Introduction
Laryngeal cancer is one of the most commonly
observed malignancies of the head and neck, and accounts for ~25%
of tumors in this region (1).
Laryngeal cancer accounts for ~1.1% of all newly diagnosed cancer
cases and for nearly 1% of all tumor-associated mortalities
worldwide (2). Among different
subtypes, laryngeal squamous cell carcinoma (LSCC) accounts for
>90% of laryngeal cancer cases, and is more prevalent in
middle-aged and elderly males (3).
Although advances have been achieved in the management of this
tumor, the survival rates of patients with LSCC have remained
unfavorable for the past 30 years (4). Therefore, it is urgent to unveil the
molecular mechanisms underlying the development of LSCC.
Zinc finger E-box binding homeobox 2 (ZEB2), a
transcription factor that belongs to the Zfh1 family, has been
found to be overexpressed in various types of cancer, including
colorectal, prostate and pancreatic cancer (5–7).
Mounting evidence has indicated that ZEB2 is involved in the
initiation and development of malignancies (8). Furthermore, it has been reported that
ZEB2 promotes the epithelial-mesenchymal transition (EMT), a
pathological process leading to specific morphological and
phenotypic alterations during cancer metastasis in tumor cells
(9,10). According to previous studies, ZEB2
was significantly upregulated in LSCC (11,12).
However, the function of ZEB2 in LSCC remains largely unknown.
In the present study, the expression of ZEB2 in LSCC
and adjacent normal tissues was investigated. In addition, the
effect of ZEB2 silencing on the proliferation, migration, invasion,
cell cycle distribution, apoptosis and EMT of LSCC cells was
examined. The results revealed that ZEB2 functioned as an oncogene
in LSCC and that targeting ZEB2 may be a promising therapeutic
target in the management of LSCC.
Patients and methods
Patients and specimens
Between January 2014 and February 2015, laryngeal
cancer tissues (n=46) and the corresponding adjacent non-tumor
tissues (n=46) were collected from laryngeal cancer patients in the
Department of Otorhinolaryngology, Head and Neck Surgery (Lihuili
Hospital, Ningbo, China). Patient characteristics are presented in
Table I. There were 21 female and
25 male patients, aged from 40–79 and the mean age was 59. All
patients were diagnosed with LSCC according to WHO criteria
(13). The research was approved
by the Institutional Review Board of Lihuili Hospital, and written
informed consent was obtained from each patient prior to
surgery.
 | Table I.Clinical characteristics of 46
laryngeal squamous cell carcinoma patients. |
Table I.
Clinical characteristics of 46
laryngeal squamous cell carcinoma patients.
| Specimen no. | Histologic
differentiation | Age (years) | TNM stage |
|---|
| 1 | Poor | 76 | T1N0M0 |
| 2 | Moderate | 53 | T4N0M0 |
| 3 | Moderate | 64 | T1N0M0 |
| 4 | Moderate | 49 | T1N0M0 |
| 5 | Poor | 64 | T4N2M0 |
| 6 | Good | 57 | T3N0M0 |
| 7 | Poor | 57 | T4N0M0 |
| 8 | Moderate | 64 | T1N0M0 |
| 9 | Moderate | 57 | T4N2M0 |
| 10 | Poor | 61 | T4N1M0 |
| 11 | Poor | 56 | T3N2M0 |
| 12 | Good | 70 | T3N0M0 |
| 13 | Good | 51 | T4N1M0 |
| 14 | Moderate | 59 | T4N1M0 |
| 15 | Poor | 68 | T2N1M0 |
| 16 | Poor | 79 | T1N0M0 |
| 17 | Poor | 55 | T4N1M0 |
| 18 | Good | 48 | T4N2M0 |
| 19 | Moderate | 59 | T3N2bM0 |
| 20 | Poor | 67 | T1N0M0 |
| 21 | Poor | 59 | T2N1M0 |
| 22 | Moderate | 64 | T1N0M0 |
| 23 | Moderate | 57 | T1N0M0 |
| 24 | Good | 52 | T1N0M0 |
| 25 | Poor | 48 | T1N0M0 |
| 26 | Poor | 60 | T1N0M0 |
| 27 | Moderate | 54 | T1N0M0 |
| 28 | Good | 40 | T2N0M0 |
| 29 | Good | 45 | T2N0M0 |
| 30 | Moderate | 63 | T3N2bM0 |
| 31 | Poor | 51 | T1N0M0 |
| 32 | Poor | 62 | T2N2MO |
| 33 | Good | 51 | T3N1M0 |
| 34 | Moderate | 59 | T4N1M0 |
| 35 | Good | 63 | T2N2M0 |
| 36 | Moderate | 74 | T3N1M0 |
| 37 | Poor | 52 | T1N0M0 |
| 38 | Poor | 72 | T4N2M0 |
| 39 | Moderate | 63 | T2N0M0 |
| 40 | Moderate | 60 | T1N0M0 |
| 41 | Good | 51 | T4N0M0 |
| 42 | Good | 51 | T3N1M0 |
| 43 | Moderate | 55 | T1N0M0 |
| 44 | Moderate | 54 | T1N0M0 |
| 45 | Poor | 66 | T1N0M0 |
| 46 | Poor | 54 | T1N0M0 |
Cell culture
Human laryngeal cancer AMC-HN8 cells (cat no.
