasing bone resorption (14).
Canonical Wnt signaling may regulate proliferation, differentiation
and function of osteoblast at multiple levels (15). In animal models, decreasing the
activity of inhibitors of the Wnt/β-catenin signaling pathway by
using antibodies against secreted frizzled protein-related protein
1, sclerostin and dickkopf WNT signaling pathway inhibitor 1
(DKK1), and small-molecule inhibitors of glycogen synthase kinase 3
β (GSK-3β) may increase bone mass and decrease the risk of
fractures (16). Therefore, the
Wnt signaling pathway represents a potential therapeutic target for
the development of novel drugs to treat osteoporosis (17). In the present study, the effects of
arbutin on MC3T3-E1 cell proliferation and differentiation were
investigated. In addition, the molecular mechanism underlying
arbutin function in inducing MC3T3-E1 cell differentiation was
examined.
Materials and methods
Chemicals
Arbutin (purity, ≥98%) was purchased from Dalian
Meilun Biotech Co., Ltd. (Dalian, China), dissolved in dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
stored at a concentration of 0.5 M. Recombinant human DKK1 was
purchased from PeproTech, Inc. (Rocky Hill, NJ, USA; cat. no.
120–30).
Cell culture
MC3T3-E1 mouse calvarial pre-osteoblasts were
purchased from The Cell Resource Center of the Shanghai Institutes
for Biological Sciences of The Chinese Academy of Sciences
(Shanghai, China), and were cultured in α-Minimum Essential Medium
(α-MEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Biological
Industries, Kibbutz Beit Haemek, Israel), 100 µg/ml streptomycin
and 100 U/ml penicillin (HyClone; GE Healthcare Life Sciences).
Cells were maintained in a cell culture incubator with 5%
CO2 at 37°C. The medium was replaced every other day.
Cells at 80% confluence were reseeded into tissue culture flasks
following treatment with 0.25% trypsin (HyClone; GE Healthcare Life
Sciences) for 1–2 min at 37°C. For osteoblastic differentiation
experiments, cells were treated with osteogenic supplement
containing 50 µg/ml L-ascorbic acid (Sigma-Aldrich; Merck KGaA) and
10 mM β-glycerophosphate disodium salt hydrate (Sigma-Aldrich;
Merck KGaA) for 9 days at 37°C. For mechanistic studies, MC3T3-E1
cells were pretreated with DKK1 (0.5 µg/ml) for 6 h at 37°C, and
were then treated with arbutin (100 µM) for 3 days at 37°C.
Cell proliferation
A Cell Counting Kit-8 assay (CCK-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was used to assess
the effects of arbutin on cell proliferation. Cells were seeded in
96-well plates at a density of 5×103 cells/well for 24 h
at 37°C. Subsequently, cells were treated with arbutin at various
concentrations (0, 10, 50 and 100 µM). After 24, 48 or 72 h, cells
were treated with a 9.1% CCK-8 solution containing 10 µl CCK-8 and
100 µl α-MEM for 1–2 h at 37°C. The optical density value of each
well was measured using a microplate reader (ELx808; BioTek
Instruments, Inc., Winooski, VT, USA) at a wavelength of 450 nm.
Relative cellular viability was calculated as the ratio between the
mean absorbance of the sample and the control.
5-ethynyl-2′-deoxyuridine (EdU)
labeling assay
The effect of arbutin on cell proliferation was
measured using an EdU Apollo®567 in vitro Imaging
kit (Guangzhou RiboBio Co., Ltd., Guangzhou, China). Cells were
inoculated in a 96-well plate at a density of 1×103
cells/well and incubated in α-MEM containing 10% FBS with 0, 10, 50
or 100 µM arbutin. Following a 72-h incubation, EdU was added to
each well at a concentration of 50 µM prior to an additional
incubation for 2 h at 37°C. Cells were washed twice with PBS and
fixed with PBS containing 4% paraformaldehyde for 30 min at room
temperature (RT). Following washing with glycine (2 mg/ml) and PBS,
cells were permeabilized with Triton X-100 (0.5%) for 10 min at RT.
Cells were incubated with 1X Apollo staining reaction liquid at RT
for 30 min in the dark. Cell nuclei were counterstained with 1X
Hoechst 33342 for 30 min at RT. EdU-positive cells were visualized
by fluorescence microscopy (magnification, ×200; Eclipse Ti; Nikon
Corporation, Tokyo, Japan) in five randomly selected fields.
Cell cycle and apoptosis analysis
MC3T3-E1 cells were seeded in six-well plates at a
density of 2×105 cells/well. Following a 24-h
incubation, cells were treated with arbutin at concentrations of 0,
10, 50 and 100 µM. Cells were harvested after 3 days at RT and
fixed with 70% ethanol for 12 h at 4°C. Cells were washed three
times with PBS and stained with propidium iodide (PI) staining
solution (Beyotime Institute of Biotechnology, Haimen, China) for
30 min at 37°C in the dark. DNA content was measured using a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) with
CellQuest Pro software (Version 5.2; BD Biosciences) and ModFit LT
software (Version 3.0; Verity Software House, Inc., Topsham, ME,
USA). For apoptosis analysis, cells treated with arbutin were
harvested and stained with fluorescein isothiocyanate-labeled
Annexin-V and PI (Dojindo Molecular Technologies, Inc.) in the dark
for 15 min at RT. The cell apoptotic rate was assessed using a
FACSCalibur flow cytometer (BD Biosciences) with CellQuest Pro
software (Version 5.2; BD Biosciences).
Alkaline phosphatase (ALP) staining
assay
Osteoblasts were seeded in 6-well plates at a
density of 5×104 cells/well incubated in α-MEM
containing osteogenic supplement and treated with 0 (control), 10,
50 or 100 µM arbutin. After 9 days at 37°C, cells were washed three
times with PBS and fixed in 4% paraformaldehyde at RT for 10 min.
Cells were rinsed three times with distilled water and subsequently
stained using the 5-bromo-4-chloro-3-indolyl phosphate/nitro blue
tetrazolium chloride ALP color development kit (Beyotime Institute
of Biotechnology) for 2 h at RT. Stained cells were imaged using a
light microscope (magnification, ×40; Eclipse Ti; Nikon
Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells were seeded in 6-well plates at a density of
2×105 cells/well. Following treatment with arbutin at
various concentrations for 3 days at 37°C, total RNA was extracted
from MC3T3-E1 cells using RNAiso Plus reagent (Takara Biotechnology
Co., Ltd., Dalian, China). In total, 1 µg RNA was reverse
transcribed into cDNA using a PrimeScript RT reagent kit with gDNA
Eraser (Takara Biotechnology Co., Ltd.), according to the
manufacturer's instructions. The reaction conditions were as
follows: 42°C for 2 min, 37°C for 15 min and 85°C for 5 sec. qPCR
was performed using equal amounts of cDNA from each sample in a
total volume of 20 µl with an ABI 7500 Fast Real-Time PCR System
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) using SYBR
premix Ex Taq II (Takara Biotechnology Co., Ltd.). The
following thermocycling conditions were used: Initial denaturation
at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and
60°C for 34 sec. Specificity of the amplification was assessed by
melting curve analysis, and β-actin served as an internal control.
Relative gene expression levels were analyzed using the
2−ΔΔCq method (18).
The sequences of the primers used were the following: Runt-related
transcription factor 2 (RUNX2), forward
5′-CCAACCGAGTCATTTAAGGCT-3′, reverse 5′-GCTCACGTCGCTCATCTTG-3′;
collagen type I α 1 chain (COL1A1), forward
5′-GCCTCCCAGAACATCACCTA-3′, reverse 5′-GCAGGGACTTCTTGAGGTTG-3′;
bone γ-carboxyglutamate protein (BGLAP), forward
5′-CGCTACCTTGGAGCCTCAGT-3′, reverse 5′-AGGCGGTCTTCAAGCCATAC-3′; Sp7
transcription factor (SP7), forward 5′-AAGGTGTACGGCAAGGCTTC-3′,
reverse 5′-CGTCAGAGCGAGTGAACCTC-3′; β-catenin, forward
5′-ATGGAGCCGGACAGAAAAGC-3′, reverse 5′-CTTGCCACTCAGGGAAGGA-3′;
β-actin, forward 5′-GGCTGTATTCCCCTCCATCG-3′, reverse
5′-CCAGTTGGTAACAATGCCATGT-3′.
Western blot analysis
MC3T3-E1 cells were seeded in 6-well plates at a
density of 2×105 cells/well. Cells were treated with
arbutin at various concentrations for 3 days and rinsed three times
with ice-cold PBS. Total cellular protein was extracted from
MC3T3-E1 cells using radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology) containing 1 mM
phenylmethylsulfonyl fluoride. Proteins were isolated following a
centrifugation at 13,800 × g for 15 min at 4°C. Protein
concentration was quantified using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). Equal amounts of
protein (20–30 µg) in each sample were separated by 10% SDS-PAGE
for 2 h at a constant voltage (110 V) and transferred onto a
polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica,
MA, USA). Membranes were blocked in TBS + Tween-20 (TBST; 20 mM
Tris-HCl, 150 mM NaCl pH 7.5 and 0.1% Tween-20) containing 5%
non-fat milk at RT for 2 h and then incubated overnight at 4°C with
appropriate primary antibodies. The antibodies used were the
following: Rabbit monoclonal anti-β-catenin (1:5,000; cat. no.
ab32572; Abcam, Cambridge, MA, USA), rabbit polyclonal anti-RUNX2
(1:1,000; cat. no. ab23981; Abcam) and mouse polyclonal
anti-β-actin (1:1,000; cat. no. AF0003; Beyotime Institute of
Biotechnology). Subsequently, membranes were washed three times
with TBST, and the PVDF membranes were incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG;
1:10,000; cat. no. ZB-2301; OriGene Technologies, Inc., Beijing,
China) or HRP-conjugated goat anti-mouse IgG (1:10,000; cat. no.
ZB-2305; OriGene Technologies, Inc.) at RT for 2 h. Proteins were
visualized using Enhanced Chemiluminescence reagents (Thermo Fisher
Scientific, Inc.) and detected with a chemiluminescence detection
system (Amersham Imager 600; GE Healthcare Life). Proteins were
quantitated using ImageJ software (version 1.52; National
Institutes of Health, Bethesda, MD, USA). Following normalization,
relative protein expression levels were calculated with β-actin as
an internal control.