0143321) were obtained from the Shanghai Cell Bank of the Chinese
Academy of Science (Shanghai, China). Cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells or tissues by
TRIzol reagent (Thermo Fisher Scientific, Inc.). The quality and
concentration of RNA was measured by NanoDrop (Thermo Fisher
Scientific, Inc.). RNA samples were reverse transcribed using cDNA
synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. qPCR was then performed
using the SYBR ExScript qRT-PCR kit (Takara Biotechnology Co.,
Ltd., Dalian, China) and conducted on an Applied Biosystems 7500
Real-Time PCR system (Thermo Fisher Scientific, Inc.). Conditions
of the PCR amplification were: 94°C (5 min), then 30 cycles at 94°C
(30 sec), 60°C (45 sec) and 72 C (45 sec) with a final extension at
72 C for 10 min.
The primer pairs used in qPCR were as follows: ZEB2
forward, 5′-AAGGAGCAGGTAATCGCAAG-3′, and reverse,
5′-GGAACCAGAATGGGAGAAACG-3′; GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the
endogenous control. The expression fold changes were calculated
using the 2−ΔΔCq method (14).
Silencing of ZEB2
ZEB2 was silenced in AMC-HN8 cells with two
different small interfering RNAs (siRNAs), which were synthesized
by GeneChem Co., Ltd. (Shanghai, China). The siRNA sequences were
as follows: si-ZEB2-1, 5′-TGAAAACATAGTCCCCAACA-3′; and si-ZEB2-2,
5′-CTGGACAACAAAAGCACT-3′. Briefly, cells were seeded at the density
of 1×106 cells/well in 6-well plates and incubated until
70% confluency was reached. On the following day, the appropriate
amount of siRNA against ZEB2 (si-ZEB2-1 and si-ZEB2-2) or negative
control siRNA (si-NC) were transfected into the cells using
Lipofectamine 3000 reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol.
Cell proliferation assay
Cell viability was evaluated by an MTT assay.
Briefly, cells were seeded in a 96-well plate (5×103
cells/well) and then treated for 24 h with or without siRNAs. A
total of 50 µl MTT solution (5 mg/ml) was added to each well, and
the cells were incubated for 4 h. The medium was then removed, and
200 µl dimethyl sulfoxide (DMSO) was added to each well.
Subsequently, the absorbance of the solutions was measured on a
BioTek microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA) at 595 nm. The relative cell viability (%) was measured by
comparing the absorbance at 595 nm with that of the vehicle
control, which was treated with 0.1% DMSO.
Cell cycle distribution analysis
Cells grown in 6-well plates (1×106
cells/well) were treated with siRNA against ZEB2 or si-NC. The
floating cells were discarded, and attached cells were harvested
and fixed in ice-cold 70% ethanol. Next, the fixed cells were
washed with phosphate-buffered saline (PBS) and resuspended in PBS
containing RNase A (20 µg/ml). The cells were fixed with 70%
ethanol and stained with propidium iodide (PI), and the cell cycle
distribution was evaluated using FACSVerse™ flow cytometer (Beckman
Coulter, Inc., Fullerton, CA, USA). Data were analyzed using FlowJo
version 10 software (FlowJo LLC, OH, USA).
Apoptosis assay
Cells grown in 6-well plates were treated with siRNA
against ZEB2 or si-NC. All the cells were harvested and fixed in
ice-cold 70% ethanol. The fixed cells were then washed with PBS,
resuspended in PBS containing RNase A (20 µg/ml) and stained with
the Annexin V-FITC/PI Apoptosis Detection kit (BD Biosciences,
Franklin Lakes, NJ, USA). Cell apoptosis was evaluated using
FACSVerse™ flow cytometer, and data were analyzed using FlowJo
version 10 software.
Cell migration and invasion
assays
For the migration assays, at 48 h post-transfection,
5×104 cells in serum-free medium were placed into the
upper chamber of a Transwell insert (24-wells plate, 8-µm pore
size; EMD Millipore, Billerica, MA, USA). For the invasion assays,
at 48 h post-transfection, 1×105 cells in serum-free
medium were placed into the upper chamber of a Transwell insert
coated with Matrigel (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Medium containing 10% FBS was added to the lower chamber
for both assays. Subsequent to incubation for 24 h, the cells
remaining on the upper membrane were removed with cotton wool.
Cells that had migrated or invaded through the membrane were
stained with methanol and 0.1% crystal violet, and counted under an
inverted microscope (Olympus Corporation, Tokyo, Japan).
Experiments were independently repeated three times.
Western blot assay
Following transfection, cells were lysed with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China). The concentration of protein
lysates were assayed by the Bradford kit (Beyotime Institute of
Biotechnology, Shanghai, China). The lysates (40 µg) were resolved
by 12% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane. The membrane was blocked with 10% skimmed-milk at room
temperature for 1 h. Subsequently, the membranes were incubated
overnight at 4°C with primary antibodies against the following:
ZEB2 (23312), obtained from Wuhan Sanying Biotechnology (Wuhan,
China); matrix metalloproteinase MMP-2 (ab37150), MMP-9 (ab58803),
E2 promoter binding factor 1 (E2F1) (ab179445) and vimentin
(ab137321), purchased from Abcam (Cambridge, MA, USA); p21 (2947),
cyclin D1 (2922), cyclin-dependent kinase (CDK)4 (12790), CDK6
(3136), caspase-3 (9662), caspase-8 (9746), caspase-9 (9502),
cleaved poly(ADP-ribose) polymerase (PARP) (9541), B-cell lymphoma
2 (Bcl-2) (4223) and Bcl-2-associated X protein (Bax) (2774), all
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA);
E-cadherin (743712) and N-cadherin (746629), from BD Biosciences;
and GAPDH (G5262), obtained from Sigma-Aldrich (Merck KGaA). The
samples were then incubated with the appropriate secondary
antibodies at room temperature for 1 h (Sigma-Aldrich; Merck KGaA).
Signals were visualized using an enhanced chemiluminescence reagent
(Pierce; Thermo Fisher Scientific, Inc.).
Statistical analyses
All the experiments were conducted at least three
times. The data were analyzed using GraphPad Prism version 6.0
software (GraphPad Software, Inc., La Jolla, CA, USA). The results
are presented as the mean ± standard deviation, and the differences
between two groups or multiple groups were measured using Student's
t-test or using analysis of variance followed by Tukey's test,
respectively. P<0.05 was considered to indicate a statistically
significant difference.