Statistical analysis
All experiments were repeated independently at least
three times. Statistical analyses were performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data are
presented as the mean ± standard deviation, and significant
differences were analyzed by one-way analysis of variance coupled
with Dunnett's post hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Arbutin promotes MC3T3-E1 cell
proliferation
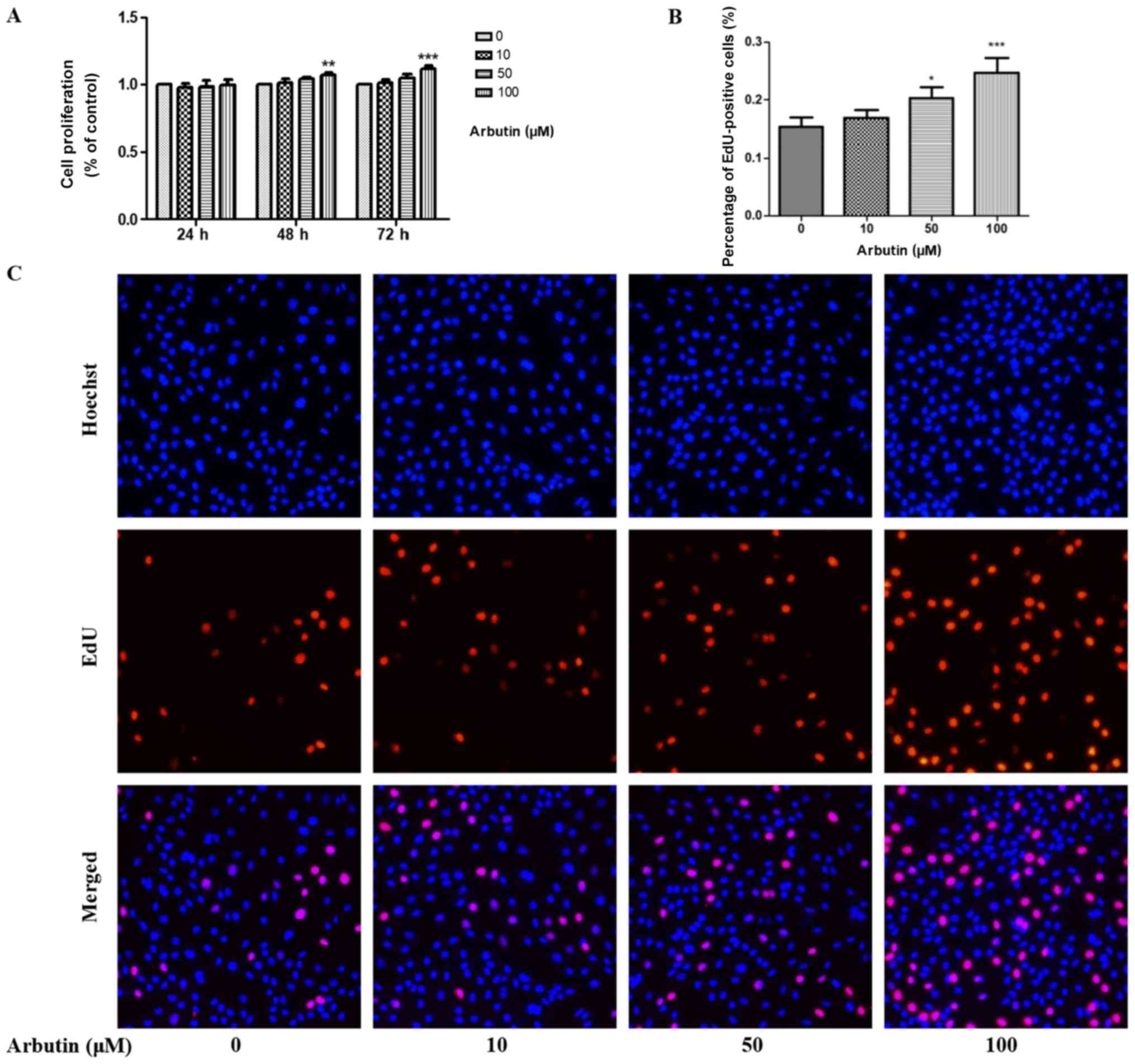
The effects of arbutin on MC3T3-E1 cell
proliferation were examined using a CCK-8 assay. Arbutin was
administered at various concentrations (0, 10, 50, and 100 µM) for
24, 48 and 72 h and CCK-8 assay was performed (Fig. 2A). After 24 h, no statistical
differences were identified in osteoblast proliferation compared
with the untreated control cells. However, MC3T3-E1 cell
proliferation was significantly increased following treatment with
arbutin at 100 µM after 48 and 72 h. EdU labeling assay was
performed after 72 h, and the results demonstrated that the
percentage of EdU-positive MC3T3-E1 cells treated with arbutin at a
concentration of 50 and 100 µM was significantly increased compared
with the untreated control (Fig. 2B
and C).
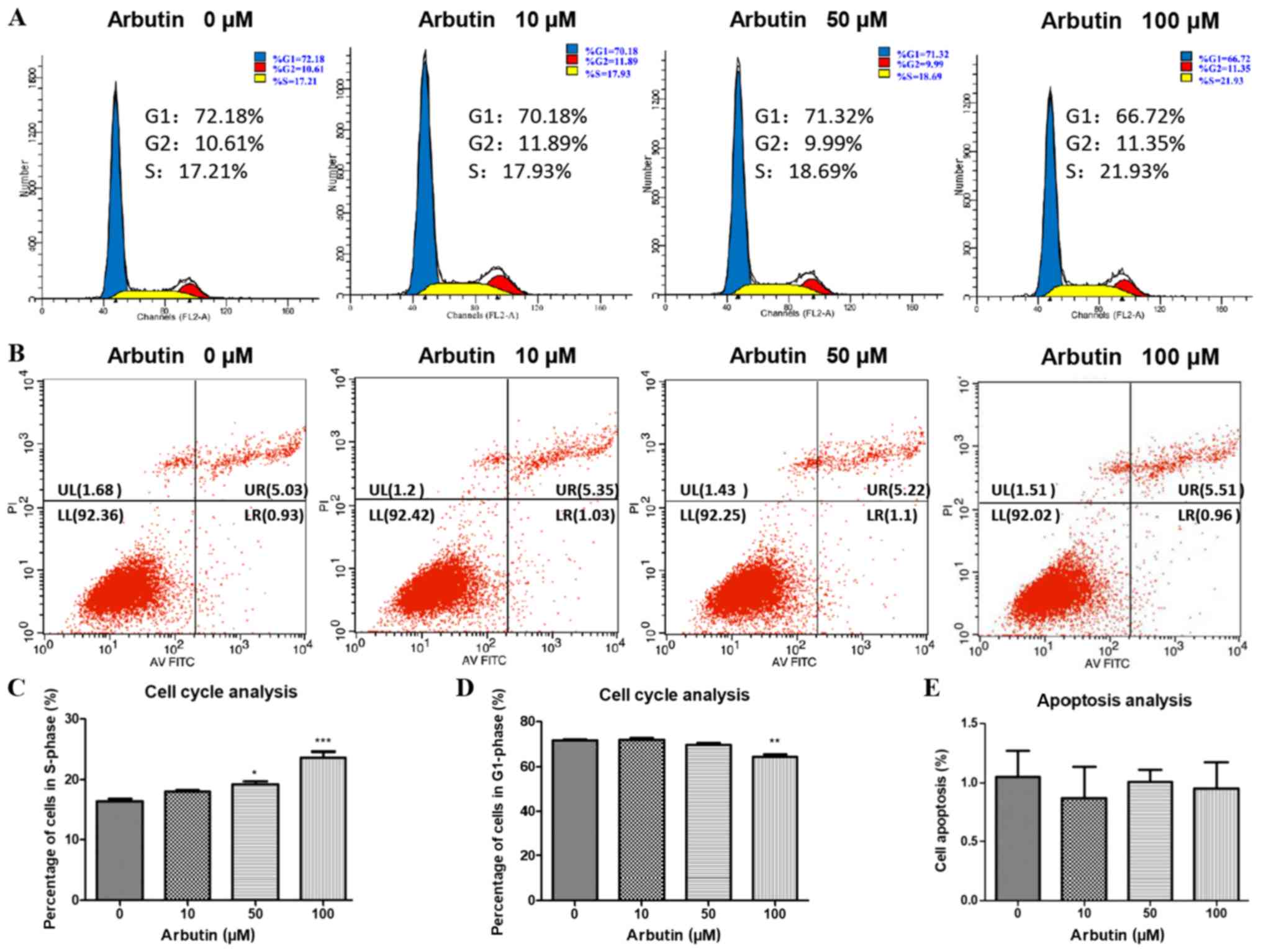
Arbutin accelerates cell cycle
progression
The effects of arbutin on cell cycle progression was