Results
ZEB2 is significantly upregulated in
laryngeal cancer tissues
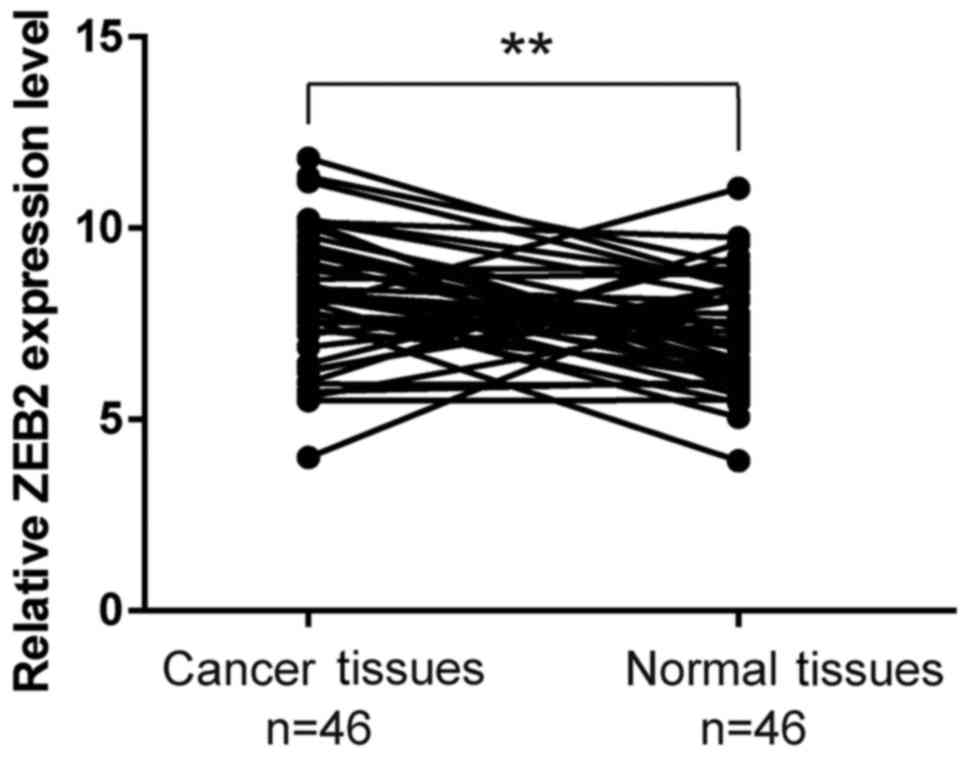
ZEB2 expression levels were investigated using
RT-qPCR assays in 46 laryngeal cancer tissues and adjacent
histologically normal tissues. The results demonstrated that ZEB2
expression was significantly higher in tumor tissues compared with
that in adjacent normal tissues (P<0.01; Fig. 1).
Silencing of ZEB2 represses laryngeal
cancer cell proliferation, migration and invasion in vitro
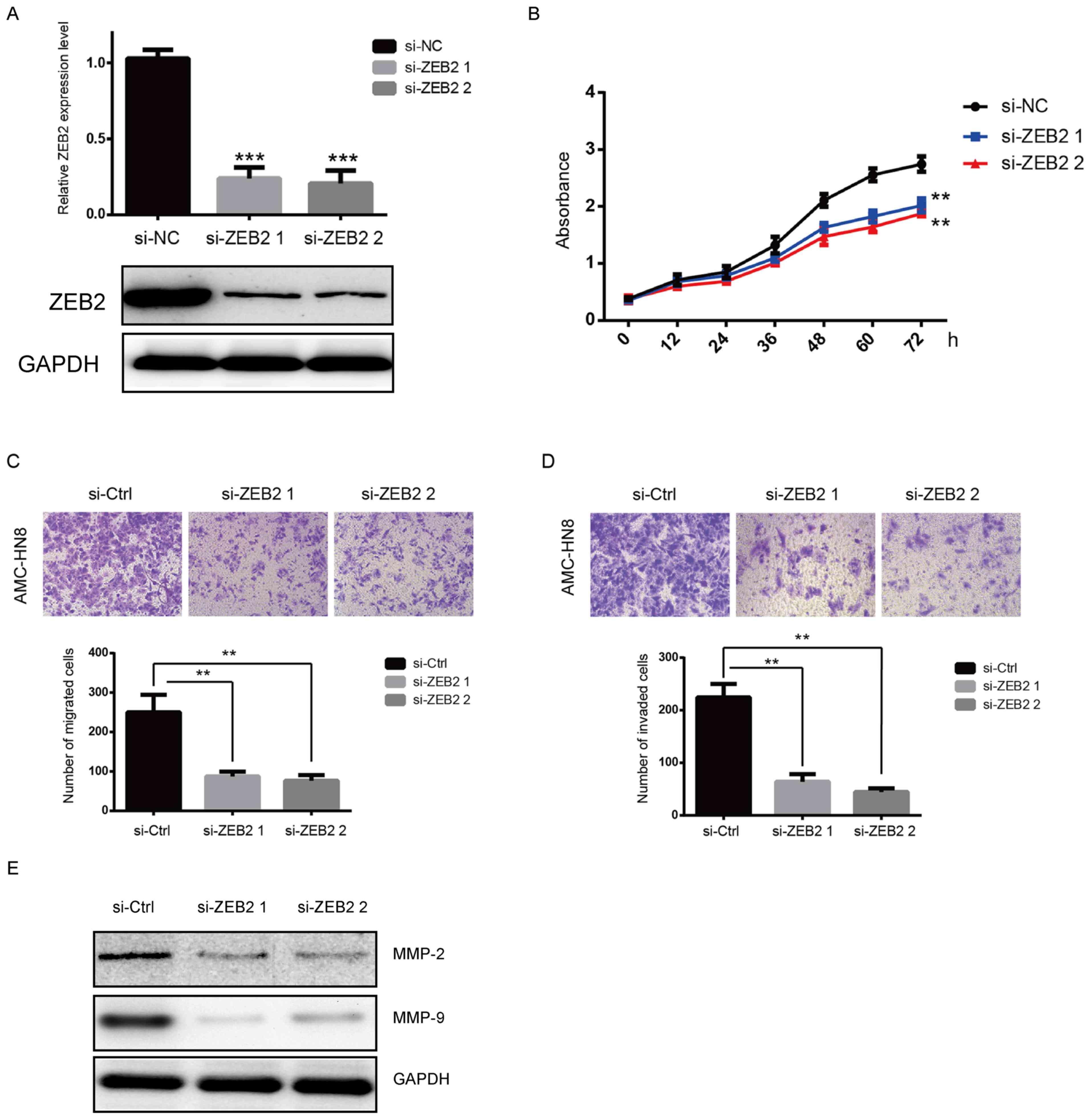
To investigate the potential role of ZEB2 in
laryngeal cancer cell proliferation, two siRNAs against ZEB2 were
transfected into laryngeal cancer AMC-HN8 cells. The RT-qPCR and
western blot analyses confirmed that the expression of ZEB2 was
effectively reduced following transfection (Fig. 2A). The MTT assay demonstrated that
the proliferation of AMC-HN8 cells was significantly suppressed by
ZEB2 downregulation, as compared with that in the si-NC group
(Fig. 2B). The role of ZEB2 in
cell migration and invasion was then further evaluated. As shown in
Fig. 2C and D, ZEB2 silencing
resulted in marked repression of the migration and invasion
abilities of AMC-HN8 cells (P<0.01). It was also observed that
the protein expression levels of MMP-2 and MMP-9 were downregulated
following silencing of ZEB2 (Fig.
2E).
Downregulation of ZEB2 induces cell
cycle arrest in laryngeal cancer cells
Furthermore, the role of ZEB2 in the cell cycle
distribution was investigated in AMC-HN8 cells using flow cytometry
analysis. As shown in Fig. 3A and
B, ZEB2 silencing caused cell cycle arrest at the
G0/G1 phase. The percentage of cells in the
G0/G1 phase significantly increased from
42.7% in the control group to 64.5 and 66.3% in cells with si-ZEB1
and si-ZEB2, respectively (P<0.01). Simultaneously, the
proportion of cells in S phase markedly decreased (Fig. 3B). To examine the molecular
mechanism responsible for the cell cycle arrest in
G0/G1 phase subsequent to ZEB2
downregulation, the expression levels of cell cycle regulatory
proteins were determined. It was observed that the expression
levels of cyclin D1, CDK4, CDK6 and E2F1 proteins were notably
reduced, while p21 levels evidently increased following the
downregulation of ZEB2. All these data suggested that ZEB2
silencing induced a G0/G1 block in laryngeal
cancer cells via altering the expression of key proteins in the
cyclin-CDK/E2F1 signaling pathway.
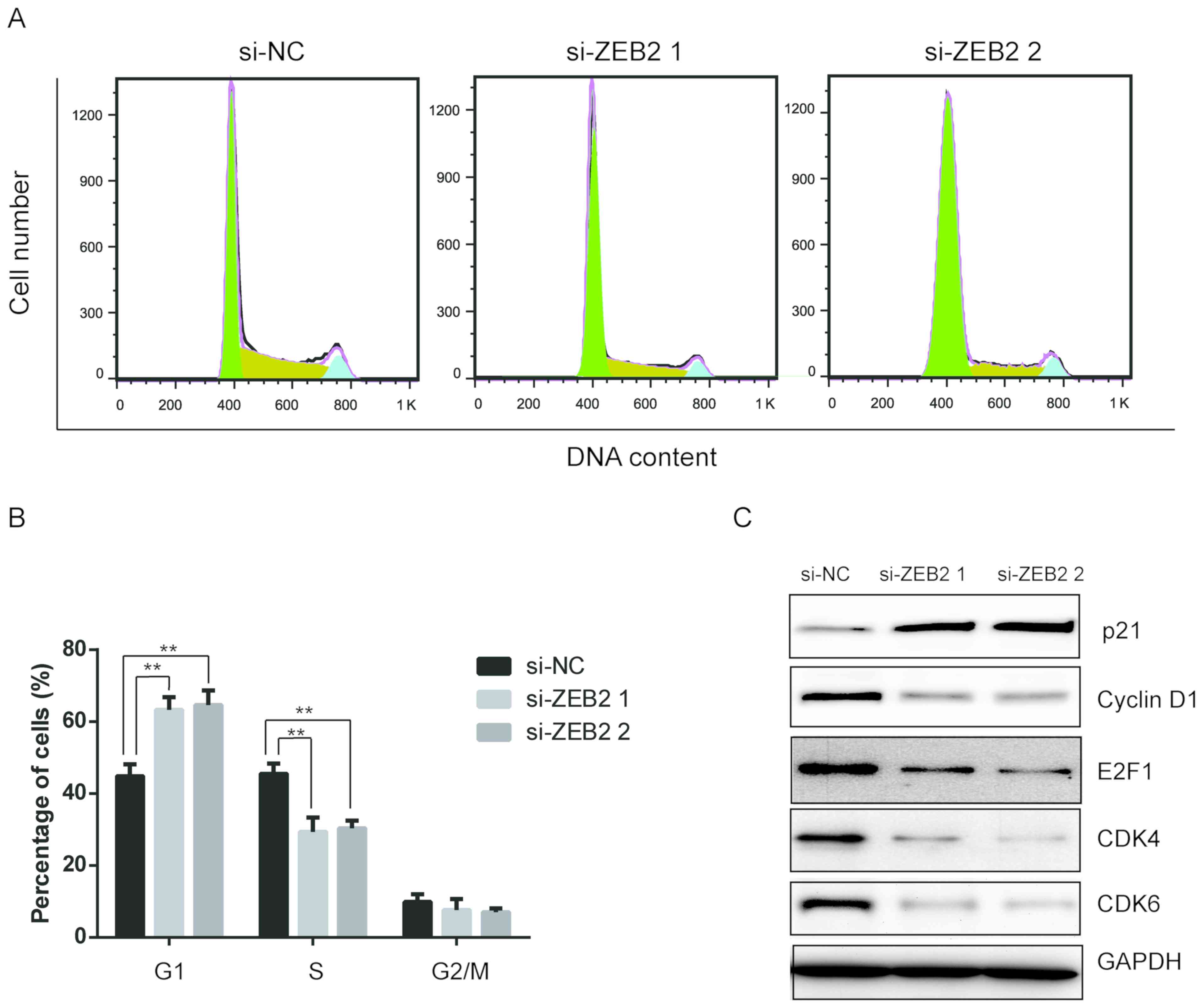 | Figure 3.Downregulation of ZEB2 induced cell
cycle arrest in laryngeal cancer cells. AMC-HN8 cells were