assessed using cell cycle analysis (Fig. 3). Treatment with arbutin at 50 and
100 µM led to an increase in the percentage of cells in S-phase
(Fig. 3A and C) and 100 µM arbutin
led to a decrease in the percentage of cells in G1-phase (Fig. 3D). No statistically significant
differences were identified in the 10 µM group compared with the
control group. The effects of arbutin on MC3T3-E1 apoptosis was
also assessed using flow cytometry (Fig. 3B and E). The rate of apoptosis was
not significantly altered following treatment with arbutin at 10,
50 and 100 µM compared with the control.
Effects of arbutin on ALP
activity
The effects of arbutin on osteoblast differentiation
was analyzed by ALP staining. After 9 days, treatment with various
concentrations of arbutin (0, 10, 50 or 100 µM) notably increased
ALP activity compared with the control group (Fig. 4A). The present findings suggested
that arbutin may increase the activity of ALP in MC3T3-E1
cells.
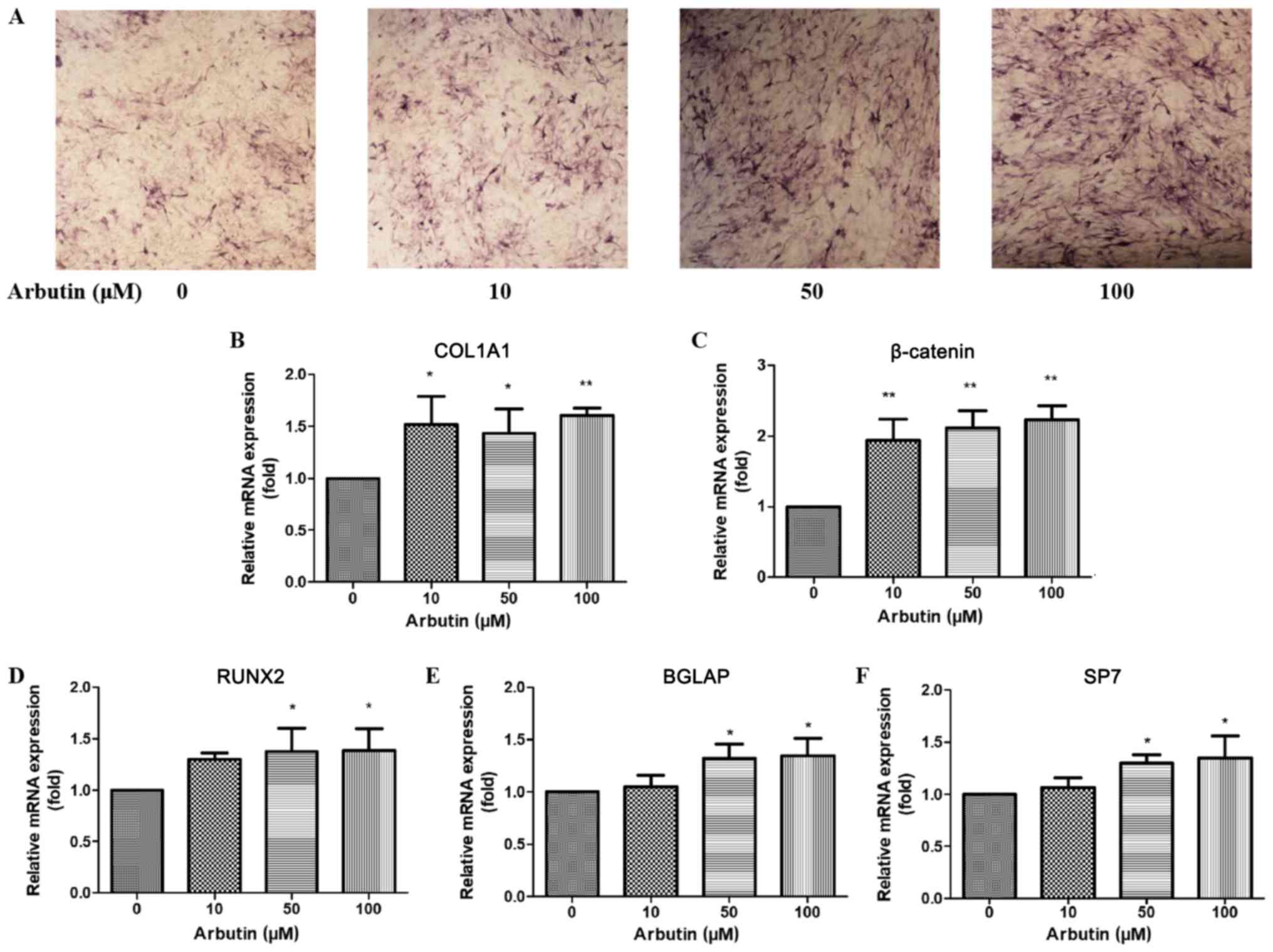 | Figure 4.Effects of arbutin on osteogenic
differentiation. (A-F) MC3T3-E1 mouse osteoblast precursor cells
were cultured in differentiation medium and treated with 0
(control), 10, 50 or 100 µM arbutin for (A) 9 or (B-F) 3 days. (A)
ALP-positive cells are stained in blue or purple. The effects of
arbutin on the mRNA expression levels of (B) COL1A1, (C) β-catenin,
(D) RUNX2, (E) BGLAP and (F) SP7 in MC3T3-E1 cells. mRNA expression
levels were assessed by reverse transcription-quantitative
polymerase chain reaction analysis; β-actin served as the internal
control. *P<0.05 and **P<0.01 vs. untreated control. ALP,
alkaline phosphatase; COL1A1, collagen type I α 1 chain; BGLAP,
bone γ-carboxyglutamate protein; SP7, Sp7 transcription factor;
RUNX2, runt-related transcription factor 2. |
Effects of arbutin on the mRNA
expression levels of COL1A1, β-catenin, RUNX2, BGLAP and SP7
The mRNA expression levels of COL1A1, β-catenin,
RUNX2, BGLAP and SP7 were assessed in MC3T3-E1 cells using RT-qPCR
following treatment with arbutin at various concentrations (0, 10,
50 or 100 µM) for 3 days. COL1A1 and β-catenin expression levels
were increased in osteoblasts treated with 10, 50 and 100 µM
arbutin compared with untreated cells (Fig. 4B and C). The expression levels of
RUNX2, BGLAP and SP7 were significantly increased following
treatment with arbutin at 50 and 100 µM (Fig. 4D-F).
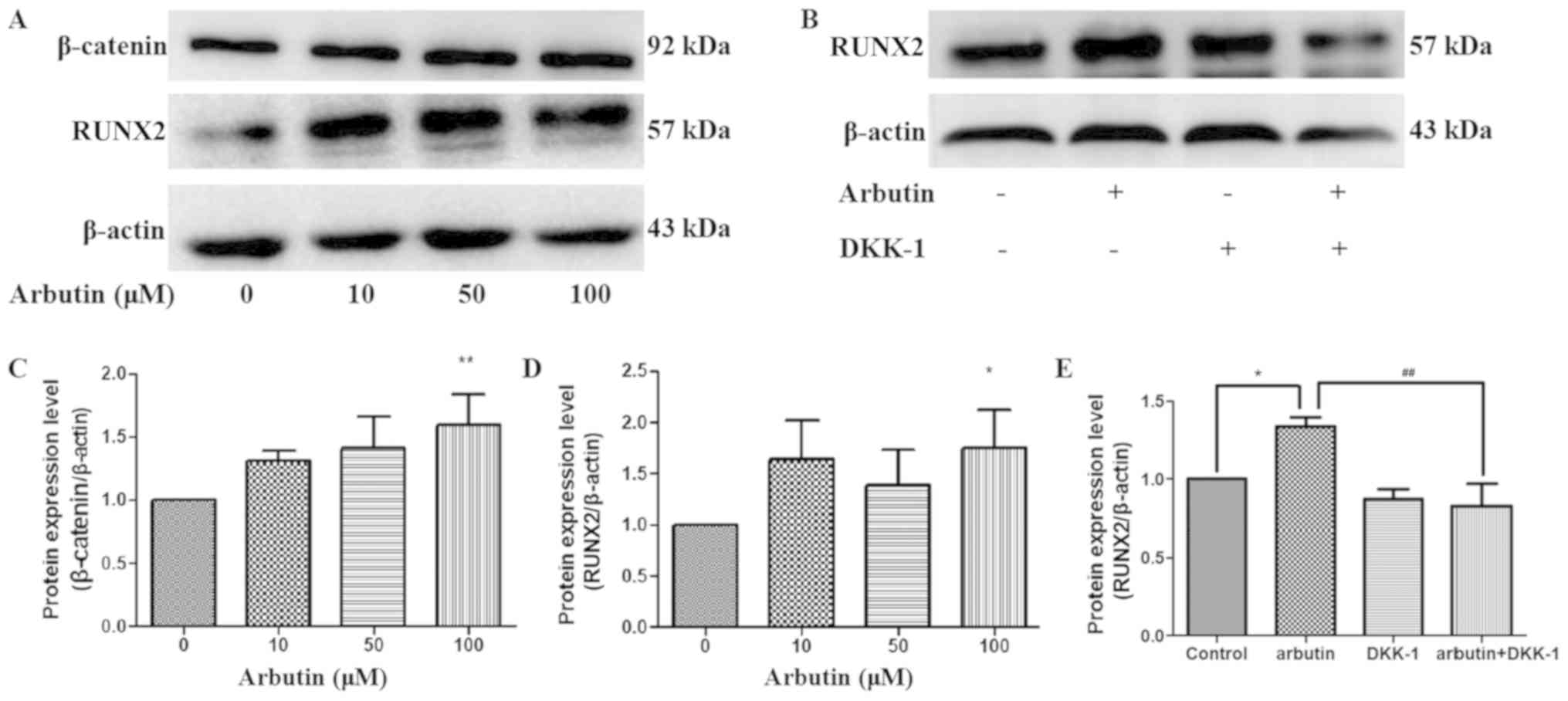
Effects of arbutin on protein
expression levels of β-catenin and RUNX2
To investigate the underlying mechanisms of
arbutin-induced osteoblast differentiation, the protein expression
levels of RUNX2 and β-catenin were examined in MC3T3-E1 cells by
western blotting (Fig. 5A and B).