transfected with si-NC or si-ZEB2 for 48 h, and then cells were
collected. (A) Cell cycle distribution was analyzed using propidium
iodide staining and flow cytometry. (B) Quantitative analysis of
cell cycle distribution. (C) Expression of cell cycle-associated
proteins, including p21, cyclin D1, E2F1, CDK4 and CDK6, were
determined by western blot assay. **P<0.01. ZEB2, zinc finger
E-box binding homeobox 2; si-, small interfering RNA; NC, negative
control; E2F1, E2 promoter binding factor 1; CDK, cyclin-dependent
kinase. |
Downregulation of ZEB2 causes
apoptosis in laryngeal cancer cells via the intrinsic pathway
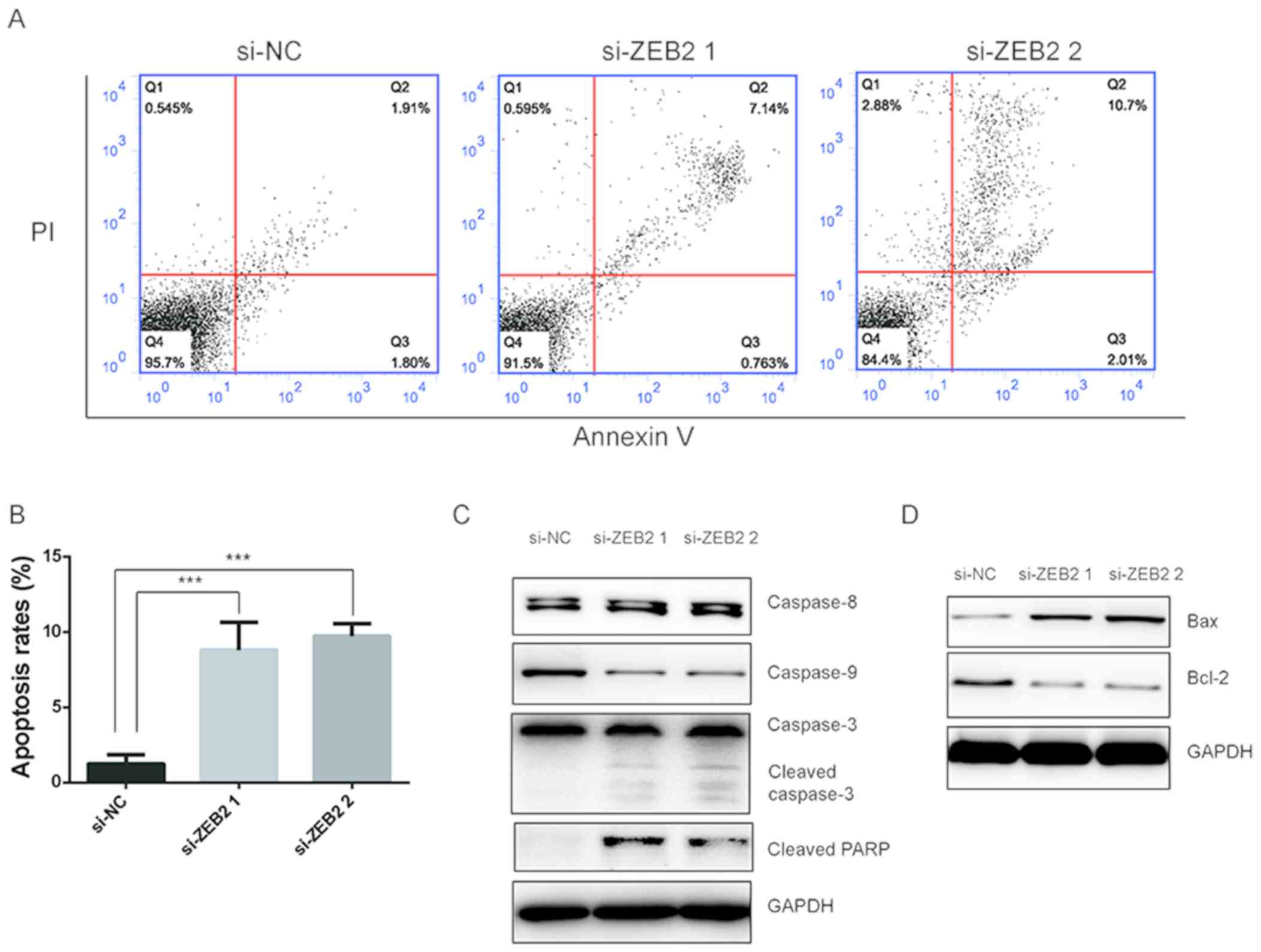
The study further investigated whether ZEB2
downregulation affected cell apoptosis using an Annexin V-FITC/PI
apoptosis detection kit. As shown in Fig. 4A and B, flow cytometry analysis
revealed that ZEB2 silencing significantly promoted the apoptosis
of AMC-HN8 cells. Cell apoptosis mainly proceeds through two
pathways, including the extrinsic pathway, which is initiated by
caspase-8, and the intrinsic pathway, which is initiated by
caspase-9 (15). To further
explore the potential role of ZEB2 in apoptosis, western blot
analysis was performed to detect the levels of associated proteins.