Treatment with arbutin at 100 µM significantly increased the
protein expression levels of β-catenin and RUNX2 in osteoblasts
compared with controls (Fig. 5C and
D, respectively). The present results suggested that arbutin
may affect osteoblast differentiation by regulating the protein
expression levels of β-catenin and RUNX2. To investigate whether
arbutin stimulates osteoblastic differentiation via the
Wnt/β-catenin signaling pathway, MC3T3-E1 cells were treated with
0.5 µg/ml DKK1 for 6 h prior to treatment with 100 µM arbutin. DKK1
significantly inhibited arbutin-induced RUNX2 protein expression
(Fig. 5E). The present results
suggested that arbutin may affect osteoblast differentiation via
the Wnt/β-catenin signaling pathway.
Discussion
Osteoporosis may result in serious fractures and
disabilities, and represents an age-associated problem worldwide
(19). Accumulating evidence
demonstrated that an imbalance between osteoclasts and osteoblasts
in bone formation and resorption may lead to osteoporosis (20). Notably, bone formation depends on
osteoblast proliferation and differentiation (21,22).
Multiple available drugs used to treat osteoporosis
are antiresorptive medications (23); however, these treatments are not
able to reverse bone loss (24).
Anabolic agents that stimulate bone formation may restore severely
damaged skeletal microstructure and loss of bone mass (24). Teriparatide is one anabolic agent
that was demonstrated to stimulate bone formation, and clinical
trials demonstrated that treatment with teriparatide decreased the
risk of new vertebral fractures and increased bone mineral density
in the hip, lumbar spine and femoral neck (25). Notably, teriparatide is an
expensive treatment. Therefore, it is important to develop novel
drugs that are able to effectively promote bone formation.
Arbutin is a cytoprotective agent and does not
exhibit significant cytotoxic effects at high concentrations
(26). Although the blood
concentration of arbutin is unclear (13), arbutin is used as a urinary
antimicrobial medicine and is considered as a safe oral agent
(27,28). Previous studies demonstrated that
arbutin may exhibit multiple activities, including skin whitening
(10,11), anti-inflammatory (29), anticancer (30) and suppression of osteoclast
differentiation (13). However,
further studies are required to investigate the effects of arbutin
on osteoblasts and its potential to be used as a novel compound for
the treatment of osteoporosis. Therefore, the present study
investigated the effects of arbutin on the proliferation and
differentiation of MCET3-E1 cells and the mechanisms underlying
arbutin function in vitro.
Bone formation is related to the number of
osteoblasts and the activity of single osteoblasts (31). The number of osteoblasts can be
increased by promoting pre-osteoblast replication or
differentiation, or by reducing cell death of mature osteoblasts
(32). In the present study,
arbutin increased proliferation of MC3T3-E1 cells without affecting
the apoptotic rate. In addition, arbutin increased cell cycle
progression by shifting cells from G1-phase to S-phase. These
results suggested that arbutin may induce osteoblastic
proliferation.
ALP is an early marker of osteogenic differentiation
(33). ALP is associated with
calcification of the skeleton during bone formation (34). In the present study, ALP staining
results suggested that osteoblasts treated with arbutin at 10, 50
and 100 µM, and with osteogenic supplement for 9 days exhibited an
increased activity of ALP, suggesting that arbutin may promote the
early differentiation of osteoblasts.
Osteogenesis has been demonstrated to be regulated
by various transcription factors, including RUNX2 and SP7, and
multiple bone-specific matrix proteins, including ALP, BGLAP and
COL1A1 (35). RUNX2 and SP7 are
important transcription factors involved in osteoblast
differentiation during bone formation (36). COL1A1 is an extracellular matrix
protein that promotes bone regeneration and osteoblast
differentiation (37). BGLAP is a
non-collagenous bone matrix protein that regulate bone turnover and
bone mineralization (38). The
present study identified that arbutin may induce the mRNA
expression levels of important osteogenic regulators, including
RUNX2, BGLAP, SP7 and COL1A1. Additionally, the expression level of
β-catenin was increased. These results suggested that arbutin may
promote osteoblastic differentiation through a mechanism involving
Wnt/β-catenin signaling.
The Wnt signaling pathway influences bone formation
during development, and bone remodeling during tissue renewal
(39). The canonical Wnt signaling
pathway was identified to be initiated by Wnt ligand signaling at
the cell surface through low-density lipoprotein receptor-related
protein 5 or 6 (Lrp5/6) and seven-pass transmembrane Frizzled
receptor (40). The interaction
between Wnt ligands and their receptor may inhibit GSK3-β in the
cytoplasm, leading to the release of β-catenin, the transcriptional
mediator of canonical Wnt signaling. Following release, β-catenin
is able to enter the nucleus, thus controlling the expression
levels of its target genes (41).
RUNX2 belongs to the Runt domain gene family and is a transcription
factor involved in osteoblastic differentiation (42). RUNX2 serves an important role in
coordinating multiple signaling pathways during osteoblast
differentiation (43). A previous
study demonstrated that canonical Wnt signaling may directly
regulate RUNX2. Specifically, the β-catenin/HNF1 homeobox A complex
may activate the expression level of RUNX2, thus promoting bone
formation (44). DKK1 is a
powerful inhibitor of the Wnt/β-catenin canonical pathway, by
binding to Lrp5/6 (45,46). The results of the present study
suggested that treatment with arbutin significantly increased the
protein expression levels of RUNX2 and β-catenin in MC3T3-E1 cells,
and DKK1 significantly decreased the protein expression level of
RUNX2. The present results suggested that arbutin may promote
MC3T3-E1 cell differentiation via the canonical Wnt/β-catenin
pathway.
Collectively, to the best of the authors' knowledge,
the present study is the first to indicate that arbutin may
stimulate proliferation and differentiation of MC3T3-E1 cells via
the Wnt/β-catenin signaling pathway. Therefore, arbutin may
represent a novel potential candidate for osteoporosis treatment.
However, further studies are required to identify the specific role
of the Wnt/β-catenin signaling pathway in arbutin-induced
osteogenesis, including the phosphorylation of β-catenin at Ser675
(47); further information
regarding the role of Wnt/β-catenin signaling may be achieved using
Wnt agonists. In the future, further studies may investigate the
ability of arbutin to promote bone formation in vivo.
Acknowledgements
Not applicable.
Funding
The present work was supported by The Major Program
of the National Nature Science Foundation of China (grant no.
81370981) and The Outstanding Scientific Fund of Shengjing
Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QF, XM and LiY conceived and designed the
experiments. XM performed the experiments and wrote the manuscript.
SL, LiY, LeY and ML analyzed the data and critically revised the
manuscript. QF supervised all research and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Das S and Crockett JC: Osteoporosis-a
current view of pharmacological prevention and treatment. Drug Des
Devel Ther. 7:435–448. 2013.PubMed/NCBI
|
|
2
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hernlund E, Svedbom A, Ivergård M,
Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B and Kanis
JA: Osteoporosis in the European Union: Medical management,
epidemiology and economic burden. A report prepared in
collaboration with the international osteoporosis foundation (IOF)
and the European federation of pharmaceutical industry associations
(EFPIA). Arch Osteoporos. 8:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cauley JA: Public health impact of
osteoporosis. J Gerontol A Biol Sci Med Sci. 68:1243–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manolagas SC and Jilka RL: Bone marrow,
cytokines, and bone remodeling. Emerging insights into the
pathophysiology of osteoporosis. N Engl J Med. 332:305–311. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baron R and Hesse E: Update on bone
anabolics in osteoporosis treatment: Rationale, current status, and
perspectives. J Clin Endocrinol Metab. 97:311–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang DZ, Hou W, Zhou Q, Zhang M, Holz J,
Sheu TJ, Li TF, Cheng SD, Shi Q, Harris SE, et al: Osthole
stimulates osteoblast differentiation and bone formation by
activation of beta-catenin-BMP signaling. J Bone Miner Res.
25:1234–1245. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Canalis E: Update in new anabolic
therapies for osteoporosis. J Clin Endocrinol Metab. 95:1496–1504.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lamien-Meda A, Lukas B, Schmiderer C,
Franz C and Novak J: Validation of a quantitative assay of arbutin
using gas chromatography in Origanum majorana and
Arctostaphylos uva-ursi extracts. Phytochem Anal.
20:416–420. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim YJ, Lee EH, Kang TH, Ha SK, Oh MS, Kim
SM, Yoon TJ, Kang C, Park JH and Kim SY: Inhibitory effects of
arbutin on melanin biosynthesis of alpha-melanocyte stimulating
hormone-induced hyperpigmentation in cultured brownish guinea pig
skin tissues. Arch Pharm Res. 32:367–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maeda K and Fukuda M: Arbutin: Mechanism
of its depigmenting action in human melanocyte culture. J Pharmacol
Exp Ther. 276:765–769. 1996.PubMed/NCBI
|
|
12
|
Wu LH, Li P, Zhao QL, Piao JL, Jiao YF,
Kadowaki M and Kondo T: Arbutin, an intracellular hydroxyl radical
scavenger, protects radiation-induced apoptosis in human lymphoma
U937 cells. Apoptosis. 19:1654–1663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omori A, Yoshimura Y, Deyama Y and Suzuki
K: Rosmarinic acid and arbutin suppress osteoclast differentiation
by inhibiting superoxide and NFATc1 downregulation in RAW 264.7
cells. Biomed Rep. 3:483–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baron R and Rawadi G: Targeting the
Wnt/beta-catenin pathway to regulate bone formation in the adult
skeleton. Endocrinology. 148:2635–2643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoeppner LH, Secreto FJ and Westendorf JJ:
Wnt signaling as a therapeutic target for bone diseases. Expert
Opin Ther Targets. 13:485–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Williams BO and Insogna KL: Where Wnts
went: The exploding field of Lrp5 and Lrp6 signaling in bone. J
Bone Miner Res. 24:171–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin X, Xiong D, Peng YQ, Sheng ZF, Wu XY,
Wu XP, Wu F, Yuan LQ and Liao EY: Epidemiology and management of
osteoporosis in the People's Republic of China: Current
perspectives. Clin Interv Aging. 10:1017–1033. 2015.PubMed/NCBI
|
|
20
|
Manolagas SC: Birth and death of bone
cells: Basic regulatory mechanisms and implications for the
pathogenesis and treatment of osteoporosis. Endocr Rev. 21:115–137.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu S, Fang T, Yang L, Chen Z, Mu S and Fu
Q: Gastrodin protects MC3T3-E1 osteoblasts from
dexamethasone-induced cellular dysfunction and promotes bone
formation via induction of the NRF2 signaling pathway. Int J Mol
Med. 41:2059–2069. 2018.PubMed/NCBI
|
|
22
|
Yun HM, Park KR, Quang TH, Oh H, Hong JT,
Kim YC and Kim EC: 2,4,5-Trimethoxyldalbergiquinol promotes
osteoblastic differentiation and mineralization via the BMP and
Wnt/β-catenin pathway. Cell Death Dis. 6:e18192015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cosman F, Nieves JW and Dempster DW:
Treatment sequence matters: Anabolic and antiresorptive therapy for
osteoporosis. J Bone Miner Res. 32:198–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uihlein AV and Leder BZ: Anabolic
therapies for osteoporosis. Endocrinol Metab Clin North Am.