Upon silencing of ZEB2, downregulation of caspase-9 and cleavage of
caspase-3 were observed, and cleavage of PARP levels were evidently
increased, while the level of caspase-8 was not affected (Fig. 4C). Furthermore, the protein
expression levels of Bcl-2 and Bax, which are key proteins that
regulate the intrinsic pathway, were respectively downregulated and
upregulated following ZEB2 downregulation. These data implied that
the intrinsic pathway was activated following the silencing of
ZEB2.
ZEB2 affects the levels of E-cadherin
proteins
The EMT has been reported to be involved in cancer
invasion and metastasis (16).
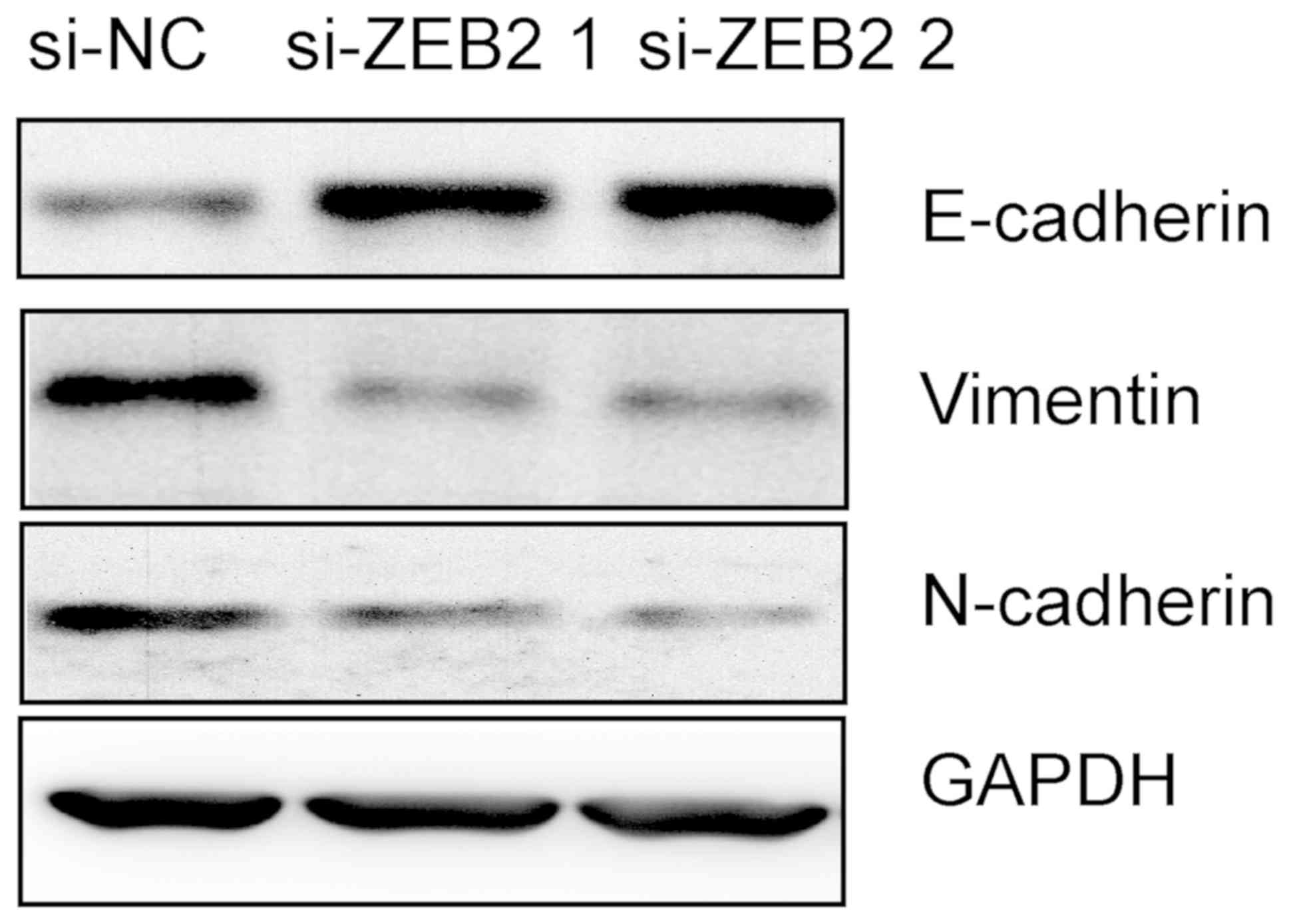
Therefore, the present study examined whether ZEB2 was able to
affect the process of EMT in AMC-HN8 cells. Western blot analysis
was performed to detect the expression levels of EMT markers,
including E-cadherin, N-cadherin and vimentin. As indicated in
Fig. 5, downregulation of ZEB2
notably induced E-cadherin expression, but repressed the expression
of N-cadherin and vimentin proteins. These results indicated that
silencing of ZEB2 inhibited the process of EMT in AMC-HN8
cells.