41:507–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura T, Sugimoto T, Nakano T,
Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H,
Nishizawa Y, et al: Randomized Teriparatide [human parathyroid
hormone (PTH) 1–34] Once-Weekly Efficacy Research (TOWER) trial for
examining the reduction in new vertebral fractures in subjects with
primary osteoporosis and high fracture risk. J Clin Endocrinol
Metab. 97:3097–3106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jurica K, Brčić Karačonji I, Mikolić A,
Milojković-Opsenica D, Benković V and Kopjar N: In vitro safety
assessment of the strawberry tree (Arbutus unedo L.) water
leaf extract and arbutin in human peripheral blood lymphocytes.
Cytotechnology. 70:1261–1278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schindler G, Patzak U, Brinkhaus B, von
Niecieck A, Wittig J, Krähmer N, Glöckl I and Veit M: Urinary
excretion and metabolism of arbutin after oral administration of
Arctostaphylos uvae ursi extract as film-coated tablets and
aqueous solution in healthy humans. J Clin Pharmacol. 42:920–927.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Genovese C, Davinelli S, Mangano K,
Tempera G, Nicolosi D, Corsello S, Vergalito F, Tartaglia E,
Scapagnini G and Di Marco R: Effects of a new combination of plant
extracts plus d-mannose for the management of uncomplicated
recurrent urinary tract infections. J Chemother. 30:107–114. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee HJ and Kim KW: Anti-inflammatory
effects of arbutin in lipopolysaccharide-stimulated BV2 microglial
cells. Inflamm Res. 61:817–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang L and Wang D, Zhang Y, Li J, Wu Z,
Wang Z and Wang D: Investigation of the pro-apoptotic effects of
arbutin and its acetylated derivative on murine melanoma cells. Int
J Mol Med. 41:1048–1054. 2018.PubMed/NCBI
|
|
31
|
Marie PJ and Kassem M: Osteoblasts in
osteoporosis: Past, emerging, and future anabolic targets. Eur J
Endocrinol. 165:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Canalis E: Management of endocrine
disease: Novel anabolic treatments for osteoporosis. Eur J
Endocrinol. 178:R33–R44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weinreb M, Shinar D and Rodan GA:
Different pattern of alkaline phosphatase, osteopontin, and
osteocalcin expression in developing rat bone visualized by in situ
hybridization. J Bone Miner Res. 5:831–842. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim YJ, Lee MH, Wozney JM, Cho JY and Ryoo
HM: Bone morphogenetic protein-2-induced alkaline phosphatase
expression is stimulated by Dlx5 and repressed by Msx2. J Biol
Chem. 279:50773–50780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kobayashi T and Kronenberg H: Minireview:
Transcriptional regulation in development of bone. Endocrinology.
146:1012–1017. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alford AI, Golicz AZ, Cathey AL and Reddy
AB: Thrombospondin-2 facilitates assembly of a type-I collagen-rich
matrix in marrow stromal cells undergoing osteoblastic
differentiation. Connect Tissue Res. 54:275–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neve A, Corrado A and Cantatore FP:
Osteocalcin: Skeletal and extra-skeletal effects. J Cell Physiol.
228:1149–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci (Landmark Ed). 19:379–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kramer I, Halleux C, Keller H, Pegurri M,
Gooi JH, Weber PB, Feng JQ, Bonewald LF and Kneissel M: Osteocyte
Wnt/beta-catenin signaling is required for normal bone homeostasis.
Mol Cell Biol. 30:3071–3085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ducy P, Schinke T and Karsenty G: The
osteoblast: A sophisticated fibroblast under central surveillance.
Science. 289:1501–1504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Franceschi RT and Xiao G: Regulation of
the osteoblast-specific transcription factor, RUNX2: Responsiveness
to multiple signal transduction pathways. J Cell Biochem.
88:446–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gaur T, Lengner CJ, Hovhannisyan H, Bhat
RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS
and Lian JB: Canonical WNT signaling promotes osteogenesis by
directly stimulating Runx2 gene expression. J Biol Chem.
280:33132–33140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Daoussis D and Andonopoulos AP: The
emerging role of Dickkopf-1 in bone biology: Is it the main switch
controlling bone and joint remodeling? Semin Arthritis Rheum.
41:170–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Taurin S, Sandbo N, Qin Y, Browning D and
Dulin NO: Phosphorylation of beta-catenin by cyclic AMP-dependent
protein kinase. J Biol Chem. 281:9971–9976. 2006. View Article : Google Scholar : PubMed/NCBI
|