Discussion
Although several therapeutic approaches have been
developed, the overall 5-year survival rates for patients with LSCC
remain unsatisfactory, largely due to metastasis and recurrence
(3). A better understanding of the
molecular mechanisms underlying the proliferation, differentiation
and progression of LSCC is vital for the development of efficient
therapeutic strategies. In the present study, the role of ZEB2 in
LSCC was investigated, and the data provide a mechanistic basis for
targeting ZEB2 in patients with LSCC.
ZEB2, a transcription factor that belongs to the ZEB
family, has been reported to be involved in the development of
various human malignancies. Knockdown of its expression may repress
the proliferation and progression of these tumors. For instance, a
recent study revealed that ZEB2 promoted tumor metastasis and was
correlated with poor prognosis in human colorectal cancer (17). In hepatocellular carcinoma, ZEB2
was reported to promote tumor growth and metastasis (18). In gastric cancer, ZEB2 may affect
the cellular response to cisplatin (19). However, few studies have examined
the roles of ZEB2 in LSCC. The present study demonstrated that ZEB2
was highly expressed in LSCC tissues as compared with adjacent
normal tissues. It was also observed that silencing of ZEB2
effectively reduced the proliferation, migration and invasion of
LSCC cells.
Mounting evidence has revealed the vital role of the
MMP family in cancer metastasis (20). Among the MMP family members, MMP-2
and MMP-9 stand out for their ability to degrade collagen IV, which
is the major extracellular component of the basement membrane
(21). The overexpression of
MMP-2/-9 is associated with an aggressive malignant phenotype and
adverse prognosis in patients with cancer (22). In the current study, ZEB2 silencing
resulted in decreased MMP-2/-9 levels, which provided an insight
into the investigation of ZEB2 on LSCC metastasis. These findings
were similar to those of previous studies, which indicated that
high ZEB2 expression levels may be positively associated with worse
tumor biological features (17,18).
The present study also demonstrated an increase in
the percentage of LSCC cells at the G1 phase following ZEB2
silencing. Cell cycle progression is regulated by a cyclin kinase
inhibitor and the cell cycle regulators p21 and p27, which are
upregulated during cell cycle arrest (23). CDKs are activated via binding to
cyclin complexes, and their activity can be repressed by cyclin
kinase inhibitors (24). When cell
cycle arrest occurs, CDKs and cyclin complexes can be repressed by
the expression of CDK inhibitors (24). The data reported in the present
study revealed that downregulation of ZEB2 led to upregulation of
p21 and downregulation of cyclin D1, E2F1, CDK4 and CDK6. Taken
together, these findings are in line with a previous study, which
also reported that downregulation of ZEB2 causes cell cycle arrest
at the G1 phase in glioma cells (25).
Silencing of ZEB2 also resulted in a significant
increase in the apoptosis in LSCC cells. There are mainly two
pathways that lead to apoptosis, namely the extrinsic and intrinsic
pathways, which are initiated by caspase-8 and caspase-9,
respectively (26). During
apoptosis, activation of caspase-8 and caspase-9 can both lead to
the activation of caspase-3. Following ZEB2 silencing, caspase-9
and caspase-3, but not caspase-8, were activated. Furthermore, the
intrinsic apoptotic pathway is known to be regulated by the Bcl-2
family members (27). In the
current study, the pro-apoptotic Bax was upregulated and the
anti-apoptotic Bcl-2 protein was downregulated following ZEB2
silencing. These findings indicated that ZEB2 affects apoptosis via
the intrinsic apoptotic pathway in LSCC cells. Notably, although
apoptosis is observed, few cells were detected at the sub-G1 phase.
This discrepancy may be due to technical reasons, since the
floating cells were discarded when the cell cycle distribution was
analyzed, whereas all cells were collected in order to measure the
apoptosis.
EMT is a biological process where epithelial cells
lose their polarity and undergo transition into a mesenchymal
phenotype (28). The occurrence of
EMT is accompanied by downregulation of the epithelial marker
protein E-cadherin, whereas mesenchymal marker proteins, such as
N-cadherin and vimentin, are upregulated. Accumulating evidence
indicates that ZEB2 can trigger EMT, thereby increasing cancer
metastasis (8,29,30).
Therefore, the present study investigated whether ZEB2 was able to
affect the EMT in LSCC cells. The results revealed higher
expression levels of E-cadherin, and lower expression levels of
N-cadherin and vimentin in the cells with downregulation of ZEB2.
These findings indicated that ZEB2 promoted the EMT in LSCC
cells.
In conclusion, the current study investigated the
level of ZEB2 mRNA expression in LSCC tissues and found it was
upregulated compared to the normal tissues. Then we investigated
the function of ZEB2 in vitro. Downregulation of ZEB2
inhibited the proliferation, migration, invasion, cell cycle
progression, apoptosis and EMT of LSCC cells. Taken together, the
results provide new evidence that ZEB2 may represent a novel
therapeutic target for the treatment of LSCC. However, the
limitations of the present study included the fact that no studies
have yet been conducted in animal models to confirm the in
vitro findings. Other limitations include the failure to
demonstrate the specific mechanism by which ZEB2 affects the
processes described herein; therefore, further research is
required.
Acknowledgements
Not applicable
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31501113 for R Yu),
Ningbo Natural Science Foundation to (grant no. 2015A610221 for Q
Li, and grant no. 2015A610177 for R Yu), and Ningbo Health Branding
Subject Fund (grant no. PPXK2018-02 for Q Li, ZS Shen).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and LM performed the experiments. ZW, GW and QH
analyzed the data. ZS and RY designed the experiments and drafted
the manuscript.
Ethics approval and consent to
participate
This project was approved by the Ethics Committee at
the Ningbo University, and all patients gave informed consent.
Patients' consent for publication
All patients agreed to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agra IM, Ferlito A, Takes RP, Silver CE,
Olsen KD, Stoeckli SJ, Strojan P, Rodrigo JP, Gonçalves Filho J,
Genden EM, et al: Diagnosis and treatment of recurrent laryngeal
cancer following initial nonsurgical therapy. Head Neck.
34:727–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kahlert C, Lahes S, Radhakrishnan P, Dutta
S, Mogler C, Herpel E, Brand K, Steinert G, Schneider M,
Mollenhauer M, et al: Overexpression of ZEB2 at the invasion front
of colorectal cancer is an independent prognostic marker and
regulates tumor invasion in vitro. Clin Cancer Res. 17:7654–7663.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanrahan K, O'Neill A, Prencipe M, Bugler
J, Murphy L, Fabre A, Puhr M, Clig Z, Murphy K and Watson RW: The
role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in
mediating docetaxel-resistant prostate cancer. Mol Oncol.
11:251–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galvan JA, Zlobec I, Wartenberg M, Lugli
A, Gloor B, Perren A and Karamitopoulou E: Expression of E-cadherin
repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells
influences tumour-budding phenotype and suggests heterogeneity of
stromal cells in pancreatic cancer. Br J Cancer. 112:1944–1950.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brabletz S and Brabletz T: The ZEB/miR-200
feedback loop-A motor of cellular plasticity in development and
cancer? EMBO Rep. 11:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Riedt T, Goossens S, Carrillo García
C, Szczepanski S, Brandes M, Pieters T, Dobrosch L, Gütgemann I,
Farla N, et al: The EMT transcription factor Zeb2 controls adult
murine hematopoietic differentiation by regulating cytokine
signaling. Blood. 129:460–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sugimoto M, Kohashi K, Itsumi M, Shiota M,
Abe T, Yamada Y, Kuroiwa K, Naito S and Oda Y: Epithelial to
mesenchymal transition in clear cell renal cell carcinoma with
rhabdoid features. Pathobiology. 83:277–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cappellesso R, Marioni G, Crescenzi M,
Guzzardo V, Mussato A, Staffieri A, Martini A, Blandamura S and
Fassina A: The prognostic role of the epithelial-mesenchymal
transition markers E-cadherin and slug in laryngeal squamous cell
carcinoma. Histopathology. 67:491–500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao S, Wang J, Xie J, Zhang T and Dong P:
Role of miR-138 in the regulation of larynx carcinoma cell
metastases. Tumour Biol. 2015.
|
|
13
|
Chu EA and Kim YJ: Laryngeal cancer:
Diagnosis and preoperative work-up. Otolaryngol Clin North Am.
41:673–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashkenazi A and Salvesen G: Regulated cell
death: Signaling and mechanisms. Annu Rev Cell Dev Biol.
30:337–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weidenfeld K and Barkan D: EMT and
stemness in tumor dormancy and outgrowth: Are they intertwined
processes. Front Oncol. 8:3812018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li MZ, Wang JJ, Yang SB, Li WF, Xiao LB,
He YL and Song XM: ZEB2 promotes tumor metastasis and correlates
with poor prognosis of human colorectal cancer. Am J Transl Res.
9:2838–2851. 2017.PubMed/NCBI
|
|
18
|
Lan T, Chang L, Wu L and Yuan Y:
Downregulation of ZEB2-AS1 decreased tumor growth and metastasis in
hepatocellular carcinoma. Mol Med Rep. 14:4606–4612. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geng DM, Kan XM and Zhang WW: Effect of
ZEB2 silencing on cisplatin resistance in gastric cancer. Eur Rev
Med Pharmacol Sci. 21:1746–1752. 2017.PubMed/NCBI
|
|
20
|
Shay G, Lynch CC and Fingleton B: Moving
targets: Emerging roles for MMPs in cancer progression and
metastasis. Matrix Biol. 44-46:200–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaudhary AK, Pandya S, Ghosh K and
Nadkarni A: Matrix metalloproteinase and its drug targets therapy
in solid and hematological malignancies: An overview. Mutat Res.
753:7–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh CY, Tsai PC, Chu CL, Chang FR, Chang
LS, Wu YC and Lin SR: Brazilein suppresses migration and invasion
of MDA-MB-231 breast cancer cells. Chem Biol Interact. 204:105–115.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bieging KT, Mello SS and Attardi LD:
Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev
Cancer. 14:359–370. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi S, Song Y, Peng Y, Wang H, Long H, Yu
X, Li Z, Fang L, Wu A, Luo W, et al: ZEB2 mediates multiple
pathways regulating cell proliferation, migration, invasion, and
apoptosis in glioma. PLoS One. 7:e388422012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu R, Yu BX, Chen JF, Lv XY, Yan ZJ, Cheng
Y and Ma Q: Anti-tumor effects of Atractylenolide I on bladder
cancer cells. J Exp Clin Cancer Res. 35:402016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu W, Yang Y, Deng G, Ma L, Wei G, Zheng
G, Han X, He D, Zhao Y, He J, et al: Vernodalol enhances
TRAIL-induced apoptosis in diffuse large B-cell lymphoma cells. Mol
Carcinog. 56:2190–2199. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh M, Yelle N, Venugopal C and Singh
SK: EMT: Mechanisms and therapeutic implications. Pharmacol Ther.
182:80–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vandewalle C, Comijn J, De Craene B,
Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F and
Berx G: SIP1/ZEB2 induces EMT by repressing genes of different
epithelial cell-cell junctions. Nucleic Acids Res. 33:6566–6578.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prislei S, Martinelli E, Zannoni GF,
Petrillo M, Filippetti F, Mariani M, Mozzetti S, Raspaglio G,
Scambia G and Ferlini C: Role and prognostic significance of the
epithelial-mesenchymal transition factor ZEB2 in ovarian cancer.
Oncotarget. 6:18966–18979. 2015. View Article : Google Scholar : PubMed/NCBI
|